Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Standard Solutions
2.4. Sample Preparation for ECD Quantitation
2.5. LC-DAD Quantitative ECD Analysis
2.6. Calibration and Quantification
2.7. Sample Preparation of Prohibited Substances
2.8. LC-MS/MS Qualitative Prohibited Substance Analysis
2.9. Method Figures of Merit
3. Results and Discussion
3.1. Sample Preparation Optimisation for Quantitation of ECD
3.2. Method Figures of Merit for Quantitation of ECD
3.2.1. Linear Dynamic Range, Linearity, LOD, LOQ
3.2.2. Selectivity
3.2.3. Repeatability, Intermediate Precision
3.2.4. Accuracy
3.3. Triple Quadrupole MS Analysis Optimisation
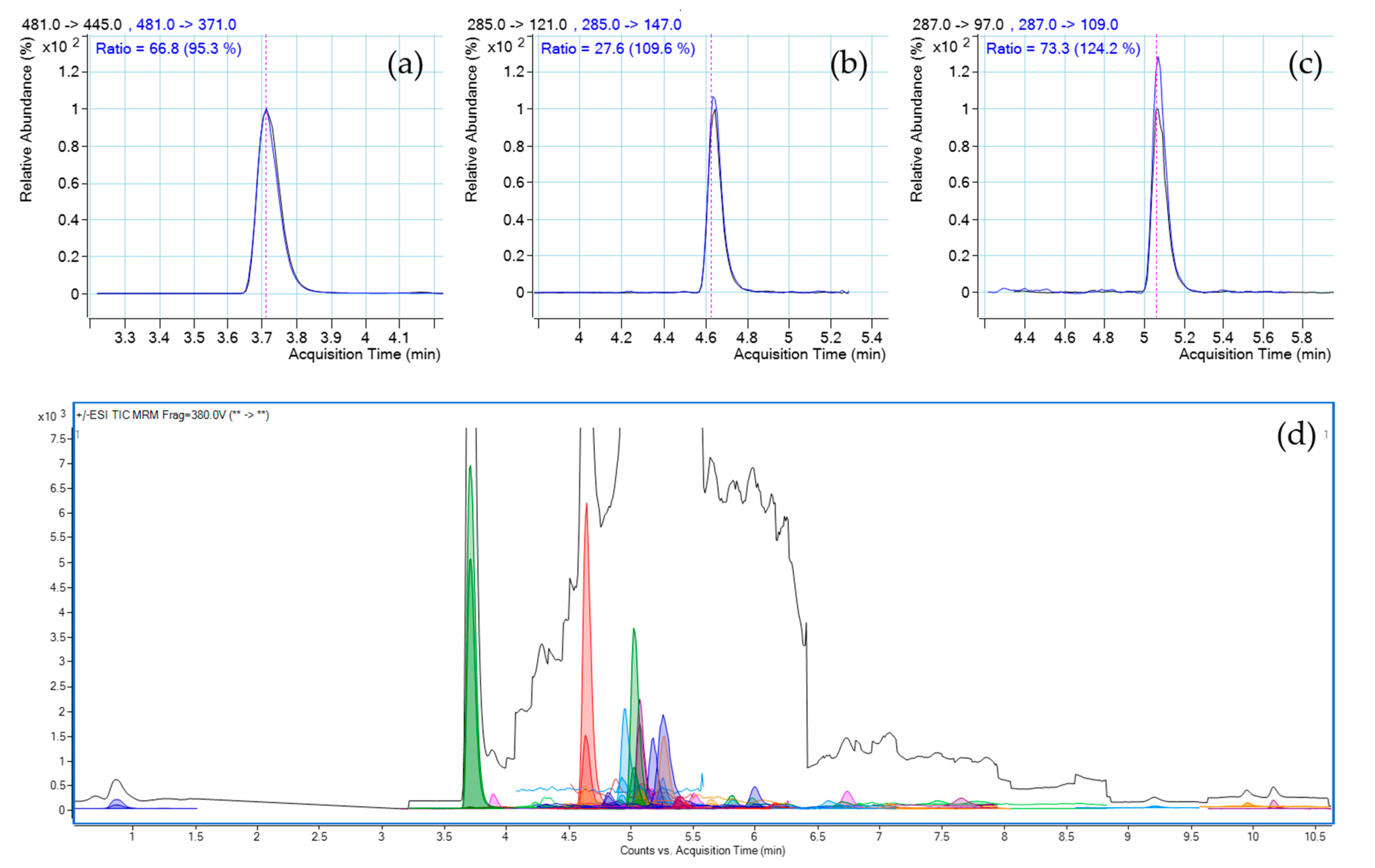
3.4. Method Figures of Merit for Limit Test of Prohibited Substances
3.5. Analysis of ECD-Containing DSs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Anti-Doping Agency. The 2020 Monitoring Program. Available online: https://www.wada-ama.org/sites/default/files/resources/files/wada_2020_english_monitoring_program_.pdf (accessed on 9 January 2023).
