Abstract
Pentachlorophenol (PCP) is a persistent organic pollutant usually present in the form of sodium salts (PCP-Na) that has been banned for many years, but it can still be detected in animal food. The present study established a method of detecting PCP-Na and its metabolites—tetrachlorocatechol (TCC), pentachlorophenol acetate (PCP-acetate), and pentachloroanisole (PCA)—in swine samples (pork, fat, liver, heart, lungs and kidney), simultaneously using liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-tandem mass spectrometry (GC-MS/MS) based on the modified QuEChERS pre-treatment method. The validation results exhibited a good sensitivity with limits of quantitation (LOQs) of 1 μg/kg–2 μg/kg. The recoveries of spiked samples were in the range of 60.5–119.9%, with a relative standard deviation (RSD) between replicates (n = 5) of between 0.70% and 12.06%.
1. Introduction
Sodium pentachlorophenolate (PCP-Na), the sodium salt of pentachlorophenol (PCP), is an organochlorine compound primarily employed in industrial wood preservation. It is categorized as a moderately toxic substance, demonstrating notable chemical and biological stability. It easily disperses through water as a carrier, exhibiting widespread diffusion, and tends to accumulate in sediments and organisms. PCP is classified by the International Agency for Research on Cancer (IARC) as a Class 2B suspected human carcinogen (IARC Class 2B). It is listed as Hazardous Substance No. 31 in the Environmental Protection Agency (EPA) list and is also classified as a Highly Hazardous Substance by the World Health Organization (WHO) [1]. PCP has been included in the Stockholm Convention and is restricted for use exclusively in utility poles. Because of their broad pesticidal efficiency spectrum and low cost, PCP and its salts were used as algicides, bactericides, fungicides, herbicides, insecticides, and molluscicides, with a variety of applications in the industrial, agricultural, and domestic fields before they were banned [2].
PCP and PCP-Na tend to accumulate in the water body environment and sediments and pollute the water environment for a long time. They have been identified in surface water, groundwater, aquaculture water, and sediments of aquatic bodies, potentially posing a threat to human health through the aquatic food chain [3]. They have been identified in livestock and poultry, food-contact papers, wooden items, and even in human blood and urine. Environmental residues of PCP-Na can infiltrate organisms via the respiratory tract and subsequently enter the food chain through bio-concentration [2]. These substances present potential risks including but not limited to carcinogenicity [4,5,6], endocrine-disrupting effects, cytotoxicity [7], reproductive toxicity [8], neurotoxicity [9], developmental toxicity [10], and genotoxicity.
As a lipophilic persistent organic pollutant, PCP shows a high pollution level in animal fat, meat, viscera, and meat products [11]. Animal-derived foods, such as pork and liver, contain various proteins, fats, and pigments, leading to a pronounced matrix effect that can impact the sensitivity of detection instruments. Currently, prevalent pretreatment techniques for PCP or PCP-Na detection in animal-derived foods encompass solid-phase extraction (SPE) [12] and QuEChERS [13], while a few methods employ liquid–liquid extraction (LLE). Among these, the QuEChERS approach is characterized by reduced organic solvent consumption, limited contamination, and ease of operation. Common instrumental detection methods for PCP-Na encompass spectrophotometry [14], gas chromatography (GC) [15], gas chromatography-tandem mass spectrometry (GC-MS/MS), liquid chromatography (LC), liquid chromatography-tandem mass spectrometry (LC-MS/MS) [16], surface-enhanced Raman spectroscopy (SERS), immunoassay, and electrochemical analysis. GC-MS/MS and LC-MS/MS methods are marked by high specificity and sensitivity, making them capable of identifying trace quantities of target compounds.
Upon entering the animal organism, PCP and PCP-Na distribute throughout various organs via the bloodstream, and through internal metabolism, generate metabolites. The primary metabolic pathways may include methylation, leading to the formation of pentachloroanisole (PCA), or hydroxylation, resulting in pentachlorophenol acetate (PCP-acetate), and could also involve dechlorination or hydroxylation, yielding tetrachlorocatechol (TCC). PCP-Na has been banned for many years, but it is still detected in animal food. In this study, a method for the simultaneous detection of PCP-Na and its metabolites (TCC, PCP-acetate, and PCA) in swine samples was established. This method provides a crucial technical approach for monitoring PCP-Na during swine farming and the subsequent sale of pork products.
2. Materials and Methods
2.1. Chemicals and Reagents
Sodium pentachlorophenolate (PCP-Na, CAS: 131-52-2), tetrachlorocatechol (TCC, CAS: 1198-55-6), pentachlorophenol acetate (PCP-acetate, CAS: 1441-02-7), and pentachloroanisole (PCA, CAS: 1825-21-4) 1000 μg/mL standard solution and QuEChERS kits for extraction with article number P-QuEChERS-EN 1101 (4 g MgSO4, 1 g NaCl, 1 g Na3Cit 2H2O, 0.5 g Na2Cit 1.5H2O) and P-QuEChERS-AOAC 1201 (1.5 g NaOAc, 6 g MgSO4) were purchased from Alta Technology (Tianjin, China). Dispersive SPE material, primary secondary amine (PSA), C18, EMR-Lipid, and graphitized carbon black (Carb-GCB) for cleaning were purchased from Agilent Technologies (Santa Clara, CA, USA). HPLC-grade methanol, acetonitrile, ethyl acetate, ammonium acetate, formic acid and acetic acid were from Thermo Fisher Scientific (Waltham, MA, USA). Analytically pure trimethylamine, sodium chloride (NaCl), anhydrous magnesium sulfate (MgSO4) and anhydrous sodium sulfate (NaSO4) were from Sinopharm Chemical Reagent (Shanghai, China).
