Abstract
Phyllanthus is a large genus of the Euphorbiaceae family, which has been widely used in traditional medicine. The current study reports the isolation of an unusual C-glycosyl flavonoid, named tenelloside, from Phyllanthus tenellus Roxb., a non-endemic plant present in Canary Islands. The chemical structure of this secondary metabolite was established employing combined spectrometric and spectroscopic techniques, including 1D and 2D NMR experiments and mass spectrometry. The absolute stereochemical configuration was determined via the comparison of experimental and calculated electronic circular dichroism spectra. In our previous work, another C-glycosylated bioactive product was isolated from another Phyllanthus species, suggesting that this class of compounds can be produced in a genus-specific manner in different geographic regions. This work represents another important report for direct future studies on the biological and chemotaxonomic potential of C-glycosylated products of the Phyllanthus genus.
1. Introduction
Natural products have been used by mankind since ancient times, and the ethnopharmacological interest in natural bioactive compounds has recently been on the rise because medicinal plants still represent a good source of novel natural therapeutic drugs [1,2,3,4,5]. Phyllanthus (Euphorbiaceae) is a large plant genus widely distributed in tropical and subtropical areas of Africa, America, and Asia [6]. Since ancient times, plants of this genus have been used in traditional medicine and ethnopharmacology for the treatment of several pathological conditions [6]. This genus, consisting of more than 700 species, can be classified into 11 subgenera. Several therapeutic properties have been attributed to plants from this genus, such as antipyretic, antibacterial, antiparasitic, anticontraceptive, and antiviral activities. Infusions of the leaves, fruits, barks, and roots of many Phyllanthus species have been used. Phyllanthus species plants have been used for the treatment of kidney and urinary disorders, intestinal infections, hypertension, malaria, diabetes, and hepatitis B. Preclinical and clinical studies carried out with the extracts of and purified compounds from these plants support most of their reported uses in traditional medicine. Species such as P. amarus, P. emblica, P. niruri, and P. urinaria are among the most investigated, exhibiting a broad pharmacological spectrum of antiviral, antimicrobial, and anti-inflammatory properties. The crude extracts of species such as Phyllanthus amarus and Phyllanthus emblica have been reported to provide antioxidant and anti-genotoxic properties [7]. Phyllanthus tenellus Roxb., commonly known as long-stalked Phyllanthus, is an annual medicinal herb traditionally used as a stone breaker to treat urolithiasis or kidney stones. This herb exhibits various pharmacological properties, including immunomodulatory, analgesic, anti-inflammatory, antimicrobial, antifungal, anti-hepatitis, antidiabetic, and antitumor activities. Additionally, the plant extract has proven effective in treating kidney, urinary bladder, and intestinal disorders [8]. The hydroalcoholic extract obtained from the leaves of this plant showed promising effects in cell cultures and mice models. P. tenellus extracts were demonstrated to be able to stimulate nitric oxide production in macrophage cultures, suggesting a direct activation effect on cells involved in inflammation processes [9,10].
Phytochemical analysis of Phyllanthus tenellus revealed the presence of medicinally important metabolites such as niranthin, nirtetralin, hinoquinine, and geranin. Niranthin demonstrates potent anti-hepatitis B surface antigen activity, while nirtetralin and nirtetralin A and B effectively suppress the secretion of hepatitis B virus (HBV). Geranin exhibits remarkable antioxidant and antihyperglycemic properties, and niranthin possesses antiviral and anticancer activities [11].
Phyllanthin and hypophyllanthin are the two most significant bioactive lignans identified in Phyllanthus species. Numerous studies have validated the cardioprotective, anti-hepatitis, antiviral, antifibrotic, anti-inflammatory, immunomodulatory, nephroprotective, and anticancer properties of phyllanthin and hypophyllanthin [11].
The Phyllanthus tenellus plant is less common than its close relatives, P. niruri and P. amarus. However, Phyllanthus tenellus has been reported to have a number of beneficial properties, including anti-hepatitis virus activity, UV protection, and immunomodulatory effects. It also has in vitro antioxidant activity, which contributes to its in vitro hepatoprotective effect. In vivo studies have shown that Phyllanthus tenellus can strengthen reduced glutathione peroxidase (GSH-Px) in the liver to promote recovery from carbon tetrachloride-induced liver damage. Additionally, Phyllanthus tenellus has antimicrobial activity and analgesic effects against neurogenic and inflammatory pain [9,11,12].
Despite its potential benefits, more research is needed to fully understand the safety of Phyllanthus tenellus. One study has shown that the ethanol extract of the aerial part of the plant is not toxic up to 2.5 g/kg body weight. However, at this dosage, rats treated with the extract exhibited depressant behavior [8,12]. This effect is thought to be due to the presence of corilagin in the extract, which inhibits prolyl oligopeptidase and acetylcholinesterase [12]. Overall, Phyllanthus tenellus is a promising plant with a variety of potential therapeutic benefits. However, more research is needed to fully understand its safety and efficacy.
The genus Phyllantus also includes Caribbean endemic plants, which are widely used by traditional medical practitioners for the treatment of different types of diseases [13]. Our recent work on the Cuban endemic Phyllanthus orbicularis Kunth elucidated the natural product responsible for the plant’s anti-inflammatory activity and identified this compound as a new C-glycoside flavonoid [14]. The scientific literature on this class of natural products still lacking, also because these compounds are very rare and their occurrence in nature is less represented with respect to their O-glycosyl-linked analogs [15,16,17,18]. The extreme interest in these compounds arises from the bioactivity profiles that C-glycosides can confer to poorly bioavailable active aglycones. Compared with common glycosyl natural products (O- and N-), C-glycosides present minimal conformational differences, with the advantage of being resistant to enzymatic or acidic hydrolysis, since the anomeric center of the acetal group is converted to an ether moiety. C-linked glycosides, as well as the enzymes involved in their metabolism (C-glycosyltranferases and C-glycosidases), are extremely rare in nature and absent in human metabolism. These features confer unique pharmacokinetic and biodistribution profiles to these molecules [19]. The bioavailability of C-glycosyl flavonoids is different from that of O-glycosyl flavonoids. While O-glycosyl flavonoids are readily absorbed in the small intestine and then deglycosylated in the liver, C-glycosyl flavonoids are not readily absorbed in the small intestine and remain intact until they reach the colon. In the colon, C-glycosyl flavonoids can be deglycosylated by bacteria to produce their aglycone forms. The presence of intact C-glycosyl flavonoids in human urine suggests that they may have some biological activity. Studies have shown that C-glycosyl flavonoids can be metabolized by the liver to produce active metabolites [20]. C-glycosylation may be a way for plants to protect flavonoids from degradation in the environment. C-glycosyl flavonoids are more stable than O-glycosyl flavonoids and are therefore less likely to be degraded by enzymes or UV radiation. The bioavailability and metabolism of C-glycosyl flavonoids is still being investigated. However, the available evidence suggests that they are a promising class of bioactive compounds with potential health benefits [19,20].