- Kwiatkowska, D.; Grucza, K.; Chajewska, K.; Konarski, P.; Wojtkowiak, K.; Drapała, A.; Wicka, M. Ecdysterone: Possible sources of origin in urine. Drug Test Anal. 2022, 36480213. [Google Scholar] [CrossRef]
- Hunyadi, A.; Herke, I.; Lengyel, K.; Báthori, M.; Kele, Z.; Simon, A.; Tóth, G.; Szendrei, K. Ecdysteroid-containing food supplements from Cyanotis arachnoidea on the European market: Evidence for spinach product counterfeiting. Sci. Rep. 2016, 6, 37322. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Ivanova, S. Comparison between the biological active compounds in plants with adaptogenic properties (Rhaponticum carthamoides, Lepidium meyenii, Eleutherococcus senticosus and Panax ginseng). Plants 2022, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Dinan, L.; Lafont, R. Effects and applications of arthropod steroid hormones (ecdysteroids) in mammals. J. Endocrinol. 2006, 191, 1–8. [Google Scholar] [CrossRef]
- Haupt, O.; Ngueu, S.T.; Diel, P.; Parr, M.K. Anabolic Effect of Ecdysterone Results in Hypertrophy of C2C12 Myotubes by an Estrogen Receptor Mediated Pathway. In Recent Advances in Dope Analysis, Proceedings of the Manfred-Donike-Workshop; 30th Cologne Workshop on Dope Analysis, 26th February to 2nd March 2012; Schänzer, W., Geyer, H., Gotzman, A., Mareck, U., Eds.; Sport und Buch Strauß: Cologne, Germany, 2012; pp. 221–225. [Google Scholar]
- Parr, M.K.; Botrè, F.; Naß, A.; Hengevoss, J.; Diel, P.; Wolber, G. Ecdysteroids: A novel class of anabolic agents? Biol. Sport 2015, 32, 169–173. [Google Scholar] [CrossRef]
- Isenmann, E.; Ambrosio, G.; Joseph, J.F.; Mazzarino, M.; Torre, X.; Zimmer, P.; Kazlauskas, R.; Goebel, C.; Botrè, F.; Diel, P.; et al. Ecdysteroids as non-conventional anabolic agent: Performance enhancement by ecdysterone supplementation in humans. Arch. Toxicol. 2019, 93, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Wilborn, C.D.; Taylor, L.W.; Campbell, B.I.; Kerksick, C.; Rasmussen, C.J.; Greenwood, M.; Kreider, R.B. Effects of methoxyisoflavone, ecdysterone, and sulfo-polysaccharide supplementation on training adaptations in resistance-trained males. J. Int. Soc. Sports Nutr. 2006, 3, 19–27. [Google Scholar] [CrossRef]
- Ambrosio, G.; Wirth, D.; Joseph, J.F.; Mazzarino, M.; Torre, X.; Botrè, F.; Parr, M.K. How reliable is dietary supplement labelling?—Experiences from the analysis of ecdysterone supplements. J. Pharm. Biomed Anal. 2020, 177, 112877. [Google Scholar] [CrossRef] [PubMed]
- Garthe, I.; Maughan, R.J. Athletes and supplements: Prevalence and perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef]
- Kozhuharov, V.R.; Ivanov, K.; Ivanova, S. Dietary supplements as source of unintentional doping. Biomed Res. Int. 2022, 2022, 8387271. [Google Scholar] [CrossRef]
