Abstract
The application of polar pesticides in agricultural production has been of great interest due to their low costs and their high effectiveness. For this reason, the possibility of their transfer to foods of animal origin is of great concern for human health. The manuscript describes the implementation and validation of an analytical method to detect polar pesticides, at regulatory levels, in three foods of animal origin, including bovine fat, chicken eggs, and cow milk. The method was fully validated to detect glyphosate, glufosinate, and their respective metabolites in the above-mentioned foods obtaining fit-for-purpose sensitivity, recoveries (76–119%), repeatability (≤20%), within-laboratory reproducibility (≤20%), and experimental measurement uncertainty less than 50% as required by the SANTE/11312/2021 criteria. Given the satisfactory results, the applicability of the method to additional molecules belonging to the same category (AMPA, cyanuric acid, ethephon, fosetyl aluminum, HEPA, maleic hydrazide, and N-acetyl-glyphosate) was also evaluated in order to meet possible future requests. Finally, the implemented method was applied to analyse samples over the period of March 2021 to August 2022 from two Italian regions (Umbria and Marche) within the national monitoring programme. In agreement with previously available data, none of the samples analysed showed the presence of glyphosate and glufosinate at levels above the legal limit.
1. Introduction
Pest control in intensive agriculture involves treatments with a variety of synthetic chemicals generically known as pesticides. These chemicals can be transferred from plants to animals via the food chain. Consequently, both contamination routes lead to bio-accumulation of pesticides in food products of animal origin such as meat, fish, fat, offal, eggs, and milk [1]. Currently, there are more than a thousand pesticides, belonging to different classes such as herbicides, fungicides, insecticides, and bactericides, and approximately 2 million tons of these products are used every year around the world [2]. Within this context, the application of polar pesticides in agricultural production has been of great interest due to their low costs and their high effectiveness.
The most known pesticide from the so-called “highly polar pesticides” class is glyphosate. Glyphosate was introduced under the industrial name of Roundup® in 1974 [3]. In agriculture, glyphosate is used extensively on crops both in the field and during the storage of cereals to reduce water content, but it is also commonly used around homes in yards, gardens, and other non-agricultural areas [4]. Microbial degradation processes in soil and water lead to aminomethyl phosphonic acid (AMPA). Glyphosate is inactivated by converting it to CO2, mainly determined by microbial processes in soil and water, and has been deeply investigated by laboratory experiments [5,6]. Glyphosate is inactivated by converting it to N-acetyl glyphosate and further N-acetyl AMPA and AMPA [7].
Besides glyphosate and its main metabolite, AMPA, other polar pesticides deserve some attention due to their widespread use.
Glufosinate (2-amino-4-(hydroxy(methyl)phosphoryl) butanoic acid) is being increasingly used to combat the increasing number of glyphosate-resistant weeds, working as an alternative herbicide to glyphosate [8]. The degradation products of glufosinate, N-acetyl glufosinate (NAG) [9] and 3-methylphosphinicopropionic acid (MPP), have similar biological and toxicological effects to the parent compound [10].
Cyanuric acid is used in disinfectants, sanitizers and bleaches, and as a chlorine stabilizer [11,12]. Ethephon (2-chloroethylphosphonic acid), an organophosphorus systemic growth regulator, is used for accelerating and controlling maturation processes of fruits and vegetables during storage and transport [13]. The study of ethephon metabolism in animals shows that it is converted to ethylene and 2-hydroxyethyl phosphonic acid (HEPA). Ethephon and HEPA residues were found in the liver and kidneys of animals [14]. Fosetyl aluminum (fosetyl—Al) is a systemic fungicide that is widely used in different crops and degrades into phosphonic acid [15]. Finally, maleic hydrazide, a plant growth regulator with some herbicidal activity, is applied to control vine growth, thereby improving grape quality [16].
In Europe, all foodstuffs intended for human consumption are subject to a maximum residue level (MRL) of pesticides in order to protect human health [17]. In Table 1, the MRLs for the pesticides belonging to the “polar class” considered in this study are summarized.

Table 1.
MRL (mg/kg) of studied pesticides in bovine fat, chicken eggs, cow milk.
For many of these polar pesticides, the MRL definition includes the presence of metabolites. Pesticide metabolites are scientifically relevant because these substances may still possess the intrinsic properties and side effects of the parent compound. For instance, the currently applicable MRL for glufosinate ammonium also includes its salts and metabolites. In the case of glyphosate, a review of MRLs was recently completed by the European Food Safety Authority (EFSA), the European authority responsible for the risk assessment associated with the consumption of this substance. Two different definitions for animal commodities were agreed upon as the basis for the MRL review: the sum of glyphosate, AMPA, and N-acetyl glyphosate for monitoring; the sum of glyphosate, AMPA, and N-acetyl glyphosate, and N-acetyl AMPA expressed as glyphosate for risk assessment. However, the lack of information about the presence of glyphosate and its metabolites in products of animal origin causes the MRLs to still be considered as tentative [18].
To fill this gap, the European Food Safety Authority (EFSA) underlines the need for confirmatory methods for glyphosate, AMPA, and N-acetyl glyphosate in fat, liver, and kidneys, as well as a confirmatory method for AMPA and N-acetyl glyphosate in all matrices [18].
In this context, multiannual control programmes for pesticide residues (MACP) are carried out by all Member States to guarantee compliance with maximum residue levels (MRLs) of pesticides and to evaluate the consumer exposure to pesticide residues in foods of plant and animal origin [19]. The Regulation (EU) 2021/601 for the current multi-year control plan (2022–2024) requires the analysis of glyphosate and glufosinate ammonium in the following foods of animal origin: cow milk and swine fat (2022), poultry fat and bovine liver (2023), and bovine fat and chicken eggs (2024). Moreover, monitoring analyses for the presence of other polar pesticides (ethephon, fosetyl aluminum) in foods of plant origin are required.
Reliable methods to enable enforcement of the regulations are therefore highly desirable; however, only a few are currently available and not proven to be applicable to foods of animal origin. A method for the detection of 11 polar pesticides in several matrices of vegetable origin (honey, grapes, and wheat) was reported by Gasparini et al. [20]. A method based on liquid chromatography–high resolution tandem mass spectrometry (LC–HRMS/MS) was developed for the determination of glyphosate in samples of gastric content in the Iberian hare (Lepus granatensis) [21]. An alternative procedure, based on electroanalytical methods [22,23] for the determination of glyphosate in different foods and environmental matrices, was also investigated, but no data on animal samples were reported.
An updated overview of the analytical methodologies available for the determination of polar pesticides in foods of animal origin was presented by Verdini et al. [24]. The review underlines the need for dedicated methods, commonly called single residue methods, covering only one or a limited number of pesticides with similar chemical characteristics.
Single residue methods for polar pesticides typically use mass spectrometry as a detection technique because these pesticides do not have chromophore or fluorophore groups. Fluorescence or photometric detectors can be used only upon derivatization procedures, which often involve long and complex steps [25,26,27].
Most methods require long and complex purification procedures to minimize matrix effects [24]. To properly compensate for any matrix effects, different approaches may be used, including the use of matrix-matched calibrants such as a standard addition to the blank matrix at the beginning of the sample preparation process or at the end of it, before instrumental injection. Alternatively, “isotopic dilution” (i.e., the addition of isotopically labelled internal standards) can be used for the same purpose.
Another issue in the analysis of polar pesticides is the lack of satisfactory retentive efficiency shown by the typical chromatographic columns used for multiresidue pesticide analyses (C18 or C8) [28]. Therefore, specific chromatography columns (anion exchange, hydrophilic interaction liquid chromatography (HILIC), porous carbon graphite (PGC), and mixed mode columns) must be used [24,29,30].