Although the plant genus Phyllanthus is not autochthonous in Europe, in recent years, the presence of Phyllanthus plants has been reported in some European islands. In particular, the presence of Phyllanthus tenellus Roxb. has been reported in the Canary Islands (Spain) and Sicily (Italy) [21]. In Europe, P. tenellus is known as a neophyte, and the many recent records in Sicily and the Canary Islands confirm that currently, P. tenellus must be considered a “naturalized European species”.
In the present work, a novel acetylated C-glycosyl flavanone from the non-endemic Canarian Phyllanthus tenellus was isolated for the first time. The compound, 6-C-(2″,4″,6″-triacetyl-β-D-glucopyranosyl)-(2S)-5,7,3′,4′-tetrahydroxyflavan-4-one, named tenelloside, was purified from the ethanolic extract via liquid–liquid partition, column chromatography, and preparative thin-layer chromatography, and its chemical structure was elucidated using combined spectroscopic and spectrometric data (i.e., NMR, HR-MS, MS/MS and UV–vis, and ECD). The isolation and characterization of this novel C-glycosyl flavonoid represent important phytochemical results and an additional chemotaxonomic report for the genus Phyllanthus. Furthermore, the continuous discovery of C-glycosyl products serves as a starting point for the study and characterization of the enzymes involved in the C-glycosylation of phenolic compounds and for the investigation of the bioactive profiles of these naturally occurring compounds.
2. Results and Discussion
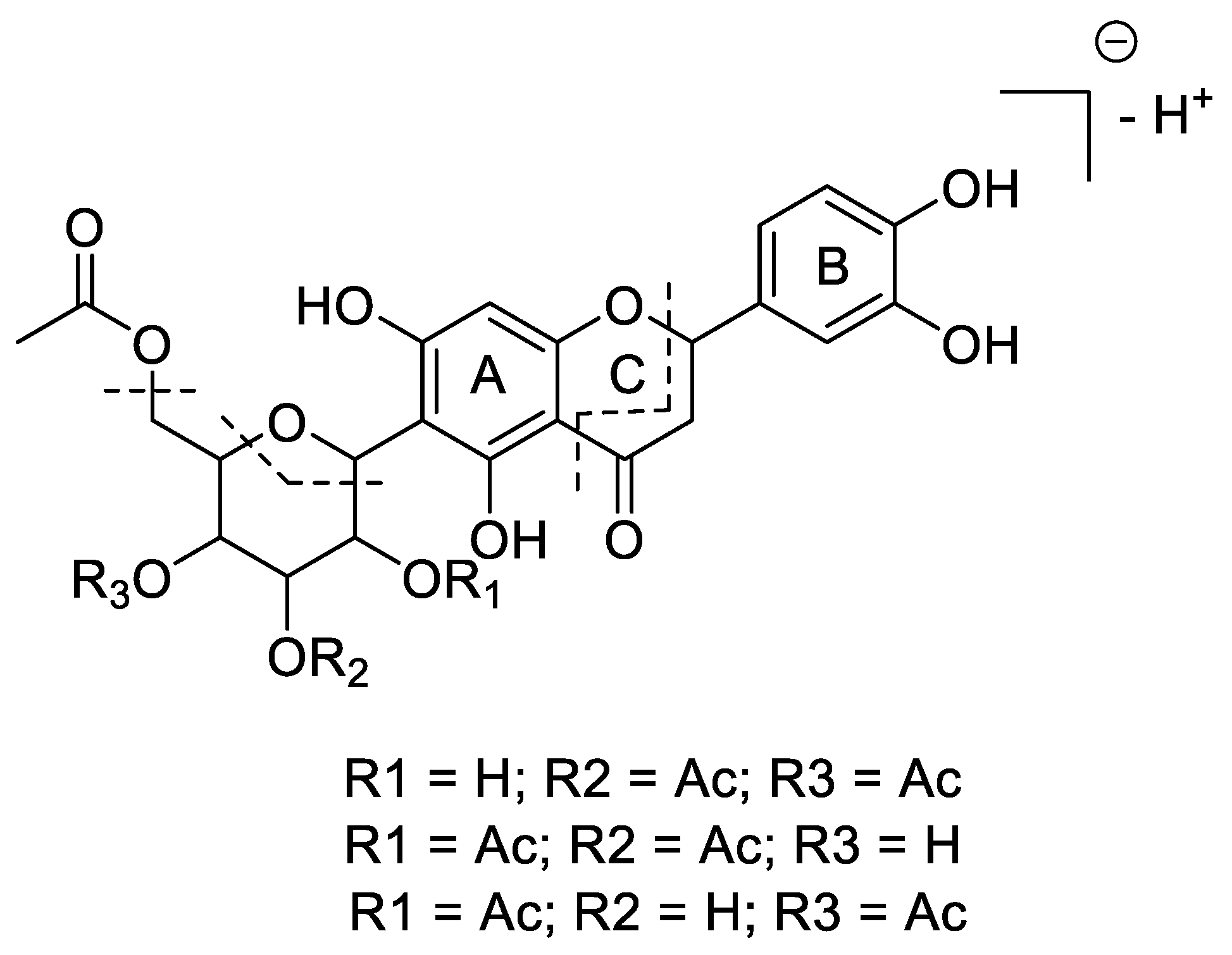
The ethanolic extract of Phyllanthus tenellus leaves was partitioned into CH2Cl2/EtOAc/H2O fractions. The EtOAc fraction was subjected to different chromatographic steps, including column chromatography and preparative TLC chromatography, to yield Compound 1 (see Experimental Section). The structure of the new compound was established as described below. The combined spectroscopic and spectrometric data display an array of signals in agreement with the presence of a C-glycosylated flavanone skeleton with three acetate, and one hexose, on penta-substituted and tri-substituted aromatic rings. The compound possesses a UV absorption maximum at λmax 290 nm, which suggests the presence of a conjugated flavonoid skeleton of flavanone. The high-resolution mass spectrometric analysis in negative mode gave rise to the C27H28O14− (m/z 575.1400, calcd. as 575.1406) molecular formula. The pseudo-molecular ion (575 m/z) was subjected to MS/MS fragmentation to produce two major fragments at 515 m/z and 311 m/z. These two fragments correspond to a loss of an acetate moiety [(M-H)-60 m/z] and a loss of a 262 m/z fragment that may be a part of a C-linked acetylated hexose moiety (the positions of the acetyl moieties were elucidated via NMR experiments as follows). The MS3 fragmentation of 311 m/z generated ions at m/z 162 and 149, resulting from the retro-Diels–Alder fragmentation of flavonoid ring C, indicating the position of the glycoside moiety to be on ring A (Supplementary Figures S1 and S2) [22]. The proposed MS/MS fragment pattern reported in Figure 1 is in agreement with the NMR-based elucidated structure.
