Determination of the Relationship between the Granulometry and Release of Polyphenols Using LC-UV, and Their Antioxidant Activity of the Pulp Powder of the Moroccan Argan Tree, “Argania spinosa L.”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Plant Material
2.3. Methods
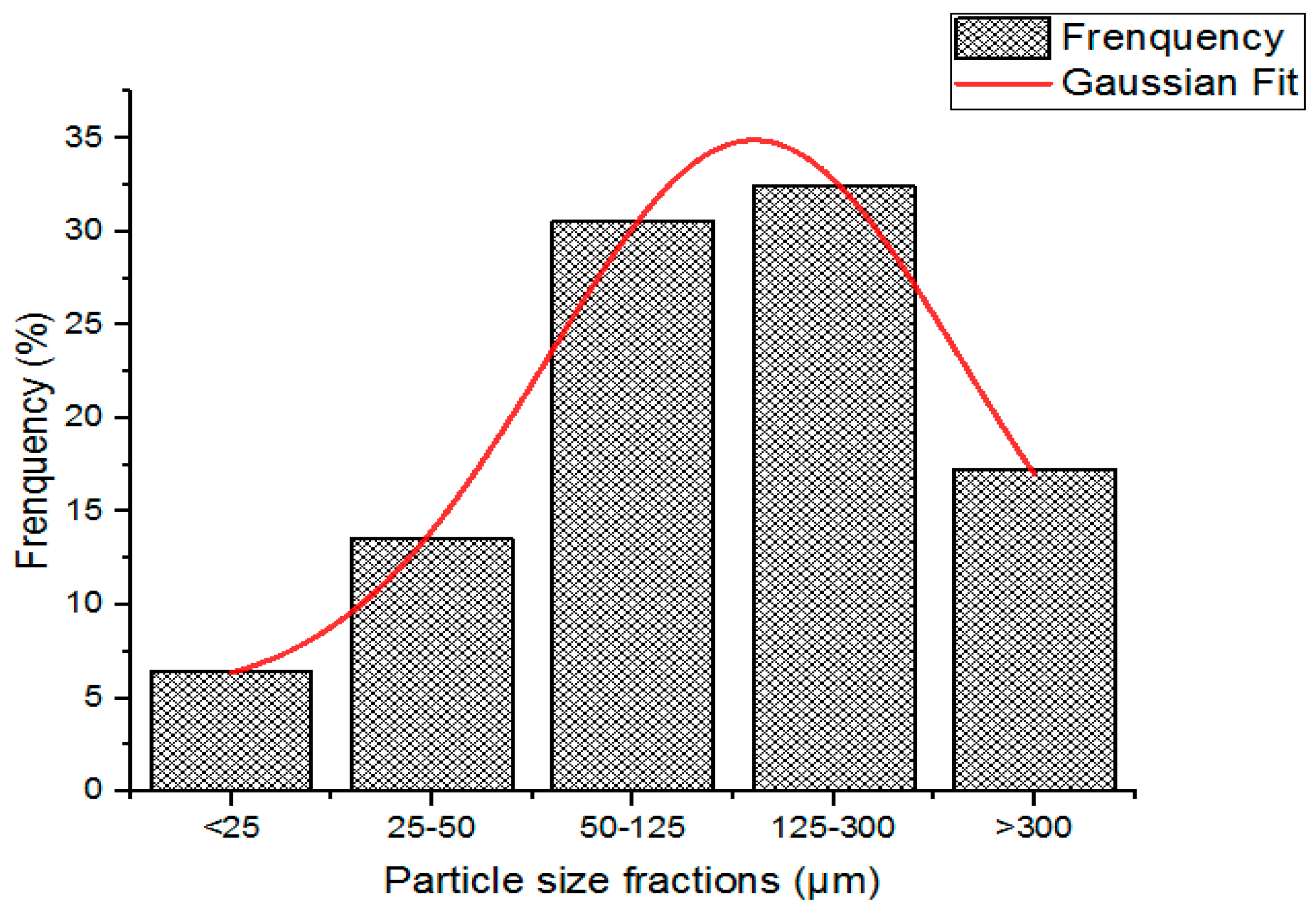
2.3.1. Particle Size Distribution
2.3.2. Determination of Sieve Powder Morphology
2.3.3. Total Polyphenol Content, Total Flavonoid Content
2.3.4. Molecular Analysis via LC-UV
2.3.5. GC-MS Analysis
2.3.6. Antioxidant Activity
2.3.7. Determination of Pigments: Chlorophylls, Carotenoids, and Pheophytins
2.3.8. Functional and Physicochemical Properties
2.4. Statistical Analysis
3. Results and Discussion
3.1. Particle Size Study
3.2. Particle Morphology (SEM Analysis)
3.3. Total Polyphenol Content and Total Flavonoid Content
3.4. Molecular Analysis by HPLC
3.5. Antioxidant Activity
3.6. GC-MS Analysis
3.7. Pigment Determination
3.8. Physicochemical Properties
3.9. Functional Properties
3.10. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashford, M. Bioavailability—Physicochemical and Dosage Form Factors. In Aulton’s Pharmaceutics; Elsevier Ltd.: Edinburgh, UK, 2013; pp. 314–333. [Google Scholar]
- Kumar, R.; Thakur, A.K.; Chaudhari, P.; Banerjee, N. Particle Size Reduction Techniques of Pharmaceutical Compounds for the Enhancement of Their Dissolution Rate and Bioavailability. J. Pharm. Innov. 2022, 17, 333–352. [Google Scholar] [CrossRef]
- Chaumeil, J. Micronization: A Method of Improving the Bioavailability of Poorly Soluble Drugs. Methods Find. Exp. Clin. Pharmacol. 1998, 20, 211–215. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Arfat, Y.A. Functional, Rheological, Microstructural and Antioxidant Properties of Quinoa Flour in Dispersions as Influenced by Particle Size. Food Res. Int. 2019, 116, 302–311. [Google Scholar] [CrossRef]
- Nabil, B.; Ouaabou, R.; Ouhammou, M.; Saadouni, L.; Mahrouz, M. Impact of Particle Size on Functional, Physicochemical Properties and Antioxidant Activity of Cladode Powder (Opuntia ficus-indica). J. Food Sci. Technol. 2020, 57, 943–954. [Google Scholar] [CrossRef]
- Rosiak, N.; Cielecka-Piontek, J.; Skibiński, R.; Lewandowska, K.; Bednarski, W.; Zalewski, P. Antioxidant Potential of Resveratrol as the Result of Radiation Exposition. Antioxidants 2022, 11, 2097. [Google Scholar] [CrossRef]
- Teleky, B.-E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly(Vinyl Alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef]
- Phupaboon, S.; Matra, M.; Prommachart, R.; Totakul, P.; Supapong, C.; Wanapat, M. Extraction, Characterization, and Chitosan Microencapsulation of Bioactive Compounds from Cannabis sativa L., Cannabis indica L., and Mitragyna speiosa K. Antioxidants 2022, 11, 2103. [Google Scholar] [CrossRef]
