Variation with In Vitro Analysis of Volatile Profiles among Aspergillus flavus Strains from Louisiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Fungal Volatile Procedure
3. Results
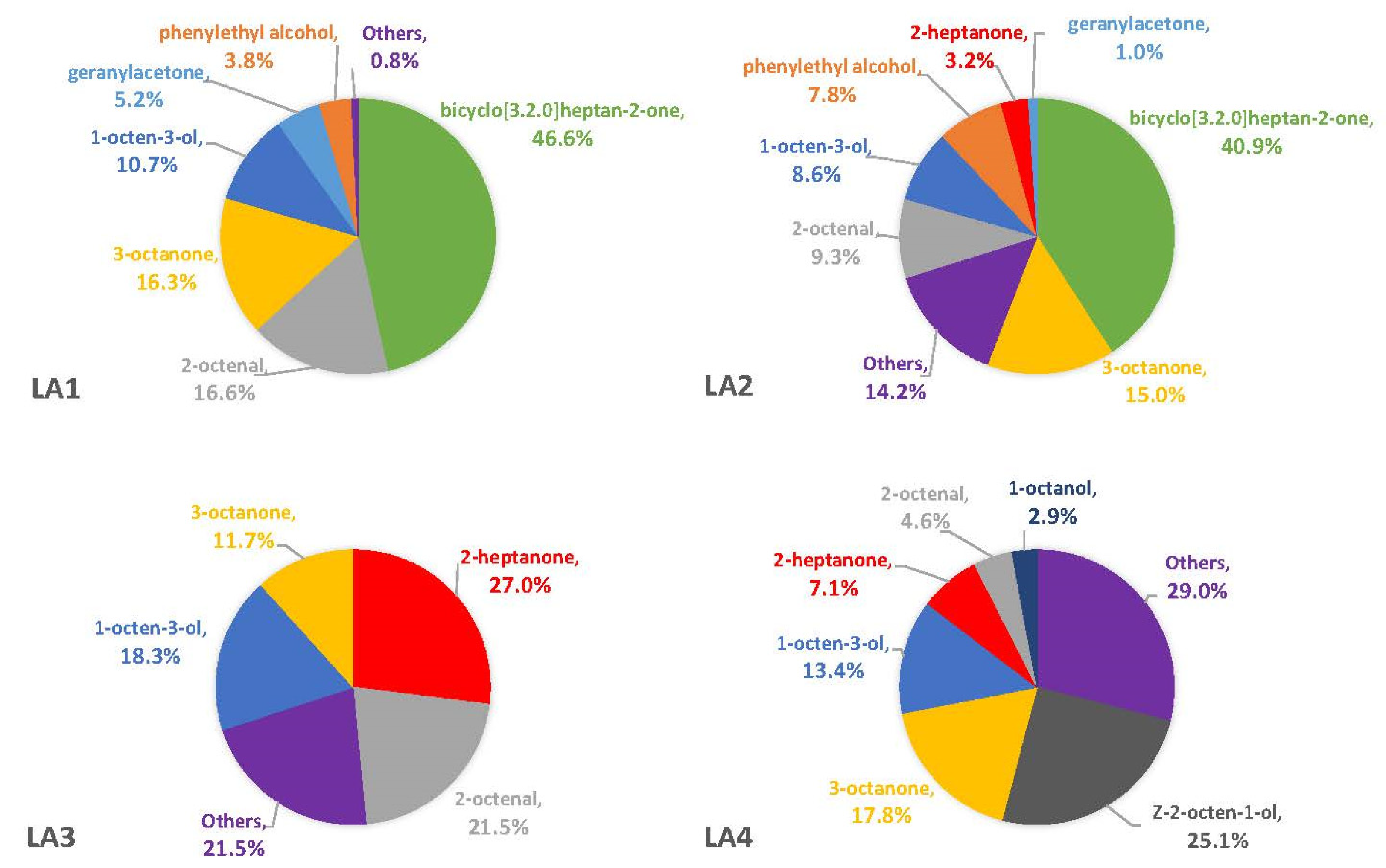
3.1. Headspace Profiles on Synthetic Media
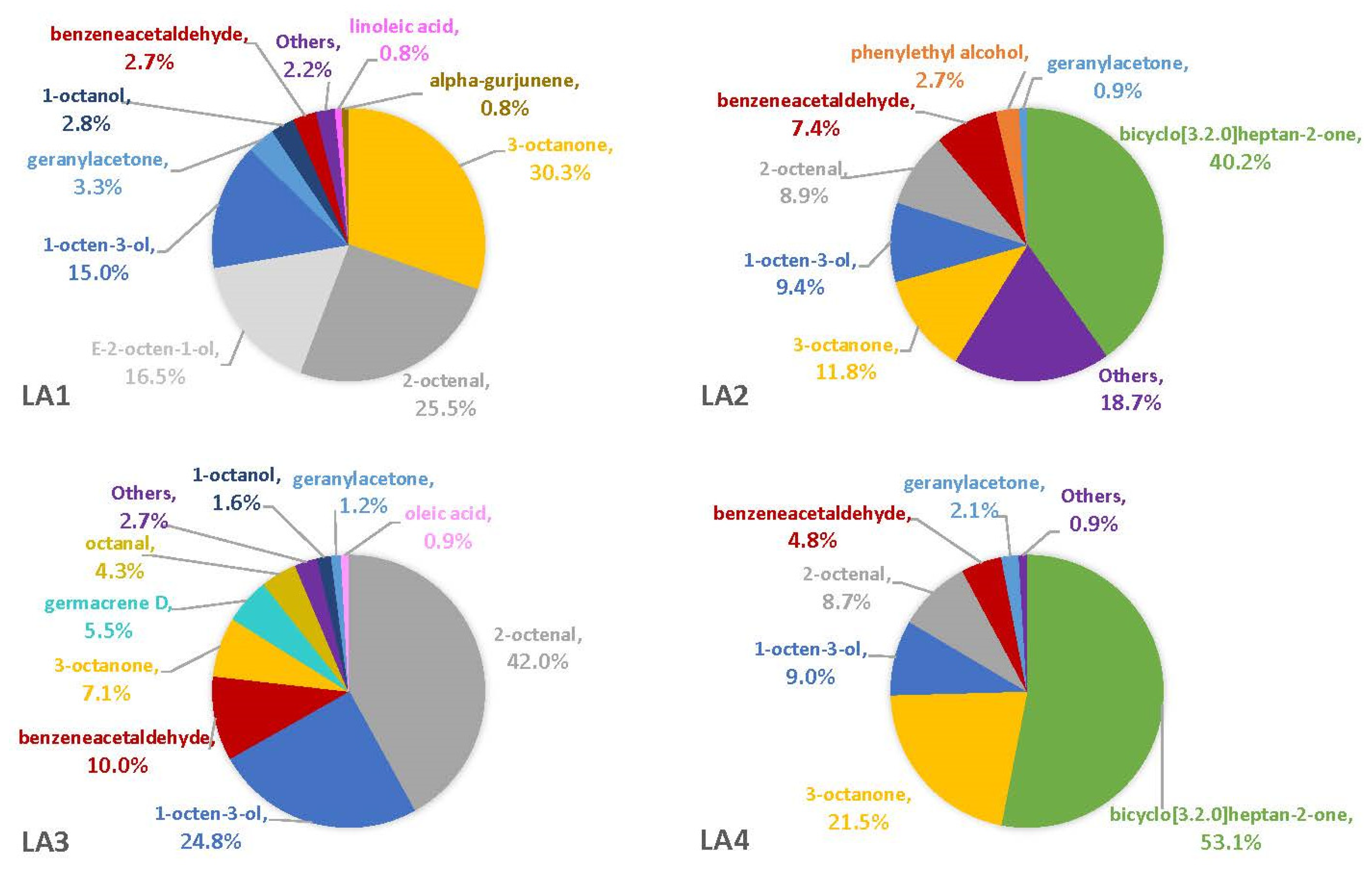
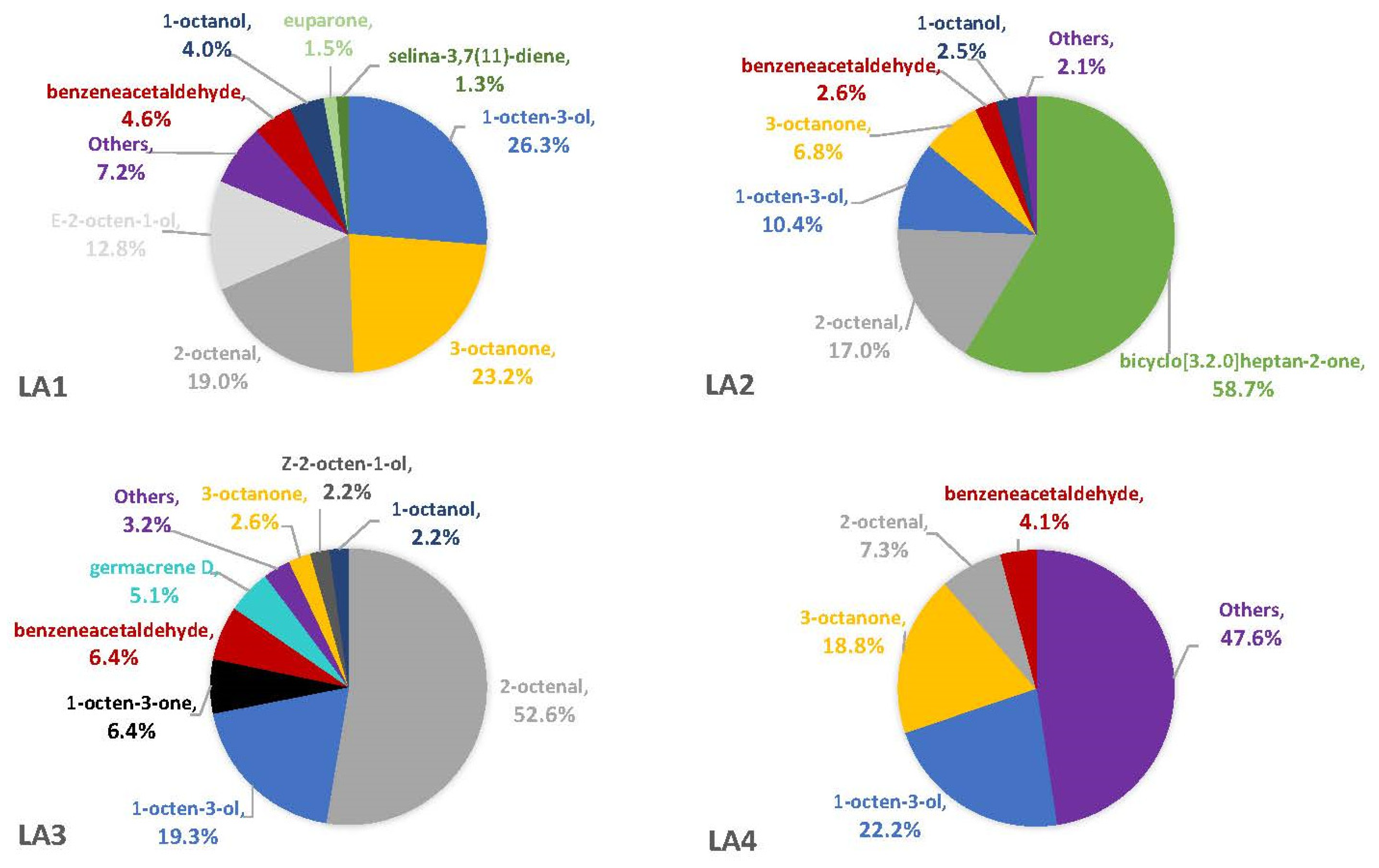
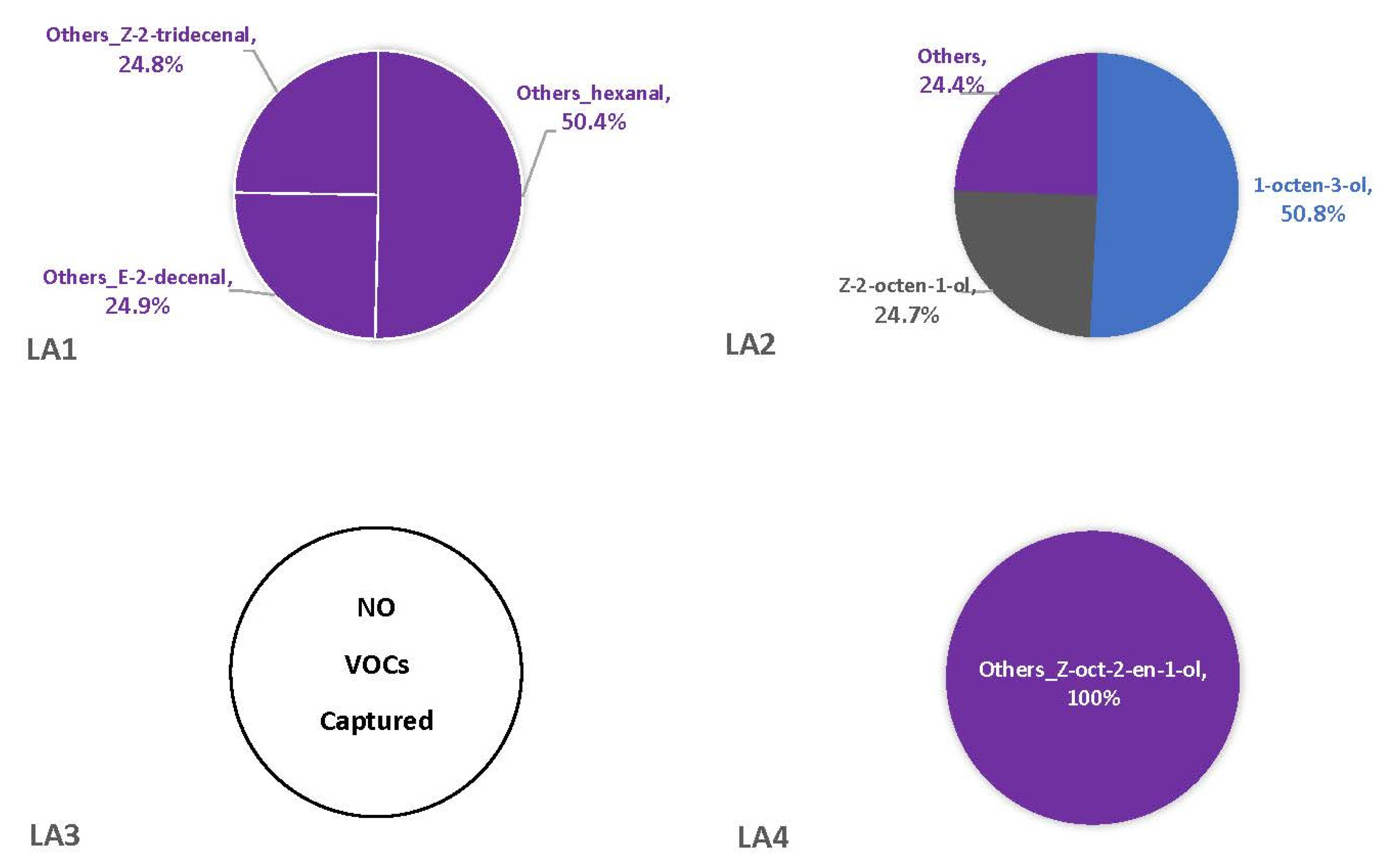
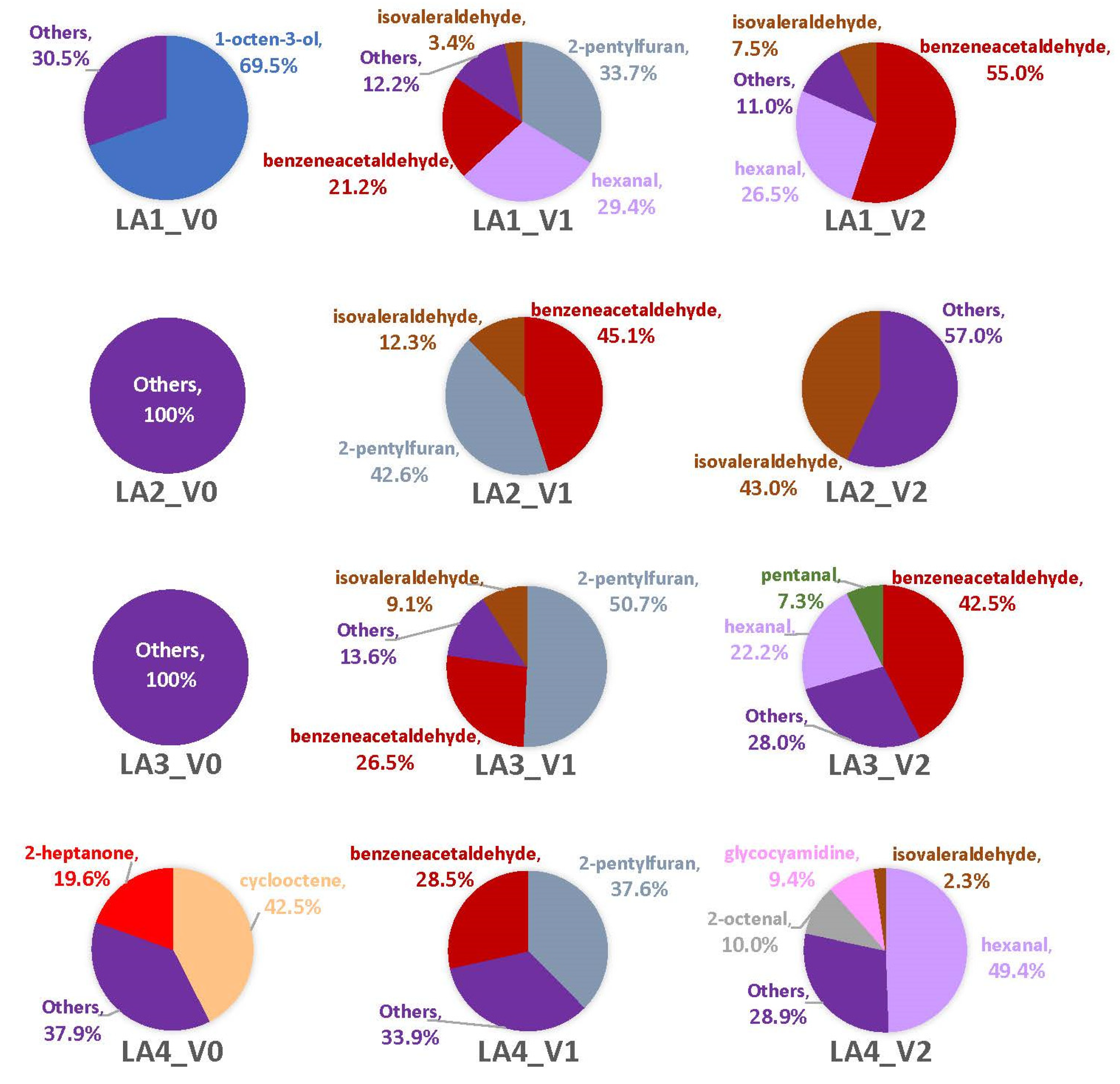
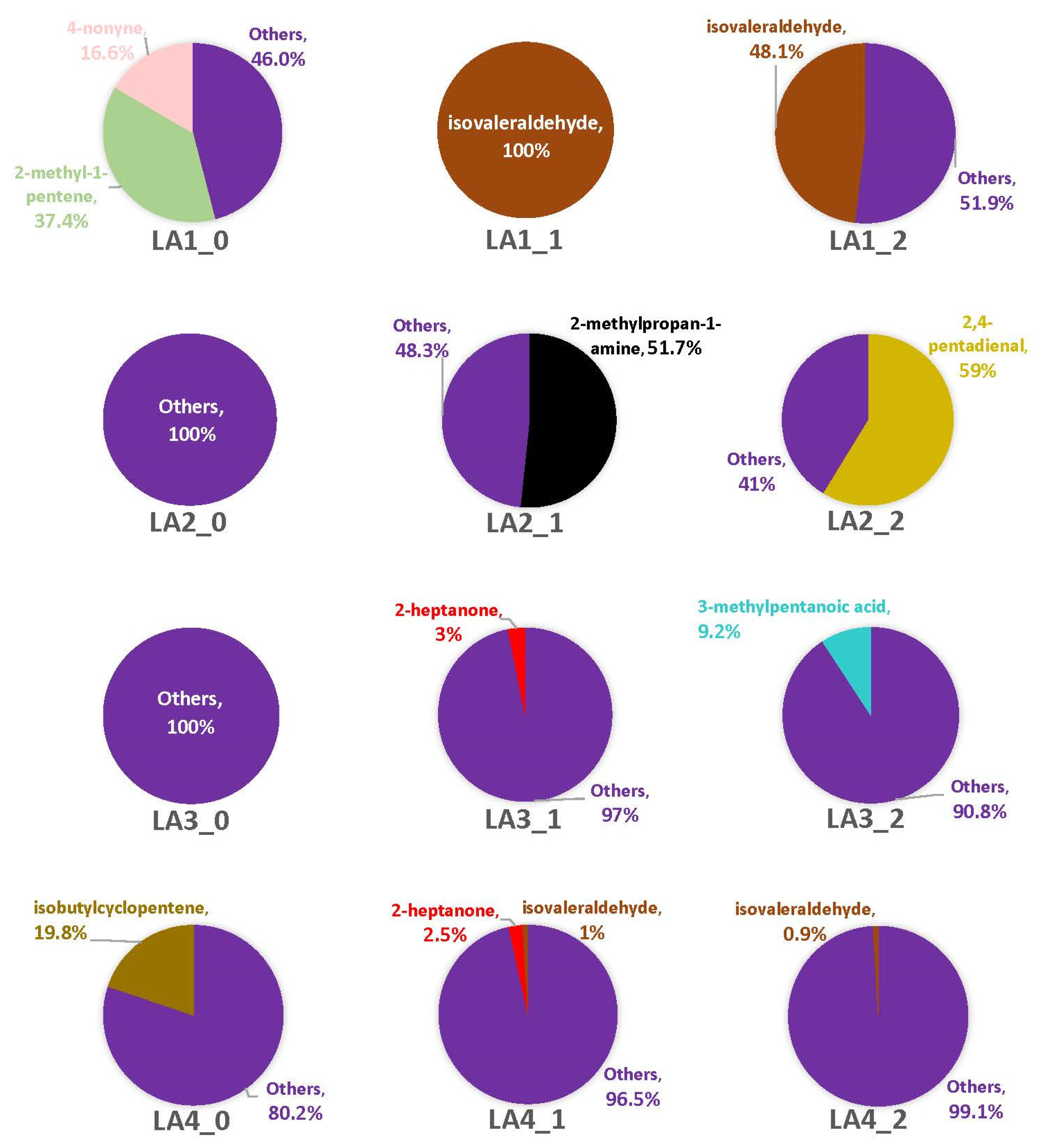
3.2. Headspace Profiles on Susceptible and Resistant Corn Genotypes
4. Discussion
4.1. Differences in Synthetic Media May Explain Observed Diversity of A. flavus VOCs
4.2. VOCs Associated with Corn Genotypes May Not Be Solely from A. flavus
4.3. LA1 Produces VOCs That Could Be Tested for Biocontrol Properties
4.4. VOCs Exist That Could Be Used to Screen for the Presence of Aflatoxigenic Aspergilli
4.5. Comparisons with Other A. flavus VOC Studies Reveal a True Diversity of Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, M.R. Elucidation of primary metabolic pathways in Aspergillus species: Orphaned research in characterizing orphan genes. Brief. Funct. Genom. 2014, 13, 451–455. [Google Scholar] [CrossRef][Green Version]
- Frisvad, J.C. Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front. Microbiol. 2015, 5, e773. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hung, R.; Yin, G.; Klich, M.A.; Grimm, C.; Bennett, J.W. Arabidopsis thaliana as bioindicator of fungal VOCs in indoor air. Mycobiology 2016, 44, 162–170. [Google Scholar] [CrossRef]
- Börjesson, T.; Stöllman, U.; Schnürer, J. Volatile metabolites produced by six fungal species compared with other indicators of fungal growth on cereal grains. Appl. Environ. Microbiol. 1992, 58, 2599–2605. [Google Scholar] [CrossRef]
- Gao, P.; Korley, F.; Martin, J.; Chen, B.T. Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. AIHA J. 2002, 63, 135–140. [Google Scholar] [CrossRef]
- Sun, D.; Wood-Jones, A.; Wang, W.; Vanlangenberg, C.; Jones, D.; Gower, J.; Simmons, P.; Baird, R.; Mlsna, T. Monitoring MVOC profiles over time from isolates of Aspergillus flavus using SPME GC-MS. J. Agric. Chem. Environ. 2014, 3, 48–63. [Google Scholar] [CrossRef]