2.2. Sample Preparation
Swine samples, including pork, fat, pig liver, heart, lung, and kidney, were obtained from Jingdong online market (Beijing, China). All the samples were homogenized before analysis, and stored at −20 °C.
Then, 5 g was weighed into a 50 mL polypropylene centrifuge tube with 10 mL of 1% acetic acid in acetonitrile, and the mixture was vortexed for 1 min. After ultrasonic extraction for 10 min, the extraction kit P-QuEChERS-EN 1101 was added and vortexed for 1 min. The samples were centrifuged at a speed of 10,000 r/min for 5 min at −4 °C. Then, 4 mL supernatant was transferred into a 10 mL tube and concentrated to less than 1 mL with nitrogen under 40 °C. This was then fixed to 1 mL with 1% acetic acid in acetonitrile. Pork and fat sample matrices were added with 120 mg of EMR. Liver, heart, lung and kidney were added with 120 mg of EMR and 25 mg of PSA. The mixture was vortexed for 1 min and filtered using a 0.22 μm PTFE filter. The filtered sample was separated into two parts. One was analyzed using LC-MS/MS, and the other (200 μL) was concentrated to dryness with nitrogen at 40 °C and then redissolved with 200 μL ethyl acetate. Finally, the mixture was vortexed and mixed for 2 min and centrifuged at a speed of 1000 r/min for 5 min, and the supernatant was analyzed using GC-MS/MS.
2.3. Instrumentation and Conditions
2.3.1. LC-MS/MS
PCP-Na and TCC analyses were performed on a Shimadzu LCMS-8050 (Kyoto, Japan) device, and data acquisition was carried out in multiple-reaction monitoring (MRM) mode. The reversed-phase separation of the analytes was performed on an Agilent ZORBAX Eclipse Plus C18 column (2.1 mm × 100 mm, 1.7 μm particle size) (Agilent Technologies Inc., Santa Clara, CA USA) with the column temperature maintained at 40 °C. The final mobile phase consisted of 0.1% formic acid and 5 mM ammonium acetate in water (A) and 0.1% formic acid in methanol (B). A gradient elution was achieved using the LC binary gradient as follows: 0–0.5 min with 40% B, 0.51–4 min with 40–100% B, 4.1–6 min with 100% B, and 6.01–8 min with 40% B at a flow rate of 0.40 mL/min.
The tandem mass spectrometer was operated in electrospray ionization (ESI) turbo ion source negative-ion mode with the interface voltage set at 3.0 kV and temperature set at 300 °C. The MRM parameters for target compounds are summarized in Table 1.

Table 1.
Multiple-reaction monitoring parameters for PCP-Na and TCC.
2.3.2. GC-MS/MS
PCP-acetate and PCA analyses were performed on a Shimadzu GCMS-TQ-8040 (Kyoto, Japan) device, and data acquisition was carried out in multiple-reaction monitoring (MRM) mode. The reversed-phase separation of the analytes was performed on an SH-Rxi-5Sil MS column (0.25 mm × 30.0 m, 0.25 μm particle size). The inlet temperature was set at 250 °C with a non-split mode. The heating program was set to an initial temperature of 140 °C and held for 2 min, then to 200 °C at a rate of 10 °C/min, and finally to 280 °C at a rate of 15 °C/min and held for 5 min.
The tandem mass spectrometer was operated in electron impact (EI) ion source mode. The ion source temperature and interface temperature were set to 230 °C and 250 °C, respectively. The solvent delay was set for 3 min. The MRM parameters are summarized in Table 2.

Table 2.
Multiple-reaction monitoring parameters for PCP-acetate and PCA.
3. Results and Discussion
During the optimization of pretreatment methods, the swine samples were divided into two groups based on their characteristics: muscle tissues (pork and fat) and viscera tissues (liver, heart, lung, kidney). Pork and liver were selected as representatives, and all samples were included in the procedure of method validation.
The data published by the State Administration for Market Regulation in China indicated that the residue levels of PCP in pork and liver ranged from 1.2 μg/kg to 54 μg/kg, with the majority being below 20 μg/kg [17]. The residue levels of PCP in seafood samples ranged from 1.08 μg/kg to 21.49 μg/kg [18]. In this study, the optimization of the sample preparation method was initially conducted using a concentration of 10 μg/kg, and the method was ultimately validated using the LOD, 2 LOD, and 10 LOD.
