Abstract
Peanut skin as an agriculture waste has poor economic value. Utilizing subcritical methanol extraction (SME) to recover catechin and epicatechin as interest compounds from peanut skin is one of the solutions to valorize the agriculture waste into more valuable products. Therefore, the objective of this research is to examine the parameter impacts on peanut skin extract recovery by subcritical methanol. Extraction was conducted under three independent variables—pressure (6 to 10 MPa), flow rate (2.5 to 7.5 mL/min) and temperature (70 to 100 °C)—with the responses of catechin and epicatechin recovery. The optimum conditions were 8 MPa, 4.39 mL/min and 79.6 °C, with catechin responses of 178.66 µg/g and epicatechin responses of 336.41 µg/g. Conditions of high pressure and temperature are optimal for epicatechin and catechin enhancement. The Chrastil model fits the solubility of catechin and epicatechin in SM effectively since it has the lowest average absolute relative deviation (AARD), which is 4.97% and 5.97%, respectively. Consequently, this method (SME) may substitute for the standard technique in extracting catechin and epicatechin.
1. Introduction
Peanuts are essential crops for the world’s largest food industry. Peanuts are commonly grown in tropical and subtropical regions, and the crop has the potential to benefit both small-scale farmers and large-scale industrial producers economically. According to reports, the average annual global peanut production is 46 million tons. Asia, the Americas and Africa are the three regions with the largest harvesting areas and yields. Most peanuts grown are utilized to produce peanut oil, peanut butter, confections, roasted peanuts, snack foods and desserts. Significant quantities of potentially polluting by-products are produced during peanut harvesting and oil extraction. However, only a fraction of these by-products are utilized as animal feed and fertilizer. A substantial amount of skin and a substantial number of peanut shells and vines are also considered agricultural waste. Since the vast majority of researchers focus on oil and kernel production, peanut by-products such as peanut skin receive little consideration [1]. Peanut skin is a waste of the peanut industry that has poor economic value. Numerous studies have shown that peanut skin is rich in bioactive compounds, particularly catechin and epicatechin, which are known for their health-promoting properties and whose application could be a new trend in health and wellness products [2,3].
Many recent studies have confirmed that peanut skin contains a wide diversity of bioactive compounds such as phenolic acids, tocopherols and flavonols [4]. The bioactive compounds present in peanut skin are epicatechin and catechin. Both of the bioactive compounds are a type of flavanol. Catechin and epicatechin are polar in nature and are plentiful in onion, grape peel, chocolate and tea [5,6]. Catechin and epicatechin have anti-HIV, anti-inflammatory, antidepressant and antihypertensive effects. Catechin ranks second in antioxidant activity compared to other naturally occurring antioxidant compounds. [7].
Numerous extraction techniques have been employed to extract biochemical and biomass products from agricultural waste; however, the most prevalent procedures are the oil press, supercritical carbon dioxide extraction, solvent extraction and ultrasound [1,3,8,9,10,11,12,13,14]. Various extraction processes for commercial applications may be too costly and take too much extraction time. In view of environmental, efficiency and economic issues, it is essential to conduct fundamental research on the use of new extraction methods in extracting the peanut skin.
Subcritical methanol extraction (SME) is capable of achieving high extraction rates in a short amount of time. SME may have various effects (pressure, temperature and flow rate) on yield. In addition, subcritical methanol is heated and kept in liquid form at a pressure that is adequate to keep it between 64.7 °C and 240 °C and 1–8.22 MPa [15]. The viscosity, dielectric constant and surface tension all drop as the temperature increases, while diffusivity improves. An ideal pressure might be applied to maintain the methanol liquid at a certain temperature [16]. Because methanol has a high affinity for polar compounds at room temperature, its use in the extraction of less polar compounds is limited. However, thermal energy weakens the hydrogen bonds between methanol molecules in the subcritical region, and the dielectric constant and polarity change over a wide range with changing temperatures and pressures [17,18]. SME’s high diffusivity enables it to quickly and efficiently permeate the substrate matrix and facilitates the extraction of target compounds into the fluid phase. The solute is desorbed from the initial binding site of the sample matrix (internal diffusion) and then eluted to the extraction solvent (external delusion) [19]. The initial desorption step or subsequent elution step may control the rate of extraction depending on the sample matrix and the conditions.
Semi-empirical models, such as those of Chrastil and Del Valle Aguilera, are preferred in determining the solubility of catechin and epicatechin. This is because those models have a good agreement between the experimental and modeling data. Hence, the parameter effect regarding the solubility of catechin and epicatechin can be evaluated and determined [20]. These models also have less adjustable parameters compared to semi-empirical and kinetic models; thus, the models easily correlated and fitted to the experimental data [21,22,23,24].
Therefore, the objective of this study is to examine the extraction parameters to obtain catechin and epicatechin from peanut skin using subcritical methanol.
2. Materials and Methods
2.1. Preparation of Raw Material
Peanut skins were purchased from G-Tach Food Industries Sdn. Bhd., Malaysia. The skins were dried for 4 h at 60 °C to decrease the moisture content below 8%. Therefore, it was possible to prevent the effects of moisture on the mass transfer and solubility. The sample was then ground at a particle size of 355 < dp < 425 µm, followed by placing it into a freezer (Liebherr EFL 3505, Germany).
2.2. Chemicals
Both epicatechin and catechin standards were obtained from Sigma-Aldrich (St. Louis, MO, USA). Ortho-phosphoric acid and methanol (analysis grade) were purchased from Fisher Scientific (Atlanta, GA, USA).