All the above-mentioned issues make the analysis of polar pesticides, especially in foods of animal origin, not very common in control laboratories.
To the best of our knowledge, only a few methods have been reported for the detection of glyphosate and of glufosinate ammonium and its metabolites (NAG, MPP) in foods of animal origin to be applied in the multi-year monitoring plans.
The manuscript describes the implementation and validation of a method to detect polar pesticides at regulatory levels in foods of animal origin. The applicability of the method to additional polar pesticides not yet included in the monitoring plan (AMPA, cyanuric acid, ethephon, fosetyl aluminum, HEPA, maleic hydrazide, and N-Acetyl Glyphosate) was also evaluated in order to cope with possible future inclusion in EC monitoring programmes. Finally, the implemented method was applied to analyse samples over the period of March 2021 to August 2022 within the national monitoring programme, from two Italian regions (Umbria and Marche).
2. Materials and Methods
2.1. Chemicals and Reagents
Reference standard solutions of AMPA, ethephon, glufosinate-ammonium, glyphosate, HEPA, maleic hydrazide, MPP, N-acetyl glyphosate in water:acetonitrile (9:1 v/v) (1000 ug/mL); fosetyl aluminum, NAG in water:acetonitrile (9:1 v/v) (100 ug/mL); isotopically labelled internal standard (ILIS) solutions of AMPA 13C 15N, cyanuric acid 3C3, ethephon D4, glufosinate D3, HEPA D4, maleic hydrazide D2, MPP D3 in water:acetonitrile (9:1 v/v) (1000 ug/mL); and reference materials of cyanuric acid, fosetyl aluminum D15 and N-Acetyl-Glyphosate-13C2,15N as a pure solid were purchased from Lab Instruments Srl (Castellana Grotte, Italy). ILIS solution of glyphosate 2-13C, 15N in water was supplied from Cambridge Isotope Laboratories, Inc. (Tewksbury, MA, USA).
Ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA) was obtained from Merck (Darmstadt, Germany) and Polygoprep™ 300-30 C18 from Macherey-Nagel GmbH & Co. KG (Düren, Germany). Methanol (MeOH) and Acetonitrile (ACN) were obtained from Carlo Erba Reagents Srl (Milan, Italy). All solvents used were of LC–MS or analytical grade. Unless otherwise specified, water purified by a Milli-Q system (Millipore, Merck KgaA, Darmstadt, Germany) was used for sample preparation and analysis.
2.2. Samples
Forty-five samples (8 samples of chicken eggs, 31 of fat, and 6 of cow milk) were collected over the period of March 2021 to August 2022 within the national monitoring programme, from two Italian regions (Umbria, Marche), and analysed by the Istituto Zooprofilattico Sperimentale of Umbria and Marche “Togo Rosati”. All samples (except for milk) were ground by a knife mill (GRINDOMIX GM 300, Restek, Haan, Germany) with dry ice. The samples were stored at −20 °C until analysis.
2.3. Reference Materials and Working Solutions
Stock solutions of cyanuric acid, N-Acetyl-Glyphosate-13C2,15N, and fosetyl-Al-D15 were prepared by dissolving the commercial crystalline pesticides in water:acetonitrile (9:1 v/v) at 1000 µg/mL and stored in plastic tube vials at 4 °C. Four working solutions (WS) were obtained by making appropriate dilutions of the stock solutions and the reference solution with acetonitrile at the following concentrations: (1) WS1: 5 µg/mL AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, Maleic hydrazide, and N-acetyl- glyphosate; (2) WS2: 1 µg/mL glufosinate ammonium, MPP, NAG; (3) WS3: 2 µg/mL glufosinate ammonium, MPP, and 0.5 µg/mL NAG; (4) WS4: 0.2 µg/mL AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, Maleic hydrazide, N-acetyl- glyphosate, and 0.04 µg/mL glufosinate ammonium, MPP, and NAG; (5) WS5: 0.05 µg/mL cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, Maleic hydrazide, N-acetyl- glyphosate; and 0.01 µg/mL glufosinate ammonium, MPP, and NAG. Mixed internal standard (ILIS) solution for spiking (WSIS1) was prepared by mixing the commercial individual ILIS stock solutions to obtain a mixture of 5 µg/mL AMPA-13C,15N cyanuric acid 3C3, ethephon D4, fosetyl al D15, glyphosate 2-13C,15N, HEPA D4, N-Acetyl-glyphosate 13C2,15N and maleic hydrazide D2, 1 µg/mL glufosinate ammonium D3, MPP D3, and NAG D3. WSIS1 was diluted (1:33.3 v/v) to prepare the WSIS2 used for the matrix-matched calibration curve.
Matrix-matched calibration solutions, including isotopically labelled internal standards, (five points for chicken eggs and six points for cow milk and bovine fat) were prepared by mixing appropriate volumes of WSIS2, WS4, and WS5 solutions, and water and blank sample extract (purified according to the clean-up procedure described in Section 2.4), as described in Table S1. The final volume of each calibrant solution was 500 μL.
2.4. Sample Preparation
2.4.1. Bovine Fat
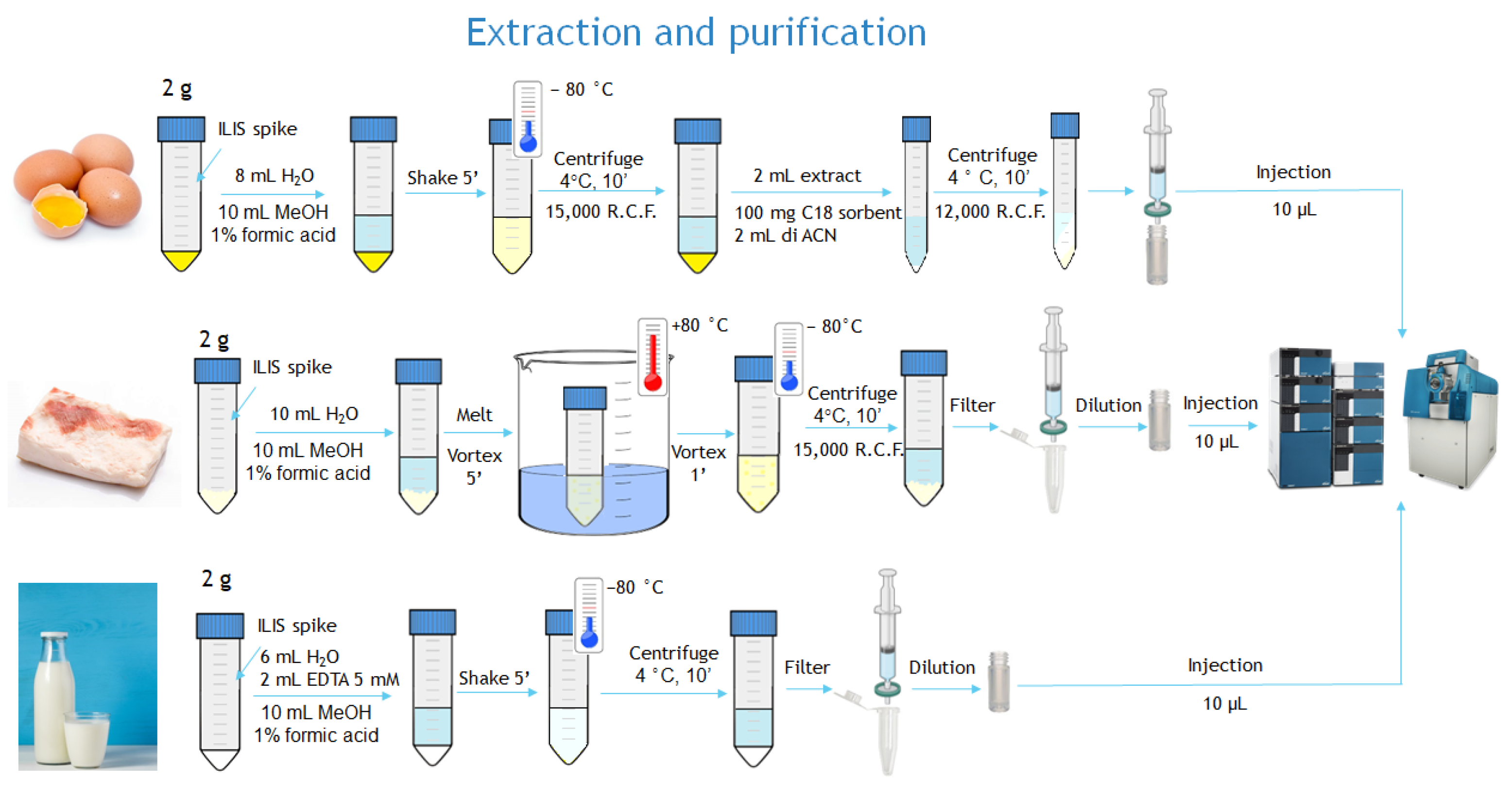
Two grams of sample were weighed in a 50 mL plastic vessel. The extraction was carried out by adding 10 mL of water and 10 mL of MeOH 1% formic acid (v/v) placing the samples in a water bath at 80 °C until the fat was completely dissolved. The samples were vortexed for 2 min and placed in the freezer at −80 °C for 15 min. After 10 min of centrifugation (15,000 g, 4 °C), the samples were filtered with PTFE filters, and then 0.25 mL of the filtered extract were added with 0.25 mL of water directly into plastic vials. Ten microliters (equivalent to 0.5 mg of sample matrix) were injected into the UPLC–QTOF system.