3.1. Optimization of Instrument Conditions
3.1.1. LC-MS/MS
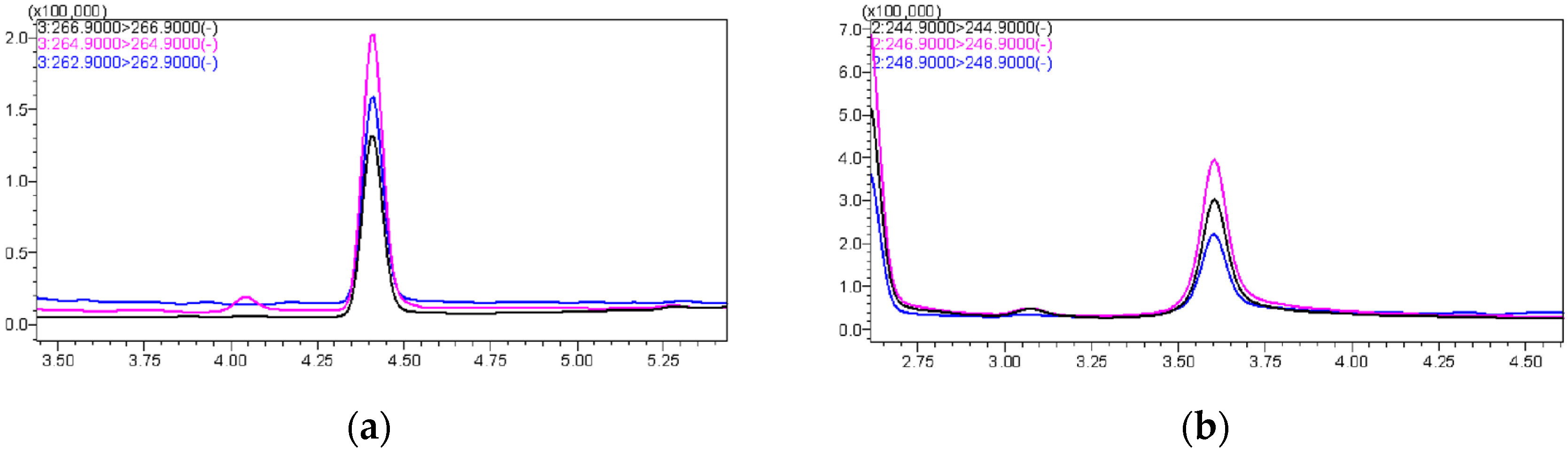
The first choice was to use methanol and acetonitrile as mobile phases separately, and the results showed that the peak area using methanol was twice that using acetonitrile; hence, methanol was selected as the organic phase. In the liquid chromatography mobile phase system, the inclusion of acid and salt can enhance the chromatographic peak shapes. Formic acid protonates the silicone silanol group within the column, thereby weakening the interaction between the silanol group and the detector, resulting in well-defined peaks with reduced tailing. Ammonium acetate enhances sensitivity by improving the ionization efficiency of the target compound. Next, different concentrations of formic acid and ammonium acetate were added to the mobile phase for testing, including 0.05% formic acid, 0.1% formic acid, 5 mmol/L ammonium acetate, and the mixture of them. The results indicated that adding 0.1% formic acid and 5 mmol/L ammonium acetate to the water, and 0.1% formic acid to methanol, yielded the best outcome, with well-defined chromatographic peaks and a high response value (Figure 1).
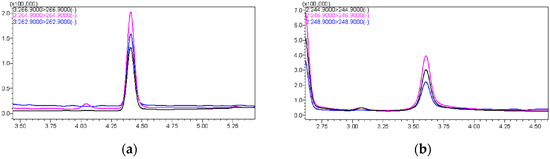
Figure 1.
(a) The chromatographic peak of PCP-Na; (b) The chromatographic peak of TCC.
3.1.2. GC-MS/MS
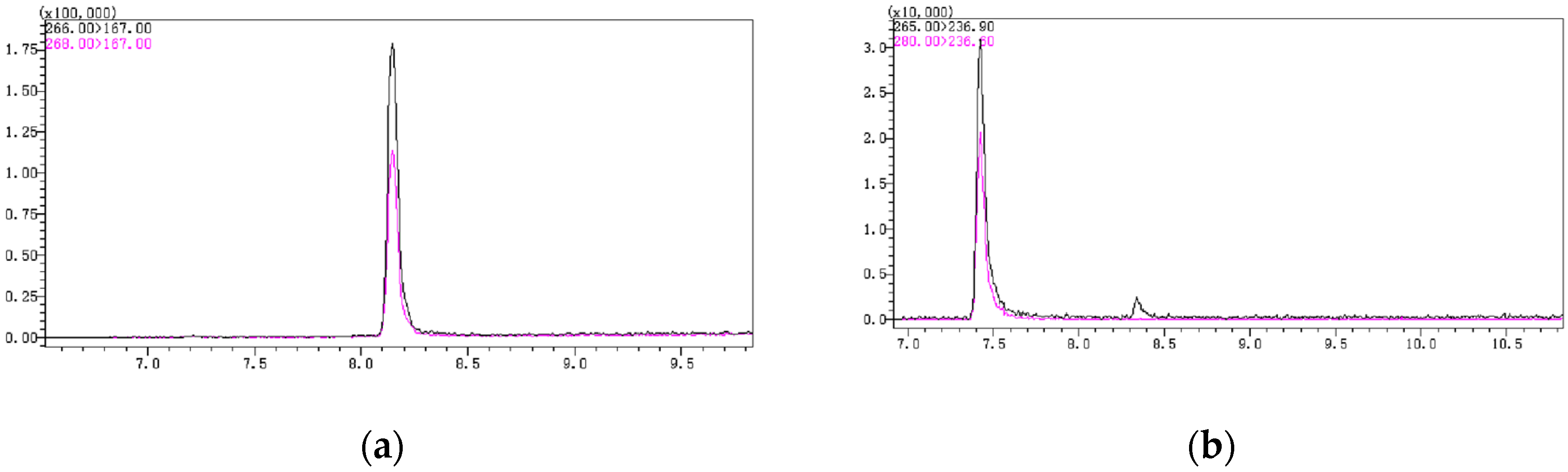
In the gas chromatography temperature program, the initial temperature and heating rate have a significant impact on the chromatographic peak shape and response. In this experiment, different initial times and heating rates were set. Finally, it was determined that an initial temperature of 140 °C, with a ramp rate of 10 °C/min up to 200 °C, followed by a ramp rate of 15 °C/min up to 280 °C, yielded better peak shapes for PCP and PCA. There was no tailing, and the response value was higher. Additionally, the two chromatographic peaks, PCP-acetate and PCA, could be well-separated without mutual interference (Figure 2).
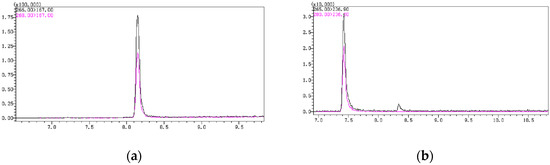
Figure 2.
(a) The chromatographic peak of PCP-acetate; (b) The chromatographic peak of PCA.