2.3. Subcritical Methanol Extraction (SME)
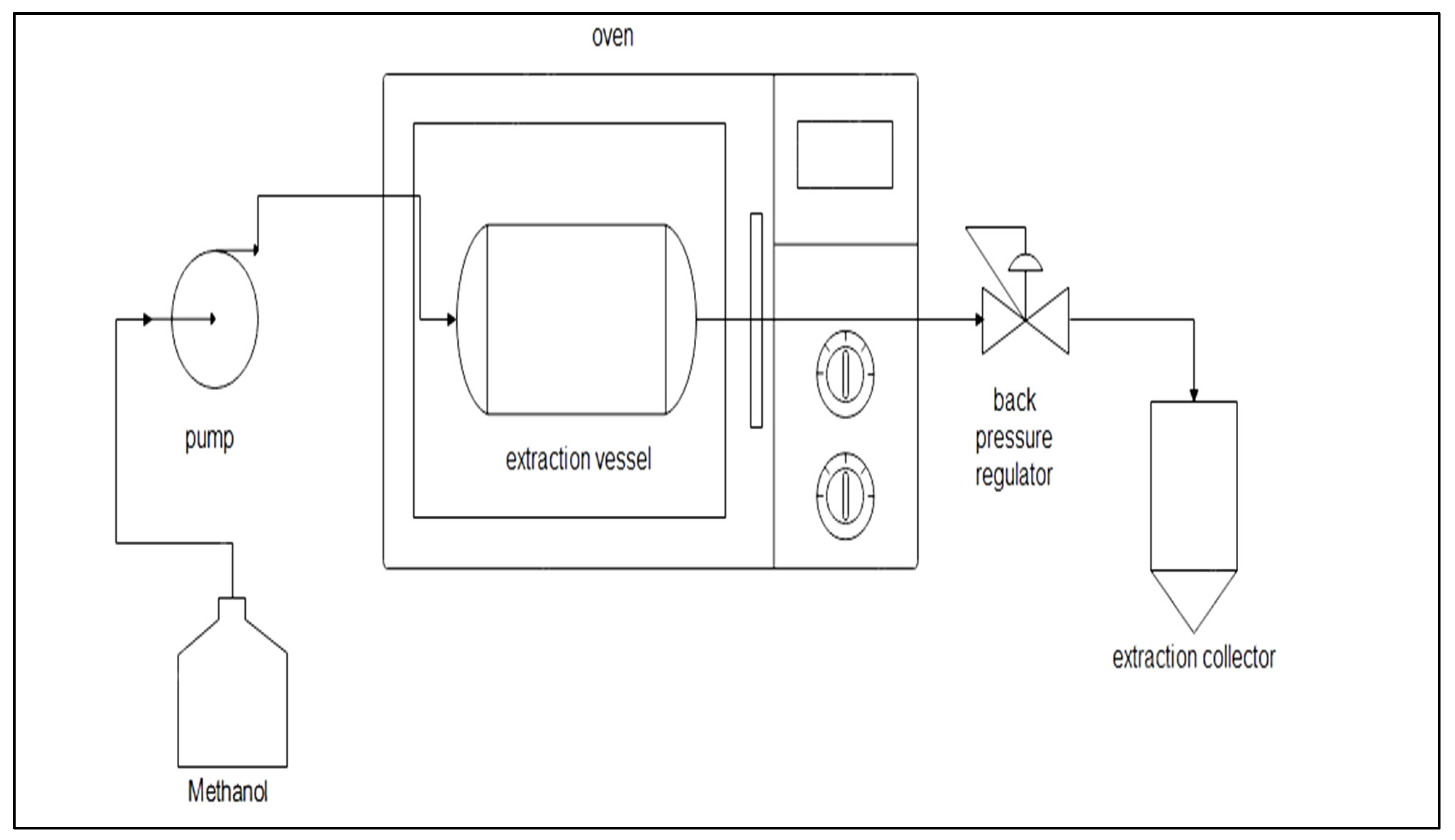
The SME consists of a high-pressure pump, 5 mL extraction vessel, back pressure regulator, pressure gauge and oven. The schematic diagram of SME is shown in Figure 1. There are three variables: temperature (70 to 100 °C), pressure (6 to 10 MPa) and flow rate (2.5 to 7.5 mL/min), as shown in Table 1. The responses of this extraction were catechin and epicatechin recovery. In total, 1 ± 0.005 g of peanut skin was put into an extraction vessel. The methanol (95%) was pumped and controlled based on the variable of flow rate. The extraction time of SME was 5 min. The oven’s temperature was set depending on the variable. The pressure was regulated using the back pressure regulator’s pressure gauge.
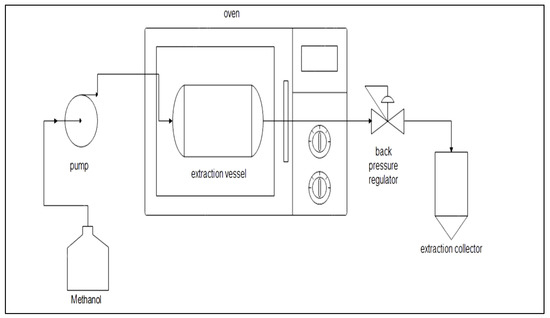
Figure 1.
The subcritical methanol extraction (SME) scheme.

Table 1.
The parameters and responses of subcritical methanol extraction.
2.4. Catechin and Epicatechin Analysis
High-performance liquid chromatography with ultraviolet-visible (HPLC-UV) (Perkin Elmer Series 200, Waltham, MA, USA) was used to analyze the epicatechin and catechin, as proposed by Poon [25] and Putra et al. [26]. The extract was injected (10 µL) into a C18 guard column (RP C18 Merck). The column temperature was set at 30 °C, and a wavelength was set at 210 nm, with a flow rate of 1 mL/min. Additionally, 0.5% ortho-phosphoric acid was used for the mobile phase.
2.5. Experimental Design for Optimization
A Box–Behnken design was formulated by “Design Expert” software (13.0.4, Minneapolis, MN, USA) to analyze the effects of variables. The pressure, temperature and flow rate were studied, as well as catechin and epicatechin as the responses. The second order was applied to correlate the data as follows:
where:
is an investigated response; is a constant; , and are coefficients of linear, quadratic and interaction terms, respectively; and are independent variables.
2.6. Semi-Emperical Modeling
2.6.1. Calculation of the Solubility of Catechin and Epicatechin
The determination of the solubility is according to Equation (2)
where is the total yield of catechin/epicatechin (g) and is the total volume of methanol (L);
2.6.2. Chrastil Model
The Chrastil model describes the equilibrium between a solute and solvent based on the assumption that temperature and density are the most influential parameters in obtaining high solubility [23]. The Chrastil equation can be formed:
where S is the solubility of solute (g/L), is the density of methanol (g/L) and T is the temperature (K). The coefficient value of represents the average number of solvent molecules in the solvato complex, and the coefficient value of defined the sum of the vaporization’s enthalpies and the solvation’s enthalpies of the solute. The coefficient value of depends on the molecular weights of the solute and solvent.