- Sun, D.; She, J.; Gower, J.L.; Stokes, C.E.; Windham, G.L.; Baird, R.E.; Mlsna, T.E. Effects of growth parameters on the analysis of Aspergillus flavus volatile metabolites. Separations 2016, 3, 13. [Google Scholar] [CrossRef]
- Li, H.; Kang, X.; Wang, S.; Mo, H.; Xu, D.; Zhou, W.; Hu, L. Early detection and monitoring for Aspergillus flavus contamination in maize kernels. Food Control 2020, 121, e107636. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.H.; Bland, J.M.; Bhatnagar, D.; Cleveland, T.E. Volatile profiles of toxigenic and non-toxigenic Aspergillus flavus using SPME for solid phase extraction. Ann. Agric. Environ. Med. 2010, 17, 301–308. [Google Scholar]
- De Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.H.; Bhatnagar, D. Volatile profiles and aflatoxin production by toxigenic and non-toxigenic isolates of Aspergillus flavus grown on sterile and non-sterile cracked corn. Ann. Agric. Environ. Med. 2012, 19, 91–98. [Google Scholar] [PubMed]
- Cary, J.W.; Gilbert, M.K.; Lebar, M.D.; Majumdar, R.; Calvo, A.M. Aspergillus flavus secondary metabolites: More than just aflatoxins. Food Saf. 2018, 6, 7–32. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E., Jr. Influence of neighboring clonal-colonies on aflatoxin production by Aspergillus flavus. Front. Microbiol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. C15H24 volatile compounds unique to aflatoxigenic strains of Aspergillus flavus. Appl. Environ. Microbiol. 1993, 59, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G. Practical considerations will ensure the continued success of pre-harvest biocontrol using non-aflatoxigenic Aspergillus flavus strains. Crit. Rev. Food Sci. Nutr. 2022, 62, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. The potential role of fungal volatile organic compounds in Aspergillus flavus biocontrol efficacy. Biol. Control 2021, 160, e104686. [Google Scholar] [CrossRef]