3.2. Optimization of Sample Extraction
3.2.1. Extraction Solvent
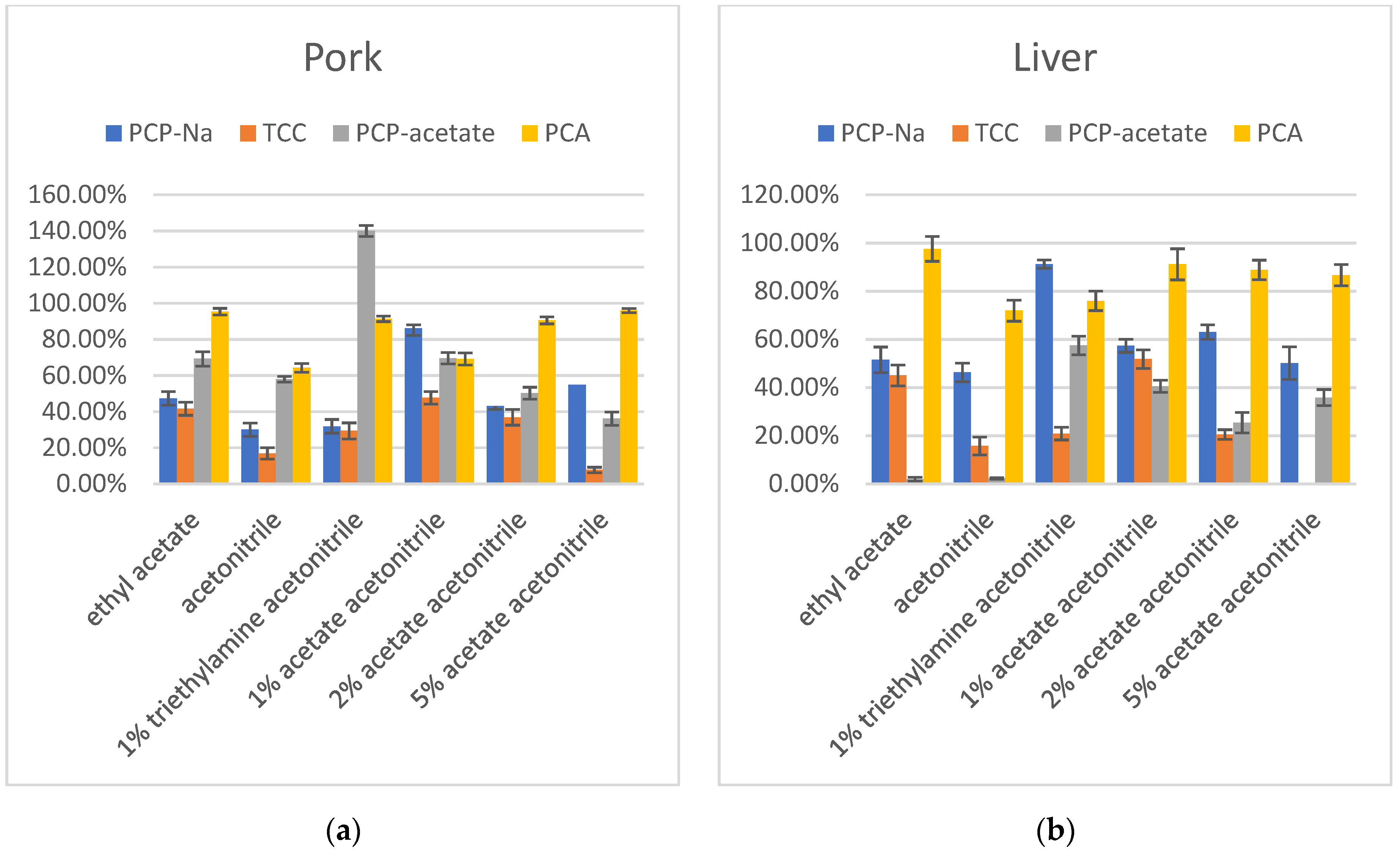
The first stage of optimization investigated using ethyl acetate, acetonitrile, 1% acetic acid in acetonitrile, and 1% triethylamine in acetonitrile as extraction solvents for PCP-Na, TCC, PCP-acetate, and PCA in pork and liver. The results indicated that when using a 1% acetic acid in acetonitrile solution as the extraction solvent, the recovery rate for PCP was the highest, falling within the range of 60–120%. TCC, on the other hand, exhibited consistently low recovery rates across all extraction solvents, with decreasing recovery rates observed as the acidity increases. Notably, the 1% acetic acid in acetonitrile solution showed relatively better recovery rates for TCC compared to other solvents. PCP-acetate demonstrated exceptionally high recovery rates under alkaline conditions, approaching 140%, indicating a strong matrix enhancement effect. The results of 1% acetic acid in acetonitrile and ethyl acetate extraction solvents showed similar recovery rates. The recoveries of PCA in ethyl acetate and 1% triethylamine in acetonitrile showed better results than in acetonitrile and 1% acetic acid in acetonitrile. Taking all factors into consideration, choosing 1% acetic acid in acetonitrile as the extraction solvent seemed to be more suitable for all compounds.
Therefore, in the next step, 2% acetic acid in acetonitrile and 5% acetic acid in acetonitrile were tested to determine if increasing the acid concentration would improve the recovery. The results showed that increasing the acid concentration in acetonitrile did not yield better results. Specifically, for TCC and PCP-acetate, the recovery decreased significantly with increasing acetic acid. Only PCA showed an increase in recovery with increasing acidity. While PCP-Na did not exhibit a consistent trend with acidity, its recovery was significantly lower when the acetic acid concentration was 2% or 5% compared to when it was 1%. Additionally, the color of the extraction solvent turned dark. Therefore, 1% acetic acid in acetonitrile was selected as the preferred extraction solvent (Figure 3).
