Comparative Study of Conventional, Microwave-Assisted and Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: The Case of Scenedesmus obliquus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Methods
2.2.1. Solid–Liquid Extraction (SLE)
2.2.2. Microwave-Assisted Extraction (MAE)
2.2.3. Supercritical Fluid Extraction (SFE)
2.3. Extract Analyses
2.4. Experimental Design, Statistical Analysis and Process Optimization
2.5. Mathematical Model of SFE Kinetics
3. Results and Discussion
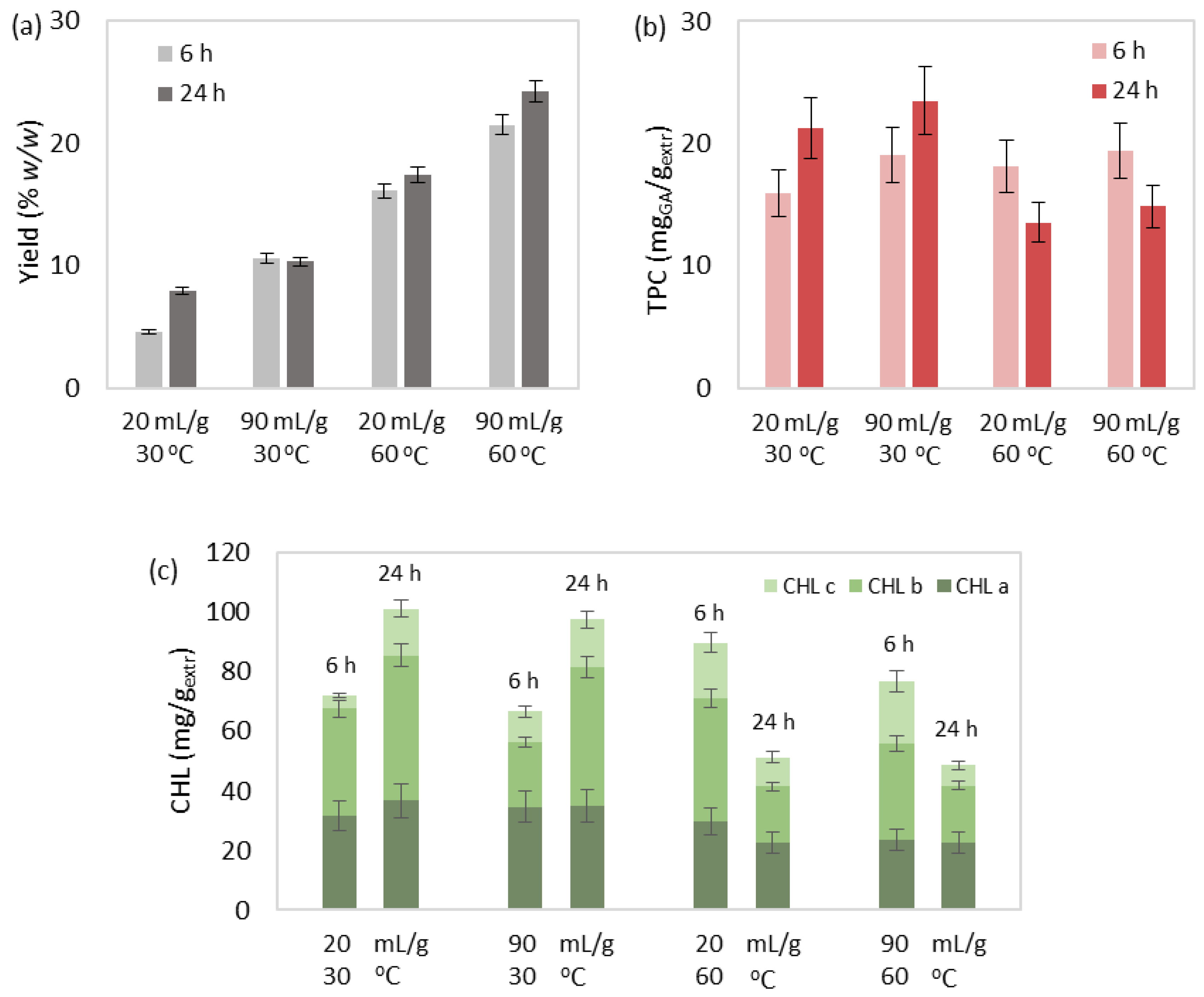
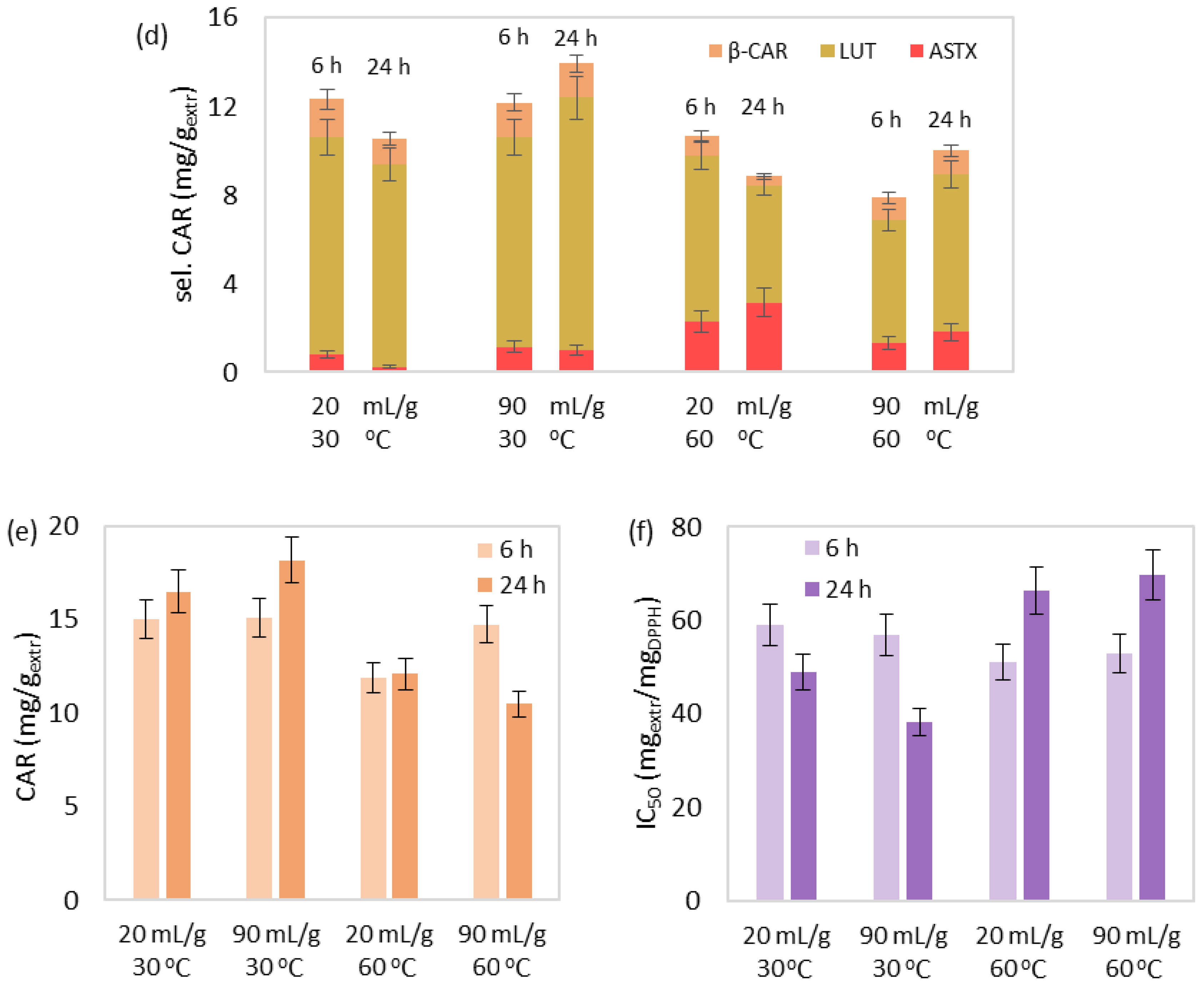
3.1. SLE of Bioactive Compounds
3.1.1. Effect Study
3.1.2. Correlation and Parameter Optimization
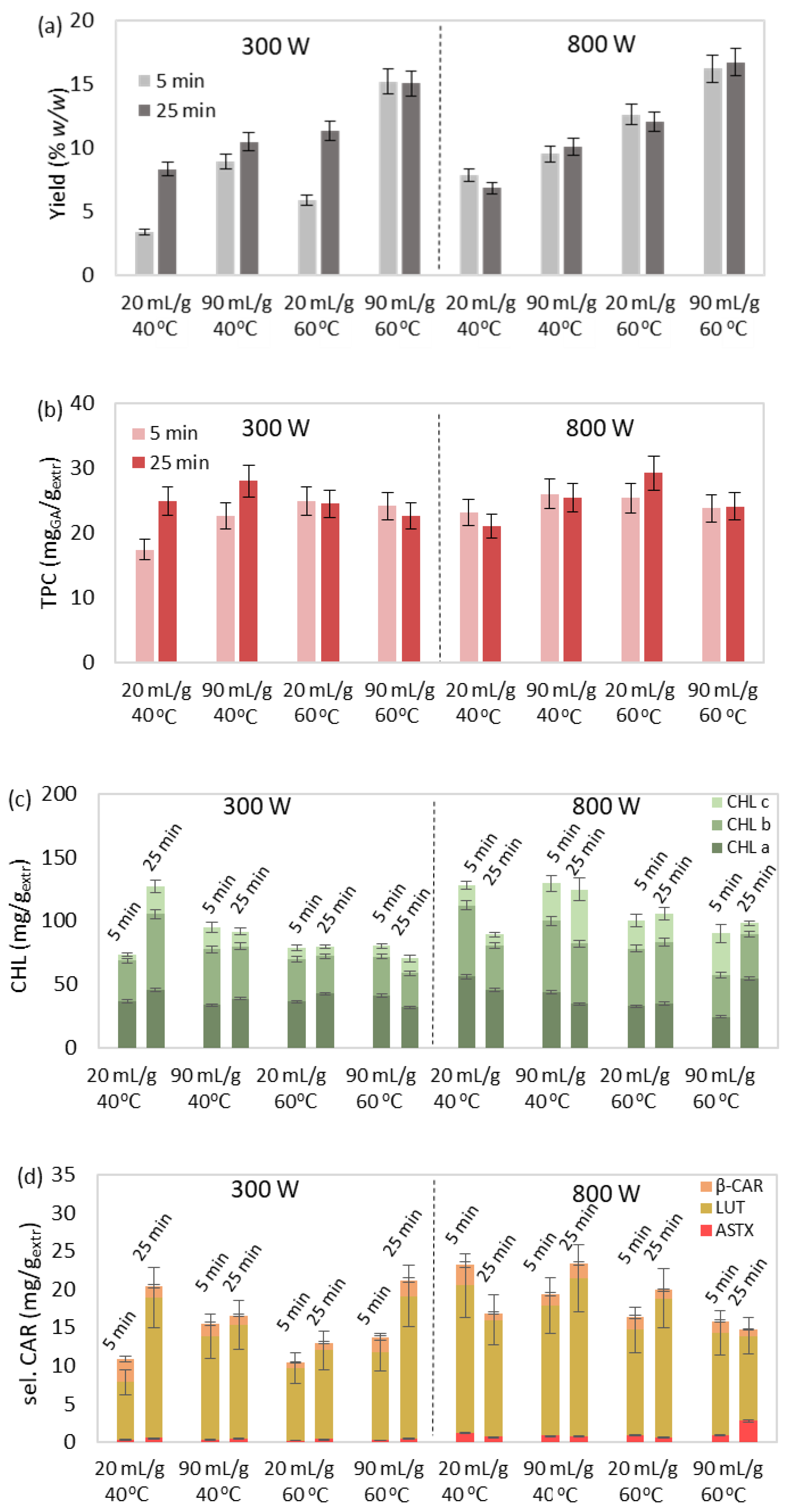
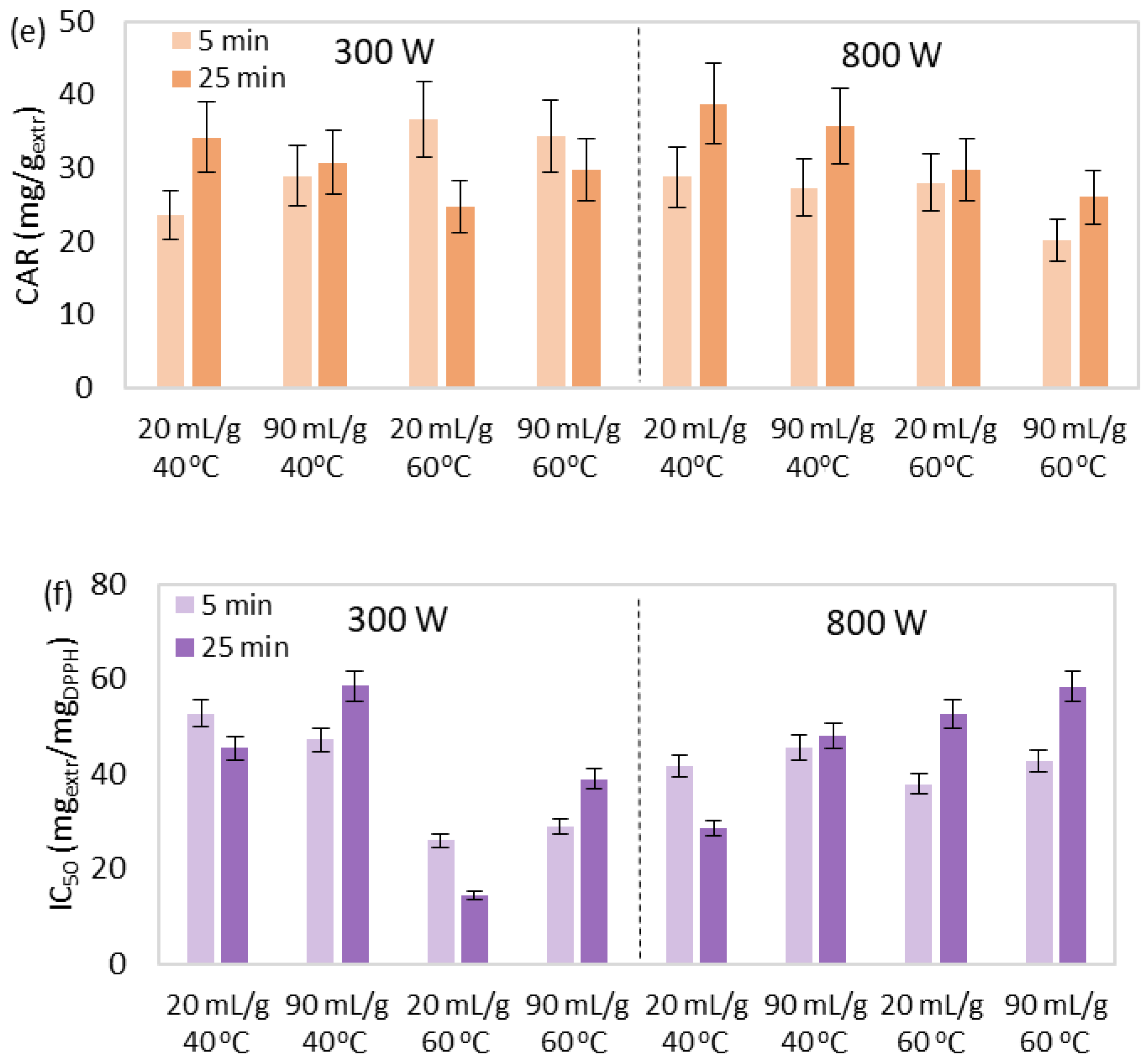
3.2. MAE of Bioactive Compounds
3.2.1. Effect Study
3.2.2. Correlation and Parameter Optimization
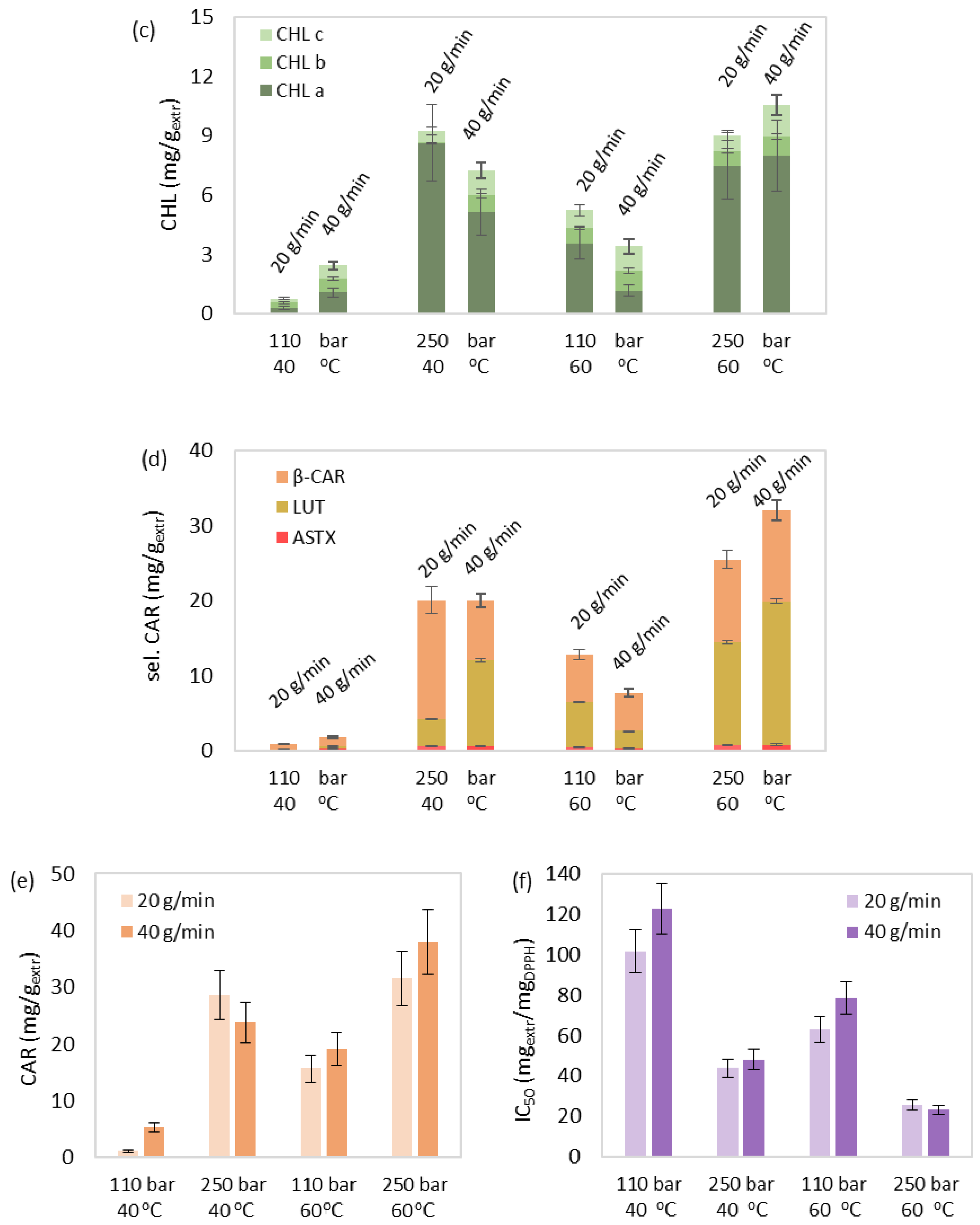
3.3. SFE of Bioactive Compounds
3.3.1. Effect Study
3.3.2. Correlation and Parameter Optimization
3.3.3. Effect of Cosolvent
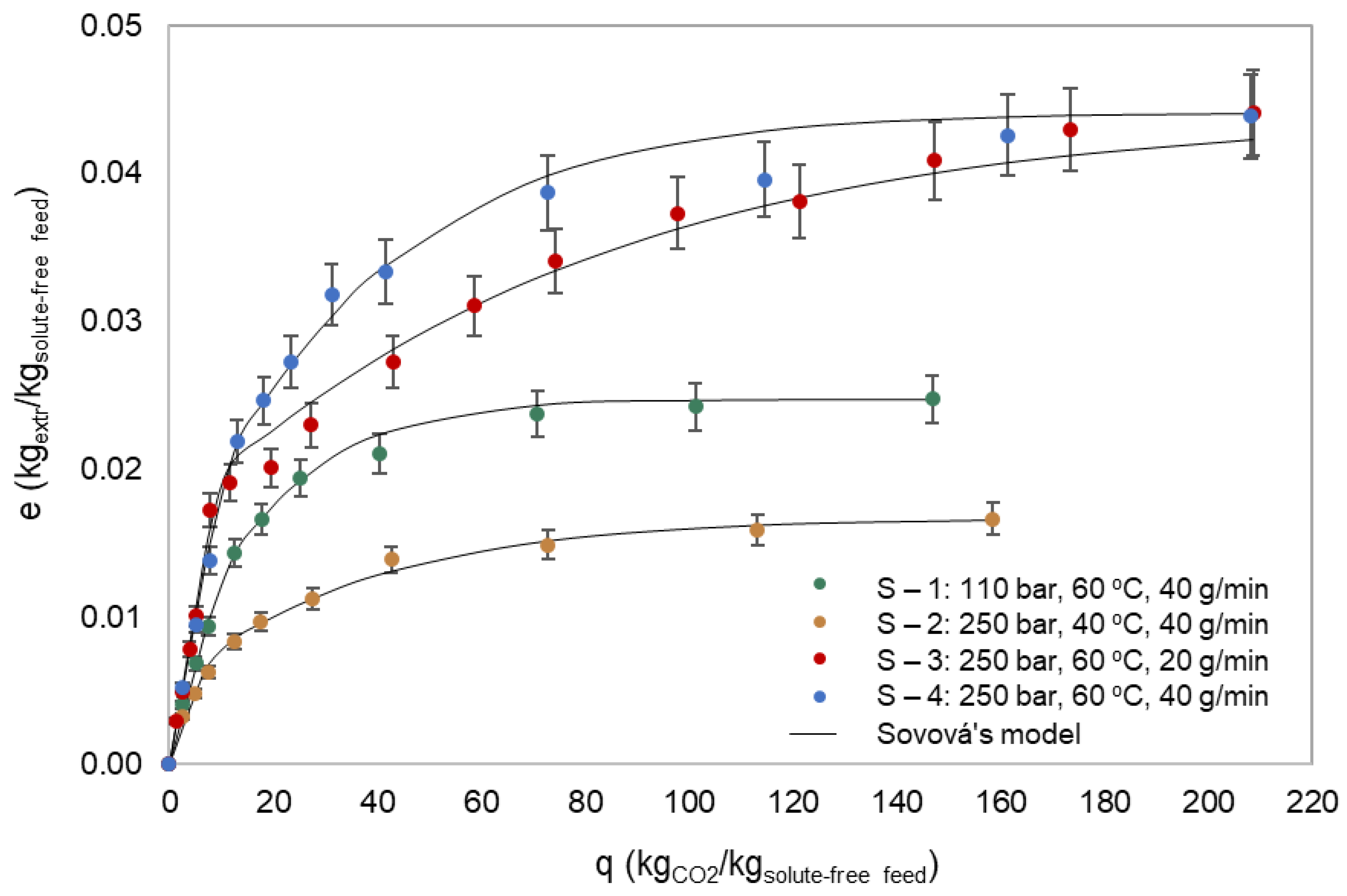
3.3.4. Kinetic Study of SFE
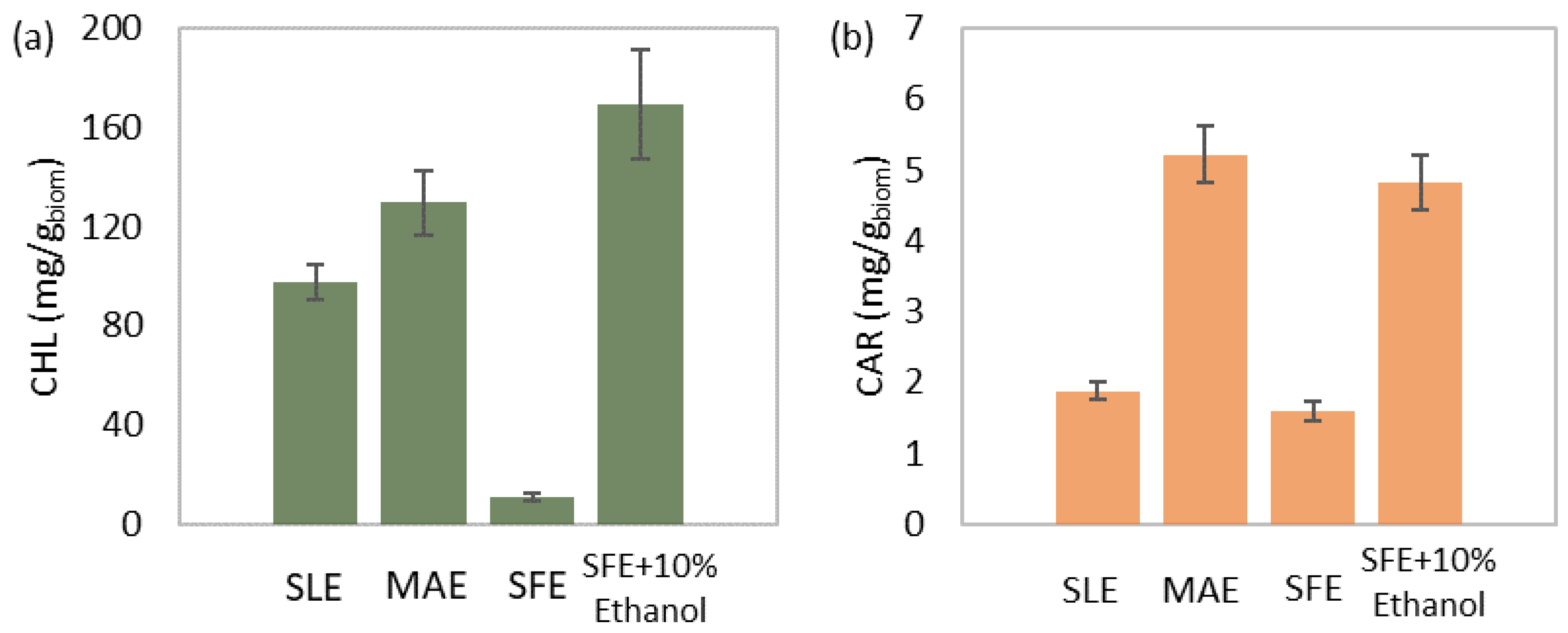
3.4. Comparison of the Proposed Extraction Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martins, R.; Sales, H.; Pontes, R.; Nunes, J.; Gouveia, I. Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review. Antioxidants 2023, 12, 328. [Google Scholar] [CrossRef]
- Priefer, C.; Jörissen, J.; Frör, O. Pathways to shape the bioeconomy. Resources 2017, 6, 10. [Google Scholar] [CrossRef]
- Vale, M.A.; Ferreira, A.; Pires, J.C.; Gonçalves, A.L. CO2 capture using microalgae. In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–405. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.-T.; Show, P.-L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vaz, B.d.S.; de Morais, E.G.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive composition, health benefits, safety and prospects as potential high-value ingredients for the functional food industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2022, 21, 1–28. [Google Scholar] [CrossRef]
- Saratale, R.G.; Ponnusamy, V.; Sirohi, R.; Piechota, G.; Shobana, S.; Dharmaraja, J.; Lay, C.h.; Saratale, G.D.; Shin, H.S.; Veermuthu, A. Microalgae cultivation strategies using cost–effective nutrient sources: Recent updates and progress towards biofuel production. Bioresour. Technol. 2022, 361, 127691. [Google Scholar] [CrossRef]