- Lauritzen, F. Dietary supplements as a major cause of anti-doping rule violations. Front. Sports Act. Living 2022, 4, 868228. [Google Scholar] [CrossRef] [PubMed]
- Geyer, H.; Parr, M.K.; Koehler, K.; Mareck, U.; Schänzer, W.; Thevis, M. Nutritional supplements cross-contaminated and faked with doping substances. J. Mass. Spectrom. 2008, 43, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Walpurgis, K.; Thomas, A.; Geyer, H.; Mareck, U.; Thevis, M. Dietary supplement and food contaminations and their implications for doping controls. Foods 2020, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Rotolo, M.C.; Di Giovannadrea, R.; Pacifici, R.; Pichini, S. A simple toxicological analysis of anabolic steroid preparations from the black market. Ann. Toxicol. Anal. 2012, 24, 67–72. [Google Scholar] [CrossRef]
- Neves, D.B.D.J.; Calda, E.D. GC-MS quantitative analysis of black market pharmaceutical products containing anabolic androgenic steroids seized by the Brazilian Federal Police. Forensic. Sci. Int. 2017, 275, 272–281. [Google Scholar] [CrossRef]
- De Cock, K.J.S.; Delbeke, F.T.; Van Eenoo, P.; Desmet, N.; Roels, K.; De Backer, P. Detection and determination of anabolic steroids in nutritional supplements. J. Pharm. Biomed. Anal. 2001, 25, 843–852. [Google Scholar] [CrossRef]
- Micalizzi, G.; Huszti, K.; Pálinkás, Z.; Mandolfino, F.; Martos, É.; Dugo, P.; Mondello, L.; Utczás, M. Reliable identification and quantification of anabolic androgenic steroids in dietary supplements by using gas chromatography coupled to triple quadrupole mass spectrometry. Drug Test. Anal. 2021, 13, 128–139. [Google Scholar] [CrossRef]
- Kozlik, P.; Tircova, B. Development of the fast, simple and fully validated high performance liquid chromatographic method with diode array detector for quantification of testosterone esters in an oil-based injectable dosage form. Steroids 2016, 115, 34–39. [Google Scholar] [CrossRef]
- Van Poucke, C.; Detavernier, C.; Van Cauwenberghe, R.; Van Peteghem, C. Determination of anabolic steroids in dietary supplements by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta. 2017, 586, 35–42. [Google Scholar] [CrossRef]
- Leaney, A.L.; Beck, P.; Biddle, S.; Brown, P.; Grace, P.B.; Hudson, C.S.; Mawson, H.D. Analysis of supplements available to UK consumers purporting to contain selective androgen receptor. Drug Test Anal. 2021, 13, 122–127. [Google Scholar] [CrossRef]
- Tircova, B.; Bosakova, Z.; Kozlik, P. Development of an UHPLC-MS/MS method for the determination of anabolic steroids currently available on the black market in the Czech Republic and Slovakia. Drug Test Anal. 2018, 11, 355–360. [Google Scholar] [CrossRef]