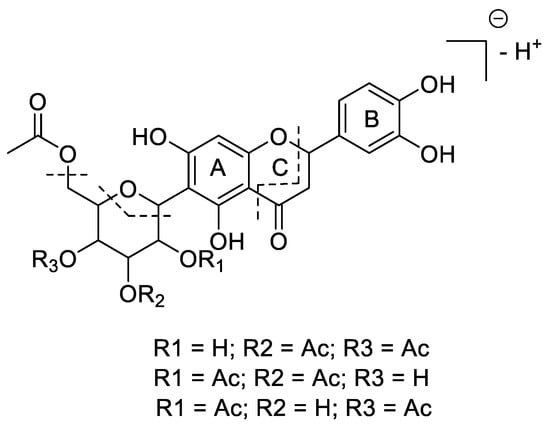
Figure 1.
Mass spectrometric fragmentation pattern and proposed structure for Compound 1 (regio-substitution of acetyl groups in sugar moiety was established via NMR experiments).
The 1H-NMR data revealed the presence of signals for three acetyl group at δ 1.82 (3H, s) and 2.09 (6H, s) and seven protons for a glycoside moiety at δ 4.91 (1H, H-1″); 5.69 (1H, t, J = 9.6 Hz, H-2″); 3.78 (1H, t, J = 9.4 Hz, H-3″); 4.98 (1H, t, J = 9.6 Hz, H-4″); 3.75 (1H, m, H-5″); 4.28 (1H, dd, J = 5.0, 12.6 Hz, Ha-6″); and 4.12 (1H, d, J = 12.6 Hz, Hb-6″).
In addition, the 1H NMR spectrum (Table 1) displayed proton signals for the flavanone skeleton, with an ABX spin system, including resonances at δ 5.72 (dd, JAX = 2.7, JBX = 13.7 Hz, H-2), 3.00 (dd, JAX = 2.7 Hz, JAB = 17.2 Hz, H-3a), and 2.78 (dd, JBX = 13.7 Hz, JAB = 17.2 Hz, H-3b). Furthermore, four protons in the aromatic range for the two phenolic rings with signals at δ 6.14 (s, H-8) and an ABX pattern with signals at δ 6.33 (1H, d, JAB = 2.0 Hz, H-2′), 6.48 (1H, dd, JAB = 2.0, JBX = 8.4 Hz, H-5′), and 7.57 (1H, d, JBX = 8.4 Hz, H-6′) were displayed, whose multiplicity and coupling constants were indicative of a penta-substituted A-ring and a 1,3,4-tri-substituted B-ring (Supplementary Figure S3).

Table 1.
1H (600 MHz) and 13C (150 MHz) NMR (δH a, CD3OD) data b of Compound 1.
The 13C NMR spectrum (Table 1) showed peaks corresponding to twenty-seven carbons (Supplementary Figure S4), which were categorized into three CH3, two sp3 CH2, six sp3 CH, four sp2 CH, eight sp2 quaternary carbons, three ester-carbonyl carbons, and one keto-carbonyl carbon via the connectivity analysis of the heteronuclear 2D experiment of HSQC, highlighting among all the 13C NMR spectroscopic data the presence of the characteristic signals for C-linked hexose at δ 103.7 (C-6) and 73.1 (CH-1″).
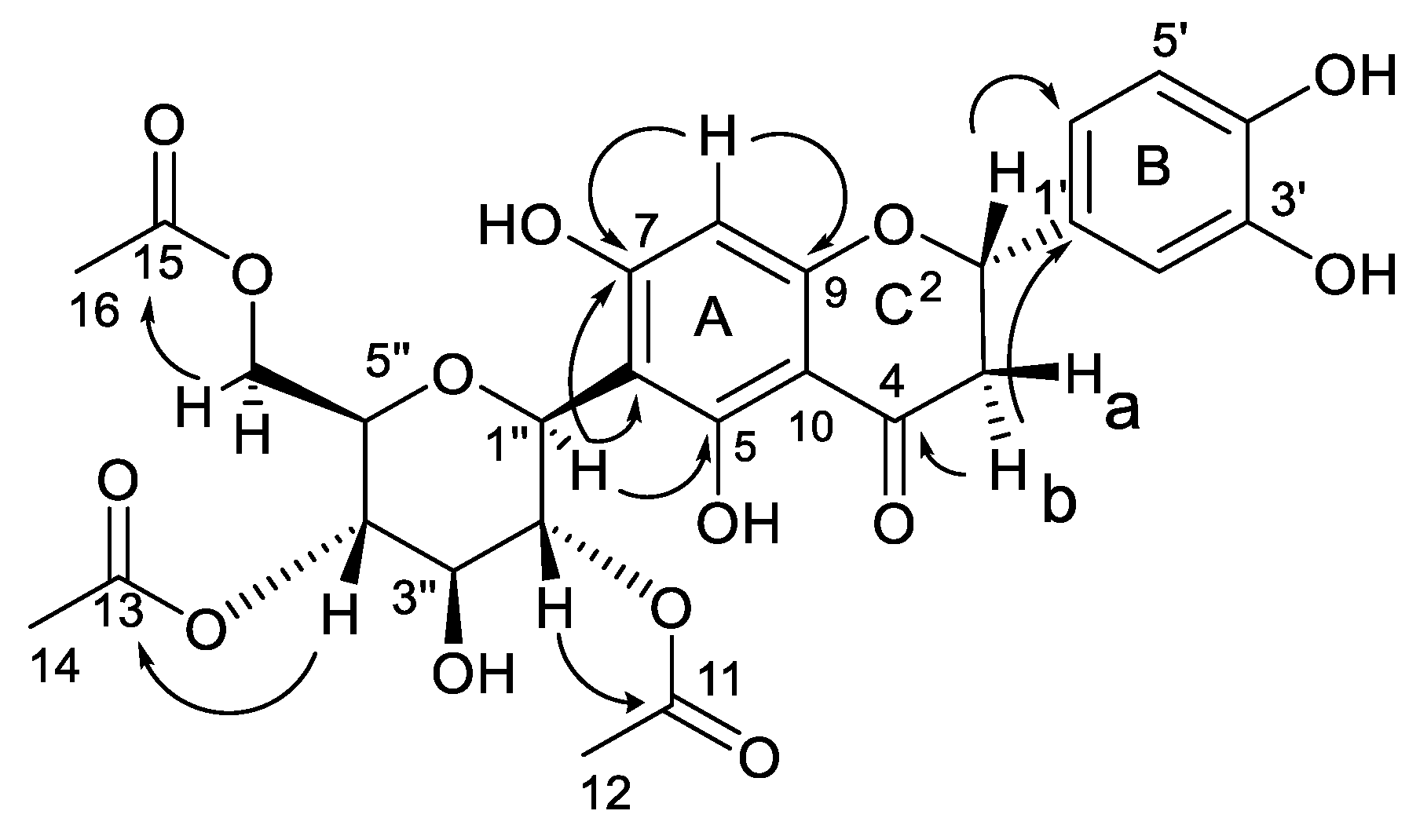