- Khatoon, H.; Rahman, N.A.; Suleiman, S.S.; Banerjee, S.; Abol-Munafi, A.B. Growth and proximate composition of Scenedesmus obliquus and Selenastrum bibraianum cultured in different media and condition. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 251–257. [Google Scholar] [CrossRef]
- Zaharieva, M.M.; Zheleva-Dimitrova, D.; Rusinova-Videva, S.; Ilieva, Y.; Brachkova, A.; Balabanova, V.; Gevrenova, R.; Kim, T.C.; Kaleva, M.; Georgieva, A. Antimicrobial and antioxidant potential of Scenedesmus obliquus microalgae in the context of integral biorefinery concept. Molecules 2022, 27, 519. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; van den Broek, L.A.M.; Houweling-Tan, B.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Green compressed fluid technologies for downstream processing of Scenedesmus obliquus in a biorefinery approach. Algal Res. 2017, 24, 111–121. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.E.T.; Martins, M.A.; Leite, M.O.; Milião, G.L.; Coimbra, J.S.R. Microalga Scenedesmus obliquus: Extraction of bioactive compounds and antioxidant activity. Rev. Ciência Agronômica 2021, 52, 1–8. [Google Scholar] [CrossRef]
- Tzima, S.; Georgiopoulou, I.; Louli, V.; Magoulas, K. Recent Advances in Supercritical CO2 Extraction of Pigments, Lipids and Bioactive Compounds from Microalgae. Molecules 2023, 28, 1410. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.; Nobre, B.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.; Palavra, A. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts; Woodhead: Sawston, UK, 2017; pp. 461–483. [Google Scholar] [CrossRef]
- Vieira, B.B.; Soares, J.; Amorim, M.L.; Bittencourt, P.V.Q.; de Cássia Superbi, R.; de Oliveira, E.B.; dos Reis Coimbra, J.S.; Martins, M.A. Optimized extraction of neutral carbohydrates, crude lipids and photosynthetic pigments from the wet biomass of the microalga Scenedesmus obliquus BR003. Sep. Purif. Technol. 2021, 269, 118711. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging technologies for the extraction of marine phenolics: Opportunities and challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Kataoka, H. Pharmaceutical Analysis| sample preparation. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and technological applications-a review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Sovová, H. Rate of the vegetable oil extraction with supercritical CO2—I. Modelling of extraction curves. Chem. Eng. Sci. 1994, 49, 409–414. [Google Scholar] [CrossRef]