- Schmidt, F.R.; Lemke, P.A.; Esser, K. Viral influences on aflatoxin formation by Aspergillus flavus. Appl. Microbiol. Biotechnol. 1986, 24, 248–252. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bhatnagar, D. Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 1994, 60, 2248–2251. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E., Jr.; Kaller, M.D. Comparison of soil and corn kernel Aspergillus flavus populations: Evidence for niche specialization. Phytopathology 2011, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. Cumulative effects of non-aflatoxigenic Aspergillus flavus volatile organic compounds to abate toxin production by mycotoxigenic aspergilli. Toxins 2022, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. The role of extrolites secreted by nonaflatoxigenic Aspergillus flavus in biocontrol efficacy. J. Appl. Microbiol. 2019, 126, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. J. Stored Prod. Res. 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Henderson, J. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Posthumus, M.A.; Dijksterhuis, J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Appl. Environ. Microbiol. 2004, 70, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kimura, T.; Tanaka, H.; Kaneko, S.; Ichii, S.; Kiuchi, M.; Suzuki, T. Analysis of volatile metabolites emitted by soil-derived fungi using head space solid-phase microextraction/gas chromatog-raphy/mass spectrometry: I. Aspergillus fumigatus, Aspergillus nidulans, Fusarium solani and Penicillium paneum. Surf. Interface Anal. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Liarzi, O.; Benichis, M.; Gamliel, A.; Ezra, D. trans-2-Octenal, a single compound of a fungal origin, controls Sclerotium rolfsii, both in vitro and in soil. Pest Manag. Sci. 2020, 76, 2068–2071. [Google Scholar] [CrossRef]
- Das, M.; Prakash, H.S.; Nalini, M.S. Antibacterial metabolites from Bipolaris specifera, an endophytic fungus from the endemic medicinal plant, Zingiber nimmonii (J. Graham) Dalzell. 3 Biotech 2020, 10, e317. [Google Scholar] [CrossRef]
- Zou, C.; Li, Z.; Yu, D. Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J. Microbiol. 2010, 48, 460–466. [Google Scholar] [CrossRef]
- Boué, S.M.; Shih, B.Y.; Carter-Wientjes, C.H.; Cleveland, T.E. Effect of soybean lipoxygenase on volatile generation and inhibition of Aspergillus flavus mycelial growth. J. Agric. Food Chem. 2005, 53, 4778–4783. [Google Scholar] [CrossRef]