The NMR data from the 2D experiments with COSY, ROESY, HSQC, and HMBC (Supplementary Figures S5–S10) permitted the accurate and full assignment of the 1H and 13C NMR chemical shifts (Table 1), substitution patterns, and relative configurations of the different functional groups in the carbon skeleton. Combination analysis of the coupling constants and the homonuclear 2D-NMR COSY experiment permitted us to identify the spin systems in the flavonoid skeleton and sugar moiety. Thus, COSY correlations established two spin systems in the aglycone, a C-CH-C-C-CH–CH spin system for the 1,3,4-trisubstituted aromatic ring B (H-2′, H-5′, and H-6′) and a CH-CH2 spin system for H-2 and H2-3 for ring C. Moreover, a CH-CH-CH-CH-CH-CH2 spin system was observed for the glycone formed by H-1″, H-2″, H-3″, H-4″, H-5″, and H2-6″. The substitution of 1 was established with an HMBC analysis (Figure 2), showing a cross-peak between the signal at δH 2.78 (H-3b) and the signals at δC 199.0 (C-4) and 119.0 (C-1′). The connectivity of the signal at δH 5.91 (H-8) with the signals at δC 166.6 (C-7) and 165.2 (C-9) supported the flavanone skeleton. Moreover, the long-range 1H-13C correlation of the methine proton at δH 4.91 (H-1″) with the signals at δC 165.0 (C-5), 103.7 (C-6), and 166.6 (C-7) located the C-linkage between aglycone and sugar. Finally, the acetate groups were located in the C-2″, C-3″, and C-6″ positions by the three-bond connectivity between the carboxyl signals of the acetate groups at δC 171.7, 171.9, and 172.8 with the signals at δH 5.69 (H-2″), 3.78 (H-3″), 4.12, and 4.28 (H2-6″), respectively.
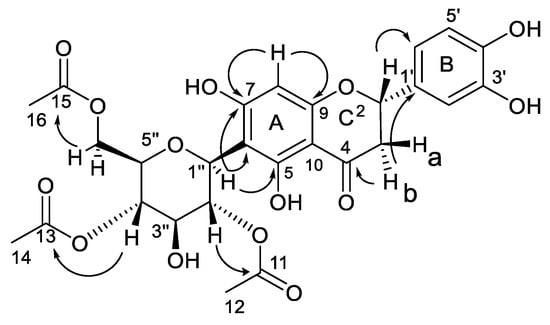
Figure 2.
Key 2D NMR HMBC correlations.
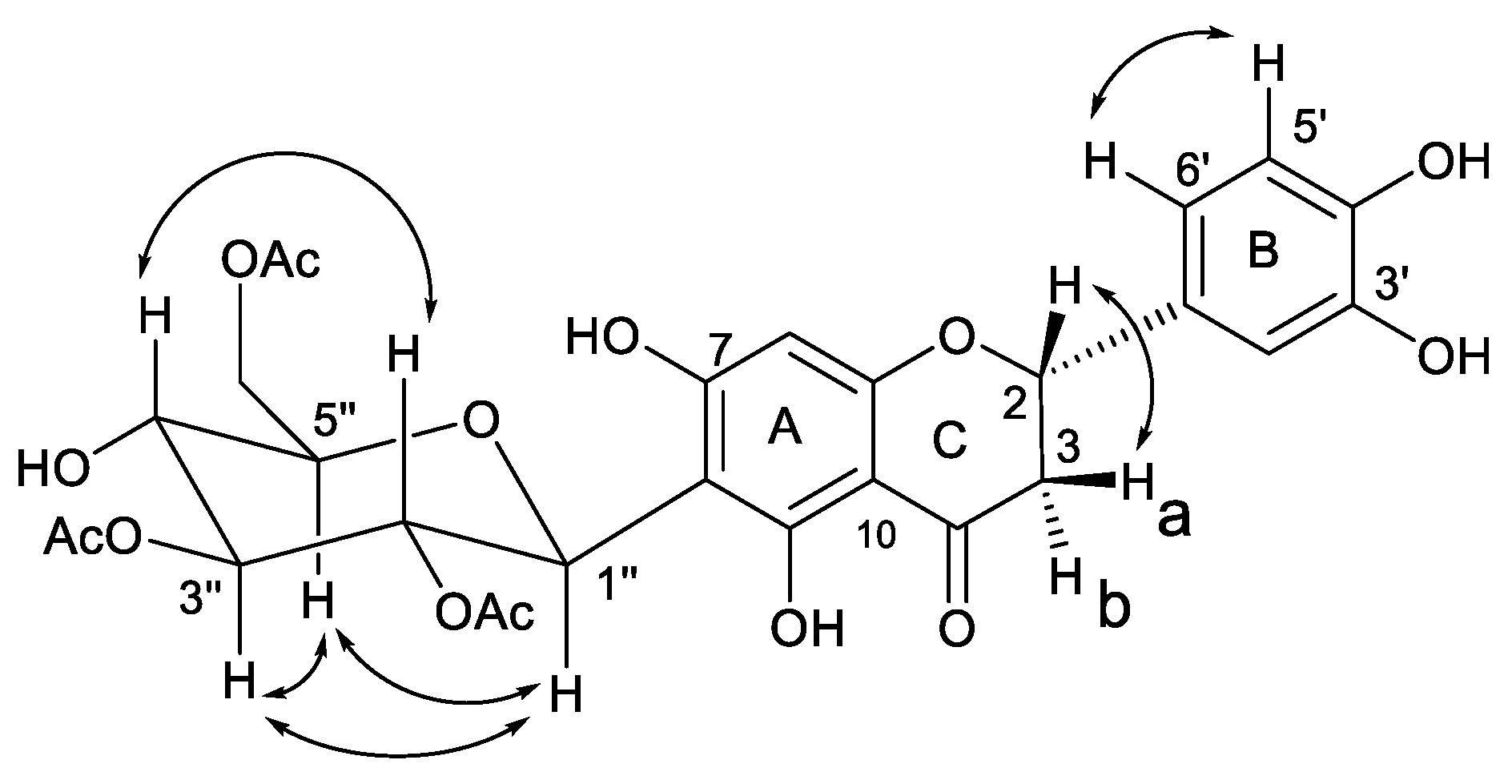
The ROESY connectivity and coupling constants of 1 allowed us to determine the relative stereochemistry. In the ROESY spectrum of 1, the correlations of H-5″ with H-3″and H-1″, and, jointly, the connectivity of H-2″ with H-4″, established the relative orientation of the glucopyranoside moiety. Moreover, a ROESY cross-peak between H-2 and H-3b evidenced the relative configuration at the C-2 position in the flavanone skeleton (Figure 3).