- Sovová, H.; Kučera, J.; Jež, J. Rate of the vegetable oil extraction with supercritical CO2—II. Extraction of grape oil. Chem. Eng. Sci. 1994, 49, 415–420. [Google Scholar] [CrossRef]
- Cha, K.H.; Lee, H.J.; Koo, S.Y.; Song, D.-G.; Lee, D.-U.; Pan, C.-H. Optimization of pressurized liquid extraction of carotenoids and chlorophylls from Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Papamichail, I.; Louli, V.; Magoulas, K. Supercritical fluid extraction of celery seed oil. J. Supercrit. Fluids 2000, 18, 213–226. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Pappa, G.D.; Louli, V.; Voutsas, E.; Magoulas, K. Experimental Design and Optimization of Recovering Bioactive Compounds from Chlorella vulgaris through Conventional Extraction. Molecules 2021, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Mantoura, R.F.C.; Wright, S.W. Phytoplankton Pigments in Oceanography: Monographs on Oceanographic Methodology; United Nations Educational, Scientific and Cultural Organizations: Paris, France, 1997. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Und Physiol. Der Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Laina, K.M.; Eleni, P.N.; Tsitseli, K.G.; Krokida, M.K. Process Design for the Extraction of Bioactive Compounds from Several Mediterranean Medicinal Plants. Chem. Eng. Trans. 2021, 86, 1327–1332. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Process Optimization of Microwave-Assisted Extraction of Chlorophyll, Carotenoid and Phenolic Compounds from Chlorella vulgaris and Comparison with Conventional and Supercritical Fluid Extraction. Appl. Sci. 2023, 13, 2740. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Reverchon, E.; Marrone, C. Supercritical extraction of clove bud essential oil: Isolation and mathematical modeling. Chem. Eng. Sci. 1997, 52, 3421–3428. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.-A.A.; Badens, E. Supercritical CO2 extraction of neutral lipids from microalgae: Experiments and modelling. J. Supercrit. Fluids 2013, 77, 7–16. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Effect of extraction parameters on the carotenoid recovery from tomato waste. Int. J. Food Sci. Technol. 2011, 46, 23–29. [Google Scholar] [CrossRef]
