Abstract
Plant secondary metabolites have a long history of potential use in managing human diseases by inhibiting enzymes that are highly expressed due to various pathogenic conditions. Prostaglandins (PGs) and leukotrienes (LTs) are proinflammatory mediators synthesized from arachidonic acid (AA) by the action of cyclooxygenases (COXs) and lipoxygenases (LOXs), respectively. Particularly, COX-2/5-LOX enzymes play a significant role in inflammatory processes and the pain associated with them. Butyrylcholinesterase (BchE) was recently suggested as a more reliable potential target for sustaining normal cholinergic function. In an attempt to identify new potential COX-2/5-LOX and BchE inhibitors, a phytochemical investigation of Launaea capitata (Spreng.) Dandy (Asteraceae) was executed. This investigation led to the isolation of a new digalactosyldiacylglycerol isomer, namely 1,2-dilinolenoyl-3-O-(α-galactopyranosyl-(1,6)-O-α-D-galactopyranosyl)-sn-glycerol (1) in addition to 1-myristoyl-2-palmitoyl-3-O-(α-galactopyranosyl-(1,6)-O-β-D-galactopyranosyl)-sn-glycerol (2), which was isolated herein for the first time from nature. The structures of the two isolates were elucidated by using 1D-, 2D-NMR, and ESI-MS spectroscopy. Compounds 1 and 2 exhibited good in vitro inhibitory activities against 5-LOX (59.01 and 21.67 μg/mL) and BchE (13.37 and 24.32 μg/mL), respectively. However, they exhibited weak inhibition of COX-2 (110.44 and 179.63 μg/mL, respectively). These inhibitory activities were explained in silico using a computational docking study. The docking results were consistent with the in vitro enzyme inhibitory activity. The lowest binding affinity for 1 and 2 was observed against COX-2 (−7.360 and −5.723 kcal/mol), whereas they exhibited greater binding affinity to 5-LOX (−8.124 and −8.634 kcal/mol), respectively, compared to its natural substrate, AA (−5.830 kcal/mol). Additionally, 1 and 2 exhibited remarkable binding affinity to BchE (−8.313 kcal/mol and −7.502 kcal/mol, respectively), which was comparable to the co-crystallized ligand, thioflavin T (−8.107 kcal/mol). This was related to the multiple and crucial hydrogen bonding interactions of these compounds with the amino acid residues in the active sites of the investigated enzymes. This study demonstrated the role of plant galactolipids as potential leads in the development of new drugs that alleviate the neuroinflammatory conditions associated with various diseases, such as Alzheimer’s disease and Type 2 diabetes mellitus.
1. Introduction
The biodiversity of natural products has significantly aided in the discovery of various drug entities and contributed to the emergence of many approved drugs [1]. Natural products, notably plant secondary metabolites, represent a rich source of potential drug leads [2,3,4]. Launaea capitata (Spreng.) Dandy is a typical annual desert plant that has a Saharo–Arabian distribution ranging from North Africa to the Arabian Peninsula and Afghanistan [5]. It belongs to the genus Launaea Cass., which is one of the largest genera of the Asteraceae family [5]. Plants of this genus grow in arid and semiarid climates, especially in the old world, including North Africa, Southwest Asia, and the Arabian Peninsula [5,6,7]. Launaea spp. are used as edible greens by the native people in these countries due to their nutritional and health benefits [8,9]. Previously reported secondary metabolic products from this genus include polyphenols (such as flavonoids, lignans, and coumarins), polyacetylenes, quinic acid derivatives, sesquiterpenoids, and triterpenoids [7,9,10,11,12,13]. A wide array of pharmacological activities has been reported for L. capitata, including anti-biofilm [14], cytotoxic [11], and antioxidant (viz., DPPH radical scavenging, hydrogen peroxide scavenging, and ferric reducing power) activities [14]. It also exhibited α-amylase inhibitory [14], antihypertensive, and muscle relaxant activities [15]. Additionally, several studies have reported on the anti-inflammatory activities and the underlying molecular mechanisms of several Launaea spp. [16,17,18,19].
Despite the beneficiary role of inflammation as a defense mechanism of the human body, it has been reported to be involved in the etiology of several severe human diseases, such as cancer, cardiovascular diseases, diabetes mellitus, and neurodegenerative diseases, such as Alzheimer’s Disease (AD) [20,21]. Nonsteroidal anti-inflammatory drugs (NSAIDs) that act by inhibiting cyclooxygenase isozymes COX-1 and COX-2 are the most commonly prescribed drugs for the treatment of inflammation [22]. Various efforts have been exerted toward the discovery of drugs that selectively inhibit the inducible COX-2 isozyme in order to avoid gastrointestinal side effects due to the use of NSAIDs. However, newly discovered COX-2 selective inhibitors (COXIBs) have also resulted in serious cardiovascular side effects, and some of them have been withdrawn from the market [23,24,25]. In addition to COXs that catalyze the oxidation of (AA) to prostaglandins (PGs), lipoxygenases (LOXs) are involved in an important metabolic pathway of AA, resulting in the production of leukotrienes (LTs) [26,27]. Therefore, there is a crucial need to find new anti-inflammatory drugs that act via the inhibition of multiple upstream targets in the arachidonic acid (AA) metabolism [22,28]. Additionally, the dual inhibition of COX-2/5-LOX has emerged as a promising strategy for the development of safer anti-inflammatory drugs through the inhibition of both PGs and LTs production [28,29]. Butyrylcholinesterase (BchE) is an important enzyme that has elevated levels during cases of systemic and neuro-inflammation that accompany serious diseases, such as AD and Type 2 diabetes mellitus [30]. It has been suggested that it performs a role in modulating inflammation through the cholinergic system [31] and was recently established as a more reliable potential target for the treatment of AD. It has been reported to be less substrate-specific than acetylcholinesterase (AchE) and to be more involved in the hydrolysis of acetylcholine (Ach) in cases of brain pathogenesis and progressive AD [32]. Thus, targeting BchE could be a better choice for maintaining the level of the neurotransmitter, ACh [33,34].
In our previous research, the in vitro inhibitory activities of some natural products against the enzymes involved in inflammation and neurodegenerative disorders were described [35,36]. This study aimed at the chromatographic purification of the main secondary metabolites of the non-polar fraction of L. capitata, spectral identification, and an in vitro evaluation of the isolated compounds as potential dual inhibitors of COX-2/5-LOX and BchE. Moreover, a computational docking study was performed to clarify the mode of the binding interaction of the isolated compounds against the three investigated target enzymes.
2. Materials and Methods
2.1. Plant Material
The plant material used in this study was taken from the whole plants of Launaea capitata (Spreng.) Dandy (Figure 1) and collected from the Riyadh region of Saudi Arabia (25°23′48.9″ N 47°14′47.6″ E) in March 2022. A voucher specimen was kept at the local herbarium of the Department of Pharmacognosy, Prince Sattam Bin Abdulaziz University (#16741). The plant was identified by Prof. Ibrahim A. Mashaly, Professor of Plant Ecology, Faculty of Sciences, Mansoura University.