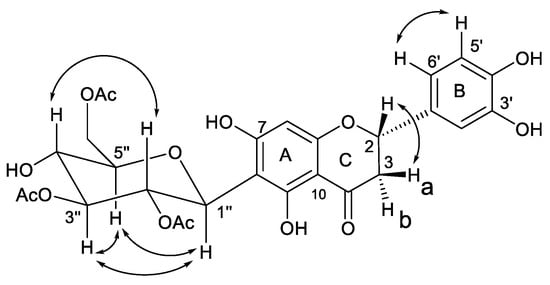
Figure 3.
Key correlations in homonuclear 2D NMR ROESY experiment.
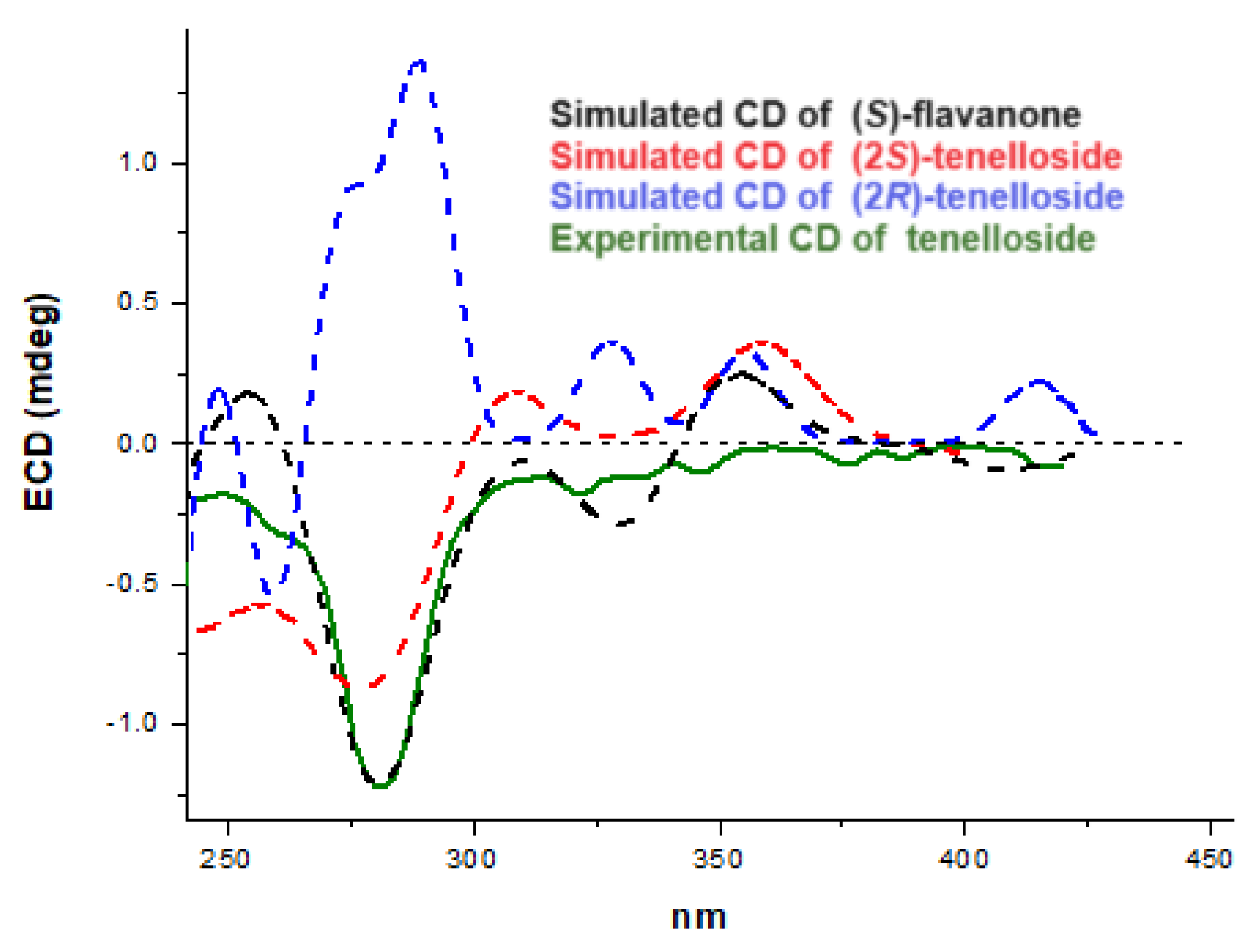
As mentioned above, the coupling constants of the H-2 hydrogen of the chiral carbon indicate an axial relative configuration of ring B. The absolute stereochemistry at this C-2 carbon was unambiguously determined by comparing the calculated and experimental electronic circular dichroism (ECD) spectra. Thus, ECD calculations were performed with the optimized enantiomer of flavanone in the (S)-configuration as well as with the most stable geometry of the tellenoside diastereomers in both the (2S)- and (2R)-configurations, using a time-dependent density functional theory method. The experimental ECD spectrum of 1 showed a negative Cotton effect (CE) at λmax 290 nm and showed good agreement with the calculated ECD data for (S)-flavanone and (2S)-tellenoside (Figure 4); therefore, the absolute configuration of 1 was determined as 2S. Our data confirmed the chiral biogenetic nature of the C-2 carbon, which involves the activity of a chalcone isomerase enzyme that stereospecifically yields the 2S enantiomer in the C-ring of this type of flavonoid [23]. All these data established the structure of this novel C-glycosylated flavanone 1 as 6-C-(2″,4″,6″-triacetyl-β-D-glucopyranosyl)-(2S)-5,7,3′,4′-tetrahydroxyflavan-4-one), which was named tellenoside.