- Radojković, M.; Zeković, Z.; Jokić, S.; Vidović, S.; Lepojević, Ž.; Milošević, S. Optimization of solid-liquid extraction of antioxidants from black mulberry leaves by response surface methodology. Food Technol. Biotechnol. 2012, 50, 167–176. [Google Scholar]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Hachemi, I.; Murzin, D.Y. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar] [CrossRef]
- Xiao, W.; Han, L.; Shi, B. Optimization of microwave-assisted extraction of flavonoid from Radix Astragali using response surface methodology. Sep. Sci. Technol. 2008, 43, 671–681. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-assisted extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; The Royal Society of Chemistry: London, UK, 2013; Volume 4. [Google Scholar] [CrossRef]
- Shang, A.; Luo, M.; Gan, R.-Y.; Xu, X.-Y.; Xia, Y.; Guo, H.; Liu, Y.; Li, H.-B. Effects of microwave-assisted extraction conditions on antioxidant capacity of sweet tea (Lithocarpus polystachyus Rehd.). Antioxidants 2020, 9, 678. [Google Scholar] [CrossRef]
- Bachtler, S.; Bart, H.-J. Increase the yield of bioactive compounds from elder bark and annatto seeds using ultrasound and microwave assisted extraction technologies. Food Bioprod. Process. 2021, 125, 1–13. [Google Scholar] [CrossRef]
- Nguyen, N.H.K.; An, N.T.D.; Anh, P.K.; Truc, T.T. Microwave-assisted extraction of chlorophyll and polyphenol with antioxidant activity from Pandanus amaryllifolius Roxb. in Vietnam. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Coimbatore, India, 8–9 April 2021; p. 012039. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Liu, C.; Sun, Y.; Lin, Z.; Liu, H. Extraction characteristics and optimal parameters of anthocyanin from blueberry powder under microwave-assisted extraction conditions. Sep. Purif. Technol. 2013, 104, 17–25. [Google Scholar] [CrossRef]
- Pan, B.; Lei, J.; Zhang, L.; Guo, Y. Research on the physical properties of supercritical CO2 and the log evaluation of CO2-bearing volcanic reservoirs. J. Geophys. Eng. 2017, 14, 1052–1060. [Google Scholar] [CrossRef]
- Machmudah, S.; Kawahito, Y.; Sasaki, M.; Goto, M. Process optimization and extraction rate analysis of carotenoids extraction from rosehip fruit using supercritical CO2. J. Supercrit. Fluids 2008, 44, 308–314. [Google Scholar] [CrossRef]