Figure 1.
Photographs of Launaea capitata; (a) the flower and (b) collected plant material used in the phytochemical study.
2.2. Chemicals and Instruments
Thin-layer chromatography was carried out on Merck silica gel 60 F254 plates (Darmstadt, Germany). Column chromatographic methods were performed using silica gel 60 with a mesh range of 70–230 (Merck, Darmstadt, Germany) using RP-C18 silica gel (Merck, Darmstadt, Germany). Solvents of reagent grade were from Loba Chemie Pvt. Ltd. (Mumbai, India). The NMR analyses were conducted using a Bruker UltraShield Plus 500 MHz spectrometer (Rheinstetten, Germany). Electrospray ionization-mass spectrometric analysis (ESI-MS) was performed using a Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific, Waltham, MA, USA).
2.3. Extraction and Purification
The dried plant material (1211.5 g) was extracted with MeOH to afford a total extract (weight: 325 g). The latter was suspended in dist. H2O (500 mL) partitioned with n-hexane (500 mL × 4 times), which was evaporated using a Rotavapor R −215/BUCHI (Vacuum controller V-850, SWITZERLAND) rotary evaporator to afford the n-hexane fraction (87.3 g). The latter was purified on a silica gel chromatographic column (CC), packed in n-hexane (100%), and eluted using gradient elution with EtOAc (from 0:100%) followed by MeOH (0:100%). The effluents (200 mL each) were evaporated and grouped based on their composition to yield 10 groups (G I: G X). Group G IX (2.2 g), eluted with EtOAc-MeOH (95:5 v/v), was purified on another silica gel CC, packed in CHCl3, eluted with gradient elution using MeOH, and the effluent (100 mL each) was further monitored using TLC. The fractions (F 41-50) eluted with CHCl3-MeOH (93:7 v/v) were purified on an RPC18 CC (1 cm × 25 cm) using MeOH-H2O (50:50 to 0:100 v/v), and the effluents (10 mL each) were monitored using RPC18 TLC (methanol 100%). The subfractions (SF 64-69) eluted with MeOH-H2O (95:5 v/v) were further purified on another RPC18 CC (1 cm × 25 cm) using MeOH-H2O (80:20 to 0:100 v/v), and the effluents (5 mL each) were collected. The eluted fractions (SSF 23-35) with MeOH-H2O (95:5 v/v) afforded compound 1 (120 mg, Rf 0.34). The subfractions (SF 70-73) eluted with MeOH-H2O (95:5 v/v) afforded compound 2 (21 mg, Rf 0.32).
2.4. Enzyme Inhibition Assays
2.4.1. BchE Inhibition Assay
The butyrylcholinesterase (BchE) in vitro inhibitory activity of the isolated compounds was determined using Ellman’s method using butyrylcholinesterase from horse serum (equine-BchE; CAS# 9001-08-5) according to the published procedure with slight modifications [37,38]. The samples were dissolved in DMSO to yield a working stock solution (10 mg/100 μL). Twelve sample concentrations (from 500 to 0.25 μg/mL) were prepared through two-fold serial dilution using Tris buffer (pH 8.0), and the maximum DMSO concentration was 0.1%. Briefly, the reaction mixture was prepared in triplicate in a 96-well plate to a final volume of 200 μL by mixing 140 μL of Tris buffer (pH 8.0), 10 μL (0.35 mM) of Ellman’s reagent (DTNB; 5,5′-dithio-bis-(2-nitrobenzoic acid), 20 μL of the different sample concentrations, and 20 μL of butyrylcholinesterase (0.22 U/mL). The reaction mixture was incubated at 37 °C for 20 min. To initiate the reaction, aliquots of 10 μL (0.5 mM) of butyrylthiocholine iodide (CAS# 1866-16-6, Sigma-Aldrich, St. Louis, MO, USA) were added. The generated color was measured at 412 nm using a microplate reader (BioTek®, Winooski, VT, USA). The reaction mixture with no added inhibitors was used as the negative control, providing the maximum absorbance (100%). Rivastigmine (CAS# 123441-03-2, Sigma-Aldrich, St. Louis, MO, USA) and Donepezil (CAS# 120011-70-3, Sigma-Aldrich, St. Louis, MO, USA) were used as the positive controls.
2.4.2. COX-2 Inhibition Assay
In vitro cyclooxygenase (COX-2) inhibitory activity was assessed by using the fluorometric method performed according to the COX-2 Inhibitor Screening Kit protocol, following the recommendations of the manufacturer (CAT # K547-100, BioVision, Milpitas, CA, USA) [39,40]. The sample solutions were prepared, as previously mentioned, to obtain twelve concentrations (from 500 to 0.25 μg/mL). Nordihydroguaiaretic acid (NDGA; CAS # 500-38-9, Sigma-Aldrich, St. Louis, MO, USA) was used as the positive control. The reaction mixture with no added inhibitors was used as the negative control. The assay was measured fluorometrically to detect prostaglandin PGG2 generated by the COX-2 enzyme at 535 nm for excitation and 587 nm for emission using a Thermo Scientific microplate reader (Waltham, MA, USA).
2.4.3. 5-LOX Inhibition Assay
In vitro 5-lipoxygenase (5-LOX) inhibitory activity was assessed by using the fluorometric method performed according to the 5-Lipoxygenase Inhibitor Screening Kit protocol, following the recommendations of the manufacturer (CAT # K980-100, BioVision, Milpitas, CA, USA) [41,42]. The sample solutions were prepared, as mentioned above, to obtain twelve concentrations (from 500 to 0.25 μg/mL). Nordihydroguaiaretic acid (NDGA; CAS # 500-38-9, Sigma-Aldrich, St. Louis, MO, USA) was used as the positive control. The reaction mixture with no added inhibitors was used as the negative control. The activity was measured by recording the fluorescence at 500 for excitation and 536 nm for emission using a Thermo Scientific microplate reader (Waltham, MA, USA).
2.5. Statistical Analysis
The results were expressed as the means (±S.D.) of the triplicates using two independent experiments. The IC50 values were calculated from the concentration–response curve prepared using GraphPad Prism software version 8.0 (San Diego, CA, USA), representing the test sample concentration that inhibited 50% of enzymes’ activity.
2.6. Docking Study
The molecular docking study was executed using Autodock vina, version 1.2.3 (Scripps Research, La Jolla, CA, USA) [43,44]. Briefly, the crystal structures for the investigated human molecular targets, including COX-2 protein (PDB code: 5IKV) [45], 5-LOX protein (PDB code: 3V99) [46], and BchE protein (PDB code: 6ESY) [47], were downloaded with the co-crystallized ligands (reference inhibitors) from the RCSB protein data bank, as presented in Table S1. The structures of the tested molecules were drawn using ChemDraw Professional version 15.0.0.106 (PerkinElmer Informatics, Inc., Waltham, MA, USA) and converted to PDB formats using Pymol software [48]. They were prepared for the docking study using Autodock tools. The protein crystal structures were also prepared using Autodock tools. The binding site coordinates were determined using a grid box around the co-crystallized ligand with the dimensions of 40 × 40 × 40 and a spacing of 0.375 Å, with the X, Y, and Z coordinates presented in Table S1. The obtained docking poses with the least RMSD values were visualized using Pymol [48].