2.4.2. Chicken Eggs
Two grams of sample were weighed in a 50 mL plastic vessel and 8 mL water were added. After vortexing the samples for 30 s, 10 mL of MeOH with 1% of formic acid water were added. The samples were mechanically shaken for 5 min and placed in the freezer at −80 °C for 15 min. After 10 min of centrifugation (15,000 g, 4 °C), 2 mL of extract were collected and placed in a centrifuge tube containing 100 mg of C18 sorbent and 2 mL of acetonitrile. Finally, the samples were vortexed for one minute and centrifuged for 10 min (12,000 g, 0 °C). The final extract was filtered with PTFE filters. Ten microliters (equivalent to 0.5 mg of sample matrix) were injected into the UPLC–QTOF system.
2.4.3. Cow Milk
Two grams of cow milk sample were extracted with 6 mL of water, 2 mL of EDTA solution (5 mM), and 10 mL of MeOH 1% formic acid (v/v) on an orbital shaker for 5 min. Subsequently, the samples were placed in a freezer at -80 ° C for 15 min and centrifuged for 10 min (15,000 g, 4 °C). The supernatant was then filtered with PTFE filters (13 mm, 0.2 µm). A quantity of 0.25 mL of the filtered extract was added with 0.25 mL of water directly into plastic vials. Ten microliters (equivalent to 0.5 mg sample) were injected into the UPLC–QTOF system.
The three sample preparation procedures are represented in Figure 1.
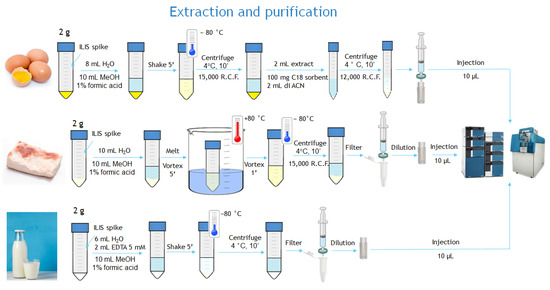
Figure 1.
Schematic of sample preparation procedures.
2.4.4. Recovery Experiments
For recovery experiments, carried out at 2 spiking levels, individual sub-samples (2 g) were spiked with the following volume of working solution: 0.02 mL of WS1 and 0.05 mL of WS2 for bovine fat; 0.02 mL of WS1 and 0.02 mL of WS2 for chicken eggs; and 0.02 mL of WS1 and 0.02 mL of WS3 for cow milk.
The mass concentrations were calculated according to the following equation:
- RF was the response factor (the peak area ratio of the relevant analyte and the corresponding internal standard in the sample test solution or the peak area of the relevant analyte (only for maleic hydrazide in the eggs and in the milk matrix);
- a was the slope of the calibration curve from calibration data, in µg − 1;
- b was the intercept of the calibration curve from calibration data;
- DF was the dilution factor of the method (20).
2.5. LC–QTOF Analysis
The LC–QTOF analysis was performed on a Triple TOF 6600 + system equipped with an electrospray ionization (ESI) interface and a UPLC Exion LC system comprising a binary pump, a degassing unit, and an auto-sampler, all from AbSCIEX (Foster City, CA, USA).
The LC column was a Hypercarb column (100 × 2.1 mm; 5 μm) equipped with a Hypercarb drop-in guard (2.1 × 10 mm; 5 µm), both by Thermo Scientific™ (Waltham, MA, USA). The column oven was set at 40 °C. The injection volume was 10 μL. The flow rate of the mobile phase was set at 200 μL/min for the first 10 min; then, it was increased to 400 μL/min throughout the gradient. Eluent A was water + 5% MeOH; eluent B was MeOH; both containing 1% acetic acid. The following gradient was used: the proportion of eluent B was increased from 0% to 30% in 10 min and kept constant for 8 min; then, it was linearly increased to 90% in 1 min and held for 3 min. For column re-equilibration, eluent B was decreased to 0% in 0.1 min and kept constant for 8 min at 200 μL/min.
The ESI (electrospray ionization) interface was used in negative ion mode, with the following settings: curtain gas at 30 psi, ion spray voltage at −4500 volts; ion source temperature at 500 °C; ion source gas 1 at 55 psi; ion source gas 2 at 65 psi, pressure interval at 0–9500 psi. The mass spectrometer was running in Product Ion mode (Analyst TF 1.8.1 AbSCIEX software). This method involves two types of scans at the same time: in the first, a “TOF MS” scan, in a predefined range of masses during which all the precursors were acquired in high resolution, is performed. The second one, called “Product ion scan”, requires the mass of the precursor to be set in low resolution, and the fragment ions generated by it are acquired in high resolution. Both kinds of ions (precursors and/or fragments) were used for identification purposes.
The main MS parameters and the exact masses used as quantifiers and qualifiers are provided as Supplementary Materials (see Supplementary Materials Tables S2 and S3). Quantification was carried out by matrix-matched calibration (see Section 2.3).
2.6. Method Validation Procedure
According to point G7 and Table 4 of the SANTE/11312/2021 document [31], the following parameters were tested for validation purposes: linearity range, LOQ, recovery, precision in repeatability conditions as RSDr, and precision in within-laboratory reproducibility conditions RSDwR.