- Mendes, R.L.; Coelho, J.P.; Fernandes, H.L.; Marrucho, I.J.; Cabral, J.M.S.; Novais, J.M.; Palavra, A.F. Applications of supercritical CO2 extraction to microalgae and plants. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1995, 62, 53–59. [Google Scholar] [CrossRef]
- Georgiopoulou, I.; Tzima, S.; Louli, V.; Magoulas, K. Supercritical CO2 Extraction of High-Added Value Compounds from Chlorella vulgaris: Experimental Design, Modelling and Optimization. Molecules 2022, 27, 5884. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Gião, M.S.; Matias, A.A.; Nunes, A.V.M.; Pintado, M.E.; Duarte, C.M.M.; Malcata, F.X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar] [CrossRef]
- Lorenzen, J.; Igl, N.; Tippelt, M.; Stege, A.; Qoura, F.; Sohling, U.; Brück, T. Extraction of microalgae derived lipids with supercritical carbon dioxide in an industrial relevant pilot plant. Bioprocess Biosyst. Eng. 2017, 40, 911–918. [Google Scholar] [CrossRef]
- Taher, H.; Al-Zuhair, S.; Al-Marzouqi, A.H.; Haik, Y.; Farid, M. Mass transfer modeling of Scenedesmus sp. lipids extracted by supercritical CO2. Biomass Bioenergy 2014, 70, 530–541. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Hasan, M. Supercritical carbon dioxide extraction of andrographolide from Andrographis paniculata: Effect of the solvent flow rate, pressure, and temperature. Chin. J. Chem. Eng. 2007, 15, 877–883. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Perez-Vega, S.; Salmeron, I.; Perez-Reyes, I.; Kwofie, E.; Ngadi, M. Influence of the Supercritical Fluid Extraction (SFE) on Food Bioactives. In Retention of Bioactives in Food Processing; Springer: Cham, Switzerland, 2022; pp. 309–340. [Google Scholar] [CrossRef]
- Yen, H.-W.; Yang, S.-C.; Chen, C.-H.; Chang, J.-S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A knowledge base for the recovery of natural phenols with different solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sens. Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Augustynska, D.; Jemioła-Rzemińska, M.; Burda, K.; Strzałka, K. Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chem. Biol. Interact. 2015, 239, 19–25. [Google Scholar] [CrossRef]
- Kavoura, D.; Kyriakopoulou, K.; Papaefstathiou, G.; Spanidi, E.; Gardikis, K.; Louli, V.; Aligiannis, N.; Krokida, M.; Magoulas, K. Supercritical CO2 extraction of Salvia fruticosa. J. Supercrit. Fluids 2019, 146, 159–164. [Google Scholar] [CrossRef]