- Syhre, M.; Scotter, J.M.; Chambers, S.T. Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 2008, 46, 209–215. [Google Scholar] [CrossRef]
- Jurjevic, Z.; Rains, G.C.; Wilson, D.M.; Lewis, W.J. Volatile metabolites associated with one aflatoxigenic and one nontoxigenic Aspergillus flavus strain grown on two different substrates. Phytopathol. Mediterr. 2008, 47, 266–271. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, Y.; Zhang, Y.; Lin, Z.; Lv, H.P. Volatile composition of Fu-brick tea and Pu-erh tea analyzed by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. LWT 2019, 103, 27–33. [Google Scholar] [CrossRef]
- Xiang, M.; Chu, J.; Cai, W.; Ma, H.; Zhu, W.; Zhang, X.; Ren, J.; Xiao, L.; Liu, D.; Liu, X. Microbial succession and interactions during the manufacture of Fu Brick Tea. Front. Microbiol. 2022, 13, e892437. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, C.; Zhao, H.; Gao, X.; Zhao, M.; Sun, W. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int. J. Food Sci. Technol. 2013, 48, 609–619. [Google Scholar] [CrossRef]
- Shi, C.; Jash, A.; Lim, L.T. Activated release of hexanal and salicylaldehyde from imidazolidine precursors encapsulated in electrospun ethylcellulose-poly(ethylene oxide) fibers. SN Appl. Sci. 2021, 3, 385. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Li, N.; Hu, Y.-S.; Cai, J.-P. Metabolomic analyses revealed multifaceted effects of hexanal on Aspergillus flavus growth. Appl. Microbiol. Biotechnol. 2021, 105, 3745–3757. [Google Scholar] [CrossRef]
- Cameron, J.N.; Carlile, M.J. Binding of isovaleraldehyde, an attractant, to zoospores of the fungus Phytophthora palmivora in relation to zoospore chemotaxis. J. Cell Sci. 1981, 49, 273–281. [Google Scholar] [CrossRef]
- Gehrig, R.; Knight, S. Formation of 2-heptanone from caprylic acid by spores of various filamentous fungi. Nature 1961, 192, 1185. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskaya, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. BioMed Res. Int. 2014, 2014, 125704. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Wilson, B.J. Aflatrem, the tremorgenic mycotoxin from Aspergillus flavus. Mycopathologia 1979, 66, 183–185. [Google Scholar] [CrossRef]