- Sobiecka, E.; Mroczkowska, M.; Olejnik, T.P. The Influence of Chlorpyrifos on the Nonenzymatic Antioxidants Content in Macrophytes Leaves. Antioxidants 2022, 11, 684. [Google Scholar] [CrossRef]
- Rodriguez, I.F.; Pérez, M.J.; Cattaneo, F.; Zampini, I.C.; Cuello, A.S.; Mercado, M.I.; Ponessa, G.; Isla, M.I. Morphological, Histological, Chemical and Functional Characterization of Prosopis alba Flours of Different Particle Sizes. Food Chem. 2019, 274, 583–591. [Google Scholar] [CrossRef]
- Savlak, N.; Türker, B.; Yeşilkanat, N. Effects of Particle Size Distribution on Some Physical, Chemical and Functional Properties of Unripe Banana Flour. Food Chem. 2016, 213, 180–186. [Google Scholar] [CrossRef]
- Goh, H.P.; Heng, P.W.S.; Liew, C.V. Comparative Evaluation of Powder Flow Parameters with Reference to Particle Size and Shape. Int. J. Pharm. 2018, 547, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Marinopoulou, A.; Biliaderis, C.G. Impact of Flour Particle Size and Hydrothermal Treatment on Dough Rheology and Quality of Barley Rusks. Food Hydrocoll. 2019, 87, 561–569. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Secondary Metabolites from Argania spinosa (L.) Skeels. Phytochem. Rev. 2002, 1, 345–354. [Google Scholar] [CrossRef]
- Charrouf, Z.; Guillaume, D. Ethnoeconomical, Ethnomedical, and Phytochemical Study of Argania spinosa (L.) Skeels. J. Ethnopharmacol. 1999, 67, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hilali, M.; Charrouf, Z.; Aziz Soulhi, A.E.; Hachimi, L.; Guillaume, D. Influence of Origin and Extraction Method on Argan Oil Physico-Chemical Characteristics and Composition. J. Agric. Food Chem. 2005, 53, 2081–2087. [Google Scholar] [CrossRef]
- Charrouf, Z.; Hilali, M.; Jauregui, O.; Soufiaoui, M.; Guillaume, D. Separation and Characterization of Phenolic Compounds in Argan Fruit Pulp Using Liquid Chromatography–Negative Electrospray Ionization Tandem Mass Spectroscopy. Food Chem. 2007, 100, 1398–1401. [Google Scholar] [CrossRef]
- Charrouf, V. Valorisation de l’arganier: Résultats et perspectives. In Proceedings of the 5e Colloque Produits Naturels d’origine Végétale, Quebec, QC, Canada, 7–9 August 2001; University of Quebec: Quebec, QC, Canada, 2002; pp. 261–270. [Google Scholar]
- El Adnany, E.M.; Elhadiri, N.; Mourjane, A.; Ouhammou, M.; Hidar, N.; Jaouad, A.; Bitar, K.; Mahrouz, M. Impact and Optimization of the Conditions of Extraction of Phenolic Compounds and Antioxidant Activity of Olive Leaves (Moroccan picholine) Using Response Surface Methodology. Separations 2023, 10, 326. [Google Scholar]