- Watson, S.B. Algal taste and odor. In Algae: Source to Treatment AWWA Manual of Water Supply Practices; AWWA Publishing: Denver, CO, USA, 2010; Volume 57, pp. 329–374. [Google Scholar]
- Amin, M.; Chetpattananondh, P.; Khan, M.N.; Mushtaq, F.; Sami, S.K. Extraction and Quantification of Chlorophyll from Microalgae Chlorella sp. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Baleli, Pakistan, 2–3 April 2018; p. 012025. [Google Scholar] [CrossRef]
- Morcelli, A.; Cassel, E.; Vargas, R.; Rech, R.; Marcílio, N. Supercritical fluid (CO2+ethanol) extraction of chlorophylls and carotenoids from Chlorella sorokiniana: COSMO-SAC assisted prediction of properties and experimental approach. J. CO2 Util. 2021, 51, 101649. [Google Scholar] [CrossRef]
- Zaripheh, S.; Erdman Jr, J.W. Factors that influence the bioavailablity of xanthophylls. J. Nutr. 2002, 132, 531S–534S. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tsao, R.; Zhang, S.; Dong, Z.; Yang, R.; Gong, J.; Pei, Y. Antioxidant activity, mutagenicity/anti-mutagenicity, and clastogenicity/anti-clastogenicity of lutein from marigold flowers. Food Chem. Toxicol. 2006, 44, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- Marquez, U.M.L.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- FiorMarkets. Global Carotenoids Market is Expected to Reach USD 3.59 Billion by 2025: Fior Markets. Available online: https://www.globenewswire.com/news-release/2019/10/15/1929461/0/en/Global-Carotenoids-Market-is-expected-to-reach-USD-3-59-billion-by-2025-Fior-Markets.html (accessed on 5 April 2023).


| Nutrients | Value (%) |
|---|---|
| Protein | 52.00 |
| Fat | 9.70 |
| Carbohydrates | 7.90 |
| Ash | 10.10 |
| Moisture | 3.90 |
| Pigments | Value (mg/gbiom) |
| Total chlorophylls | 31.79 |
| Total carotenoids | 6.43 |
| Chemical Name | Supplier | Origin | CAS-RN | Purity |
|---|---|---|---|---|
| Carbon dioxide | TAE Hellas SA | Athens Greece | 124-38-39 | 99.5% |
| Anhydrous sodium carbonate | Fisher Scientific International Inc. | Pittsburgh PA, USA | 497-19-8 | 99.5% |
| Orthophosphoric acid | “ | “ | 7664-38-2 | 85.4% |
| Water | “ | “ | 7732-18-5 | |
| Methanol | “ | “ | 67-56-1 | ≥99.8% |
| Ethanol | “ | “ | 64-17-5 | ≥99.8% |
| Tert-butyl-methyl ether (MTBE) | “ | “ | 1634-04-4 | ≥99.5% |
| Ethyl acetate | “ | “ | 141-78-6 | ≥99.9% |
| Gallic acid | “ | “ | 149-91-7 | 98% |