2.6.3. Del Valle Aguilera
The DVA model was developed from the Chrastil model, where the addition of one adjustable parameter maximizes the temperature dependence [24]. The Del Valle–Aguilera equation is shown in Equation (4):
where S is the solubility of solute (g/L), is the density of methanol (g/L) and T is the temperature (K). The coefficient value of represents the average number of solvent molecules in the solvato complex related to the density and the coefficient value of , defined as −ΔH, where ΔH is the sum of the of vaporization’s enthalpies and the solvation’s enthalpies of the solute. The coefficient value of depends on the quadratic effect of temperature, and the coefficient value of is related to the molecular weights of the solute and solvent.
2.6.4. Average Absolute Relative Deviation (AARD) and Coefficient of Determination (R2)
The equation of the AARD between the model and the experiment is presented below:
In Equation (6), n is the number of data points, is the solvation power of the calculated data and is the solvation power of the experimental data. Meanwhile, Equation (6) shows the equation of R2.
From the above equation, represents the residual data of calculated and experimental data. It is a measure of the discrepancy between the data and an estimation model. is the variance of the data.
3. Results and Discussions
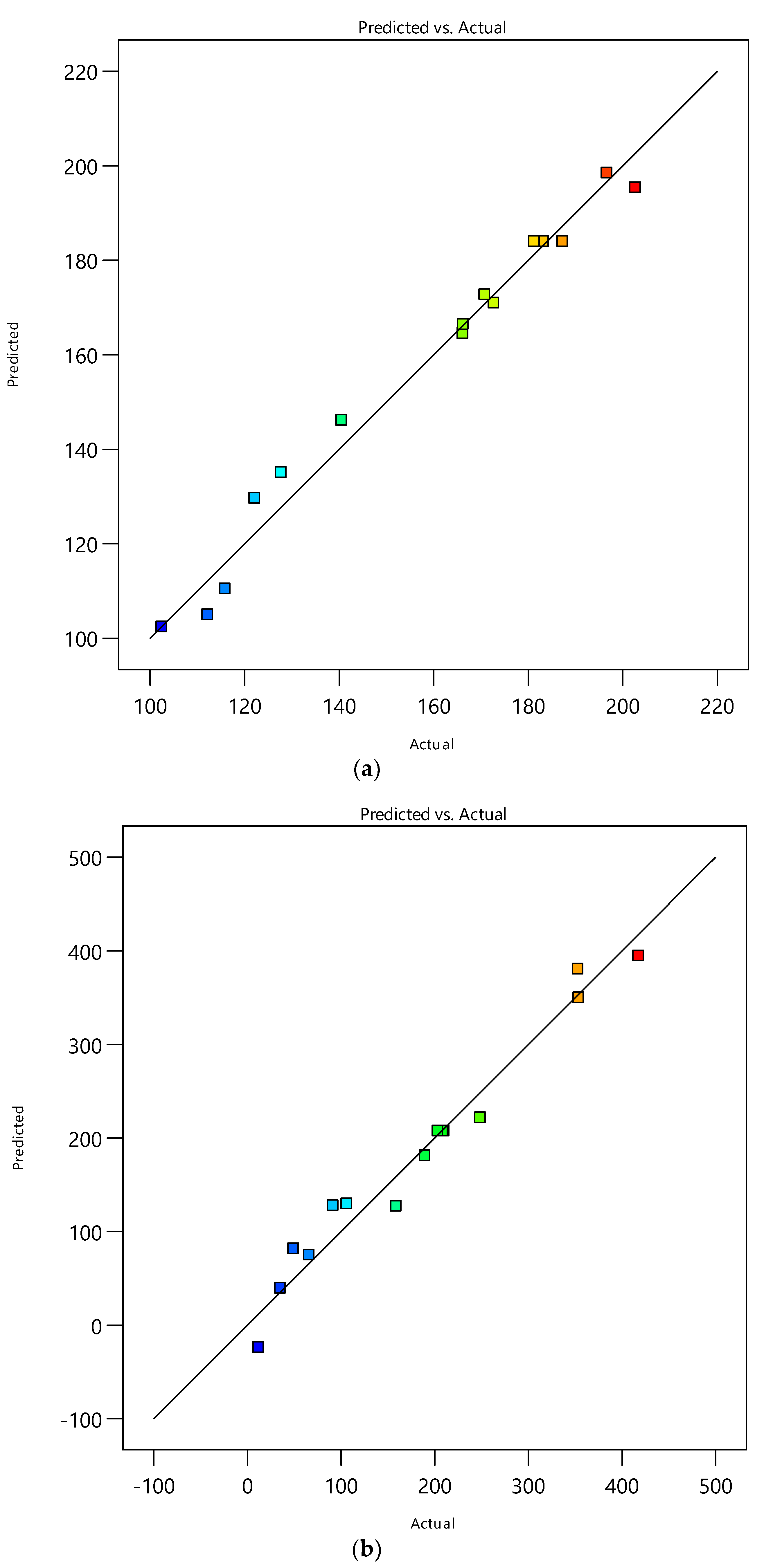
Catechin and epicatechin were extracted from peanut skin using SME. To enhance catechin and epicatechin recovery, response surface methodology (RSM) was used. Table 1 presents the factors and the responses of SME. Analysis of variance (ANOVA) has been used to determine the significance of the model in correlating the experimental data. The ANOVA table for catechin and epicatechin is shown in Table 2 and Table 3, respectively. The optimization value of SME is shown in Table 4. The predicted and actual values for the catechin and epicatechin recovery by SME are shown in Figure 2.

Table 2.
ANOVA table of catechin recovery by SME.

Table 3.
ANOVA table of epicatechin recovery by SME.

Table 4.
The predicted and observed parameters and responses for SME.