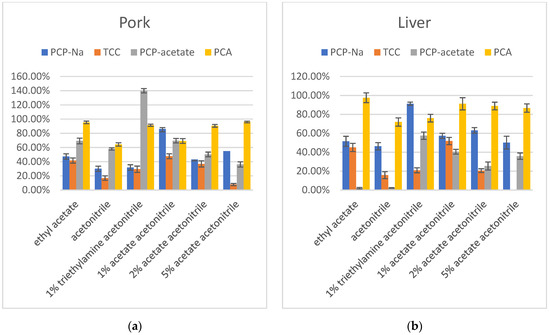
Figure 3.
(a) Recovery in extraction solvent optimization of PCP-Na, TCC, PCP-acetate, and PCA in pork (n = 3); (b) Recovery in extraction solvent optimization of PCP-Na, TCC, PCP-acetate, and PCA in liver (n = 3).
3.2.2. Extraction Salts
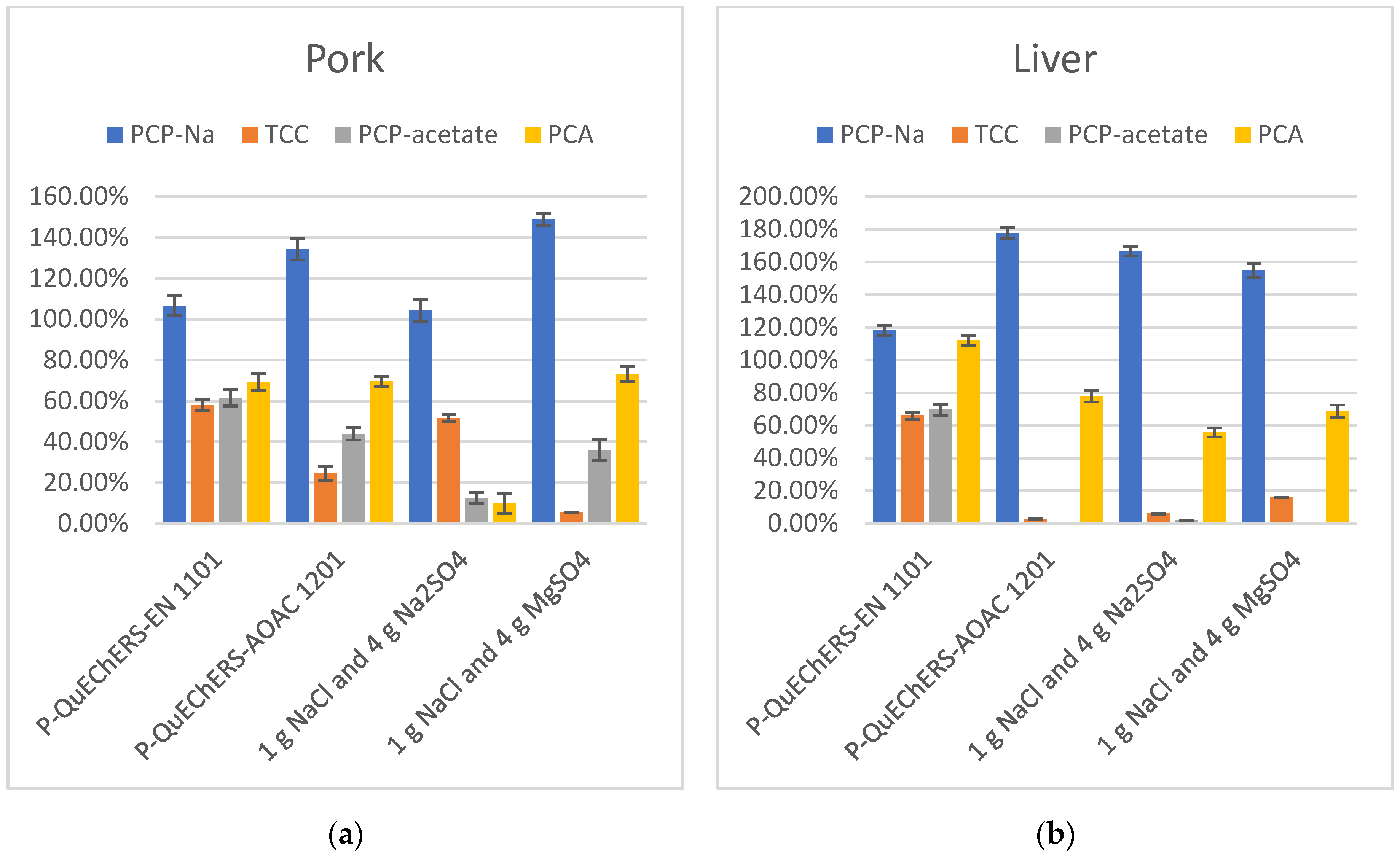
In the extraction process, the addition of salts like Na3Cit, Na2Cit, and NaOAc to the extraction solution can provide a buffering environment, creating favorable conditions for the simultaneous extraction of various target compounds with different properties. NaCl can separate the organic phase from the aqueous phase through salting-out, while Na2SO4 and MgSO4 can remove water content. Therefore, this experiment investigated the addition of four different combinations of salts to the extraction solution, including QuEChERS kits P-QuEChERS-EN 1101, QuEChERS kits P-QuEChERS-AOAC 1201, 1 g NaCl and 4 g Na2SO4, and 1 g NaCl and 4 g MgSO4. The recovery results in the extraction solution containing buffering salts were better than those without buffering salts, where the target compounds could be more stable and more fully extracted in the presence of the buffer salts. Only in the presence of NaCl, Na2SO4, and MgSO4 could the target compounds not be fully extracted, and they may even be lost under certain conditions, such as during the extraction of PCP-acetate in liver samples. Additionally, the combination of the buffering salts Na3Cit and Na2Cit outperforms option NaOAc in terms of extraction efficiency. Overall, the best recovery of the target compounds when the buffer salts were found when using QuEChERS kits P-QuEChERS-EN 1101 with 4 g MgSO4, 1 g NaCl, 1 g Na3Cit 2H2O, and 0.5 g Na2Cit 1.5H2O added to the extract. (Figure 4).