- Valdes, J.J.; Cameron, J.E.; Cole, R.J. Aflatrem: A tremorgenic mycotoxin with acute neurotoxic effects. Environ. Health Perspect. 1985, 62, 459–463. [Google Scholar] [CrossRef]
- Josselin, L.; De Clerck, C.; De Boevre, M.; Moretti, A.; Jijakli, M.H.; Soyeurt, H.; Fauconnier, M.-L. Volatile organic compounds emitted by Aspergillus flavus strains producing or not aflatoxin B1. Toxins 2021, 13, 705. [Google Scholar] [CrossRef]
- Calvo, A.M.; Hinze, L.L.; Gardner, H.W.; Keller, N.P. Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus spp. Appl. Environ. Microbiol. 1999, 65, 3668–3673. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, A.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Carradori, S.; D’Antonio, M.; Orlando, G.; Cairone, F.; Cesa, S.; Filippi, A.; Fraschetti, C.; Zengin, G.; et al. Chemical and bioinformatics analyses of the anti-leishmanial and anti-oxidant activities of hemp essential oil. Biomolecules 2021, 11, 272. [Google Scholar] [CrossRef]
- Kaminśki, E.; Libbey, L.M.; Stawicki, S.; Wasowicz, E. Identification of the predominant volatile compounds produced by Aspergillus flavus. Appl. Microbiol. 1972, 24, 721–726. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Marriott, P.J.; Zhang, X.; Wang, S.; Li, J.; Ma, L. Chemometric analysis of the volatile compounds generated by Aspergillus carbonarius strains isolated from grapes and dried vine fruits. Toxins 2018, 10, 71. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal volatile organic compounds: More than just a funky smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef]
- Karbin, S.; Rad, A.B.; Arouiee, H.; Jafarnia, S. Antifungal activities of the essential oils on post-harvest disease agent Aspergillus flavus. Adv. Environ. Biol. 2009, 3, 219–225. [Google Scholar]