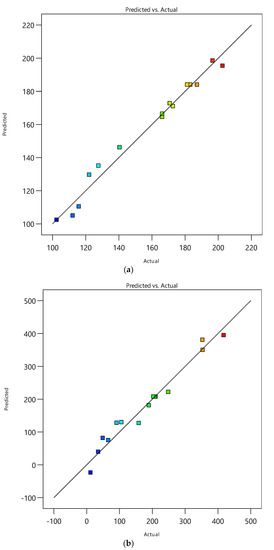
Figure 2.
Predicted vs. actual responses: (a) Catechin, (b) Epicatechin.
The parameter-fixing experiments were carried out based on an analysis of previous research, preliminary data and the limitation apparatus. The maximum pressure was chosen at 8 MPa to maintain the subcritical phase/region of methanol [15]. The maximum temperature was increased to 100 °C to avoid the degradation of these compounds [2]. In order to avoid the solvent channeling effect and low residence time, the flow rate of solvents was set to 7.5 mL/min [27]. The extraction time was set to 5 min to avoid the long-process heat during the extraction.
3.1. Process Effects and Statistical Data Regarding Catechin Recovery
The parameters of temperature, pressure and flow rate were investigated using a second-order model to predict the catechin recovery. An ANOVA table was used to determine if the model successfully fitted the experimental data. In addition, the quadratic model was selected because the satisfactory coefficient (R2) was more than 0.80 and the p-value was less than 0.05, as shown in Table 2. Furthermore, the F-value of the model (27.44) was higher than the F-table, indicating that the model was significant in fitting the experimental data. According to Table 2, the linear coefficient of pressure (A), temperature (B), flow rate (C) and interaction between the AC and the quadratic flow rate (C2) had a statistically significant (p < 0.05) effect on catechin recovery. The quadratic model is shown in Equation (7).
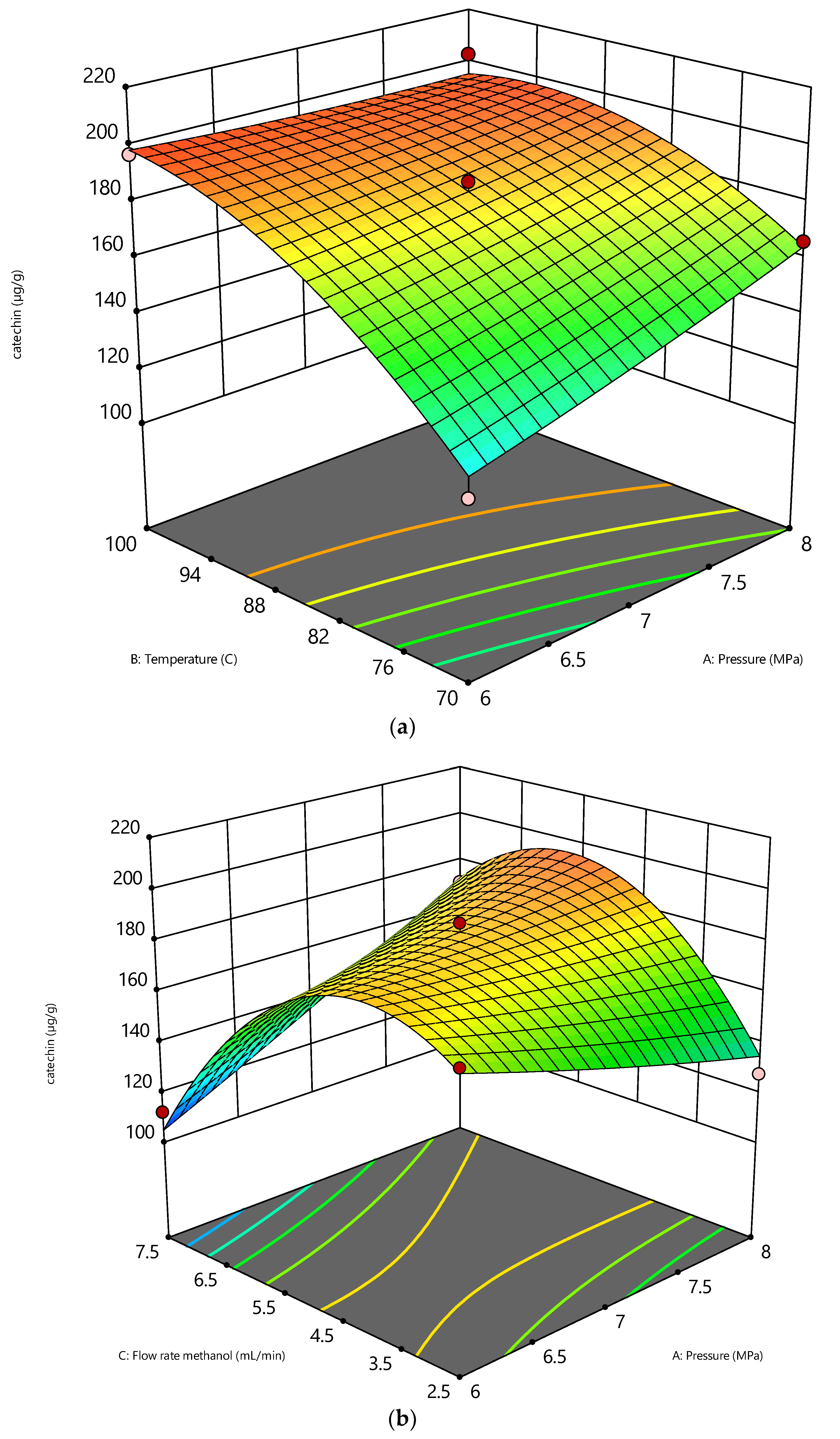
At a constant flow rate of 5 mL/min and pressure of 6 and 8 MPa, catechin recovery is significantly increased by increasing the temperature, as shown in Figure 3a. Catechin is significantly soluble in methanol due to the vapor pressure of catechin [26]. The liquid form of catechin changes to vapor and is easily soluble in methanol. Furthermore, increasing the temperature from 80 to 100 °C will boost the solvents’ diffusivity. The high diffusivity of solvents increases their solvating capacity for the target compounds [28]. The bioactive compounds will be affected by high temperatures, and this condition will increase the probability of the degradation of interest compounds [29]. This condition can be avoided by a short extraction time, whereas the heat transfer between the solvent and the solute can be reduced [30]. A higher temperature of methanol will change the polarity of the solvent, whereas a higher temperature will increase the acidity of methanol [31]. The surface pore of peanut skin is easily broken by the acid condition; thus, the recovery of catechin can be enhanced by subcritical methanol.