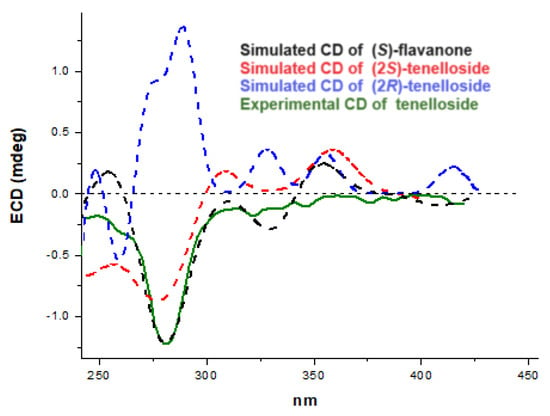
Figure 4.
Experimental circular dichroism of tenelloside (solid line) and relevant calculated spectra of (S)-flavanone (dashed black line) and (S)- and (R)-tenelloside (dashed red and blue lines).
Tenelloside is the triacetyl C-6-glycosylated derivative of eridictyiol, a known active flavanone found in “yerba santa” (Eriodictyon californicum (Hook. and Arn.) Torr.), a plant native to North America [24]. As mentioned before, C-linked glycosidic phenols are a very unique class of compounds with future potential in different fields. The scientific literature on this class of natural products is still poor, also because these compounds are very rare, and their occurrence in nature is less represented with respect to their O-glycosyl-linked analogs. These modifications of plant secondary metabolites are biochemically and evolutionally not well studied and characterized. Enzymes involved in C-glycosyl bond formation are very little known, as minor amounts of C-glycosides are formed as minor byproducts of O-glycosylation [19,20]. The isolation and characterization of the novel C-glycosyl flavonoid tenelloside represent important phytochemical results and an additional chemotaxonomical report for the genus Phyllanthus. Furthermore, the continuous discovery of C-glycosidic products serves as a starting point for the study and characterization of the enzymes involved in the C-glycosylation of phenolic compounds. Phyllanthus tenellus has been used in traditional medicine for different purposes, and C-glycosyl flavonoids such as tenelloside represent promising bioactive compounds. The discovery of tenelloside in the Phyllanthus tenellus plant described herein is significant for several reasons.
Pharmaceutical potential: C-glycoside flavonoids, including tenelloside, exhibit exceptional stability compared with common glycosides (O- and N-), making them resistant to enzymatic and acidic hydrolysis. This enhanced stability translates into increased bioavailability and longer half-lives in vivo, contributing to their potential therapeutic efficacy.
Chemotaxonomic marker: Tenelloside serves as a valuable chemotaxonomic marker for distinguishing effective Phyllanthus tenellus plants from closely related species.
Drug quality analysis: Tenelloside can be employed as a tool for drug quality analysis, ensuring the authenticity and efficacy of Phyllanthus tenellus-based remedies.
The presence of tenelloside in the Phyllanthus tenellus plant offers promising avenues for the research and development of novel therapeutic agents with enhanced stability and bioavailability.
3. Methods and Materials
3.1. Plant Material
The Phyllanthus tenellus Roxb. plant materials were collected in Tenerife, Puerto de la Cruz, Jardín de Aclimatación de La Orotava (harvested outside the greenhouses) (UTM: 349671/3143739) by Alfredo Reyes Betancort and Cristina González Montelongo (27 June 2019). The aerial parts were spread on a tray, turned over intermittently, and air-dried at room temperature (22 ± 4 °C) for two weeks. The dried plant materials were ground and stored until extraction.
3.2. Compound Isolation
An amount of 150 g of ground, dry aerial parts of P. tenellus were mixed with 1.5 L (10 volumes, w/v) of ethanol (98% EtOH). After an initial 15 min of ultrasonication in a water bath, the plant material was extracted two times for 2 h with 1.5 L of ethanol under magnetic stirring at room temperature without sonication and one last time overnight (15 h) at room temperature with the same procedure. The extract was filtered, and the solvent was reduced under reduced pressure using a rotary evaporator to 30 mL. To the resulting suspension, 300 mL of H2O was added, and it was partitioned via liquid–liquid extraction, first with dichloromethane (DCM, 3 × 300 mL) and then with ethyl acetate (EtOAc, 3 × 300 mL). The organic fractions were concentrated under reduced pressure at 40 °C in a rotary evaporator to give DCM (1.0 g) and EtOAc (1.5 g) fractions, whereas the aqueous residue was lyophilized, providing the aqueous fraction (1.94 g). The EtOAc fraction (1.5 g) was subjected to silica gel column chromatography using an increasing polarity gradient (CH2Cl2:MeOH from 0:10 to 10:0) to produce 60 sub-fractions of 20 mL each (F1-F60). The sub-fractions F16-F17-F18 were combined based on their TLC profiles (A6, 38 mg) and further purified with preparative TLC silica gel using the CH2Cl2:MeOH mobile phase (9:1) to afford 26 mg of the pure compound (Rf, 0.4). Different grades of silica gel 60 were employed for the column chromatography and thin-layer chromatography (TLC) separations. Particle sizes of 15–40 and 63–200 μm were used for column chromatography, while silica gel 60 F254 was utilized for analytical and preparative TLC. Spots were visualized using ultraviolet (UV) light and by spraying silica gel plates with a mixture of water, sulfuric acid, and acetic acid (1:4:20) and heating the plates. All solvents employed were of analytical grade and obtained from Panreac, Barcelona, Spain.