3. Results and Discussion
3.1. Identification of the Isolated Compounds
3.1.1. Identification of Compound 1
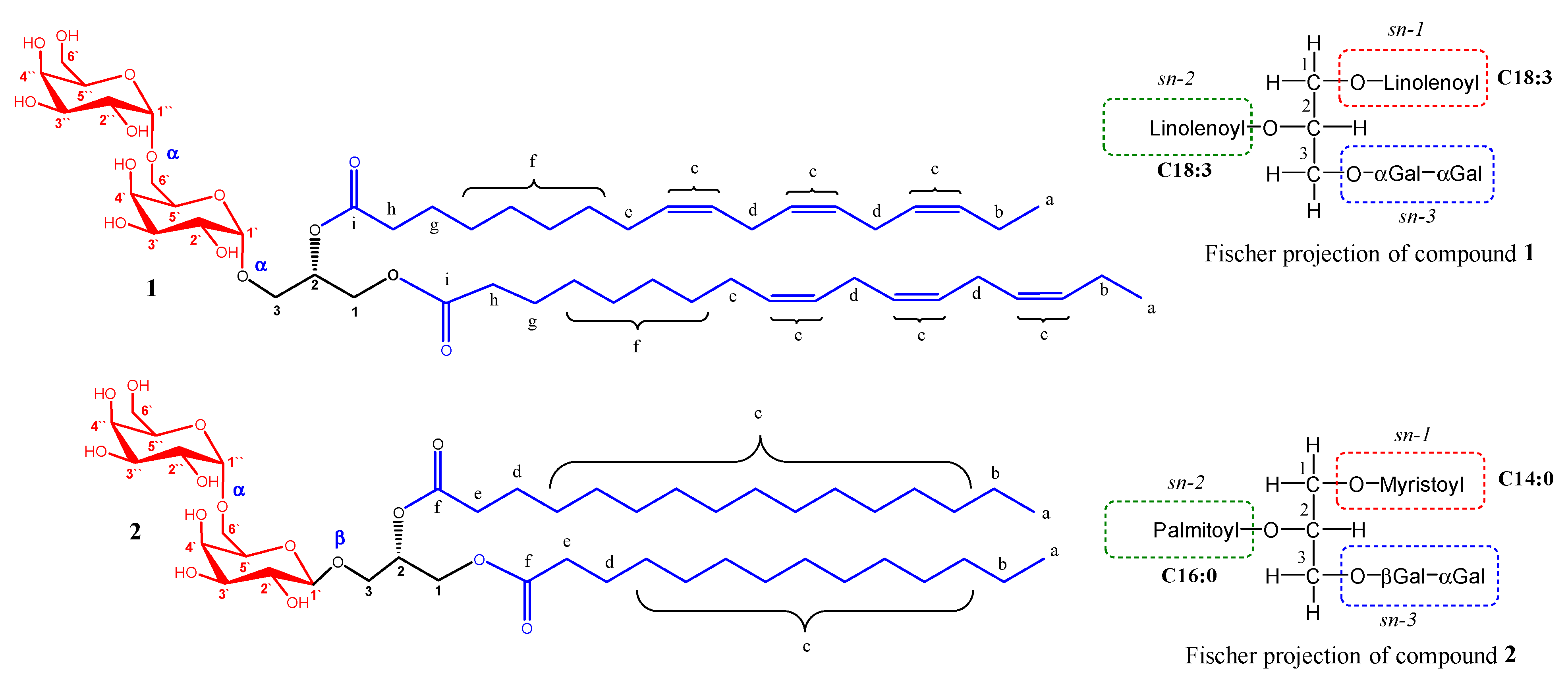
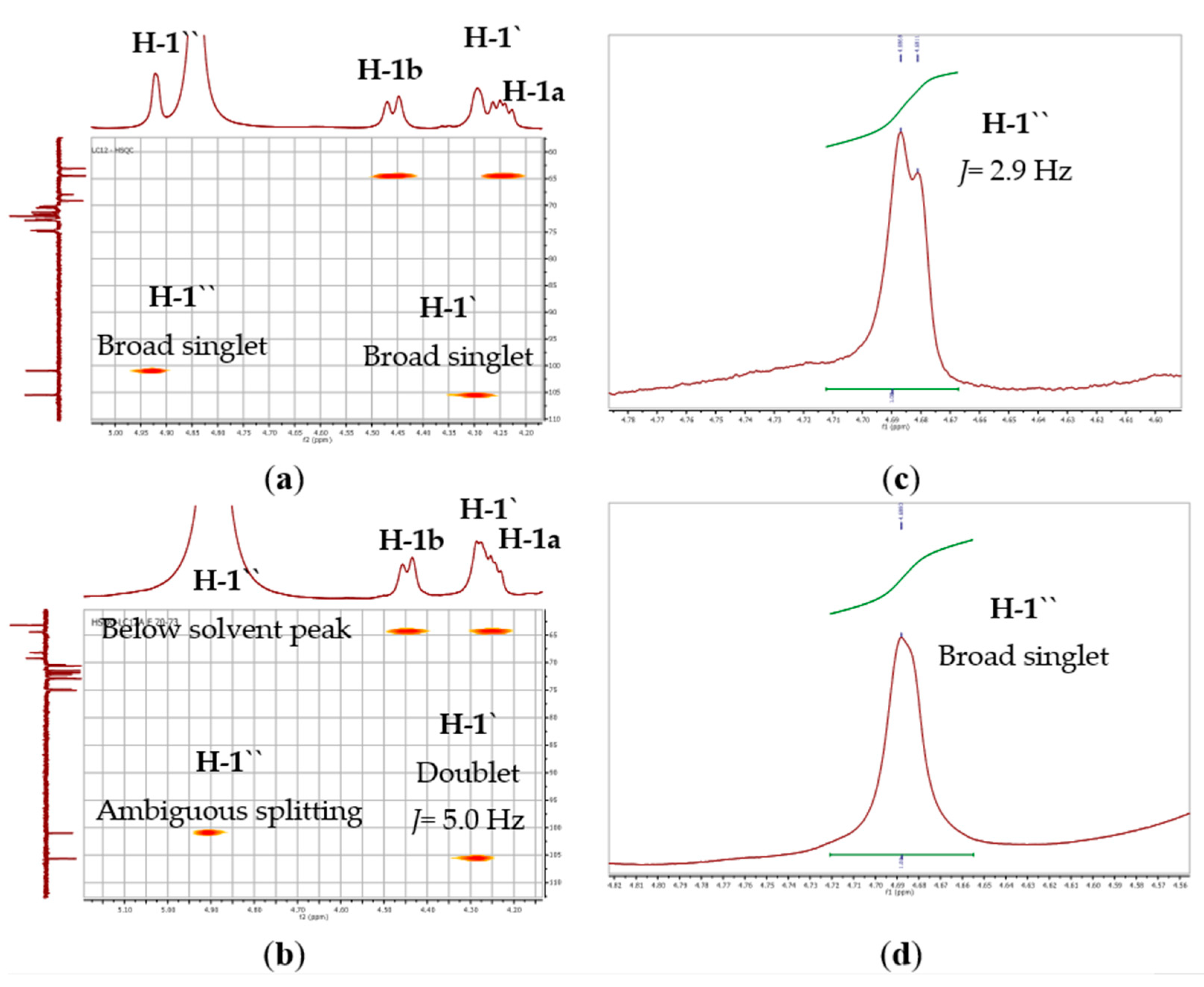
The ESI-MS spectra of compound 1 (Figure 2 and Figure S7) were consistent with the molecular formula C51H84O15, which was deduced from the pseudomolecular ion peak at m/z 959.5715 for [M+Na]+ (Calcd. 959.5708). The 1H and 13C NMR data of 1 (Table 1, Figures S1 and S2) revealed the presence of a methyl triplet at δH 0.97 (6H, J= 7.6 Hz; δC 14.8, H-a), a broad singlet at δH 1.34–1.38 (16H; δC 30.1–30.6, H-f), three aliphatic proton signals resonating at δH 2.06 (8H; δC at 21.4, H-b and 28.1, H-e), δH 2.33 (4H; δC 34.8 and 35.0, H-h), and δH 2.81 (8H; δC 26.4, 26.5, H-d), and twelve olefinic protons resonating at δH 5.32-5.41 (δC 128.1, 128.7, 129.0, 129.1, 130.9, and 132.6; H-c). The 13C NMR data of 1 (Table 1, Figure S2) showed the presence of two signals for two ester carbonyl groups at δC 174.3 and 174.6 (C-i), which further suggested the presence of two identical tri-unsaturated acyl chains with a total of six “cis” double bonds. These acyl chains were confirmed as linolenoyl based on the ESI-MS spectra (Figures S8–S10), which showed a characteristic peak in the positive mode at m/z 261.1312 for a linolenoyl fragment (Calcd. 261.2218) and two additional peaks in the negative mode at m/z 277.0563 for a linolenate fragment (Calcd. 277.2168) and 675.3194 for [M-acyl]− (Calcld. 675.3592) [49]. The presence of digalacotosyl moiety was evident from the DEPT135 spectrum that displayed two anomeric carbon signals at δC 105.0 and 100.4 (C-1′ and C-1′′, respectively) and eight oxymethine signals at δC 69.6-74.3 (2′-5′ and 2′′-5′′). In addition, four oxymethylene signals were found, of which two methylene signals at δC 67.4 and 62.5 were assigned to C-6′ and C-6′′ of the digalactose moiety, respectively. The other two oxymethylene signals at δC 68.6 (C-3; δH 3.97, m and 3.76, m), and 63.9 (C-1; δH 4.44, brd, J= 11.7 Hz and δH 4.24, dd, J= 11.6, 6.9 Hz), in addition to an oxymethine at δC 72.2 (CH, C-2; δH 5.27, m), were assigned to the sn-2 position of the glycerol moiety. These findings were consistent with the spectral data of digalactosyl–diacylglycerides [50,51,52]. The configurations of the digalactose moieties were assigned based on the splitting pattern of the anomeric protons. The anomeric proton of the first galactosyl moiety (H-1′) appeared as a broad singlet at δH 4.29 (Figure 3a), which established an α-linkage with the glycerol moiety. Additionally, the anomeric proton of the second galactosyl moiety (H-1′′) appeared as a broad singlet at δH 4.92, which confirmed an α-linkage between 1′′ and 6′ of the two galactose entities. To avoid the accidental overlapping of H-1′′ with the solvent peak, the 1H NMR experiment was repeated in DMSO-d6 (Figure 3b), which confirmed the previous results as H-1′′ was displayed as a doublet (J= 2.9 Hz) at δH 4.68. All of the proton signals were assigned based on the correlations deduced from the HSQC spectrum (Figure S5), and the connectivity of the different moieties was confirmed by the HMBC spectrum (Figure S6). Significant HMBC correlations were found between the proton at δH 4.24 (Ha-1, dd, J= 11.6, 6.9 Hz) and 4.45 (Hb-1, d, J= 11.7 Hz) of glycerol with the carbonyl ester signal at 174.3, 174.6 (C-i), confirming the diacylglyceride structure.
Figure 2.
Structures of compounds 1 and 2 isolated from Launaea capitata.

Table 1.
1H (500 MHz, CD3OD) and 13C (125 MHz) NMR spectral data of compound 1 (J in Hz).
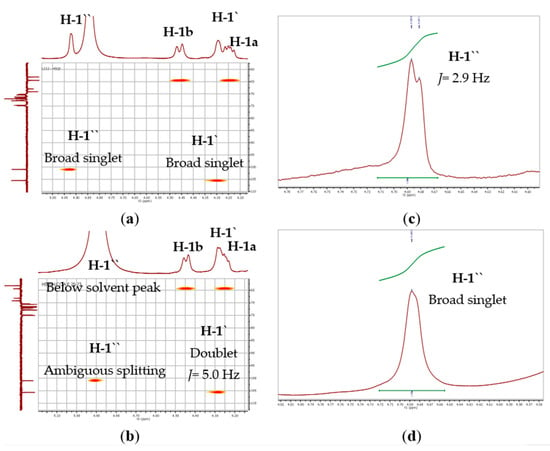
Figure 3.
HSQC expansions (4.10–5.10 ppm) of; (a) compound 1 and (b) compound 2 in CD3OD; and 1H NMR expansions of; (c) compound 1 (δH 4.60–4.78) and (d) compound 2 (δH 4.56–4.82) in DMSO-d6, revealing the splitting patterns and coupling constant “J” values (Hz) for the anomeric protons of galactose moieties, H-1′ and H-1′′ in both solvents.
Thus, compound 1 was confirmed as the new structure, 1,2-dilinolenoyl-3-O-(α-galactopyranosyl-(1,6)-O-α-D-galactopyranosyl)-sn-glycerol (Figure 2). To the best of our knowledge, this is the first report of this compound from nature; however, other isomers with different configurations at the two anomeric carbons were previously reported in the literature, including 1,2-dilinolenoyl-3-O-(α-galactopyranosyl-(1,6)-O-β-D-galactopyranosyl)-sn-glycerol [50,53,54] and 1,2-dilinolenoyl-3-O-(β-galactopyranosyl-(1,6)-O-β-D-galactopyranosyl)-sn-glycerol [55].