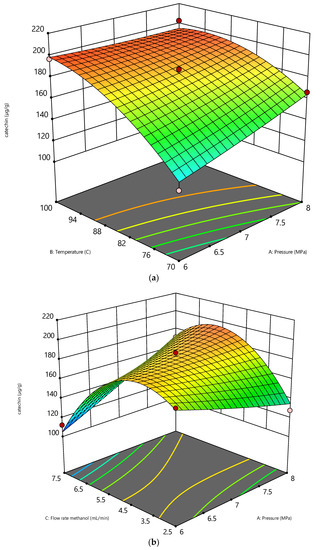
Figure 3.
The effect of SME variables on catechin (µg/g): (a) effect of pressure vs temperature (b) effect of pressure vs flowrate.
As shown in Figure 3b, a decrease in flow rate was a significant factor in enhancing the catechin recovery. The fact that the extraction rate was affected by the water flow rate (Figure 4) indicated that the extraction behavior was influenced by the internal mass transfer of the compounds from the solid phase’s surface to the methanol phase [16]. Furthermore, a low flow rate of methanol will increase the residence time of the solvent; thus, the mass transfer between the solute and solvent will increase. Therefore, the solvent flow rate is another important factor that needs to be considered while conducting the experiment.
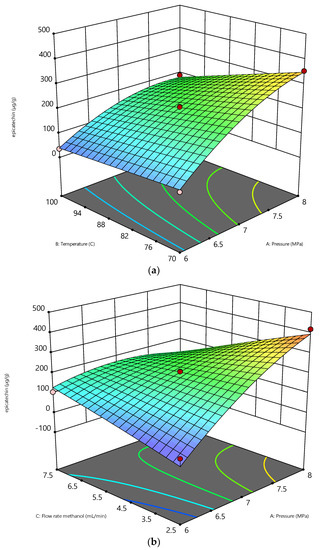
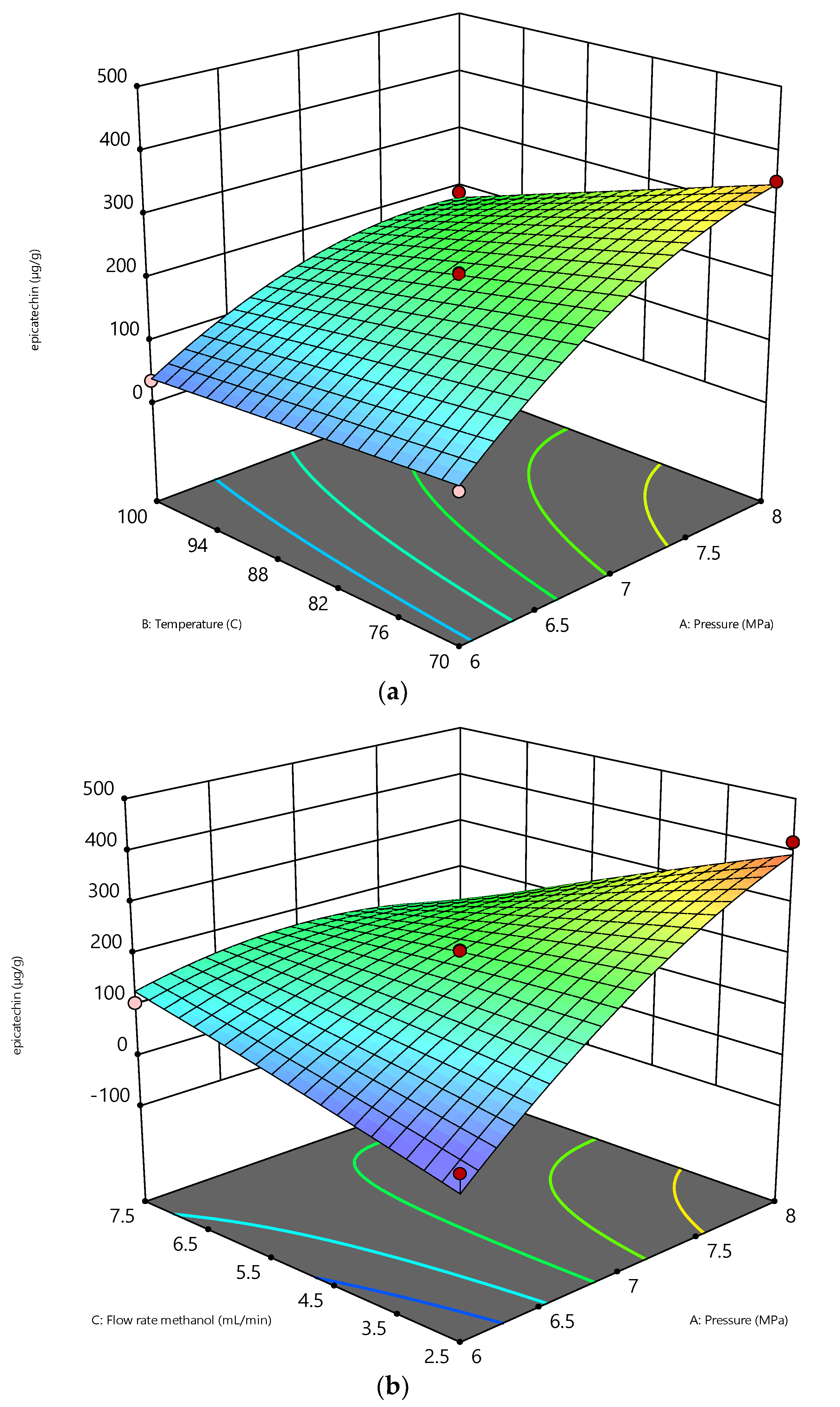
Figure 4.
The variables’ effects on epicatechin (µg/g): (a) effect of pressure vs temperature (b) effect of pressure vs flowrate.