2.6.1. Calibration Curves and Linearity Ranges
Calibration curves were built as follows. Calibrant solutions were prepared as described in Section 2.3. In each of the targeted animal foods, the following calibration ranges were evaluated:
- (i)
- Bovine fat: 0–0.040 mg/kg for AMPA, ethephon, fosetyl Al, and glyphosate; 0–0.020 mg/kg for cyanuric acid, HEPA, and maleic hydrazide; 0–0.010 mg/kg for N-Acetyl-glyphosate; and 0–0.008 mg/kg for glufosinate ammonium, MPP, and NAG.
- (ii)
- Cow milk: 0–0.020 mg/kg for AMPA, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl-glyphosate; 0–0.040 mg/kg for cyanuric acid and ethephon; 0–0.008 mg/kg for glufosinate ammonium; and 0–0.004 mg/kg for MPP and NAG.
- (iii)
- Chicken eggs: 0–0.020 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl- glyphosate; and 0–0.004 mg/kg for glufosinate ammonium, MPP, and NAG.
These ranges were chosen taking into account a dilution factor of 20 during the extraction/purification process of all samples.
Calibration curves were obtained by plotting for each analyte the Response factor (RF) versus the concentration (µg/kg). The RF was calculated as the ratio between the peak area of the natural analyte and the relative ILIS for all pesticides, except maleic hydrazide in chicken eggs and cow milk, for which external calibration was used.
For linearity evaluation, calibration curves (prepared as described in Section 2.3) were injected on three different days spread over two weeks.
The Ordinary Least Squares (OLS) method including the point (0; 0) was used to obtain the calibration function. For each calibration point, the deviation of back-calculated concentration (BCC), that is, the deviation of calculated concentration by the calibration function (Cmeasured) from the true concentration (Ctrue), was evaluated according the following expression: (Cmeasured − Ctrue) × 100/Ctrue). According to the SANTE/11312/2021 document [31], the calibration curve was considered linear when, for each point, the deviation of BCC from true concentration was ≤±20%.
2.6.2. LOQ, Recovery, Repeatability, and Within-Laboratory Reproducibility
Evaluation of analytical parameters was carried out by analysing blank samples of bovine fat, chicken eggs, and cow milk fortified at two concentration levels as reported in Table 2. Level 1 was the estimated LOQ, while level 2 was set at 5 × LOQ for all pesticides in all matrices except 2.5 × LOQ for glufosinate ammonium and 10 × LOQ for MPP and NAG in cow’s milk.

Table 2.
Validation levels for all pesticides in each matrix. Level 1 = LOQ, Level 2 = 5 × LOQ for all pesticides in all matrices except 2.5 × LOQ for glufosinate ammonium and 10 × LOQ for MPP and NAG in cow’s milk.
The spiking levels set for LOQ were equal to or lower than the MRL based on different commodity/analyte combinations, as defined in the Regulation EC 396/2005 and subsequent amendments [17]. Furthermore, in the case of glufosinate and its metabolites, the spiking levels were set considering the complex definition of the MRL expressed as the sum of the parent compound and related metabolites, each multiplied by their respective conversion factor (the ratio of the molecular mass of the parent pesticide to the molecular mass of the metabolite).
For the two concentration levels as defined above, validation experiments confirmed the compliance with performance criteria (recoveries in the range of 70–120% and precision values lower than or equal to 20%).
For the calculation of intra-laboratory reproducibility (RSDwR), blank samples of bovine fat, chicken eggs, and cow milk were fortified at 0.1 mg/kg for glyphosate and at 0.05 mg/kg for glufosinate ammonium, MPP, and NAG and analysed by 3 different operators on 3 different days each (n = 9).
2.6.3. Measurement Uncertainty
According to the approach described in appendix C of the SANTE document, relative expanded measurement uncertainty (U′) was calculated, as required by ISO/IEC 17025 [32], using intra-laboratory validation/QC data [31].
The relative expanded measurement uncertainty was calculated by applying a coverage factor k = 2 (level of confidence of approximately 95%) to the relative combined uncertainty (u′) which was calculated using the within-laboratory reproducibility relative standard deviation (RSDwR) and the method and the laboratory bias.
The method and laboratory bias were calculated by recovery experiments carried out in an intra-laboratory reproducibility study according to the following equation:
where the meanbias equals the mean of the corresponding bias and the SD.Pbias equals the population standard deviation of the corresponding bias (calculated with the function stdev.p in excel) obtained in the intra-laboratory reproducibility study.
To assess compliance with the legislation regarding the MRL exceedances, a default value of uncertainty of 50%, as stated by the SANTE criteria, should be applied. To apply this default parameter, labs must demonstrate to have an experimental U′ value of ≤50%.
3. Results and Discussion
3.1. Set-Up of the Sample Pretreatment Method
Two methods were considered as starting points as they were the only ones available in the literature involving the determination of several polar pesticides: (i) the Quick Method for the Analysis of Numerous Highly Polar Pesticides in Foods Involving Extraction with Acidified Methanol and LC–HRMS Measurement in Foods of Animal Origin (QuPPe AO) [30] and (ii) the procedure developed by Herrera et al. [33].
The application of both procedures to the analysis of the targeted polar pesticides in the foods of animal origin included in the Regulation (EU) 2021/601 [19] showed some limitations. Specifically, peak splitting of AMPA, ethephon, and fosetyl was observed in cow milk analysed in the QuPPe AO method due to the large amount of matrix injected on the column (0.05 g). The dilution of the final test sample in order to decrease the amount of matrix injected on the column and to avoid peak splitting was evaluated; however, this resulted in a significant decrease in sensitivity. On the other hand, the protocol by Herrera et al. [33] foresaw a higher dilution factor, which was not compatible with the sensitivity of the equipment used in the study.
To set up a protocol suitable for implementation by laboratories not equipped with high end instrumentation, some modifications to the above-mentioned procedures, such as speed and time of centrifugation, the adding of additional purification steps, and different dilution factors, were tested and applied in order to obtain a good compromise between sensitivity and the matrix effect and to improve LOQ for the selected analytes. Finally, a 20-fold dilution factor was chosen in the implemented method to reduce the matrix effect.
3.2. In-House Verification of Method Performance
Data obtained from in-house validation are summarized in Table 3.

Table 3.
In-house analytical performances of the LC–MS/MS method for polar pesticides in bovine fat, chicken eggs, and cow milk including spiking levels of validation (level 1, level 2), and related recovery % and repeatability (RSDr), spiking level of within-laboratory reproducibility study and related recovery (n = 6) and within-laboratory reproducibility (RSDWLR).
Full in-house validation was performed for the four molecules required by the coordinated control plan, namely glyphosate, glufosinate, MPP, and NAG, including the evaluation of linearity ranges, recovery rates (%), limit of quantification (LOQ), repeatability (RSDr), and within-laboratory reproducibility (RSDwR).
In addition, to explore the extension of the method’s scope to the analysis of further molecules such as AMPA, cyanuric acid, ethephon, fosetyl aluminum, HEPA, maleic hydrazide, and N-acetyl glyphosate, the following parameters were validated: linearity ranges, recovery rates (%), repeatability (RSDr), and LOQ.
The linearity study, using the back-calculated concentrations method, provided fully satisfactory results for all the polar pesticides analysed.
The evaluation of method precision, in repeatability conditions, revealed that in all cases RSDr was lower than 20%, meeting the SANTE requirements for each of the analytes at the relevant tested concentrations. Moreover, satisfactory recovery values were obtained, ranging from 76% to 119%, in compliance with acceptability criteria established by the SANTE criteria [31].
According to the Regulation EC 396/2005 and subsequent amendments [17], quantification limits suitable for enforcement of the legal limit shall be equal to the MRL if there is an asterisk in the value of the MRL, or lower than the MRL if there is not an asterisk (see Table 1).