3.1.2. Identification of Compound 2
The ESI–MS data of compound 2 (Figure S17) were consistent with the molecular formula of C45H84O15, as deduced from the pseudomolecular ion peak at m/z 847.4663 for [M-OH]+ (Calcld. 847.5783). The 1H, 13C NMR, and DEPT135 spectra of 2 (Table 2 and Figures S11–S13) were closely related to that of compound 1 and indicated a digalactosyl diacylglyceride. However, it differed in the absence of the signals corresponding to the olefinic double bonds at δH 5.31–5.41. It showed the presence of a methyl triplet at δH 0.90 (J = 6.3 Hz; δC 15.0, H-a), three broad singlets located at δH 1.31–1.36 (40H; δC 30.5–33.5, H-c), δH 1.31 (4H; δC 24.2, overlapped, H-b), and δH 1.62 (4H; 26.3 and 26.4, H-d), and a quartet at δH 2.34 (J = 6.7 Hz; 35.4 and 35.5, H-e). These findings suggested the presence of two saturated fatty acid chains. The ESI-MS spectra of 2 (Figures S17–S20) showed typical fragments for a C14:0-C16:0 diacylglyceride. This was revealed from the peaks at m/z 643.4547 for [M-OH-Myrisoyl+Na]+ (Calcld. 643.3669) and m/z at 523.3611 for [M-digalactosyl]+ fragment (Calcld. 523.4726) in the positive mode. Additionally, the negative mode showed a peak at m/z at 311.1689 for the [plamitoyl-glyceride-H2O]− fragment (Calcld. 311.2586). Therefore, the acyl chains were assigned as 1-myristoyl, 2-palmitoyl digalactoglyceride (Figure 2). The appearance of H-1′ as a doublet (J = 5.0 Hz) at δH 4.28 indicated that the galactosyl linkage with the sn-3 position of glycerol is in the β-configuration. However, due to the coincidence of the anomeric proton signal of the second galactose moiety (H-1′′) with the solvent signal at δH 4.90 (Figure 3c), the 1H NMR experiment was repeated using DMSO-d6 as a solvent (Figure 3d), which revealed a broad singlet at δH 4.69 corresponding to H-1′′, confirming an α-linkage between the two galactose moieties. The connectivity of the different moieties was established by using the HMBC spectrum (Figure S16). The most important HMBC correlations were found between the proton signals at δH 5.27 (H-2), 4.44 (Hb-1), and 4.23 (Ha-1) with the carbonyl ester groups at δC 175.1/175.5 (C-f).

Table 2.
1H (500 MHz, CD3OD) and 13C (125 MHz, CD3OD) NMR spectral data of compound 2 (J in Hz).
Thus, compound 2 was confirmed to be 1-myristoyl-2-palmitoyl-3-O-(α-galactopyranosyl-(1,6)-O-β-D-galactopyranosyl)-sn-glycerol. It is worth noting that this is the first isolation of this compound from nature. However, the synthesis of this structure has been previously described in the literature [56].
3.2. Enzymes Inhibitory Activities
Plant galactolipids, including digalactosyldiacylglycerol, are natural compounds that form the bulk of the thylakoid membranes of chloroplasts in plants [51]. They are potential health-promoting compounds widely distributed in the plant kingdom [57]. Several reports have demonstrated that they possess various pharmacological activities, including their role in the inhibition of pancreatic lipase [58], platelet-activating factors [59], cancer cell migration [60], hyperlipidemia [56], inflammation [61], and filariasis [62]. Galactolipids have been suggested to be responsible for the anti-inflammatory activity of Rosa canina and contribute to its well-documented effects on arthritis [57]. The incidence of galactolipids in animals is restricted only to the central and peripheral nervous systems, where they are implicated in myelin development [52,63]. They are among the major classes of compounds involved in the development of myelin, which forms the bulk of the white matter in the brain [63,64,65]. While their deficiency would contribute to the pathology of AD [64]. The structural similarities of the fatty acids involved in the formation of the two isolated galactolipids (1 and 2) to the structure of AA inspired us to investigate their inhibitory activity against enzymes acting on AA to produce proinflammatory mediators. Therefore, they were tested as dual inhibitors of COX-2/5-LOX enzymes, which catalyze the first steps of AA metabolism, leading to the progression of inflammation. Moreover, 2-Arachidonoylglycerol, which could be close in structure to the investigated galactolipids, was reported to be a substrate to BchE [66]. Therefore, we investigated the inhibitory activity of the isolated galactolipids against COX-2, 5-LOX and BchE enzymes.
Compound 2 exhibited the dual inhibition of both 5-LOX (21.67 ± 1.75 μg/mL) and BchE (24.32 ± 1.29 μg/mL), which can be useful in ameliorating the systemic and neuro-inflammation associated with AD and T2DM, respectively. Compound 1 exhibited the dual inhibition of 5-LOX and BchE, with better BchE inhibitory activity (13.37 ± 0.98 μg/mL) compared to compound 2. However, it exhibited lower 5-LOX inhibition (59.01 μg/mL) than compound 2. On the other hand, both 1 and 2 exhibited weak COX-2 inhibitory activity (110.44 ± 3.75 and 179.63 ± 8.14 μg/mL, respectively), as can be seen in Table 3.

Table 3.
IC50 (μg/mL) values of the isolated compounds against COX-2, 5-LOX, and BchE enzymes.
3.3. Docking Study
We performed a computational docking study to reveal the in silico binding mode of the interaction of the isolated compounds against the three tested target enzymes. The obtained results were consistent with the in vitro enzyme inhibitory activity. While they showed the lowest binding affinity to the COX-2 enzyme, with docking scores of −7.360 and −5.723 kcal/mol for compounds 1 and 2, respectively (Table 4), both 1 and 2 showed greater binding affinity to 5-LOX compared to its native substrate, arachidonic acid (−5.830 kcal/mol, Table 4). Compound 2 showed greater binding affinity to 5-LOX (−8.634 kcal/mol) than compound 1 (−8.124 kcal/mol), which explained its lower IC50 in the in vitro inhibition assay against 5-LOX.

Table 4.
Docking scores of the isolated compounds (1 and 2) against COX-2, 5-LOX, and BchE enzymes using AutoDock Vina 1.2.3.