- Parr, M.K.; Ambrosio, G.; Wuest, B.; Mazzarino, M.; Torre, X.; Sibilia, F.; Joseph, J.F.; Diel, P.; Botrè, F. Targeting the administration of ecdysterone in doping control samples. Forensic. Toxicol. 2020, 38, 172–184. [Google Scholar] [CrossRef]
- Grucza, K.; Wicka, M.; Drapała, A.; Kwiatkowska, D. Determination of Ecdysterone in Dietary Supplements and Spinach by Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Separations 2022, 9, 8. [Google Scholar] [CrossRef]
- Dinan, L.; Guibout, B.L.; Lafont, R. Small-scale analysis of phytoecdysteroids in seeds by HPLC-DAD-MS for the identification and quantification of specific analogues, dereplication and chemotaxonomy. Phytochem. Anal. 2020, 31, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Napierała, M.; Nawrot, J.; Gornowicz-Porowska, J.; Florek, E.; Moroch, A.; Adamski, Z.; Kroma, A.; Miechowicz, I.; Nowak, G. Separation and HPLC Characterization of Active Natural Steroids in a Standardized Extract from the Serratula coronata Herb with Antiseborrheic Dermatitis Activity. Int. J. Environ. Res. Public Health 2020, 17, 6453. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Method A Laboratory Guide to Method Validation and Related Topics. 2nd ed. Available online: https://www.eurachem.org/images/stories/Guides/pdf/MV_guide_2nd_ed_EN.pdf/ (accessed on 13 December 2022).

| Compound | MRM 1 | MRM 2 | RT | ||
|---|---|---|---|---|---|
| Transition | CE | Transition | CE | ||
| Ractopamine | 302 > 164 | 15 | 302 > 284 | 10 | 0.86 |
| β-ecdysterone | 481 > 445 | 20 | 481 > 371 | 15 | 3.70 |
| Anastrasole | 294 > 225 | 25 | - | - | 3.89 |
| Adrenosterone | 301 > 121 | 30 | 301 > 257 | 22 | 4.29 |
| 1,4-androstadiene-3,17-dione | 285 > 121 | 20 | 285 > 147 | 10 | 4.63 |
| 19-norandrostendione | 273 > 197 | 20 | 273 > 255 | 20 | 4.77 |
| RAD 140 | 394 > 223 | 5 | 394 > 170 | 35 | 4.80 |
| Trenbolone | 271 > 253 | 20 | 271 > 199 | 25 | 4.81 |
| 9(10)-dehydronandrolone | 273 > 161 | 25 | 273 > 135 | 30 | 4.89 |
| Boldenone | 287 > 121 | 20 | 287 > 135 | 20 | 4.91 |
| Fluoxymesterone | 337 > 281 | 20 | 337 > 241 | 25 | 4.98 |
| Ostarine | 388 > 118 | 35 | 388 > 269 | 20 | 5.02 |
| Oxandrolone | 289 > 229 | 20 | 289 > 121 | 25 | 5.06 |
| Nandrolone | 275 > 109 | 30 | 275 > 145 | 30 | 5.06 |
| 4(5)-androstene-3,17-dione | 287 > 97 | 25 | 287 > 109 | 25 | 5.06 |
| Metribolone | 285 > 227 | 20 | 285 > 267 | 20 | 5.07 |
| Methylclostebol | 337 > 319 | 15 | - | - | 5.07 |
| Exemestane | 297 > 121 | 25 | 297 > 279 | 10 | 5.10 |
| Methandienone | 301 > 121 | 20 | 301 > 149 | 20 | 5.16 |
| 4-androstene-3,6,17-trione | 301 > 149 | 20 | 301 > 107 | 35 | 5.16 |
| Gestrinone | 309 > 199 | 30 | 309 > 241 | 15 | 5.25 |
| Testosterone | 289 > 97 | 25 | 289 > 109 | 25 | 5.38 |
| 1-androstendione | 287 > 185 | 15 | 287 > 203 | 15 | 5.43 |