Therefore, the estimated LOQs for all analytes, corresponding to the first level of validation, were fit for purpose for the official control of the regulated analytes. Given the satisfactory performance obtained, the method has been accredited according to the ISO/IEC 17025 [31] standard for the detection of four polar pesticides required in the multi-year control plan (glyphosate, glufosinate ammonium, MPP, and NAG). To this aim, an intra-laboratory reproducibility study and the calculation of measurement uncertainty were performed.
Data obtained from the intra-laboratory reproducibility study of samples from bovine fat, chicken eggs, and cow milk confirmed the accuracy of the analytical method. Recovery values ranged from 91 to 105% with RSDwR values lower than 20%. Data are reported in Table 3 for the three matrices.
The experimental relative expanded measurement uncertainties (U′) of glyphosate, glufosinate ammonium, MPP, and NAG were between 33 and 46%, 39 and 48%, and 15 and 27% for the above-mentioned pesticides in bovine fat, chicken eggs, and cow milk, respectively. Therefore, the obtained experimental values were lower than the maximum expanded uncertainty stated by the SANTE criteria [31] which report a maximum acceptable value U′ = 50% for pesticides. Experimental U′ values for each molecule for the three matrices included in the study are reported in Table S4.
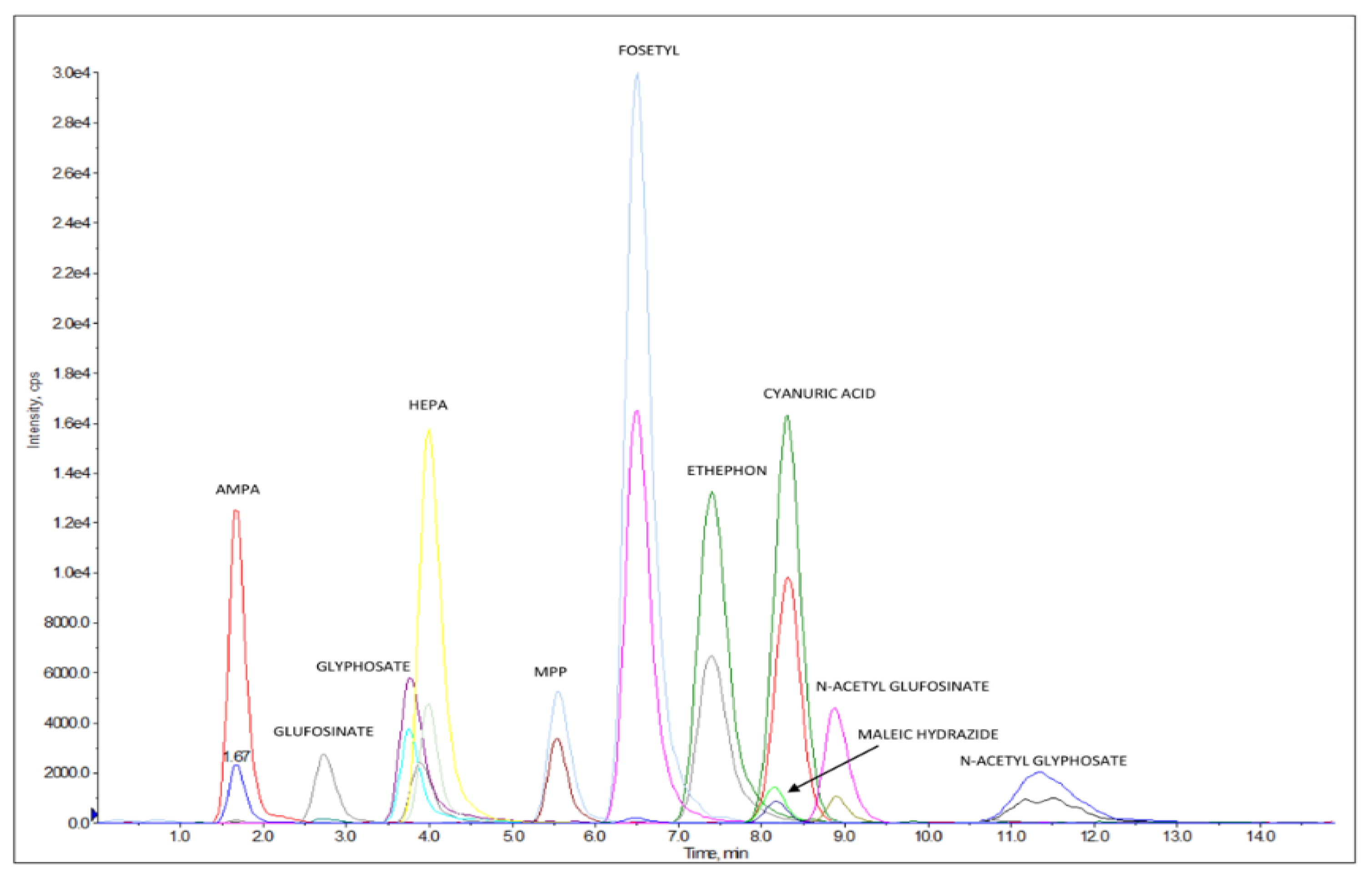
Figure 2 reports an LC–MS/HRMS chromatogram of a bovine fat sample spiked at 0.040 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, N-Acetyl- glyphosate; at 0.008 mg/kg for glufosinate ammonium, MPP, and NAG; at 0.006 mg/kg AMPA-13C,15N, cyanuric acid 3C3, ethephon D4, fosetyl Al D15, glyphosate 2-13C,15N, HEPA D4, and N-Acetyl-glyphosate 13C2,15N; and at 0.0012 mg/kg for glufosinate ammonium D3, MPP D3, and NAG D3.
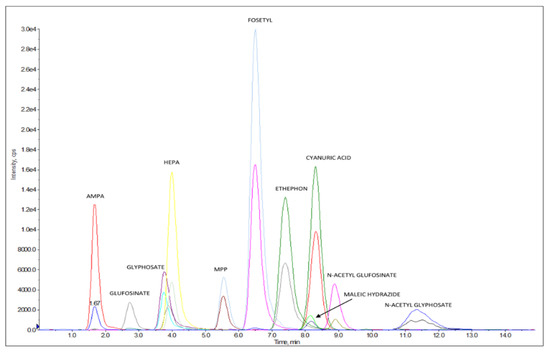
Figure 2.
Chromatogram of bovine fat extract spiked at 0.040 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl-glyphosate, and at 0.008 mg/kg for glufosinate ammonium, MPP, and NAG. Concentration levels for ILIS were 0.006 mg/kg for AMPA-13C,15N, cyanuric acid 3C3, ethephon D4, fosetyl Al D15, glyphosate 2-13C,15N, HEPA D4, and N-Acetyl-glyphosate 13C2,15N, and the levels were 0.0012 mg/kg for glufosinate ammonium D3, MPP D3, and NAG D3. For each native pesticide and relative ILIS, one ion is reported.