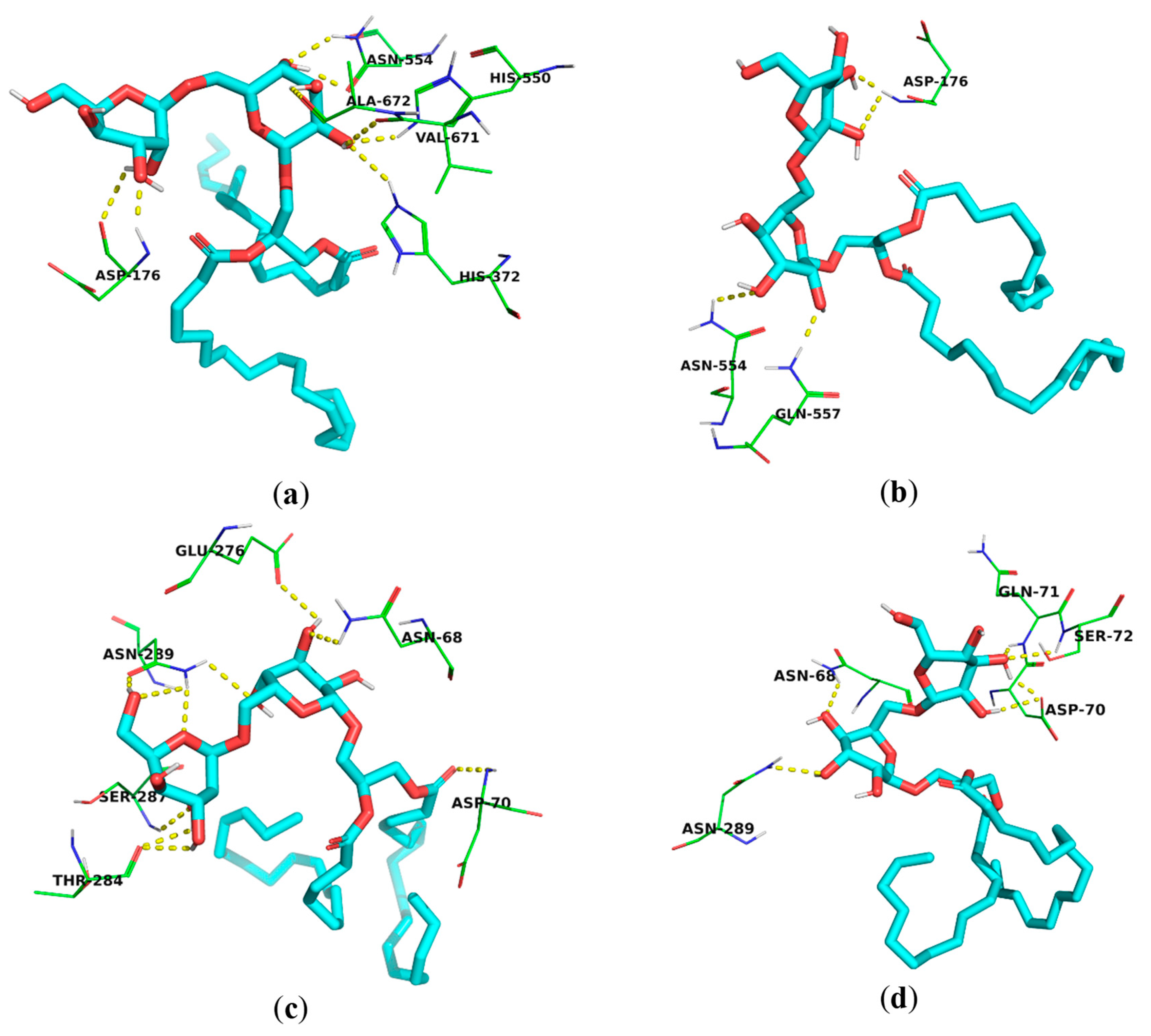
Both compounds showed multiple hydrogen-bonding interactions with several amino acid residues in the active site of 5-LOX, including Asp-176 and Asn-554, in addition to other amino acids [67,68] (Figure 4a,b). Both compounds 1 and 2 exhibited remarkable binding affinity to BchE (−8.313 and −7.502 kcal/mol, respectively), comparable to that of the co-crystallized ligand, thioflavin T (−8.107 kcal/mol). Both compounds 1 and 2 exhibited hydrogen bonding with Asp-70 amino acid, which is essential for the BchE activity [47], potentially justifying their low IC50 values in the in vitro BchE inhibition assay (13.37 ± 0.98 and 24.32 ± 1.29 μg/mL, respectively). In addition, they also showed H-bonding to other amino acid residues, such as the Asn-289, Asn-68, Glu-276, and Gln-71 of the BchE active site [69] (Figure 4c,d).
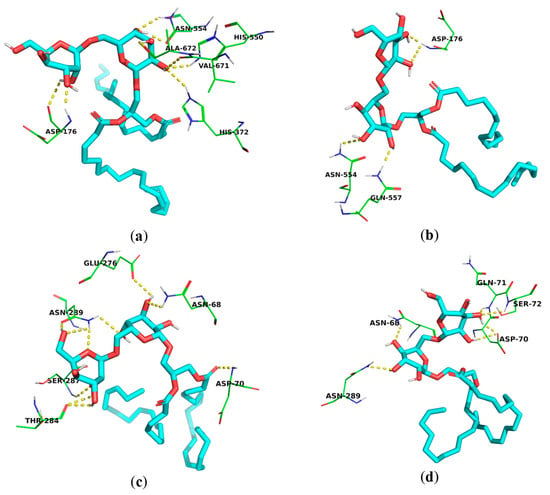
Figure 4.
Molecular binding models of the isolated galactolipids within the active sites of the 5-LOX and BchE enzymes obtained using AutoDock Vina (a) binding mode of compound 1 within the active site of the 5-LOX enzyme; (b) binding mode of compound 2 within the active site of the 5-LOX enzyme; (c) binding mode of compound 1 within the active site of the BchE enzyme; (d) and binding mode of compound 2 within the active site of the BchE enzyme.
4. Conclusions
In this study, the isolation of two digalactosyldiacylglycerol derivatives (i.e., plant galactolipids) from Launaea capitata (Spreng.) Dandy, for the first time from nature, has been reported. It is worth noting that plant galactolipids have been demonstrated by several studies to possess various medicinal properties, such as anti-inflammatory, antihyperlipidemic, antiplatelet aggregation, antimetastatic, and other properties. Although the isolated galactolipids exhibited weak in vitro inhibition of the COX-2 enzyme, they exhibited a remarkable inhibition of 5-LOX and BchE enzymes, which was further supported by a computational docking study against the crystal structures of their proteins. The obtained results suggested the beneficial effects of the isolated galactolipids in ameliorating both systemic inflammation and neuroinflammation associated with several diseases, such as Alzheimer’s disease and Type 2 diabetes mellitus. Future studies are suggested to further explore the role of the isolated galactolipids in relation to myelin development and other molecular mechanisms involved in inflammation and additionally investigate their potential use as enzyme inhibitors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10020083/s1, Figure S1–S20: the spectral data of the isolated molecules, including 1D and 2D NMR and ESI–MS. Table S1. The PDB retrieved codes of the crystal structures of utilized enzymes’ proteins and the used grid box coordinates in docking studies.