- Mourjane, A.; Hanine, H.; El Adnany, E.M.; Ouhammou, M.; Hidar, N.; Nabil, B.; Boumendjel, A.; Bitar, K.; Mahrouz, M. Energetic Bio-Activation of Some Organic Molecules and Their Antioxidant Activity in the Pulp of the Moroccan Argan Tree «Argania spinosa L.». Molecules 2022, 27, 3329. [Google Scholar] [CrossRef]
- Tranchant, J. Chromatographie en Phase Gazeuse, 3rd ed.; Masson: Paris, France, 1982. [Google Scholar]
- Abdel-Hameed, E.-S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, Nutritionals and Antioxidant Properties of Two Prickly Pear Cactus Cultivars (Opuntia ficus indica Mill.) Growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef]
- Boutakiout, A.; Elothmani, D.; Hanine, H.; Mahrouz, M.; Le Meurlay, D.; Hmid, I.; Ennahli, S. Effects of Different Harvesting Seasons on Antioxidant Activity and Phenolic Content of Prickly Pear Cladode Juice. J. Saudi Soc. Agric. Sci. 2018, 17, 471–480. [Google Scholar] [CrossRef]
- Lfitat, A.; Zejli, H.; Bousraf, F.Z.; Bousselham, A.; El Atki, Y.; Gourch, A.; Lyoussi, B.; Abdellaoui, A. Comparative Assessment of Total Phenolics Content and in Vitro Antioxidant Capacity Variations of Macerated Leaf Extracts of Olea europaea L. and Argania spinosa (L.) Skeels. Mater. Today Proc. 2021, 45, 7271–7277. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, Identification and Quantification of Carotenoids and Chlorophylls in Dietary Supplements Containing Chlorella vulgaris and Spirulina platensis Using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Cheng, Y.F.; Bhat, R. Functional, Physicochemical and Sensory Properties of Novel Cookies Produced by Utilizing Underutilized Jering (Pithecellobium jiringa Jack.) Legume Flour. Food Biosci. 2016, 14, 54–61. [Google Scholar] [CrossRef]
- Baljeet, S.Y.; Yadav, R.; Yadav, R. Studies on Functional Properties and Incorporation of Buckwheat Flour for Biscuit Making. Int. Food Res. J. 2010, 17, 1067–1076. [Google Scholar]
- Odoemelam, S.A. Functional Properties of Raw and Heat Processed Jackfruit (Artocarpus heterophyllus) Flour. Pak. J. Nutr. 2005, 4, 366–370. [Google Scholar] [CrossRef]
- Adebowale, Y.A.; Adeyemi, I.A.; Oshodi, A.A. Functional and Physicochemical Properties of Flours of Six Mucuna Species. Afr. J. Biotechnol. 2005, 4, 1461–1468. [Google Scholar]
- Association of Official Analytical Chemists. AOAC Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2007. [Google Scholar]
- Astello-García, M.G.; Cervantes, I.; Nair, V.; Santos-Díaz, M.d.S.; Reyes-Agüero, A.; Guéraud, F.; Negre-Salvayre, A.; Rossignol, M.; Cisneros-Zevallos, L.; Barba de la Rosa, A.P. Chemical Composition and Phenolic Compounds Profile of Cladodes from Opuntia spp. Cultivars with Different Domestication Gradient. J. Food Compos. Anal. 2015, 43, 119–130. [Google Scholar] [CrossRef]