- Cleveland, T.E.; Carter-Wientjes, C.H.; De Lucca, A.J.; Boué, S.M. Effect of soybean volatile compounds on Aspergillus flavus growth and aflatoxin production. J. Food Sci. 2009, 74, H83–H87. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.; Skourti, A.; Nika, E.P.; Boukouvala, M.C.; Kavallieratos, N.G. Five natural compounds of botanical origin as wheat protectants against adults and larvae of Tenebrio molitor L. and Trogoderma granarium Everts. Environ. Sci. Pollut. Res. 2021, 28, 42763–42775. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Li, X.-D.; Li, X.; Li, X.-M.; Yin, X.-L.; Wang, B.-G. Antimicrobial bisabolane-type sesquiterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Nat. Prod. Res. 2021, 35, 4265–4271. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a sesquiterpene from the essential oil extract of Opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Zellagui, A.; Gherraf, N.; Rhouati, S. Chemical composition and antibacterial activity of the essential oils of Ferula vesceritensis Coss et Dur. leaves, endemic in Algeria. Org. Med. Chem. Lett. 2012, 2, e31. [Google Scholar] [CrossRef]
- Becker, E.M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.; Dias, R.B.; Soares, M.B.; Prata, A.P.N.; Rocha, C.A.G.; Costa, E.V.; Bezerra, D.P. Antitumor properties of the essential oil from the leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.S.; Cai, J.P. Heptanal inhibits the growth of Aspergillus flavus through disturbance of plasma membrane integrity, mitochondrial function and antioxidant enzyme activity. LWT 2022, 154, e112655. [Google Scholar] [CrossRef]
- Gardini, F.; Lanciotti, R.; Guerzoni, M.E. Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. Lett. Appl. Microbiol. 2001, 33, 50–55. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Carter-Wientjes, C.H.; Boué, S.; Bhatnagar, D. Volatile trans-2-hexenal, a soybean aldehyde, inhibits Aspergillus flavus growth and aflatoxin production in corn. J. Food Sci. 2011, 76, M381–M386. [Google Scholar] [CrossRef]
- Todokoro, T.; Negoro, H.; Kotaka, A.; Hata, Y.; Ishida, H. Aspergillus oryzae FaeA is responsible for the release of ferulic acid, a precursor of off-odor 4-vinylguaiacol in sake brewing. J. Biosci. Bioeng. 2022, 133, 140–145. [Google Scholar] [CrossRef]
- Upadhyay, P.; Singh, N.K.; Tupe, R.; Odenath, A.; Lali, A. Biotransformation of corn bran derived ferulic acid to vanillic acid using engineered Pseudomonas putida KT2440. Prep. Biochem. Biotechnol. 2020, 50, 341–348. [Google Scholar] [CrossRef]