Author Contributions
F.M.A.B. established the concept, designed, and conducted the experiments, interpreted the data, and wrote the original draft. A.E.S. analyzed the data and wrote the original draft. M.H.E. performed the docking experiments, analyzed the data, and wrote the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, grant number (IF-PSAU-2021/03/17920).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials at https://www.mdpi.com/article/10.3390/separations10020083/s1.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through project number (IF-PSAU-2021/03/17920).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kilian, N. Revision of Launaea Cass. (Compositae, Lactuceae, Sonchinae). Englera 1997, 17, 1–478. [Google Scholar] [CrossRef]
- Daur, I. Plant flora in the rangeland of western Saudi Arabia. Pak. J. Bot. 2012, 44, 23–26. [Google Scholar]
- Elsharkawy, E.R. Isolation of phytoconstituents and evaluation of anticancer and Antioxidant potential of Launaea mucronata (Forssk.) Muschl. subsp. Pak. J. Pharm. Sci. 2017, 30, 399–405. [Google Scholar]
- Al-Fatimi, M. Wild edible plants traditionally collected and used in southern Yemen. J. Ethnobiol. Ethnomed. 2021, 17, 49. [Google Scholar] [CrossRef]
- Khalil, H.E.; Aldakheel, T.S.; AlAhmed, A.; Emeka, P.M.; Kandeel, M. Anti-proliferative activity of leaves of Launaea capitata Asteraceae: Phytochemical, cytotoxicity and in silico studies. Trop. J. Pharm. Res. 2020, 19, 2129–2136. [Google Scholar] [CrossRef]
- Mansour, R.M.A.; Ahmed, A.A.; Saleh, N.A.M. Flavone glycosides of some Launaea species. Phytochemistry 1983, 22, 2630–2631. [Google Scholar] [CrossRef]
- Emad, F.; Khalafalah, A.K.; El Sayed, M.A.; Mohamed, A.H.; Stadler, M.; Helaly, S.E. Three new polyacetylene glycosides (PAGs) from the aerial part of Launaea capitata (Asteraceae) with anti-biofilm activity against Staphylococcus aureus. Fitoterapia 2020, 143, 104548. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Parveen, S.; Riaz, N.; Tahir, M.N.; Ashraf, M.; Afzal, I.; Ali, M.S.; Malik, A.; Jabbar, A. New bioactive natural products from Launaea nudicaulis. Phytochem. Lett. 2012, 5, 793–799. [Google Scholar] [CrossRef]
- Cheriti, A.; Belboukhari, M.; Belboukhari, N.N.B.; Djeradi, H.H.D. Phytochemical and biological studies on Launaea Cass. Genus ( Asteracea) from Algerian sahara. Curr. Top. Phytochem. 2012, 11, 67–80. [Google Scholar]
- Hossain, J.S.; El-Sayed, M.; Aoshima, H. Antioxidative and anti-α-amylase activities of four wild plants consumed by pastoral nomads in Egypt. Orient. Pharm. Exp. Med. 2009, 9, 217–224. [Google Scholar] [CrossRef]
- Tariq, M.; Mossa, J.S.; Al-yahya, M.A.; Al-meshal, I.A.; Al-badr, A.A. Phytochemical and Biological Screening of Saudi Medicinal Plants Part-10* A Study on Saudi Plants of Family Compositae. Int. J. Crude Drug Res. 1987, 25, 17–25. [Google Scholar] [CrossRef]
- Nguyen, T.Q.C.; Binh, T.D.; Kusunoki, R.; Pham, T.L.A.; Nguyen, Y.D.H.; Nguyen, T.T.; Kanaori, K.; Kamei, K. Effects of Launaea sarmentosa Extract on Lipopolysaccharide-Induced Inflammation via Suppression of NF-κB/MAPK Signaling and Nrf2 Activation. Nutrients 2020, 12, 2586. [Google Scholar] [CrossRef]
- Asif, M.; Mahrukh; Saadullah, M.; Yaseen, H.S.; Saleem, M.; Yousaf, H.M.; Khan, I.U.; Yaseen, M.; Shams, M.U. Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology 2020, 28, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Akimat, E.K.; Omwenga, G.I.; Moriasi, G.A.; Ngugi, M.P. Antioxidant, Anti-Inflammatory, Acute Oral Toxicity, and Qualitative Phytochemistry of The Aqueous Root Extract of Launaea cornuta (Hochst. Ex Oliv. & Hiern.). J. Evid. Based Complement. Altern. Med. 2021, 26, 2515690X211064585. [Google Scholar] [CrossRef]
- Lamia, S.; Belboukhari, N.; Aminata, K.; Sulaiman, M.; Yakoubi, M.; Sekkoum, K.; Abdelkrim, C. Investigation of The Analgesic and Anti-Inflammatory Activities of Launaea Nudicaulis From Southwest of Algeria. Biomed. J. Sci. Technol. Res. 2021, 23, 17173–17178. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Proschak, E.; Steinhilber, D.; Rovati, G.E. Two-pronged approach to anti-inflammatory therapy through the modulation of the arachidonic acid cascade. Biochem. Pharmacol. 2018, 158, 161–173. [Google Scholar] [CrossRef]
- Sharma, J.N.; Jawad, N.M. Adverse effects of COX-2 inhibitors. Sci. World J. 2005, 5, 629–645. [Google Scholar] [CrossRef]
- Rainsford, K. Anti-inflammatory drugs in the 21st century. In Inflammation in The Pathogenesis of Chronic Diseases; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–27. [Google Scholar]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 inhibitors: Road from success to controversy and the quest for repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Medchemcomm 2018, 9, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Ethiraj, K.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Meshram, M.A.; Bhise, U.O.; Makhal, P.N.; Kaki, V.R. Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR. Eur. J. Med. Chem. 2021, 225, 113804. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets (Formerly Curr. Drug Targets CNS Neurol. Disord.) 2014, 13, 1432–1439. [Google Scholar]
- Ramachandra, G.; Lakshmi, G. Influence of butyrylcholinesterase on the course of COVID-19. Biomed. Rev. 2021, 32, 37–46. [Google Scholar]
- Dighe, S.N.; Deora, G.S.; De la Mora, E.; Nachon, F.; Chan, S.; Parat, M.-O.; Brazzolotto, X.; Ross, B.P. Discovery and structure–activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Kiewert, C.; Duysen, E.G.; Lockridge, O.; Greig, N.H.; Klein, J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 2007, 100, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Abdel Bar, F.M.; Sameti, M.; Foudah, A.I.; Haque, A.; Elsbaey, M. In vitro and in silico inhibition of COX-2 and 5-LOX by beta-carboline alkaloids from the seeds of Peganum harmala L. S. Afr. J. Bot. 2022, 147, 926–936. [Google Scholar] [CrossRef]
- Soliman, A.F.; Abdel Bar, F.M.; Sallam, A.; Galala, A.A. New neuroprotective sesquiterpene lactate esters from carotol biotransformation. S. Afr. J. Bot. 2023, 153, 163–171. [Google Scholar] [CrossRef]