| Δ9(11)-methyltestosterone | 301 > 283 | 20 | 301 > 147 | 25 | 5.46 |
| LGD4033 | 383 > 267 | 20 | 383 > 337 | 5 | 5.63 |
| Norclostebol | 309 > 143 | 40 | 309 > 291 | 15 | 5.77 |
| Mibolerone | 303 > 149 | 30 | 303 > 117 | 50 | 5.77 |
| 3,5-androstadiene-7,17-dione (Arimistane) | 285 > 81 | 30 | 285 > 107 | 25 | 5.77 |
| 17α-methyltestosterone | 303 > 109 | 30 | 303 > 97 | 30 | 5.78 |
| 5α-androst-1-en-17β-ol-3-one (1-testosterone) | 289 > 187 | 20 | 289 > 205 | 15 | 5.89 |
| Methenolone | 303 > 83 | 20 | 303 > 187 | 25 | 5.95 |
| Epitestosterone | 289 > 97 | 25 | 289 > 109 | 25 | 6.14 |
| Bolasterone | 317 > 97 | 25 | 317 > 107 | 40 | 6.19 |
| Clostebol | 323 > 143 | 25 | 323 > 131 | 30 | 6.20 |
| Stanozolol | 329 > 81 | 55 | 329 > 95 | 55 | 6.20 |
| Tetrahydrogestrinone | 313 > 199 | 35 | 313 > 159 | 25 | 6.58 |
| Norethandrolone | 303 > 285 | 15 | 303 > 109 | 30 | 6.68 |
| Calusterone | 317 > 97 | 25 | 317 > 123 | 20 | 6.72 |
| Trenbolone acetate | 313 > 253 | 20 | 313 > 271 | 15 | 6.80 |
| Stenbolone | 303 > 187 | 25 | - | - | 7.27 |
| Etiocholanolone | 273 > 255 | 10 | 291 > 255 | 10 | 7.44 |
| Norbolethone | 317 > 299 | 15 | 317 > 245 | 20 | 7.65 |
| Androsterone | 273 > 255 | 10 | 291 > 273 | 10 | 7.74 |
| Danazol | 338 > 120 | 25 | 338 > 310 | 30 | 7.83 |
| Methylstenbolone | 317 > 201 | 20 | 317 > 145 | 35 | 8.53 |
| Testosterone acetate | 331 > 97 | 20 | 331 > 109 | 30 | 9.19 |
| Testosterone propionate | 345 > 97 | 20 | 345 > 109 | 30 | 9.99 |
| GW501516 | 454 > 257 | 30 | 454 > 188 | 50 | 10.15 |
| Testosterone-d3 (ISTD) | 292 > 97 | 25 | 292 > 109 | 25 | 5.38 |
| Method Figures of Merit | |||||
|---|---|---|---|---|---|
| linearity | concentration range (µg/mL) | 1.0–50.0 | |||
| slope | 6.51 ± 0.09 | ||||
| intercept | 1.18 ± 0.23 | ||||
| correlation coefficient | 0.9999 | ||||
| LOD (µg·g−1) | 35 | ||||
| LOQ (µg·g−1) | 115 | ||||
| intra-day precision (CV%) | sample 1 | sample 2 | sample 3 | sample 4 | |
| day 1 (n = 6) | 1.77 | 3.24 | 1.64 | 4.36 | |
| day 2 (n = 6) | 3.40 | 1.63 | 3.20 | 4.93 | |
| day 3 (n = 6) | 2.36 | 1.98 | 2.49 | 4.86 | |
| intermediate precision (n = 18, CV%) | 1.75 | 0.43 | 1.17 | 0.79 | |
| recovery (%) | 70 | 91 | 90 | 90 | |
| 73 | 91 | 93 | 100 | ||
| 71 | 93 | 93 | 97 | ||
| Sample Code | Sample Type | Product Description | Source | Detected WADA-Prohibited Substances (S1, S4) | ECD Content (mg·g−1) | Measured ECD Content (mg) Per·Capsule or Tablet | Labeled ECD Content (mg) Per Capsule or Tablet | Daily Dose |
|---|---|---|---|---|---|---|---|---|
| A1 | capsule | Dietary supplement with Cyanotis arachnoidea extract | webshop | n.d. | 52.5 | 23.6 | 135 | 3 |
| A2 | webshop | 4(5)-androstene-3,17-dione | 13.2 | 8.2 | ||||