2″,4″,6″-6-C-triacetyl-β-D-glucopyranosyl-(2S)-3′,4′,5,7-tetrahydroxyflavan-4-one (tenelloside). Yellow amorphous solid. [α]25D−27.3 (c 0.5; MeOH). CD (c 0.001; MeOH) UV-vis λmax; MeOH: 290 nm. 1H NMR and 13C NMR in CD3OD (600 MHz, see Table 1). HRESIMS [M − H]− calculated for C27H27O14−: 575.1406, experimental 575.1400.
3.3. NMR and Mass Spectrometry (MS/MS and High-Resolution MS)
The optical rotations were determined with a Perkin Elmer 241 (Perkin Elmer, Waltham, USA, MA) automatic polarimeter in CH3OH at 300 °K, and the [α]D values are given in 10−1 deg cm2/g. One-dimensional and two-dimensional NMR spectra were acquired with a Bruker Avance 600 spectrometer (Bruker, Billerica, USA, MA) with Bruker-provided pulse sequences, using CD3OD as the solvent. Chemical shifts were reported in δ (ppm) relative to TMS as the internal reference.
High-resolution (HR) mass spectra were obtained using an Orbitrap mass spectrometer (Q Exactive, Thermo Scientific, Bremen, Germany) equipped with an electrospray ionization (ESI) source operating in negative mode. The instrument was operated under the following conditions: a capillary temperature of 275 °C; a spray voltage of 3.5 kV; a sheath gas (nitrogen) of 10 arbitrary units; a capillary voltage of 65 V; and a tube lens of 125 V. Mass spectrometry data were collected using Xcalibur software (Version 2.2, Thermo Scientific, Bremen, Germany). The tandem mass spectrometry experiments (CID MS/MS) were performed with a linear ion trap mass spectrometer (LTQ mass spectrometer, Thermo Scientific) equipped with an ESI source (negative mode) and a syringe pump. The ESI source operating conditions were as follows: ion spray voltage = 4.0 kV; sheath gas = 5 (arbitrary scale); sweep gas = 5 (arbitrary scale); capillary temperature = 275 °C; capillary voltage = 10 V; and tube lens voltage = 55 V. Samples were diluted in MeOH and were infused into the ESI source via a syringe pump at a flow rate of 5 μL/min. Collision-induced dissociation (CID) was employed for fragmentation, and the collision energy was varied between 5% and 20% (arbitrary units of helium flow).
3.4. Calculated and Experimental Electronic Circular Dichroism (ECD)
The three-step in silico procedure employed to assign the absolute stereochemical configuration of flavanone and Compound 1 was as follows:
- Conformational search: Molecular mechanics (MM) calculations were performed on flavanone in the (S)-configuration, as well as on the structures of 1 with both the (2S)- and (2R)-configurations. The search identified the most representative conformations within an energetic window of 3 kcal/mol. For flavanone, 8 conformations were selected; for (2S)-tellenoside, 24 conformations were selected; and for (2R)-tellenoside, 29 conformations were selected.
- Energetic stability assessment: The energetic stability of all the conformations selected in step 1 was assessed using the Hartree–Fock 3-21G level of theory as a single-point energy evaluation.
- ECD simulation: Quantum-mechanical simulations of electronic circular dichroism (ECD) were performed for the most stable conformations of flavanone and the ones of one in both the (2S)- and (2R)-configurations.
The conformational search was conducted using MM calculations with the MMFF94 force field in the SPARTAN 10 program. The conditions were as follows: All rotatable bonds were varied. A maximum energy gap of 40 kJ × mol−1 was imposed for retained conformations. Conformers were considered duplicates if their R2 value was at least 0.9. The single-point energy evaluations were also performed using the SPARTAN 10 program. The ECD spectra were simulated using the Amsterdam Density Functional (ADF) package at the BLYP level of theory with the QZ4P Large Core basis set. The solvent was methanol, and 50 singlet excitations were taken into account. The diagonalization method was Davidson, the velocity representation was used, and the scaling factor was 0.96. The peak width was 25.
4. Conclusions
The isolation of a new C-glycosylated compound represents important data to add to the literature given the rarity of these compounds in nature. In this case, the isolated compound tenelloside is a C-glycosyl flavanone with the sugar portion containing three acetyl groups, a feature that makes the product much more lipophilic than a non-acetylated glycoside. The compound was isolated from a plant species of the Phyllanthus genus present in the Canary Islands (Phyllanthus tenellus Roxb., Tenerife). This species is also present in other European sub-tropical islands such as Sicily in Italy, although it is not an endemic plant of either Tenerife or Sicily. The presence of C-glycosidic flavonoids in other Phyllanthus genus plants had already been documented by our group in 2019 in the plant Phyllanthus orbicularis, isolating and characterizing a novel C-glycosylated compound with anti-inflammatory activity [14]. The new C-glycosylated product tenelloside present in Phyllanthus tenellus suggests the presence of C-glycosides in the genus Phyllanthus as chemo-taxonomic and probably genus-specific biomarkers. Furthermore, given the powerful biological activity of flavonoids and the extremely interesting pharmacokinetic potential of C-glycosyl products, future studies will be aimed at investigating the biological properties of this new product to gain more insights into the ethnopharmacology of Phyllanthus plants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations11010015/s1. Figure S1: MS2 515 m/z [M-H]−; Figure S2. MS3 [515 → 311] m/z; Figure S3. 1H-NMR spectrum of tenelloside; Figure S4. 13C-NMR of tenelloside; Figure S5. COSY of tenelloside; Figure S6. HSQC of tenelloside; Figure S7. HMBC of tenelloside (part 1); Figure S8. HMBC of tenelloside (part 2); Figure S9. ROESY of tenelloside; Figure S10. ROESY of tenelloside (zoom).
Author Contributions
Conceptualization, A.F. and I.L.B.; methodology, A.F., I.A.J.D. and C.G.M.; software, A.F. and M.P.; validation, I.L.B., I.A.J.D. and A.F.; formal analysis, C.V.; investigation, A.F.; resources, A.F. and I.L.B.; data curation, C.P.R., I.L.B. and I.A.J.D.; writing—original draft preparation, A.F.; writing—review and editing, I.L.B., I.A.J.D., M.P., C.V. and A.F.; visualization, A.F.; supervision, I.L.B. and I.A.J.D.; project administration, A.F.; funding acquisition, A.F. and I.L.B. All authors have read and agreed to the published version of this manuscript.