Machmudah, et al. [32] found that increasing the flow rate not only shortened the residence period but also increased the number of solvent molecules in contact with the solute. Consequently, this enhanced the intermolecular contact between the solvent and the solute, hence enhancing solute dissolution. Increasing the flow rate increased the mass transfer, while intraparticle diffusion resistance predominated. The yields reduced, as indicated by the flow rate increasing because it decreases the mass transfer and surface velocity. The major disadvantage of utilizing greater flow rates is an increase in the amount of the extract. As a result, the final extract’s concentration will be lower [33,34]. The extraction time and extract concentration are two crucial aspects that must be taken into consideration when choosing the appropriate flow rate in practice. Higher concentrated extracts and shorter extraction times are preferred [35]. Lachos-Perez et al. [36] also found that increasing the flow rate may increase the extraction yields of heat-sensitive components, since the exposure period of extracted components decreases with increasing continuous phase flow rates.
Figure 3b shows that raising the pressure at a flow rate of 5 mL/min and a constant temperature of 70 °C enhances the catechin recovery. The diffusivity of methanol increased as the pressure increased at a constant temperature. As a result, the increased contact of the solvent molecules aids in the solute’s dissolution. Increasing the diffusivity improves the extraction’s solubility [37]. Furthermore, an increase in viscosity with pressure was found to be strongly dependent on the molecular structure [38]. Cheng, Xue, Yu, Du and Yang [16] found that pressure has a minimal influence on the dielectric constant of the solvent instead of the temperature in the subcritical region. The temperature is adjusted to control the dielectric constant to mimic various organic solvents. Applying pressure during the extraction process has the primary benefit of keeping the methanol in a liquid condition when its temperature is higher than the boiling point, which allows it to behave favorably throughout the extraction process [39,40].
By forcing the solvent to enter the solid pores and rupture the matrix, the pressure in the SWE aids in the solubilization of the analytes by enhancing the mass transfer of the solutes to the solvent [30]. Additionally, the pressure reduces the development of air bubbles in the matrix when combined with high extraction temperatures [41]. These conditions improve the analyte’s solubility and the sample matrix’s desorption kinetics [42].
3.2. Process Effects and Statistical Data on Epicatechin Recovery
The variables of temperature, pressure and flow rate were examined using a quadratic model. An ANOVA table was used to determine if the quadratic model fits the experimental data, including the treatment factors, their interactions and the quadratic coefficients, as shown in Table 3. In addition, the quadratic model was based on R2 being more than 0.80, and the p-value was less than 0.05. According to Table 3, the linear pressure (A), the temperature (B) and the interaction of AC and BC had a statistically significant (p < 0.05) effect on epicatechin recovery. The quadratic model is shown in Equation (8).
Figure 4a shows that increasing the pressure from 6 to 8 MPa is significant to enhancing the epicatechin recovery at constant temperatures (70 °C and 100 °C), and a constant flow rate (2.5 mL/min) enhanced the recovery of epicatechin. This is similar to the results of catechin recovery in that an increasing pressure will increase the diffusivity of the solvent in extracting the interest compounds. The pressure has a minimal influence on the dielectric constant instead of the temperature [43]. Adjusting the temperature of water to manipulate its dielectric constant mimics the properties of several organic solvents. The fundamental benefit of applying pressure during the extraction process is retaining the water in a liquid state while its temperature is above the boiling point, allowing it to behave favorably throughout the extraction process [39,40].
By forcing the solvent to enter the solid pores and rupture the matrix, the pressure in the SME aids in the solubilization of the epicatechin by enhancing the mass transfer [30]. High pressure also reduces the formation of air bubbles in the matrix when combined with high extraction temperatures, which is advantageous in the process in preventing the solvent from reaching the solute [41]. These conditions improve the epicatechin’s solubility and the sample matrix’s desorption kinetics [42]. At a constant flow rate of 5 mL/min and a pressure of 6 MPa, there was a slight increase in the temperature, which enhances the epicatechin recovery. This is due to the vapor/sublimation pressure state being dominated throughout the extraction process. The epicatechin is easily diluted in the solvent [44,45,46]. Certain methanol properties change when the temperature and pressure vary; for instance, as the temperature rises, the polarity of subcritical methanol decreases [9]. Consequently, the polarity of epicatechin may be distinguished. In addition, a rise in temperature reduces methanol’s viscosity, hence increasing its diffusivity. As the viscosity of the solvent decreases, the solubility of the epicatechin rises. These changes in the thermodynamic characteristics increase the solubility of epicatechin in methanol by decreasing its viscosity and surface tension, hence increasing the diffusivity and mass transfer rate [47].
Similar results have occurred with catechin recovery, where an increase in the flowrate from 2.5 to 7.5 mL/min at a constant pressure of 6 MPa decreases the epicatechin recovery, as shown in Figure 4b. The reason for this is that the low methanol flow rate affects the high extraction rate of epicatechin. The internal mass transfer of epicatechin from the solid phase’s surface to the methanol phase affected the extraction behavior. A slow methanol flow rate will lengthen the residence time of the solvent required to extract epicatechin [16,48]. Increased flow rates enabled larger volumes of solvent to come into contact with the plant material, which led to an increased extraction of inactive metabolites and the dilution of bioactive content, resulting in decreased epicatechin recovery.
A similar effect was seen in relation to lengthening the extraction duration, which resulted in reduced overall phenolic and flavonoid levels as a result of the enhanced simultaneous extraction of additional components in addition to the primary target compounds. Carr et al. [49] also discovered that dynamic extraction is often quicker than static extraction due to the continual presence of a new solvent increasing the mass transfer driving force. The selection of one technique over another will depend on whether a lengthy exposure to the solvent at a high temperature may cause degradation (in the case of static extraction) or whether the product must be substantially concentrated in the extract mixture (in the case of dynamic extraction).
However, there are contradictive results under the conditions of increasing the flowrate from 2.5 to 7.5 mL/min at a constant pressure of 4 MPa. The fast flowrate methanol increased the epicatechin recovery, whereas the extraction behavior was influenced by the external mass transfer of the epicatechin from the solid phase’s surface to the methanol phase [50]. High flow rates might predominantly improve the extraction capability because they increase the total methanol volume and shorten the residence times, which inhibit the formation of degradation products [36,51].