- León-Martínez, F.M.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J. Spray Drying of Nopal Mucilage (Opuntia ficus-indica): Effects on Powder Properties and Characterization. Carbohydr. Polym. 2010, 81, 864–870. [Google Scholar] [CrossRef]
- Yu, D.; Chen, J.; Ma, J.; Sun, H.; Yuan, Y.; Ju, Q.; Teng, Y.; Yang, M.; Li, W.; Fujita, K.; et al. Effects of Different Milling Methods on Physicochemical Properties of Common Buckwheat Flour. Lebensm.-Wiss. Technol. 2018, 92, 220–226. [Google Scholar] [CrossRef]
- Tong, L.-T.; Gao, X.; Lin, L.; Liu, Y.; Zhong, K.; Liu, L.; Zhou, X.; Wang, L.; Zhou, S. Effects of Semidry Flour Milling on the Quality Attributes of Rice Flour and Rice Noodles in China. J. Cereal Sci. 2015, 62, 45–49. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, B.-C.; Sun, H.-J.; Zhang, J.-G.; Sun, Y.-H.; Wei, Z.-J. Physicochemical Properties and Adsorption of Cholesterol by Okra (Abelmoschus esculentus) Powder. Food Funct. 2015, 6, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process Preserv. 2017, 41, e13203. [Google Scholar]
- Razboršek, M.I.; Ivanovi’c, M.; Kolar, M. Validated stability-indicating GC-MS method for characterization of forced degradation products of trans-caffeic acid and trans-ferulic acid. Molecules 2021, 26, 2475. [Google Scholar]
- Zhao, C.; Liu, Y.; Lai, S.; Cao, H.; Guan, Y.; San Cheang, W.; Liu, B.; Zhao, K.; Miao, S.; Rivière, C.; et al. Effects of domestic cooking process on the chemical and biological properties of dietary phytochemicals. Trends Food Sci. Technol. 2019, 85, 55–66. [Google Scholar]
- Stavchansky, S.; Gowan, W.G. Evaluation of the Bioavailability of a Solid Dispersion of Phenytoin in Polyethylene Glycol 6000 and a Commercial Phenytoin Sodium Capsule in the Dog. J. Pharm. Sci. 1984, 73, 733–736. [Google Scholar] [CrossRef]
- Santos, F.A.; Frota, J.; Arruda, B.; Melo, T.; Castro Brito, G.; Chaves, M.; Rao, V. Antihyperglycemic and hypolipidemic effects of α,β-amyrin, a triterpenoid mixture from Protium heptaphyllum in the mouse. Lipids Health Dis. 2012, 11, 98. [Google Scholar]
- Ramírez-Moreno, E.; Córdoba-Díaz, D.; de Cortes Sánchez-Mata, M.; Díez-Marqués, C.; Goñi, I. Effect of Boiling on Nutritional, Antioxidant and Physicochemical Characteristics in Cladodes (Opuntia ficus indica). LWT-Food Sci. Technol. 2013, 51, 296–302. [Google Scholar] [CrossRef]
- Khales, A.; Baaziz, M. Quantitative and Qualitative Aspects of Peroxidases Extracted from Cladodes of Opuntia ficus indica. Sci. Hortic. 2005, 103, 209–218. [Google Scholar] [CrossRef]
- Qiu, Y.-K.; Chen, Y.-J.; Pei, Y.-P.; Hisashi, M.; Masayuki, Y. New constituents from the fresh stems of Opuntia dillenii. J. Chin. Pharm. Sci. 2003, 12, 1–5. [Google Scholar]