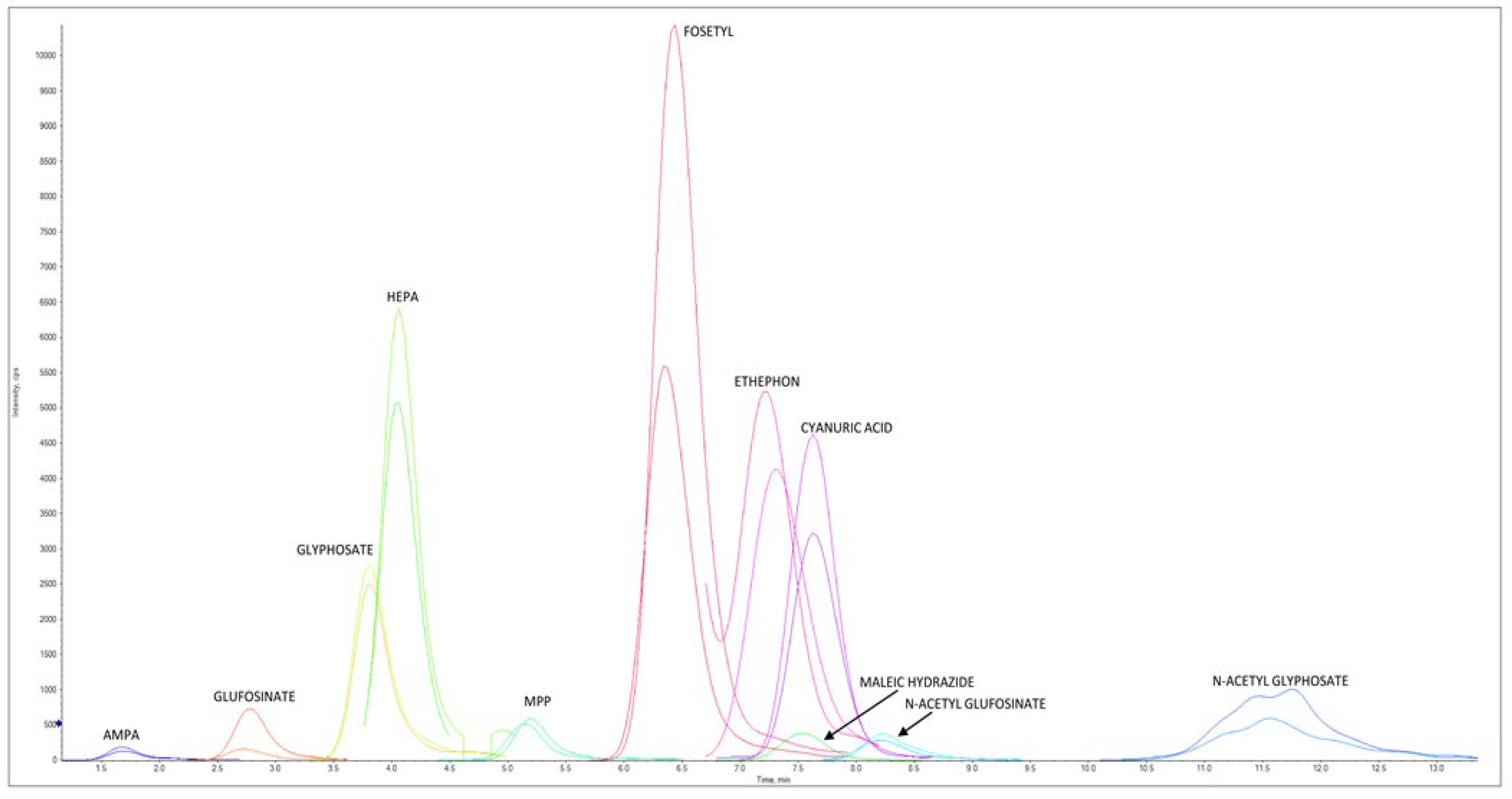
Figure 3 reports an LC–MS/HRMS chromatogram of a chicken egg sample spiked at 0.020 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl- glyphosate, at 0.004 mg/kg for glufosinate ammonium, MPP, and NAG, at 0.006 mg/kg AMPA-13C,15N, cyanuric acid 3C3, ethephon D4, fosetyl Al D15, glyphosate 2-13C,15N, HEPA D4, and N-Acetyl-glyphosate 13C2,15N, and at 0.0012 mg/kg for glufosinate ammonium D3, MPP D3, and NAG D3.
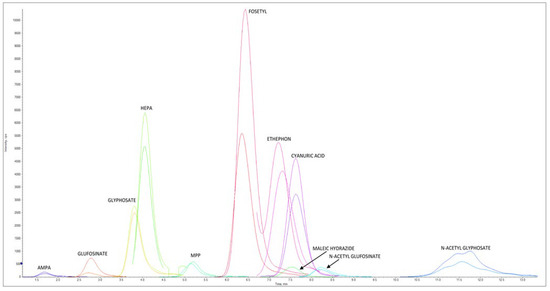
Figure 3.
LC–MS/HRMS chromatogram of chicken egg extract spiked at 0.020 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl-glyphosate and at 0.004 mg/kg for glufosinate ammonium, MPP, and NAG. Concentration levels for ILIS were 0.006 mg/kg for AMPA-13C,15N, cyanuric acid 3C3, ethephon D4, fosetyl Al D15, glyphosate 2-13C,15N, HEPA D4, and N-Acetyl-glyphosate 13C2,15N, and the levels were 0.0012 mg/kg for glufosinate ammonium D3, MPP D3, and NAG D3. For each native pesticide and relative ILIS, one ion is reported.
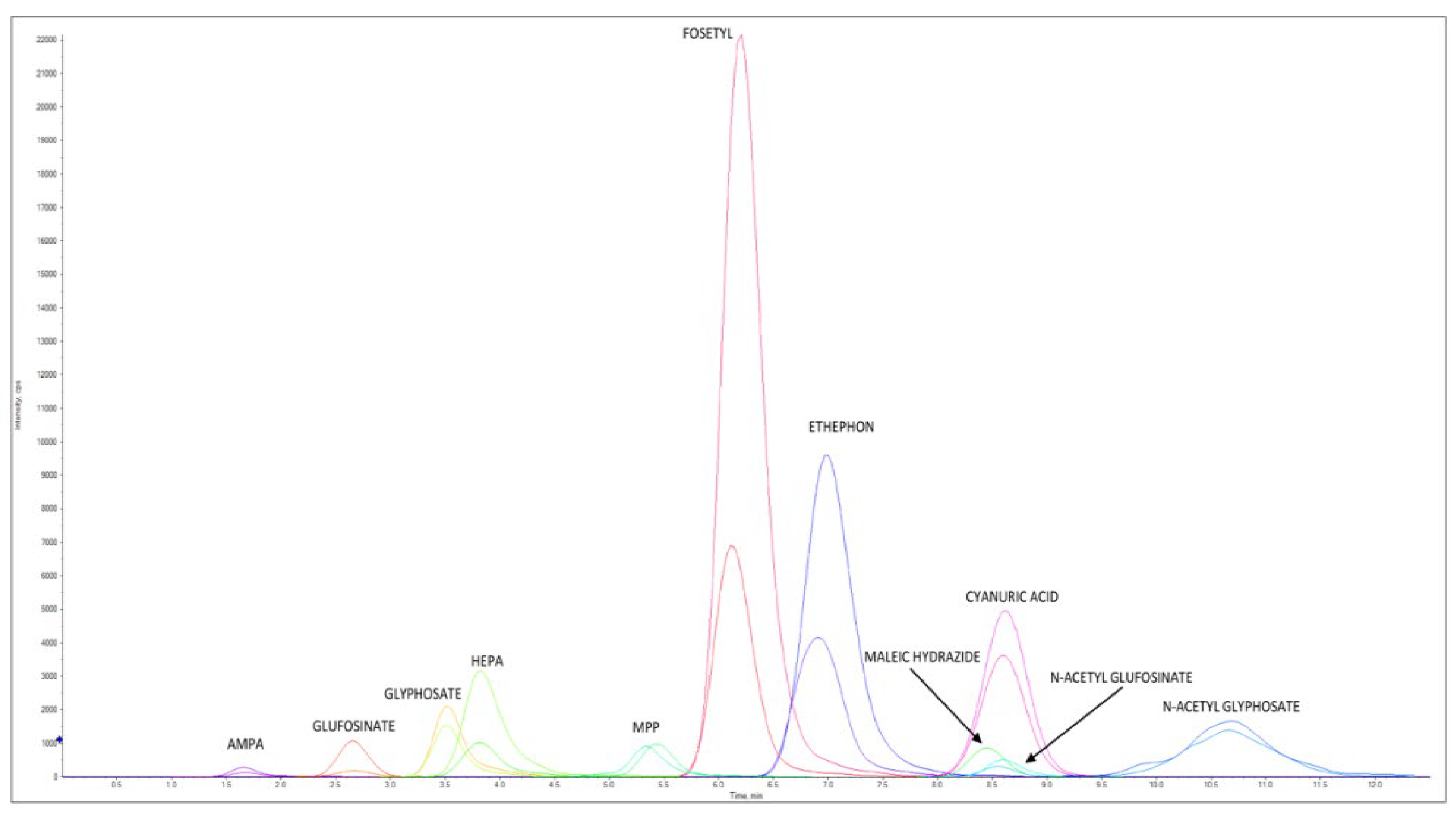
Figure 4 reports an LC–MS/HRMS chromatogram of a cow milk sample spiked at 0.040 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl- glyphosate, at 0.008 mg/kg for glufosinate ammonium, MPP, and NAG, at 0.006 mg/kg AMPA-13C,15N, cyanuric acid 3C3, ethephon D4, fosetyl Al D15, glyphosate 2-13C,15N, HEPA D4, and N-Acetyl-glyphosate 13C2,15N, and at 0.0012 mg/kg for glufosinate ammonium D3, MPP D3, and NAG D3.