| Astaxanthin | Acros Organics BVBA | Antwerp, Belgium | 472-61-7 | ≥98% |
| Lutein | Extrasynthese SAS | Lyon, France | 127-40-2 | ≥92% |
| β-carotene | Sigma Aldrich Co. | Saint Louis, MO, USA | 7235-40-7 | 99% |
| 2,2-diphenyl-1-picrylhydrazyl (DPPH•) | “ | “ | 1898-66-4 | 95% |
| Folin–Ciocalteu reagent | Carlo Erba Reagents SAS | Milan, Italy | 12111-13-16 |
| Time (min) | MTBE (% v/v) | Methanol (% v/v) | aq. Phosphoric Acid, 1% v/v (% v/v) |
|---|---|---|---|
| 0 | 15 | 81 | 4 |
| 15 | 30 | 66 | 4 |
| 23 | 80 | 16 | 4 |
| 27 | 80 | 16 | 4 |
| 27.1 | 15 | 81 | 4 |
| 35 | 15 | 81 | 4 |
| Run | T (°C) | t (h) | R (mLsolv/gbiom) | Yield (% w/w) | TPC (mgGA/gextr) | CHL (mg/gextr) | sel. CAR (mg/gextr) | CAR (mg/gextr) | IC50 (mgextr/mgDPPH) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 6 | 20 | 4.62 | 15.93 | 72.12 | 12.32 | 15.02 | 58.93 |
| 2 | 30 | 6 | 90 | 10.61 | 19.04 | 66.68 | 12.18 | 15.08 | 56.88 |
| 3 | 30 | 24 | 20 | 7.89 | 21.22 | 101.17 | 10.53 | 16.49 | 48.92 |
| 4 | 30 | 24 | 90 | 10.33 | 23.47 | 97.46 | 13.94 | 18.17 | 38.06 |
| 5 | 45 | 15 | 55 | 13.27 | 13.06 | 83.66 | 12.31 | 13.01 | 56.52 |
| 6 | 45 | 15 | 55 | 14.19 | 9.88 | 90.95 | 11.78 | 13.97 | 51.61 |
| 7 | 45 | 15 | 55 | 13.07 | 10.84 | 98.76 | 10.41 | 15.36 | 62.27 |
| 8 | 60 | 6 | 20 | 16.14 | 18.16 | 89.74 | 10.67 | 11.87 | 50.99 |
| 9 | 60 | 6 | 90 | 21.52 | 19.39 | 76.75 | 7.88 | 14.74 | 52.86 |
| 10 | 60 | 24 | 20 | 17.42 | 13.54 | 51.31 | 8.84 | 12.09 | 66.29 |
| 11 | 60 | 24 | 90 | 24.27 | 14.86 | 48.49 | 10.00 | 10.47 | 69.66 |
| Run | Τ (°C) | P (W) | R (mLsolv/gbiom) | t (min) | Yield (% w/w) | TPC (mgGA/gextr) | CHL (mg/gextr) | sel. CAR (mg/gextr) | CAR (mg/gextr) | IC50 (mgextr/mgDPPH) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 300 | 20 | 5 | 3.43 | 17.41 | 73.38 | 10.89 | 23.60 | 52.83 |
| 2 | 40 | 800 | 20 | 5 | 7.82 | 23.10 | 78.92 | 10.42 | 28.76 | 41.58 |
| 3 | 40 | 300 | 90 | 5 | 8.91 | 22.70 | 94.77 | 15.43 | 28.91 | 47.19 |
| 4 | 40 | 800 | 90 | 5 | 9.52 | 25.99 | 80.24 | 13.63 | 27.26 | 45.58 |
| 5 | 40 | 300 | 20 | 25 | 8.31 | 24.92 | 127.09 | 20.42 | 34.17 | 45.45 |
| 6 | 40 | 800 | 20 | 25 | 6.85 | 21.09 | 79.68 | 12.98 | 38.75 | 28.67 |
| 7 | 40 | 300 | 90 | 25 | 10.47 | 28.05 | 91.59 | 16.54 | 30.72 | 58.64 |
| 8 | 40 | 800 | 90 | 25 | 10.07 | 25.42 | 70.34 | 21.19 | 35.67 | 47.97 |