- Kang, Y.-R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, Characterization, and Functional Properties of Chlorophylls, Pheophytins, and Zn-Pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Mathlouthi, M. Water Content, Water Activity, Water Structure and the Stability of Foodstuffs. Water Determ. Food-Chall. Anal. 2001, 12, 409–417. [Google Scholar] [CrossRef]
- Becker, L.; Zaiter, A.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, E.; Scher, J.; Dicko, A. Improvement of Antioxidant Activity and Polyphenol Content of Hypericum perforatum and Achillea millefolium Powders Using Successive Grinding and Sieving. Ind. Crops Prod. 2016, 87, 116–123. [Google Scholar] [CrossRef]
- Becker, L.; Zaiter, A.; Petit, J.; Karam, M.-C.; Sudol, M.; Baudelaire, E.; Scher, J.; Dicko, A. How Do Grinding and Sieving Impact on Physicochemical Properties, Polyphenol Content, and Antioxidant Activity of Hieracium pilosella L. Powders? J. Funct. Foods 2017, 35, 666–672. [Google Scholar] [CrossRef]
- Cassia, R.; Azevedo, P.; Pereira, G.; Araújo, M. Development and Validation of Dissolution Test for Captopril in Capsules by HPLC. Rev. Bras. Ciênc. Farm. 2008, 44, 261–269. [Google Scholar]
- Appiah, F.; Patrick, K.; Sebe, A.T.; Asibuo, J. Physicochemical and Functional Properties of Seeds of Three Local Cowpea (Vigna unguilata L. Walp) Cultivars in Ghana. Acta Hortic. 2011, 911, 437–446. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, Q.-X.; Qiao, M.; Ma, F.-F.; Thakur, K.; Wei, Z.-J. Effect of Superfine Grinding on Properties of Vaccinium bracteatum Thunb Leaves Powder. Food Sci. Biotechnol. 2017, 26, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J. Effect of Particle Size and Temperature on Rheology and Creep Behavior of Barley β-d-Glucan Concentrate Dough. Carbohydr. Polym. 2014, 111, 89–100. [Google Scholar] [CrossRef]
- Costa, F.O.; Pais, A.A.C.C.; Sousa, J.J.S. Analysis of Formulation Effects in the Dissolution of Ibuprofen Pellets. Int. J. Pharm. 2004, 270, 9–19. [Google Scholar] [CrossRef]
- Ndife, J.; Kida, F.; Fagbemi, S. Production and Quality Assessment of Enriched Cookies from Whole Wheat and Full Fat Soya. Eur. J. Food Sci. Technol. 2014, 2, 19–28. [Google Scholar]





| Particle Size (µm) | >300 | 300–125 | 125–50 | 50–25 | ≤25 |
|---|---|---|---|---|---|
| Protocatechuic acid | 4.44 ± 0.78 | 15.57 ± 0.72 | 36.37 ± 0.82 | 108.87 ± 0.86 | 278.90 ± 2.07 |
| Caffeic acide | ND | ND | ND | ND | ND |
| Ferulic acid | 6.31 ± 0.49 | 34.58 ± 0.22 | 38.36 ± 0.32 | 43.96 ± 0.22 | ND |
| Hesperidin | ND | ND | ND | ND | ND |
| Salicylic acid | ND | ND | ND | ND | ND |
| Vanillic acid | ND | ND | ND | ND | ND |
| Catechin | 56.92 ± 1.03 | 259.11 ± 1.21 | 221.02 ± 0.91 | 170.82 ± 1.02 | 122.59 ± 1.24 |
| Chorogenic acid | 64.90 ± 0.92 | 350.99 ± 1.11 | 300.98 ± 1.17 | 199.12 ± 0.86 | 125.70 ± 1.28 |