| VOC: IUPAC (Synonym) a | CAS No. | R.T. | Medium b |
|---|---|---|---|
| heptan-2-one (2-heptanone) | 000110-43-0 | 7.65 | Y 2,3,4 |
| oct-1-en-3-ol (1-octen-3-ol) | 003391-86-4 | 10.49 c | Y 1,2,3,4, M 1,2,3,4, Z 1,2,3,4, C 2 |
| octan-3-one (3-octanone) | 000106-68-3 | 10.70 | Y 1,2,3,4, M 1,2,3,4, Z 1,2,3,4 |
| 2-phenylacetaldehyde (benzeneacetaldehyde) | 000122-78-1 | 12.49 | M 1,2,3,4, Z 1,2,3,4 |
| trans-oct-2-enal (2-octenal) | 002363-89-5 | 12.93 | Y 1,2,3,4, M 1,2,3,4, Z 1,2,3,4 |
| cis-oct-2-en-1-ol (cis-2-octen-1-ol) | 026001-58-1 | 13.25 c | Y 4, Z 3, C 2 |
| bicyclo [3.2.0]heptan-2-one (same or similar) | 029268-42-6 | 13.30 | Y 1,2, M 4, Z 2 |
| octan-1-ol (1-octanol) | 000111-87-5 | 13.34 | Y 4, M 1,3, Z 1,2,3 |
| 2-phenylethanol (phenylethyl alcohol) | 000060-12-8 | 14.66 | Y 1,2, M 2 |
| (5E)-6,10-dimethylundeca-5,9-dien-2-one (geranylacteone) | 003796-70-1 | 20.87 | Y 1,2, M 1,2,3,4 |
| trans-oct-2-en-1-ol (trans-2-octen-1-ol) | 018409-17-1 | 13.20 c | M 1, Z 1 |
| 1-(5-acetyl-6-hydroxy-1-benzofuran-2-yl)ethanone (euparone) * | 053947-86-7 | 23.42 | Z 1 |
| (1aR,4R,7bS)-1,1,4,7-tetramethyl-1a,2,3,4,4a,5,6,7b-octahydrocyclopropa[e]azulene (α-gurjunene) * | 000489-40-7 | 23.65 | M 1 |
| (4aR,8aR)-5,8a-dimethyl-3-propan-2-ylidene-1,2,4,4a,7,8-hexahydronaphthalene (selina-3,7(11)-diene) * | 006813-21-4 | 23.66 | Z 1 |
| (9Z,12Z)-octadeca-9,12-dienoic acid (linoleic acid) * | 000060-33-3 | 26.97 | M 1 |
| oct-1-en-3-one (1-octen-3-one) | 004312-99-6 | 10.28 c | Z 3 |
| octanal (caprylaldehyde) * | 000124-13-0 | 11.22 | M 3 |
| (1E,6E,8S)-1-methyl-5-methylidene-8-propan-2-ylcyclodeca-1,6-diene (germacrene D) * | 023986-74-5 | 21.39 | M 3, Z 3 |
| cis-octadec-9-enoic acid (oleic acid) | 000112-80-1 | 26.98 | M 3 |
| VOC: IUPAC (Synonym) a | CAS No. | R.T. | Corn Variety and Time Point b |
|---|---|---|---|
| 3-methylbutanal (isovaleraldehyde) | 000590-86-3 | 0.95 | V1 2,3, V2 1,2,4, M1 1,4, M2 1,2,4 |
| hexanal (caproaldehyde) | 000066-25-1 | 4.34 | V1 1, V2 1,3,4 |
| heptan-2-one (2-heptanone) | 000110-43-0 | 7.65 | V0 4, M1 3,4 |
| 2-phenylacetaldehyde (benzeneacetaldehyde) | 000122-78-1 | 12.49 | V1 1,2,3,4, V2 1,3 |