Zaidul, et al. [52] also stated that the flow rate has a significant impact on the extraction’s mass transfer, which may be divided into a diffusion-controlled region and a solubility-controlled phase. A reduced solvent flow enhances extraction effectiveness, especially in the solubility-controlled area, and reduces the total solvent mass needed to extract a given quantity of an extract. In the solubility-controlled zone, the extract production was directly proportional to the quantity of methanol required for extraction at lower pressures.
3.3. Multiple Responses Optimization and Comparison with the Previous Study
Multiple optimizations were carried out to determine the optimal conditions for the multiple responses (catechin and epicatechin). The optimal conditions were 8 MPa, 4.39 mL/min and 79.6 °C, with catechin responses of 178.66 µg/g and epicatechin responses of 336.41 µg/g. The validation of the SME extraction optimization is seen in Table 4, where the difference between the predicted and actual data is less than 10%. Consequently, the optimization data may be used in the scale-up operation.
Putra, Rizkiyah, Zaini, Machmudah and Yunus [26] observed that the impacts of the pressure and ethanol rate considerably improved the recovery of catechin and epicatechin by supercritical carbon dioxide (ScCO2) extraction. At 21.86 MPa, 332.23 K and 0.17 mL/min, the highest concentrations of catechin (752.03 μg/g) were achieved. According to the findings, SME has less catechin than ScCO2 extraction. To extract catechin, supercritical carbon dioxide uses a higher-pressure solvent, which increases the density and diffusivity of the solvent. However, a higher pressure results in greater production and safety expenses. As a consequence, this technique (SME) may be substituted for the standard method of catechin extraction due to the lower process and safety costs.
3.4. Semi-Empirical Models for the Solubility of Catechin and Epicatechin
Catechin and epicatechin’s solubility varied from 1.55 × 10−3 to 6.65 × 10−3 g/L and from 2.41× 10−4 to 1.42 × 10−2 g/L, respectively, as shown in Table 5. The experimental solubility and predicted data for the solubility of catechin and epicatechin in subcritical methanol are also shown in Table 5. Table 6 shows the correlation data of the models fitted to the solubility of catechin and epicatechin and the AARD of the model. Catechin and epicatechin’s solubility data were satisfactorily correlated using the Del Valle Aguilera (DVa) model due to the lower percentage of the AARD. the DVa model data were employed to assess the solubility characteristics of the catechin and epicatechin compounds instead of the Chrastil model.

Table 5.
Experimental and calculated solubility of catechin and epicatechin in subcritical methanol.

Table 6.
Correlation data of the models fitted to the solubility of catechin and epicatechin.
The coefficient value of a is negative for catechin and epicatechin in this study, showing that an endothermic reaction (+ΔH) is the best condition to promote solubility. This is because the positive value of ΔH indicates that a high-heat procedure is required to accelerate the reaction. As a result, as the temperature rises, the solvation power of subcritical water increases, allowing the epicatechin and catechin to be extracted. The coefficient of k is not significant in this study, which is related to the pressure/density due to the incompressible solvent. The Chrastil/DVa model is commonly used for a compressible solvent such as supercritical carbon dioxide. Therefore, the temperature effect is significant to enhancing the solubility of catechin/epicatechin in subcritical methanol.
4. Conclusions
Several recent works of research have revealed that peanut skin contains a broad variety of bioactive compounds, including phenolic acids, tocopherols and flavonols, that have remarkable dietary and medicinal applications. Catechin and epicatechin are the beneficial substances found in peanut skin. Subcritical methanol extraction (SME) is capable of achieving high extraction rates of catechin and epicatechin in a short amount of time. Depending on the operational circumstances, SME may have various effects on the product yield and quality. The optimal conditions were 8 MPa, 4.39 mL/min and 79.6 °C, with a catechin response at 178.66 µg/g and an epicatechin response at 336.41 µg/g. Catechin and epicatechin’s solubility varied from 1.55 × 10−3 to 6.65 × 10−3 g/L and from 2.41 × 10−4 to 1.42 × 10−2 g/L, respectively. The Chrastil model fits catechin and epicatechin’s solubility in SM efficiently since it has the lowest average absolute relative deviation (AARD), which are 4.97% and 5.97%, respectively. Higher temperatures and slower flow rate conditions were suitable for extracting the catechin and epicatechin using SME. This method (SME) may become an alternative method for substituting the conventional technique for extracting catechin and epicatechin.
Author Contributions
Methodology, A.H.A.A.; software, D.N.R.; validation, D.N.R.; writing—original draft preparation, N.R.P.; writing—review and editing, A.H.A.A. and A.P.; visualization, N.R.P.; supervision, M.A.C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the Professional Development Research University grant (R.J130000.7113.05E53) from Universiti Teknologi Malaysia for supporting this work.
Data Availability Statement
Data are available on request from the authors.