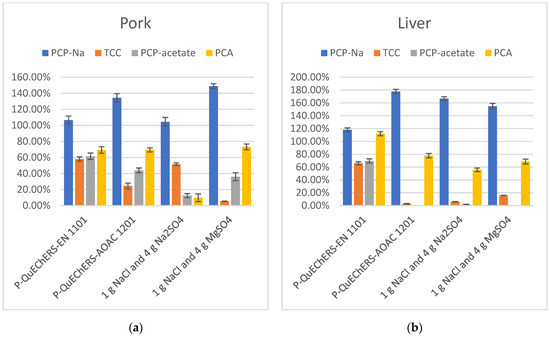
Figure 4.
(a) Recovery in extraction salts optimization of PCP-Na, TCC, PCP-acetate, and PCA in pork (n = 3); (b) Recovery in extraction salts optimization of PCP-Na, TCC, PCP-acetate, and PCA in liver (n = 3).
3.2.3. Purification Procedure
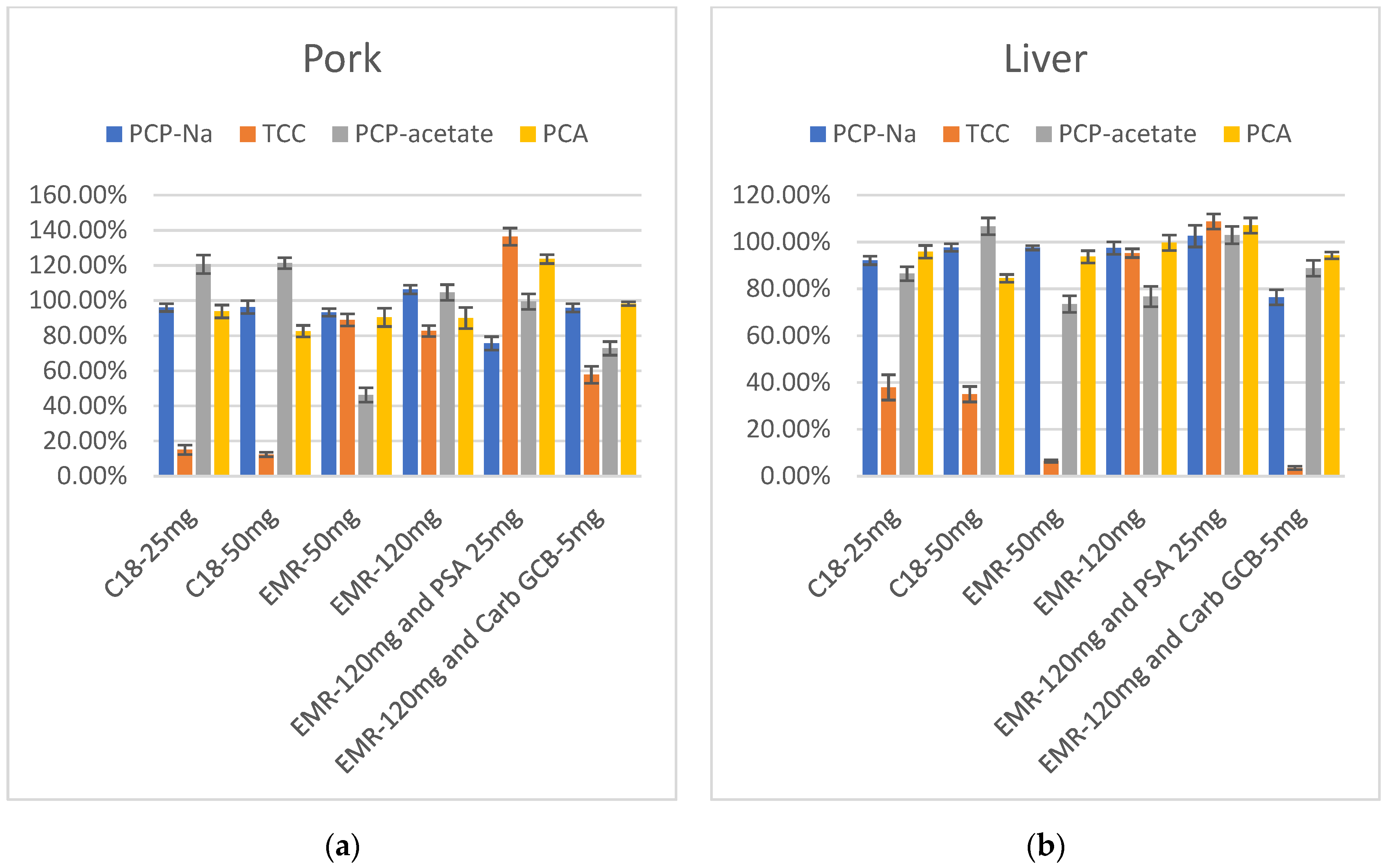
Swine samples contain high levels of fats and proteins, as well as numerous pigments in viscera tissues. Acetonitrile, as a widely used extraction solvent, is not only capable of extracting various types of drugs but also serves as an effective method for precipitating proteins from the matrix of animal samples. Additionally, the addition of acid to acetonitrile can enhance protein precipitation. Although an acidic acetonitrile solution can prevent a large number of proteins from dissolving into the extraction solvent, other impurities such as fats and pigments can also be introduced into the solvent. In order to minimize the impact of these impurities on the detection of target compounds and to employ a relatively simple and time-efficient purification step, this experiment employs a dispersion solid-phase extraction method to investigate adsorbents that are effective in removing fats and pigments. This study investigated six groups of purification agents, including traditional fat-removing filler C18 and newly developed specialized fat-removing filler EMR-Lipid; PSA (which effectively removes fatty acids); organic acids; some polar pigments and sugars; and Carb-GCB, which has strong adsorption effects on pigments, as well as their combinations.