| 2-pentylfuran (2-amylfuran) | 003777-69-3 | 10.45 | V1 1,2,3,4 |
| 2-methylpent-1-ene (2-methyl-1-pentene) | 000763-29-1 | 6.60 | M0 1 |
| oct-1-en-3-ol (1-octen-3-ol) | 003391-86-4 | 10.49 c | V0 1 |
| non-4-yne (4-nonyne) * | 020184-91-2 | 11.70 | M0 1 |
| 2-methylpropan-1-amine (isobutylamine) * | 000078-81-9 | 4.34 | M1 2 |
| (2E)-penta-2,4-dienal (2,4-pentadienal) * | 000764-40-9 | 4.36 | M2 2 |
| pentanal (valeraldehyde) * | 000110-62-3 | 0.96 | V2 3 |
| 3-methylpentanoic acid (3-methylvaleric acid) * | 000105-43-1 | 7.33 | M2 3 |
| 1-(2-methylpropyl)cyclopentene (isobutylcyclopentene) * | 053098-47-8 | 11.71 | M0 4 |
| cyclooctene (same or similar) * | 000931-88-4 | 12.87 | V0 4 |
| 2-amino-1,4-dihydroimidazol-5-one (glycocyamidine) | 000503-86-6 | 12.69 | V2 4 |
| VOC (IUPAC) | Synonym or Common Name |
|---|---|
| (4aR,8aR)-5,8a-dimethyl-3-propan-2-ylidene-1,2,4,4a,7,8-hexahydronaphthalene | selina-3,7(11)-diene |
| 1-(5-acetyl-6-hydroxy-1-benzofuran-2-yl)ethanone | euparone |
| non-4-yne | 4-nonyne |
| VOC (IUPAC) | Synonym or Common Name |
|---|---|
| heptan-2-one | 2-heptanone |
| cis-oct-2-en-1-ol | cis-2-octen-1-ol |
| oct-1-en-3-one | 1-octen-3-one |
| 2-amino-1,4-dihydroimidazol-5-one | glycocyamidine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, G.G.; Lloyd, S.W. Variation with In Vitro Analysis of Volatile Profiles among Aspergillus flavus Strains from Louisiana. Separations 2023, 10, 157. https://doi.org/10.3390/separations10030157
Moore GG, Lloyd SW. Variation with In Vitro Analysis of Volatile Profiles among Aspergillus flavus Strains from Louisiana. Separations. 2023; 10(3):157. https://doi.org/10.3390/separations10030157
Chicago/Turabian StyleMoore, Geromy G., and Steven W. Lloyd. 2023. "Variation with In Vitro Analysis of Volatile Profiles among Aspergillus flavus Strains from Louisiana" Separations 10, no. 3: 157. https://doi.org/10.3390/separations10030157
APA StyleMoore, G. G., & Lloyd, S. W. (2023). Variation with In Vitro Analysis of Volatile Profiles among Aspergillus flavus Strains from Louisiana. Separations, 10(3), 157. https://doi.org/10.3390/separations10030157