- Obregon, A.D.; Schetinger, M.R.; Correa, M.M.; Morsch, V.M.; da Silva, J.E.; Martins, M.A.; Bonacorso, H.G.; Zanatta, N. Effects per se of organic solvents in the cerebral acetylcholinesterase of rats. Neurochem. Res. 2005, 30, 379–384. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzym. Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Lee, C.; Liao, J.; Chen, S.; Yen, C.; Lee, Y.; Huang, S.; Huang, S.; Lin, C.; Chang, V.H. Fluorine-Modified Rutaecarpine Exerts Cyclooxygenase-2 Inhibition and Anti-inflammatory Effects in Lungs. Front. Pharmacol. 2019, 10, 91. [Google Scholar] [CrossRef]
- Yoon, S.H.; Cho, D.Y.; Choi, S.R.; Lee, J.Y.; Choi, D.K.; Kim, E.; Park, J.Y. Synthesis and biological evaluation of salicylic acid analogues of celecoxib as a new class of selective cyclooxygenase-1 inhibitor. Biol. Pharm. Bull. 2021, 44, 1230–1238. [Google Scholar] [CrossRef]
- Yarla, N.S.; Pathuri, G.; Gali, H.; Terzyan, S.; Panneerselvam, J.; Chandrakesan, P.; Scotti, M.T.; Houchen, C.; Madka, V.; Rao, C.V. Discovery and Development of a Novel mPGES-1/5-LOX Dual Inhibitor LFA-9 for Prevention and Treatment of Chronic Inflammatory Diseases. J. Inflamm. Res. 2020, 13, 1261–1278. [Google Scholar] [CrossRef]
- Shaaban, M.; Kamal, A.; Faggal, S.; Farag, N.; Aborehab, N.; El-Sahar, A.; Mohamed, K. Design, synthesis, and biological evaluation of new pyrazoloquinazoline derivatives as dual COX-2/5-LOX inhibitors. Archiv. Pharm. 2020, 353, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Rui, Z.; Neau, D.B.; Waight, M.T.; Bartlett, S.G.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663. FASEB J. 2012, 26, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.L.; Brazzolotto, X.; Macdonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Guella, G.; Frassanito, R.; Mancini, I. A new solution for an old problem: The regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid. Commun. Mass Spectrom. 2003, 17, 1982–1994. [Google Scholar] [CrossRef]
- Bartels, D.; Dörmann, P. Plant Lipids: Methods and Protocols. In Methods in Molecular Biology; Walker, J.M., Ed.; Springer Nature Humana Press: Bonn, Germany, 2021; Volume 2295. [Google Scholar]
- Dörmann, P. Galactolipids in Plant Membranes; eLS, Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2013; pp. 1–7. [Google Scholar]
- Douce, R.; Joyard, J. Plant galactolipids. In Lipids: Structure and Function; Elsevier: Amsterdam, The Netherlands, 1980; pp. 321–362. [Google Scholar]
- Cateni, F.; Falsone, G.; Zilic, J.; Bonivento, P.; Zacchigna, M.; Žigon, D.; Sosa, S.; Altinier, G. Glyceroglycolipids from Euphorbia nicaeensis All. with antiinflamatory activity. ARKIVOC 2004, 2004, 54–65. [Google Scholar] [CrossRef]
- Wu, J.; Long, L.; Song, Y.; Zhang, S.; Li, Q.; Huang, J.; Xiao, Z. A new unsaturated glycoglycerolipid from a cultured marine dinoflagellate Amphidinium carterae. Chem. Pharm. Bull. 2005, 53, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Seki, K.; Ohnishi, M.; Ito, S.; Fujino, Y. Structure of novel glyceroglycolipids in Adzuki bean (Vigna angularis) seeds. Biochem. Cell Biol. 1990, 68, 59–64. [Google Scholar] [PubMed]
- Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of digalactosyl diacylglycerols and their structure-inhibitory activity on human lanosterol synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159–162. [Google Scholar] [CrossRef]
- Christensen, L.P. Galactolipids as potential health promoting compounds in vegetable foods. Recent Pat. Food Nutr. Agric. 2009, 1, 50–58. [Google Scholar] [CrossRef]
- Watanabe, D.; Kerakawati, R.; Morita, T.; Nakamura, T.; Ueno, K.; Kumamoto, T.; Nakanishi, W.; Ishikawa, T.; Uzawa, J.; Seki, H.; et al. Isolation of β-Sitosterol and Digalactopyranosyl-diacylglyceride from Citrus hystrix, a Thai Traditional Herb, as Pancreatic Lipase Inhibitors. Heterocycles 2009, 78, 1497–1505. [Google Scholar] [CrossRef]
- Fan, G.-j.; Kim, S.; Han, B.H.; Han, Y.N. Glyceroglycolipids, a novel class of platelet-activating factor antagonists from Kalimeris indica. Phytochem. Lett. 2008, 1, 207–210. [Google Scholar] [CrossRef]
- Seo, E.J.; Wu, C.F.; Ali, Z.; Wang, Y.H.; Khan, S.I.; Walker, L.A.; Khan, I.A.; Efferth, T. Both Phenolic and Non-phenolic Green Tea Fractions Inhibit Migration of Cancer Cells. Front. Pharmacol. 2016, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-m.; Yu, K.; Xia, Y.; Shine, M.B.; Wang, C.; Navarre, D.; Kachroo, A.; Kachroo, P. Mono- and Digalactosyldiacylglycerol Lipids Function Nonredundantly to Regulate Systemic Acquired Resistance in Plants. Cell Rep. 2014, 9, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Misra, S.; Gupta, J.; Misra-Bhattacharya, S. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite, Brugia malayi. Eur. J. Med. Chem. 2012, 50, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.; Popko, B. Galactolipids are molecular determinants of myelin development and axo–glial organization. Biochim. Biophys. Acta Gen. Subj. 2002, 1573, 406–413. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of lipids in Alzheimer’s disease pathology and potential therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Dupree, J.L.; Suzuki, K.; Popko, B. Galactolipids in the formation and function of the myelin sheath. Microsc. Res. Tech. 1998, 41, 431–440. [Google Scholar] [CrossRef]
- Barricklow, J.; Blatnik, M. 2-Arachidonoylglycerol is a substrate for butyrylcholinesterase: A potential mechanism for extracellular endocannabinoid regulation. Arch. Biochem. Biophys. 2013, 536, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Saura, P.; Maréchal, J.D.; Masgrau, L.; Lluch, J.M.; González-Lafont, À. Computational insight into the catalytic implication of head/tail-first orientation of arachidonic acid in human 5-lipoxygenase: Consequences for the positional specificity of oxygenation. Phys. Chem. Chem. Phys. 2016, 18, 23017–23035. [Google Scholar] [CrossRef]
- Sobeh, M.; Mamadalieva, N.Z.; Mohamed, T.; Krstin, S.; Youssef, F.S.; Ashour, M.L.; Azimova, S.S.; Wink, M. Chemical profiling of Phlomis thapsoides (Lamiaceae) and in vitro testing of its biological activities. Med. Chem. Res. 2016, 25, 2304–2315. [Google Scholar] [CrossRef]
- Uzairu, S.M.; Tijani, Y.; Gadaka, M.A.; Modu, B.; Watafua, M.; Ahmad, H.A.; Zakariya, U.A.; Ibrahim, A.; Daja, A.; Zanna, H.; et al. Kinetics and computational study of butyrylcholinesterase inhibition by methylrosmarinate: Relevance to Alzheimer’s disease treatment. Heliyon 2022, 8, e10613. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).