The results showed that after purification with C18, the recovery rates of TCC were low in both pork and liver extracts, and increasing the amount of C18 had no significant impact on the results. Following purification with 50 mg EMR-Lipid, TCC showed improved recovery in muscle extract but remained low in liver extract, while PCP-acetate exhibited relatively low recovery. Increasing the amount of EMR-Lipid to 120 mg led to a notable improvement in the recovery of TCC in liver extract. PCP-acetate also showed significant improvement, especially in the pork extract. At this point, the recovery rates of these four target compounds—PCP-Na and its metabolites TCC, PCP-acetate, and PCA— in both pork and liver fell within the range of 60–120%. However, due to the dark color of liver extract, large-volume and long-term injection can contaminate the instrument and affect sensitivity. Therefore, the consideration of adding PSA or Carb-GCB to reduce the impact of pigments is necessary. PSA and Carb-GCB were separately mixed with EMR-Lipid for the purification of the extracts. The color of the liver extract became lighter in both cases. When PSA and 120 mg of EMR-Lipid were combined for purification, the recovery rates of the target compounds in liver were quite satisfactory. However, when Carb-GCB was mixed with 120 mg of EMR-Lipid, there was a significant decrease in the recovery rate of TCC. Therefore, the final purification method selected was 120 mg of EMR-Lipid for purifying the pork, while a combination of 120 mg of EMR-Lipid and 25 mg of PSA was used for purifying the liver samples (Figure 5).
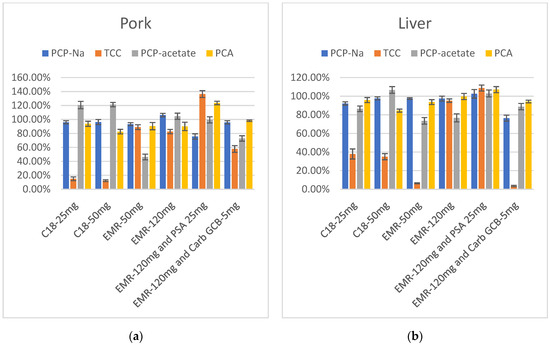
Figure 5.
(a) Recovery in purification optimization of PCP-Na, TCC, PCP-acetate, and PCA in pork (n = 3); (b) Recovery in purification optimization of PCP-Na, TCC, PCP-acetate, and PCA in liver (n = 3).
3.3. Method Validation
3.3.1. Accuracy and Precision
The accuracy of methods for detecting target compound residues is generally evaluated using the recovery rate. Spiked negative blank samples with the target component were analyzed, and five replicates were performed to calculate the mean recovery. Precision was determined by analyzing the relative standard deviation (RSD) of five replicate samples. The recoveries of PCP-Na, TCC, PCP-acetate, and PCA in muscle tissues (pork and fat) and viscera tissues (liver, heart, lung, kidney) ranged from 70.8 to 117.1%, 60.5 to 119.9%, 62.1 to 104.4%, 70.9 to 118.6%, 62.0 to 117.0%, and 60.3 to 118.8%, respectively. The RSDs for these compounds also ranged from 2.78 to 11.06%, 4.01 to 9.75%, 2.57 to 12.06%, 2.37 to 10.54%, 0.07 to 11.49% and 2.33 to 7.14%, respectively (Table 3).

Table 3.
Recovery and RSD of PCP-Na, TCC, PCP-acetate, and PCA in swine samples.
3.3.2. Linearity and Matrix Effect
The linearity of the developing method was investigated by calibration matrix curves with six concentration levels from 1 μg/kg to 200 μg/kg and evaluated by calculating the correlation coefficient (R2). The result showed good linearity with R2 ≥ 0.99 (Table 4).

Table 4.
Linear equations and R2 of PCP-Na, TCC, PCP-acetate, and PCA in swine samples.
The matrix effect is an important factor in the detection method used to assess the impact of different sample matrices on the ion intensity of the target compounds. It is classified into the enhancement effect and the suppression effect based on the influence on the instrument response signal. In this study, the matrix effect was represented by the slope of the matrix standard curve divided by the slope of the pure solvent standard curve. The results showed that most exhibited a clear enhancement effect, except for TCC, PCP-acetate, and PCA in the kidney, as well as PCP-acetate in the heart and lungs, which showed suppression effects. Due to the strong matrix effects observed for each target compound in different matrices, this method relies on matrix standard curves for quantitation.
3.3.3. Sensitivity
The limit of detection (LOD) was determined by calculating the three-fold signal-to-noise ratio (S/N) for the determination of standards and spiked samples. The LODs for the four target compounds were 0.5 μg/kg in all samples, including pork, fat, liver, heart, lungs, and kidney.
The limits of quantitation (LOQ) were determined by calculating the ten-fold signal-to-noise ratio (S/N) for the determination of standards and spiked samples. Additionally, the recovery needs to fall within the range of 60–120%. Therefore, the LOQs for PCP, PCP-acetate, and PCA were 1 μg/kg in all samples. The LOQs for TCC were 1 μg/kg in pork, fat, and lung, and 2 μg/kg in liver, heart, and kidney.
4. Conclusions
This study established a method for simultaneously detecting the residues of PCP-Na and its metabolites, TCC, PCP-acetate, and PCA, in swine tissues by adding recoveries to blank samples. By optimizing instrumental parameters, extraction solutions, buffering salts, and purification adsorbents, an efficient and resource-saving detection method has been developed. With this method, the metabolites of PCP-Na in pork and pig visceral samples can be detected and analyzed. The method provides a technical means to further explore the PCP-Na residues and metabolism in animal-derived food. This method is simple to operate, has good sensitivity, and covers a wide range of sample types. It not only helps to comprehensively analyze the residual status of PCP but also provides a reference for other related toxicological evaluations and in-vivo metabolic distribution studies.