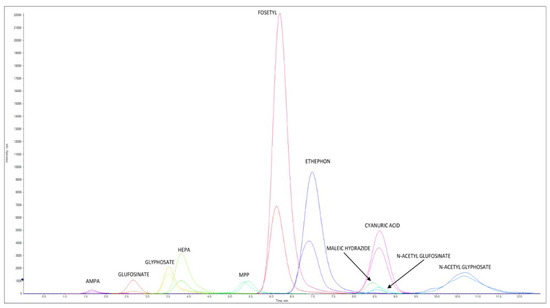
Figure 4.
LC–MS/HRMS chromatogram of cow milk extract spiked at 0.040 mg/kg for AMPA, cyanuric acid, ethephon, fosetyl Al, glyphosate, HEPA, maleic hydrazide, and N-Acetyl-glyphosate and at 0.008 mg/kg for glufosinate ammonium, MPP, and NAG. Concentration levels for ILIS were 0.006 mg/kg for AMPA-13C,15N, cyanuric acid 3C3, ethephon D4, fosetyl Al D15, glyphosate 2-13C,15N, HEPA D4, and N-Acetyl-glyphosate 13C2,15N and at 0.0012 mg/kg for glufosinate ammonium D3, MPP D3, and NAG D3. For each native pesticide and relative ILIS, one ion is reported.
3.3. Application of the Implemented Method for Official Control Purposes
The validated and accredited method was used for the analysis of 45 real samples of foods of animal origin, i.e., 8 samples of chicken eggs, 31 of fat (11 of bovine fat, 12 of swine fat, and 8 of poultry fat), and 6 of cow milk. The data obtained represent the first results of a monitoring plan in two regions located in central Italy (Umbria and Marche). Glufosinate and its metabolites and glyphosate were never detected in any of the analysed samples.
Data described in this study were in line with occurrence data reported in the literature, where glyphosate has rarely been detected in cow milk, including infant formula milk; and never above the MRL [34,35,36]. Few studies have been carried out regarding the occurrence of polar pesticides in foods of animal origin required by the monitoring plan. Only one manuscript reported the quantification of glyphosate in different samples of animal origins (3 milk, 1 egg, and 15 samples of meat and fish). The results showed the presence of glyphosate in two samples of meat and fish only, but neither one was above the established MRL [37].
4. Conclusions
In this study, we developed and validated an LC–QTOF method for the detection of 11 polar pesticides in three matrices of animal origin—eggs, milk, and fat. The extraction procedure is simple and does not require special purification steps. Good validation parameters, according to the SANTE document [31], were obtained for all molecules. For this reason, the method can be considered reliable and sensitive for routine monitoring of polar pesticides. No contaminated samples were found when the method was applied in the annual monitoring programme. These findings were in agreement with the literature data. The implementation of the accredited method in routine analysis will provide data useful for re-evaluating risk assessment studies in foods of animal origin. An inter-laboratory comparison (in progress) will provide data on method transferability to other laboratories and/or other mass spectrometers such as triple quadruple detectors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10010044/s1. Table S1. Preparation of matrix-matched calibration solutions. Table S2. MS parameters for pesticide quantification Table S3. Exact masses of precursor ion (*) extract by TOF MS experiment and exact masses of product ion used for quantification (1) and qualification (2). Table S4. Relative expanded measurement uncertainty (U′) data. Table S5. AMPA—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S6. Cyanuric acid—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S7. Ethephon—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S8. Fosetyl—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S9. Glyphosate—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S10. HEPA—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S11. Maleic hydrazide—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S12. N acetyl glyphosate—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S13. Glufosinate ammonium—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S14. MPP—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682. Table S15. NAG—Verification of linearity using the method specified in C17 of the document SANTE/2019/12682.