| Epicathechin | 0.65 ± 0.28 | 601.65 ± 1.21 | 432.19 ± 1.33 | 291.05 ± 1.36 | 109.59 ± 1.06 |
| Vanillin | 6.98 ± 0.59 | ND | ND | ND | ND |
| p-Coumaric acid | ND | ND | ND | ND | ND |
| Sinapic acid | ND | ND | ND | 32.86 ± 0.75 | 43.76 ± 0.55 |
| Naringin | ND | ND | ND | ND | ND |
| Rutin | 88.36 ± 0.92 | 522.58 ± 1.10 | 369.78 ± 1.02 | 288.96 ± 1.16 | 211.78 ± 1.44 |
| Quercetin | ND | 56.97 ± 0.27 | 46.77 ± 0.43 | 28.99 ± 0.63 | 17.77 ± 0.31 |
| Kaempferol | 62.12 ± 1.22 | 63.07 ± 0.09 | 99.27 ± 0.29 | 179.55 ± 0.89 | 304.39 ± 1.81 |
| Totale | 290.70 | 1904.52 | 1544.14 | 1344.18 | 1290.71 |
| Compound | RT (min) | Mol. Formula | >300 | <300; >125 | <125; >50 | <50; >25 | <25 |
|---|---|---|---|---|---|---|---|
| Cis-p-mentha-1(7),8-dien-2-ol (%) | 7.82 | C10H16O | ND | ND | 0.36 ± 0.01 | 0.39 ± 0.01 | 0.36 ± 0.01 |
| Retinal (%) | 11.20 | C20H28O | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.09 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Lycophyll (%) | 22.77 | C40H56O2 | 0.01 | 0.01 | 0.02 | 0.04 | 0.04 |
| Androstatriene, 3-hydroxy-17-oxo (%) | 46.26 | C19H24O2 | 0.02 ± 0.00 | ND | ND | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Carotene, 3,4-didehydro-1,2-dihydro-1 m Ethoxy (%) | 14.54 | C41H58O | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 |
| Olean-12-en-3-ol, acetate (%) | 49.82 | C32H52O2 | 1.55 ± 0.03 | 3.1 ± 0.12 | 4.32 ± 0.11 | 1.16 ± 0.05 | 1.18 ± 0.02 |
| Amyrin (%) | 49.78 | C30H50O | 0.18 ± 0.01 | 0.2 ± 0.03 | 0.21 ± 0.01 | 0.08 ± 0.00 | 0.04 ± 0.00 |
| 2-Hydroxychalcone (%) | 34.13 | C15H12O2 | ND | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Ethyl iso-allocholate (%) | 21.88 | C26H44O5 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| Spirost-8-en-11-one, 3-hydroxy (%) | 51.78 | C27H40O4 | 0.01 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.00 | 0.03± 0.00 |
| Betulin (%) | 28.91 | C30H50O2 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.06 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.00 |
| Lupeol (%) | 45.97 | C30H50O | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Octamethyl-docosahydropicene-3,13-diol (%) | 46.13 | C30H52O2 | 0.07 ± 0.00 | 0.01 ± 0.00 | ND | 0.02± 0.00 | 0.06 ± 0.00 |
| Betulinaldehyde (%) | 46.28 | C30H48O2 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.08 ± 0.00 | 0.04 ± 0.00 |
| Carbenoxolone (%) | 46.32 | C34H50O7 | ND | 0.07 ± 0.00 | ND | ND | 0.07 ± 0.00 |
| Astaxanthin (%) | 14.05 | C31H50O3 | ND | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.01 ± 0.00 |
| Urs-12-en-28-oic acid, 3-hydroxy-, methyl ester (%) | 46.24 | C30H50O2 | 0.05 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 | 0.06 ± 0.00 | ND |
| Size Fractions (µm) | <25 | 25–50 | 50–125 | 125–300 | >300 |