Author Contributions
Conceptualization, J.Q., X.Y. and Y.Q.; methodology, Q.J., M.C., Y.L., Y.X. and J.Q.; validation, M.C., M.L. and X.Y.; formal analysis, Q.J., Y.L. and F.M.; investigation, Y.L., F.M., Y.X., Y.Q., X.Y. and J.Q.; resources, Y.Q. and X.Y.; data curation, Q.J. and M.L.; writing—original draft preparation, M.C.; writing—review and editing, Q.J. and J.Q.; visualization, Q.J., M.L. and M.C.; supervision, F.M. and Y.Q.; project administration, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Risk Assessment Project for Agro-product Quality and Safety with grant number GJFP20210301.
Data Availability Statement
Not applicable.
Acknowledgments
The research was supported by Key Laboratory of Feed and Livestock and Poultry Products Quality & Safety Control, Ministry of Agriculture and Rural Affairs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Farhadi, K.; Farajzadeh, M.; Matin, A.; Hashemi, P. Dispersive liquid-liquid microextraction and liquid chromatographic determination of pentachlorophenol in water. Open Chem. 2009, 7, 369–374. [Google Scholar] [CrossRef]
- WHO. Environmental Health Criteria 71-Pentachlorophenol; WHO: Geneva, Switzerland, 1987. [Google Scholar]
- Mäenpää, K.A.; Penttinen, O.-P.; Kukkonen, J.V.K. Pentachlorophenol (PCP) bioaccumulation and effect on heat production on salmon eggs at different stages of development. Aquat. Toxicol. 2004, 68, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.S.; Jones, S. Pentachlorophenol and Cancer Risk: Focusing the Lens on Specific Chlorophenols and Contaminants. Environ. Health Perspect. 2008, 116, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; International Agency for Research on Cancer Monograph Working Group. Carcinogenicity of pentachlorophenol and some related compounds. Lancet Oncol. 2016, 17, 1637–1638. [Google Scholar] [CrossRef] [PubMed]
- Spalding, J.W.; French, J.E.; Stasiewicz, S.; Furedi-Machacek, M.; Conner, F.; Tice, R.R.; Tennant, R.W. Responses of transgenic mouse lines p53(+/−) and Tg center dot AC to agents tested in conventional carcinogenicity bioassays. Toxicol. Sci. 2000, 53, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, N.; Mahmood, R. Protective effect of catechin on pentachlorophenol-induced cytotoxicity and genotoxicity in isolated human blood cells. Environ. Sci. Pollut. Res. 2020, 27, 13826–13843. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chaube, R. Toxicological Impact of Pentachlorophenol on the Hepatic and Reproductive Activity of the Stinging Catfish Heteropneustes fossilis. Aquac. Stud. 2019, 19, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Markovich, Z.R.; Hartman, J.H.; Ryde, I.T.; Hershberger, K.A.; Joyce, A.S.; Ferguson, P.L.; Meyer, J.N. Mild pentachlorophenol-mediated uncoupling of mitochondria depletes ATP but does not cause an oxidized redox state or dopaminergic neurodegeneration in Caenorhabditis elegans. Curr. Res. Toxicol. 2022, 3, 100084. [Google Scholar] [CrossRef] [PubMed]
- Namit, A.; Dowell, W.; Matiasek, S.; Webster, J.; Stachura, D.L. Pentachlorophenol has significant adverse effects on hematopoietic and immune system development in zebrafish (Danio rerio). PLoS ONE 2022, 17, e0265618. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Mortimer, D.; Rose, M.; Smith, F.; Steel, Z.; Panton, S. Recently listed Stockholm convention POPs: Analytical methodology, occurrence in food and dietary exposure. Sci. Total Environ. 2019, 678, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chen, S.; Song, A.H.; Huang, J.C.; Cheng, Y.M.; Liu, J.; Wang, Y. Establishment of trace pentachlorophenol and its sodium salt in animal-origin foods by automated solid phase extraction-coupled with ultra performance liquid chromatography-tandem mass spectrometry. Food Mach. 2019, 7, 80–86. [Google Scholar]
- Wang, L.Z.; Fang, E.H.; Wang, C.J.; Chen, Y.; Lin, Z.X.; Xu, D.M. Determination of trace pentachlorophenol and its sodium salt in animal-origin foods by QuEChERS-ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2018, 36, 518–522. [Google Scholar] [CrossRef]
- Awawdeh, A.M.; Harmon, H.J. Spectrophotometric detection of pentachlorophenol (PCP) in water using water soluble porphyrins. Sens. Actuators B Chem. 2005, 106, 234–242. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Park, S.; Lee, J.; Choi, J. Gas chromatographic determination of phenol in fish tissues as a phenyl acetate derivative following solvent extraction of acidified samples. Acta Chromatogr. 2020, 32, 64–67. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, C.; Yang, G.; Zhang, S.; Qian, X. Determination of sodium pentachlorophenoxide residues in animal-derived foods by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Agric. Biotechnol. 2022, 11, 99–101. [Google Scholar]
- State Administration for Market Regulation. Available online: https://spcjsac.gsxt.gov.cn/ (accessed on 22 October 2022).
- Yan, X.; Zhao, Q.; Yan, Z.; Chen, X.; He, P.; Li, S.; Fang, Y. Determination of Pentachlorophenol in Seafood Samples from Zhejiang Province Using Pass-Through SPE-UPLC-MS/MS: Occurrence and Human Dietary Exposure Risk. Molecules 2023, 28, 6394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).