Funding
This research was supported by FEBS, EMBO, and “Tomas de Iriarte” grants to A.F. and by an MCIN/AEI/10.13039/501100011033/FEDER, UE grant (PID2022-136549OB-100) to I.L.B.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors want to thank María Daniela Gómez Expósito for her administrative and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and Characterization of Bioactive Compounds from Plant Resources: The Role of Analysis in the Ethnopharmacological Approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B. Ethnopharmacology and Drug Discovery. J. Ethnopharmacol. 2005, 100, 50–52. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, J.; Qin, J.; Yang, M. Screening Techniques for the Identification of Bioactive Compounds in Natural Products. J. Pharm. Biomed. Anal. 2019, 168, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Conrado, A.B.; Mosca, L.; Fontana, M. Chemistry and Biochemistry of Sulfur Natural Compounds: Key Intermediates of Metabolism and Redox Biology. Oxidative Med. Cell. Longev. 2020, 2020, 8294158. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Swamy, M.K.; Sinniah, U.R. Natural Bio-Active Compounds. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Springer: Singapore, 2019; pp. 1–608. [Google Scholar] [CrossRef]
- Mao, X.; Wu, L.-F.; Guo, H.-L.; Chen, W.-J.; Cui, Y.-P.; Qi, Q.; Li, S.; Liang, W.-Y.; Yang, G.-H.; Shao, Y.-Y.; et al. The Genus Phyllanthus: An Ethnopharmacological, Phytochemical, and Pharmacological Review. Evid. Based Complement. Altern. Med. 2016, 2016, 7584952. [Google Scholar] [CrossRef] [PubMed]
- Gowrishanker, B.; Vivekanandan, O.S. In Vivo Studies of a Crude Extract of Phyllanthus amarus L. in Modifying the Genotoxicity Induced in Vicia faba L. by Tannery Effluents. Mutat. Res. Toxicol. 1994, 322, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.K.; Yong, C.Y.; Faruq, U.; Ong, H.K.; Amin, Z.B.M.; Ho, W.Y.; Sharifudin, S.; Jaganath, I.B. In Vivo Toxicity and Antioxidant of Pressurize Hot Water Phyllanthus tenellus Roxb. Extracts. BMC Complement. Med. Ther. 2021, 21, 86. [Google Scholar] [CrossRef]
- Ignácio, S.R.N.; Ferreira, J.L.P.; Almeida, M.B.; Kubelka, C.F. Nitric Oxide Production by Murine Peritoneal Macrophages In Vitro and In Vivo Treated with Phyllanthus tenellus Extracts. J. Ethnopharmacol. 2001, 74, 181–187. [Google Scholar] [CrossRef]
- Hidayah, N.; Jusoh, M.; Subki, A.; Keong Yeap, S.; Yap, K.C.; Jaganath, I.B. Pressurized Hot Water Extraction of Hydrosable Tannins from Phyllanthus tenellus Roxb. BMC Chem. 2019, 13, 134. [Google Scholar] [CrossRef]
- Nikule, H.A.; Nitnaware, K.M.; Chambhare, M.R.; Kadam, N.S.; Borde, M.Y.; Nikam, T.D. In-Vitro Propagation, Callus Culture and Bioactive Lignan Production in Phyllanthus tenellus Roxb: A New Source of Phyllanthin, Hypophyllanthin and Phyltetralin. Sci. Rep. 2020, 10, 10668. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Peng, W.-H.; Cheng, H.-Y.; Chen, F.-N.; Lai, M.-T.; Chiu, T.-H. Hepatoprotective Effect of Phyllanthus in Taiwan on Acute Liver Damage Induced by Carbon Tetrachloride. Am. J. Chin. Med. 2006, 34, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Ihantola-Vormisto, A.; Summanen, J.; Kankaanranta, H.; Vuorela, H.; Asmawi, Z.M.; Moilanen, E. Anti-Inflammatory Activity of Extracts from Leaves of Phyllanthus emblica. Planta Medica 1997, 63, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Francioso, A.; Franke, K.; Villani, C.; Mosca, L.; D’Erme, M.; Frischbutter, S.; Brandt, W.; Sanchez-Lamar, A.; Wessjohann, L. Insights into the Phytochemistry of the Cuban Endemic Medicinal Plant Phyllanthus orbicularis: Fideloside, a Novel Bioactive 8-C-glycosyl 2,3-Dihydroflavonol. Molecules 2019, 24, 2855. [Google Scholar] [CrossRef] [PubMed]
- Bililign, T.; Griffith, B.R.; Thorson, J.S. Structure, Activity, Synthesis and Biosynthesis of Aryl-C-Glycosides. Nat. Prod. Rep. 2005, 22, 742–760. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, M.; Nidetzky, B. C-Ribosylating Enzymes in the (Bio)Synthesis of C-Nucleosides and C-Glycosylated Natural Products. ACS Catal. 2023, 13, 15910–15938. [Google Scholar] [CrossRef]
- Chua, L.S.; Abdullah, F.I.; Awang, M.A. Potential of Natural Bioactive C-Glycosyl Flavones for Antidiabetic Properties. Stud. Nat. Prod. Chem. 2020, 64, 241–261. [Google Scholar] [CrossRef]
- Tegl, G.; Nidetzky, B. Leloir Glycosyltransferases of Natural Product C-Glycosylation: Structure, Mechanism and Specificity. Biochem. Soc. Trans. 2020, 48, 1583–1598. [Google Scholar] [CrossRef]
- Franz, G.; Grun, M. Chemistry, Occurrence and Biosynthesis of C-Glycosyl Compounds in Plants. Planta Medica 1983, 47, 131–140. [Google Scholar] [CrossRef]
- Courts, F.L.; Williamson, G. The Occurrence, Fate and Biological Activities of C-Glycosyl Flavonoids in the Human Diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef]
- Khamar, H.; Benkhnigue, O.; Douira, A.; Zidane, L.; Touhami, A.O. Phyllanthus tenellus Roxb. (Phyllanthaceae), a Newly Naturalising Species in Morocco. Check List 2022, 18, 411–417. [Google Scholar] [CrossRef]
- Tureček, F.; Hanus, V. Retro-Diels-Alder Reaction in Mass Spectrometry. Mass Spectrom. Rev. 1984, 3, 85–152. [Google Scholar] [CrossRef]
- Forkmann, G.; Heller, W. Biosynthesis of Flavonoids. Compr. Nat. Prod. Chem. 1999, 1, 713–748. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Avula, B.; Lee, J.; Upton, R.; Khan, I.A. Chemical Characterization and Quantitative Determination of Flavonoids and Phenolic Acids in Yerba Santa (Eriodictyon Spp.) Using UHPLC/DAD/Q-ToF. J. Pharm. Biomed. Anal. 2023, 234, 115570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).