|---|---|---|---|---|---|
| Moisture (%) | 6.41 ± 0.57 | 6.57 ± 0.12 | 6.76 ± 0.09 | 5.31 ± 0.07 | 5.68 ± 0.40 |
| Ash (%) | 6.19 ± 0.27 | 5.63 ± 0.11 | 4.63 ± 0.26 | 4.44 ± 0.45 | 4.22 ± 0.30 |
| pH | 4.52 ± 0.03 | 4.39 ± 0.06 | 4.34 ± 0.04 | 3.99 ± 0.05 | 3.85 ± 0.08 |
| Titrable acidity (% citric acide) | 3.66 ± 0.05 | 3.71 ± 0.07 | 3.45 ± 0.07 | 3.13 ± 0.07 | 3.09 ± 0.03 |
| soluble solide (°Brix) | 4.23 ± 0.06 | 5.93 ± 0.06 | 7.07 ± 0.06 | 9.33 ± 0.06 | 4.97 ± 0.12 |
| WAC (%) | 14.17 ± 0.29 | 15.99 ± 0.71 | 17.65 ± 0.38 | 22.43 ± 0.40 | 26.18 ± 0.17 |
| OAC (%) | 8.22 ± 0.19 | 10.11 ± 0.11 | 14.19 ± 0.32 | 16.26 ± 0.23 | 16.43 ± 0.38 |
| SC (mL) | 14.77 ± 0.25 | 14.50 ± 0.50 | 14.33 ± 0.58 | 16.67 ± 0.58 | 16.17 ± 0.29 |
| LGC (%) | 20.00 ± 0.00 | 18.00 ± 0.00 | 16.00 ± 0.00 | 14.00 ± 0.00 | 14.00 ± 0.00 |
| BD (g/cm3) | 0.31 ± 0.02 | 0.34 ± 0.01 | 0.42 ± 0.01 | 0.60 ± 0.01 | 0.62 ± 0.01 |
| WSI (g/100 g) | 47.63 ± 0.37 | 43.61 ± 0.42 | 44.12 ± 0.75 | 39.84 ± 0.69 | 33.52 ± 0.47 |
| FC (%) | 39.83 ± 0.76 | 29.00 ± 1.00 | 15.33 ± 1.15 | 15.18 ± 1.29 | 13.67 ± 0.28 |
| FS (%) | 18.23 ± 0.25 | 14.10 ± 0.36 | 8.33 ± 0.58 | 6.88 ± 0.34 | 7.00 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourjane, A.; Hanine, H.; El Adnany, E.M.; Ouhammou, M.; Bitar, K.; Mahrouz, M.; Boumendjel, A. Determination of the Relationship between the Granulometry and Release of Polyphenols Using LC-UV, and Their Antioxidant Activity of the Pulp Powder of the Moroccan Argan Tree, “Argania spinosa L.”. Separations 2023, 10, 542. https://doi.org/10.3390/separations10100542
Mourjane A, Hanine H, El Adnany EM, Ouhammou M, Bitar K, Mahrouz M, Boumendjel A. Determination of the Relationship between the Granulometry and Release of Polyphenols Using LC-UV, and Their Antioxidant Activity of the Pulp Powder of the Moroccan Argan Tree, “Argania spinosa L.”. Separations. 2023; 10(10):542. https://doi.org/10.3390/separations10100542
Chicago/Turabian StyleMourjane, Ayoub, Hafida Hanine, El Mustapha El Adnany, Mourad Ouhammou, Khalid Bitar, Mostafa Mahrouz, and Ahcene Boumendjel. 2023. "Determination of the Relationship between the Granulometry and Release of Polyphenols Using LC-UV, and Their Antioxidant Activity of the Pulp Powder of the Moroccan Argan Tree, “Argania spinosa L.”" Separations 10, no. 10: 542. https://doi.org/10.3390/separations10100542
APA StyleMourjane, A., Hanine, H., El Adnany, E. M., Ouhammou, M., Bitar, K., Mahrouz, M., & Boumendjel, A. (2023). Determination of the Relationship between the Granulometry and Release of Polyphenols Using LC-UV, and Their Antioxidant Activity of the Pulp Powder of the Moroccan Argan Tree, “Argania spinosa L.”. Separations, 10(10), 542. https://doi.org/10.3390/separations10100542