Author Contributions
Conceptualization, V.M.T.L. and I.P.; methodology, I.P.; validation, E.V.; formal analysis, E.V. and L.F.; investigation, E.V.; resources, I.P.; data curation, B.C. and E.V.; writing—original draft preparation, B.C. and E.V.; writing—review and editing, V.M.T.L., I.P. and L.F.; supervision, I.P.; project administration, I.P.; funding acquisition, I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministero della Salute (Italian Ministry of Health) (Grant number: IZS UM 07/19 RC).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Castillo, M.; Carbonell, E.; González, C.; Miralles-Marco, A. Pesticide Residue Analysis in Animal Origin Food: Procedure Proposal and Evaluation for Lipophilic Pesticides. In Pesticides—Recent Trends in Pesticide Residue Assay; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. RTP 2000, 31 Pt 1, 117–165. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, O.K.; Gimsing, A.L. Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: A review. Pest Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef]
- Nørskov, N.P.; Jensen, S.K.; Sørensen, M.T. Robust and highly sensitive micro liquid chromatography–tandem mass spectrometry method for analyses of polar pesticides (glyphosate, aminomethylphosfonic acid, N-acetyl glyphosate and N-acetyl aminomethylphosfonic acid) in multiple biological matrices. J. Chromatogr. A 2019, 1605, 360343. [Google Scholar] [CrossRef]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef]
- Müller, B.P.; Zumdick, A.; Schuphan, I.; Schmidt, B. Metabolism of the herbicide glufosinate-ammonium in plant cell cultures of transgenic (rhizomania-resistant) and non-transgenic sugarbeet (Beta vulgaris), carrot (Daucus carota), purple foxglove (Digitalis purpurea) and thorn apple (Datura stramonium). Pest Manag. Sci. 2001, 57, 46–56. [Google Scholar] [CrossRef]
- Aris, A.; Leblanc, S. Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada. Reprod. Toxicol. 2011, 31, 528–533. [Google Scholar] [CrossRef]
- Dorne, J.L.; Doerge, D.R.; Vandenbroeck, M.; Fink-Gremmels, J.; Mennes, W.; Knutsen, H.K.; Vernazza, F.; Castle, L.; Edler, L.; Benford, D. Recent advances in the risk assessment of melamine and cyanuric acid in animal feed. Toxicol. Appl. Pharmacol. 2013, 270, 218–229. [Google Scholar] [CrossRef]
- Long, L.; Bu, Y.; Chen, B.; Sadiq, R. Removal of urea from swimming pool water by UV/VUV: The roles of additives, mechanisms, influencing factors, and reaction products. Water Res. 2019, 161, 89–97. [Google Scholar] [CrossRef]
- El-Okazy, A.M. The Effects of Combination of Gibberellic Acid-3 (GA3) and Ethephon (2-Chloroethyl Phosphonic Acid) (Plant Growth Regulators) on Some Physiological Parameters in Mice. J. Egypt. Public Health Assoc. 2008, 83, 67–86. [Google Scholar]
- Yamada, Y. ETHEPHON (106). In Proceedings of the Pesticide Residue in Food 2015, Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group, Geneva, Switzerland, 15–24 September 2015. [Google Scholar]
- Hacker, K.; Benkenstein, A.; Eichorn, E.; Kolberg, D.; Wildgrube, C.; Scherbaum, E.; Anastassiades, M. Fosetyl and Phosphonic Acid—Residue Situation and Some Interesting Facts. In Proceedings of the EPRW 2016, The Evagoras Lanitis Center, Limassol, Cyprus, 24–27 May 2016. [Google Scholar]
- Chamkasem, N. Determination of glyphosate, maleic hydrazide, fosetyl aluminum, and ethephon in grapes by liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2017, 65, 7535–7541. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Union 2005, 70, 1–17. [Google Scholar]
- European Food Safety Authority (EFSA). Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005–revised version to take into account omitted data. EFSA J. 2019, 17, e05862. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/601 of 13 April 2021 concerning a coordinated multiannual control programme of the Union for 2022, 2023 and 2024 to ensure compliance with maximum residue levels of pesticides and to assess the consumer exposure to pesticide residues in and on food of plant and animal origin. Off. J. Eur. Union L 127 2021, 64, 29. [Google Scholar]
- Gasparini, M.; Angelone, B.; Ferretti, E. Glyphosate and other highly polar pesticides in fruit, vegetables and honey using ion chromatography coupled with high resolution mass spectrometry: Method validation and its applicability in an official laboratory. J. Mass Spectrom. 2020, 55, e4624. [Google Scholar] [CrossRef]
- Martinez-Haro, M.; Chinchilla, J.M.; Camarero, P.R.; Viñuelas, J.A.; Crespo, M.J.; Mateo, R. Determination of glyphosate exposure in the Iberian hare: A potential focal species associated to agrosystems. Sci. Total Environ. 2022, 823, 153677. [Google Scholar] [CrossRef]
- Zambrano-Intriago, L.A.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Araújo, A.N.; Montenegro, M.C. Challenges in the design of electrochemical sensor for glyphosate-based on new materials and biological recognition. Sci. Total Environ. 2021, 793, 148496. [Google Scholar] [CrossRef]
- Zambrano-Intriago, L.A.; Amorim, C.G.; Araújo, A.N.; Gritsok, D.; Rodríguez-Díaz, J.M.; Montenegro, M.C. Development of an inexpensive and rapidly preparable enzymatic pencil graphite biosensor for monitoring of glyphosate in waters. Sci. Total Environ. 2023, 855, 158865. [Google Scholar] [CrossRef]
- Verdini, E.; Pecorelli, I. The Current Status of Analytical Methods Applied to the Determination of Polar Pesticides in Food of Animal Origin: A Brief Review. Foods 2022, 11, 1527. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, B.; Yuan, D.; Ma, J. A simple method for the determination of glyphosate and aminomethylphosphonic acid in seawater matrix with high performance liquid chromatography and fluorescence detection. Talanta 2016, 161, 700–706. [Google Scholar] [CrossRef] [PubMed]
- García de Llasera, M.P.; Gómez-Almaraz, L.; Vera-Avila, L.E.; Peña-Alvarez, A. Matrix solid-phase dispersion extraction and determination by high-performance liquid chromatography with fluorescence detection of residues of glyphosate and aminomethylphosphonic acid in tomato fruit. J. Chromatogr. A 2005, 1093, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hogendoorn, E.A.; Ossendrijver, F.M.; Dijkman, E.; Baumann, R.A. Rapid determination of glyphosate in cereal samples by means of pre-column derivatisation with 9-fluorenylmethyl chloroformate and coupled-column liquid chromatography with fluorescence detection. J. Chromatogr. A 1999, 833, 67–73. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Nobile, M.; Panseri, S.; Arioli, F. Detection of glyphosate and its metabolites in food of animal origin based on ion-chromatography high resolution mass spectrometry (IC-HRMS). Food Addit. Contam. Part A 2019, 36, 592–600. [Google Scholar] [CrossRef]
- Dias, J.; Lopez, S.; Mol, H.; de Kok, A. Influence of different hydrophilic interaction liquid chromatography stationary phases on method performance for the determination of highly polar anionic pesticides in complex feed matrices. J. Sep. Sci. 2021, 44, 2165–2176. [Google Scholar] [CrossRef]
- Anastassiades, M.; Kolberg, D.I.; Eichhorn, E.; Wachtler, A.K.; Benkenstein, A.; Zechmann, S.; Mack, D.; Wildgrube, C.; Barth, A.; Sigalov, I.; et al. Quick Method for the Analysis of Numerous Highly Polar Pesticides in Food Involving Extraction with Acidified Methanol and LC-MS/MS Measurement. In Food of Animal Origin (QuPPe-AO-Method), Version 3.2. 2019. Available online: https://www.eurl-pesticides.eu/userfiles/file/meth_QuPPe_AO_V3_2.pdf (accessed on 5 January 2023).
- SANTE/11312/2021: Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed Implemented by 1 January 2022. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/SANTE_11312_2021.pdf (accessed on 24 November 2022).
- UNI CEI EN ISO/IEC 17025:2018; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2018.
- Herrera López, S.; Dias, J.; de Kok, A. Analysis of highly polar pesticides and their main metabolites in animal origin matrices by hydrophilic interaction liquid chromatography and mass spectrometry. Food Control 2020, 115, 107289. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; Young, A.E. Detection of dietary DNA, protein, and glyphosate in meat, milk, and eggs. J. Anim. Sci. 2017, 95, 3247–3269. [Google Scholar] [CrossRef]
- Schnabel, K.; Schmitz, R.; von Soosten, D.; Frahm, J.; Kersten, S.; Meyer, U.; Breves, G.; Hackenberg, R.; Spitzke, M.; Dänicke, S. Effects of glyphosate residues and different concentrate feed proportions on performance, energy metabolism and health characteristics in lactating dairy cows. Arch. Anim. Nutr. 2017, 71, 413–427. [Google Scholar] [CrossRef]
- Von Soosten, D.; Meyer, U.; Hüther, L.; Dänicke, S.; Lahrssen-Wiederholt, M.; Schafft, H.; Spolders, M.; Breves, G. Excretion pathways and ruminal disappearance of glyphosate and its degradation product aminomethylphosphon. J. Dairy Sci. 2016, 99, 5318–5324. [Google Scholar] [CrossRef]
- Zoller, O.; Rhyn, P.; Rupp, H.; Zarn, J.A.; Geiser, C. Glyphosate residues in Swiss market foods: Monitoring and risk evaluation. Food Addit. Contam. Part B Surveill. 2018, 11, 83–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).