Acknowledgments
The authors would like to acknowledge the Professional Development Research University grant from Universiti Teknologi Malaysia for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nepote, V.; Grosso, N.; Guzman, C.A. Extraction of antioxidant components from peanut skins. Grasas Aceites 2007, 53. [Google Scholar] [CrossRef]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Rodriguez-Amado, I.; Agregán, R.; Munekata, P.E.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT 2018, 88, 1–8. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Ruslan, M.S.H.; Mohd Azizi, C.; Idham, Z.; Morad, N.A.; Ali, A. Parametric evaluation for extraction of catechin from Areca catechu Linn seeds using supercritical CO2 extraction. J. Teknol. 2015, 74, 87–92. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical water extraction of phenolic compounds from onion skin wastes (Allium cepa cv. Horcal): Effect of temperature and solvent properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Yian, L.N.; Ramli, W.D.; Yunus, M.A.C. Optimization of supercritical carbon dioxide and co-solvent ethanol extraction of wasted peanut skin using response surface methodology. In Proceedings of the MATEC Web of Conferences, Semarang, Indonesia, 14 March 2018; p. 02005. [Google Scholar]
- Bai, L.-S.; Yang, Y.; Lv, D.-D. Microwave extraction of total flavonoids in peanut skins. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2012, 35, 977–980. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Marcus, Y. Extraction by subcritical and supercritical water, methanol, ethanol and their mixtures. Separations 2018, 5, 4. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Kinetic study of subcritical water extraction of flavonoids from citrus unshiu peel. Sep. Purif. Technol. 2020, 250, 117259. [Google Scholar] [CrossRef]
- Wu, G.; Dong, H.; Li, J.; Guo, L.; Cheng, Y.; Geng, Y.; Wang, X. Extraction of parishin B and parishin C from Gastrodiae Rhizoma by subcritical water technology. J. Ind. Eng. Chem. 2022, 108, 280–287. [Google Scholar] [CrossRef]
- Kubátová, A.; Jansen, B.; Vaudoisot, J.-F.; Hawthorne, S.B. Thermodynamic and kinetic models for the extraction of essential oil from savory and polycyclic aromatic hydrocarbons from soil with hot (subcritical) water and supercritical CO2. J. Chromatogr. A 2002, 975, 175–188. [Google Scholar] [CrossRef]
- Putra, N.R.; Idham, Z.B.; Machmudah, S.; Ruslan, M.S.H.b.; Che Yunus, M.A. Extraction of peanut skin oil by modified supercritical carbon dioxide: Empirical modelling and optimization. Sep. Sci. Technol. 2018, 53, 2695–2703. [Google Scholar] [CrossRef]
- Esquıvel, M.; Bernardo-Gil, M.; King, M. Mathematical models for supercritical extraction of olive husk oil. J. Supercrit. Fluids 1999, 16, 43–58. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer Science & Business Media: Berlin, Germany, 2013; Volume 4. [Google Scholar]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1988, 27, 1551–1553. [Google Scholar] [CrossRef]
- Poon, G. Analysis of catechins in tea extracts by liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. A 1998, 794, 63–74. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Machmudah, S.; Yunus, M.A.C. Solubility of catechin and epicatechin from Arachis Hypogea skins wastes by using supercritical carbon dioxide-ethanol and its optimization. J. Food Meas. Charact. 2021, 15, 2031–2038. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: Optimization and modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Rasidek, N.A.M.; Nordin, M.F.M.; Tokuyama, H.; Nagatsu, Y.; Mili, N.; Zaini, A.S.; Idham, Z.; Yunus, M.A.C. Subcritical water-based pectin from banana peels (Musa Paradisiaca Cv. Tanduk) as a natural gelation agent. Mater. Today Proc. 2021, 47, 1329–1335. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Veza, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L.; Yunus, M.A.C. Solubilization and Extraction of Valuable Compounds from Peanut skin in Subcritical Water. J. Food Process. Preserv. 2022, 46, e17005. [Google Scholar] [CrossRef]
- Zaini, A.S.; Putra, N.R.; Idham, Z.; Mohd Faizal, A.N.; Che Yunus, M.A.; Mamat, H.; Abdul Aziz, A.H. Comparison of alliin recovery from Allium sativum L. using Soxhlet extraction and subcritical water extraction. ChemEngineering 2022, 6, 73. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sa-Nogueira, I.; Simoes, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Zaini, A.; Putra, N.; Idham, Z.; Norodin, N.M.; Rasidek, N.M.; Yunus, M.C. Mini Review: Extraction of Allicin from Allium sativum using Subcritical Water Extraction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012023. [Google Scholar] [CrossRef]
- Ciftci, D.; Saldaña, M.D. Hydrolysis of sweet blue lupin hull using subcritical water technology. Bioresour. Technol. 2015, 194, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Martinez-Jimenez, F.; Rezende, C.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Subcritical water hydrolysis of sugarcane bagasse: An approach on solid residues characterization. J. Supercrit. Fluids 2016, 108, 69–78. [Google Scholar] [CrossRef]
- Ko, M.-J.; Cheigh, C.-I.; Chung, M.-S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Griest, E.M.; Webb, W.; Schiessler, R.W. Effect of pressure on viscosity of higher hydrocarbons and their mixtures. J. Chem. Phys. 1958, 29, 711–720. [Google Scholar] [CrossRef]
- Pillot, M.; Lebeau, B.; Nouali, H.; Daou, T.J.; Patarin, J.; Ryzhikov, A. High pressure intrusion of water and LiCl aqueous solutions in hydrophobic KIT-6 mesoporous silica: Influence of the grafted group nature. Microporous Mesoporous Mater. 2019, 280, 248–255. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Vatai, T.; Škerget, M.; Knez, Ž.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Rambabu, K.; AlYammahi, J.; Thanigaivelan, A.; Bharath, G.; Sivarajasekar, N.; Velu, S.; Banat, F. Sub-critical water extraction of reducing sugars and phenolic compounds from date palm fruit. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Idham, Z.; Qomariyah, L.; Yunus, M.A.C. Extraction rate of Valuable Compounds from Peanut Skin Waste by Ethanol-Assisted Supercritical Carbon Dioxide: Modelling and Optimization. Malays. J. Fundam. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Redzuan, S.; Ho, C.Y.; Idham, Z.; Yusuf, S.; Putra, N.R.; Yunus, M.A.C.; Mansor, S.; Ruslan, M.S.H. Optimization of Anthocyanins Extracts from Roselle (Hibiscus sabdarifa) Petals Using Ultrasonic-Assisted Extraction Method. In Proceedings of the 3rd International Conference on Separation Technology, Johor, Malaysia, 15–16 August 2020; Springer: Berlin/Heidelberg, Germany, 2021; pp. 295–309. [Google Scholar]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Idham, Z.; Zaini, M.A.A.; Yunus, M.A.C.; Aziz, A.H.A. Optimization and solubilization of interest compounds from roselle in subcritical ethanol extraction (SEE). Alex. Eng. J. 2022, 65, 59–74. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Idham, Z.; Veza, I.; Qomariyah, L.; Yunus, M.A.C. Optimization and modelling in flavonoid and phenolic compounds recovery from peanut skin by subcritical water. Biomass Convers. Biorefinery 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Mayanga-Torres, P.; Lachos-Perez, D.; Rezende, C.; Prado, J.; Ma, Z.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Valorization of coffee industry residues by subcritical water hydrolysis: Recovery of sugars and phenolic compounds. J. Supercrit. Fluids 2017, 120, 75–85. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Nik Norulaini, N.A.; Mohd Omar, A.K.; Smith, R.L. Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel. J. Food Eng. 2007, 79, 1007–1014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).