| A3 | NFCSO | 4(5)-androstene-3,17-dione | 7.98 | 4.9 | ||||
| B1 | capsule | Dietary supplement with Rhaponticum carthamoides extract | webshop | n.d. | 27.6 | 9.3 | 200 | 3 × 1 |
| C1 | capsule | Dietary supplement with Rhaponticum carthamoides extract | webshop | n.d. | 8.03 | 4.7 | 300 | 1 |
| D1 | capsule | Dietary supplement with Rhaponticum carthamoides extract | webshop | n.d. | n.d. | n.d. | 200 | 1 |
| E1 | capsule | Dietary supplement with with Maca extract, L-arginine, L-ornithine hydrochloride and zink | webshop | n.d. | 3.41 | 3.4 | 2.5 | 2–3 |
| E2 | capsule | NFCSO | n.d. | 2.51 | 2.5 | |||
| F1 | capsule | Dietary supplement with Spinacia oleracea extract and L-leucinnal | webshop | n.d. | 39 | 34.1 | 100 | 1–2 |
| G1 | capsule | Dietary supplement with Cyanotis vaga extract | webshop | n.d. | 96.8 | 26.9 | 250 | 4 |
| H1/1 | capsule | Dietary supplement with Cyanotis vaga and other herbal extracts, vitamins, minerals, amino acids and substances with anabolic effect | NFCSO | n.d. | n.d. | n.d. | not defined | 1 |
| H1/2 | capsule | n.d. | 28.5 | 18.5 | not defined | 3 | ||
| H1/3 | tablet | n.d. | n.d. | n.d. | not defined | 3 | ||
| H1/4 | tablet | n.d. | n.d. | n.d. | not defined | 1 | ||
| H2/1 | capsule | webshop | n.d. | n.d. | n.d. | not defined | 1 | |
| H2/2 | capsule | 1,4-androstene-3,17-dione | 29.9 | 19.4 | not defined | 3 | ||
| H2/3 | tablet | n.d. | n.d. | n.d. | not defined | 3 | ||
| H2/4 | tablet | n.d. | n.d. | n.d. | not defined | 1 | ||
| I1 | capsule | Dietary supplement with Spinacia oleracea and Yam extracts, gamma-oryzanol and vitamins | webshop | n.d. | n.d. | n.d. | 25 | 3 |
| I2 | capsule | NFCSO | n.d. | 14.2 | 14.2 | 25 | 3 | |
| J1 | tablet | Dietary supplement with Leuzea carthamoides extract | webshop | 4(5)-androstene-3,17-dione, 1,4-androstene-3,17-dione | 1.08 | 10.8 | 15 | 2–4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pálinkás, Z.; Békési, D.; Utczás, M. Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements. Separations 2023, 10, 242. https://doi.org/10.3390/separations10040242
Pálinkás Z, Békési D, Utczás M. Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements. Separations. 2023; 10(4):242. https://doi.org/10.3390/separations10040242
Chicago/Turabian StylePálinkás, Zoltán, Dániel Békési, and Margita Utczás. 2023. "Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements" Separations 10, no. 4: 242. https://doi.org/10.3390/separations10040242
APA StylePálinkás, Z., Békési, D., & Utczás, M. (2023). Quantitation of Ecdysterone and Targeted Analysis of WADA-Prohibited Anabolic Androgen Steroids, Hormones, and Metabolic Modulators in Ecdysterone-Containing Dietary Supplements. Separations, 10(4), 242. https://doi.org/10.3390/separations10040242