| 9 | 50 | 550 | 55 | 15 | 15.21 | 26.72 | 113.95 | 11.84 | 22.34 | 44.68 |
| 10 | 50 | 550 | 55 | 15 | 12.97 | 21.68 | 115.46 | 17.73 | 31.69 | 42.70 |
| 11 | 50 | 550 | 55 | 15 | 14.27 | 23.19 | 95.94 | 17.80 | 26.79 | 39.10 |
| 12 | 60 | 300 | 20 | 5 | 5.86 | 24.97 | 127.99 | 23.19 | 36.60 | 26.01 |
| 13 | 60 | 800 | 20 | 5 | 12.61 | 25.40 | 100.18 | 16.39 | 28.00 | 37.87 |
| 14 | 60 | 300 | 90 | 5 | 15.18 | 24.13 | 129.46 | 19.34 | 34.32 | 28.88 |
| 15 | 60 | 800 | 90 | 5 | 16.18 | 23.83 | 90.27 | 15.84 | 20.12 | 42.65 |
| 16 | 60 | 300 | 20 | 25 | 11.31 | 24.48 | 89.29 | 16.86 | 24.74 | 14.45 |
| 17 | 60 | 800 | 20 | 25 | 12.05 | 29.23 | 105.49 | 19.94 | 29.70 | 52.67 |
| 18 | 60 | 300 | 90 | 25 | 15.02 | 22.64 | 124.91 | 23.38 | 29.68 | 38.98 |
| 19 | 60 | 800 | 90 | 25 | 16.70 | 24.09 | 98.09 | 14.75 | 25.98 | 58.46 |
| Run | Τ (οC) | P (bar) | F (gsolv/min) | Cosolvent (% w/w) | Yield (% w/w) | TPC (mgGA/gextr) | CHL (mg/gextr) | sel. CAR (mg/gextr) | CAR (mg/gextr) | IC50 (mgextr/mgDPPH) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | 110 | 20 | 0 | 0.98 | 1.93 | 0.76 | 0.86 | 1.14 | 101.74 |
| 2 | 40 | 110 | 40 | 0 | 1.10 | 0.78 | 2.43 | 1.76 | 5.33 | 122.77 |
| 3 | 40 | 250 | 20 | 0 | 1.37 | 9.35 | 9.22 | 20.06 | 28.65 | 43.91 |
| 4 | 40 | 250 | 40 | 0 | 1.63 | 10.07 | 7.23 | 20.02 | 23.78 | 48.05 |
| 5 | 50 | 180 | 30 | 0 | 2.90 | 5.68 | 4.78 | 18.90 | 21.97 | 44.86 |
| 6 | 50 | 180 | 30 | 0 | 2.55 | 4.71 | 4.11 | 17.53 | 29.63 | 48.05 |
| 7 | 50 | 180 | 30 | 0 | 2.76 | 4.11 | 4.46 | 18.39 | 25.19 | 39.06 |
| 8 | 60 | 110 | 20 | 0 | 1.97 | 2.79 | 5.24 | 12.81 | 15.62 | 62.79 |
| 9 | 60 | 110 | 40 | 0 | 2.41 | 2.48 | 3.40 | 7.72 | 19.11 | 78.48 |
| 10 | 60 | 250 | 20 | 0 | 4.20 | 12.06 | 9.00 | 25.44 | 31.51 | 25.59 |
| 11 | 60 | 250 | 40 | 0 | 4.22 | 11.06 | 10.54 | 32.04 | 37.93 | 23.18 |
| SFE+10% ethanol | 60 | 250 | 40 | 10 | 9.75 | 17.96 | 168.81 | 37.90 | 49.43 | 15.25 |
| Run | P (bar) | T (°C) | F (g/min) | (kgsolute/kgsolv) | (kgsolute/kgsolute-free feed) | (kgsolute/kgsolute-free feed) | 102 (s−1) | 104 (s−1) | AAD * (%) |
|---|---|---|---|---|---|---|---|---|---|
| S – 1 | 110 | 60 | 40 | 0.0013 | 0.025 | 0.014 | 6.66 | 5.08 | 3.89 |
| S – 2 | 250 | 40 | 40 | 0.0010 | 0.017 | 0.010 | 2.56 | 2.46 | 4.71 |
| S – 3 | 250 | 60 | 20 | 0.0021 | 0.044 | 0.026 | 2.56 | 0.59 | 5.09 |
| S – 4 | 250 | 60 | 40 | 0.0021 | 0.044 | 0.026 | 2.56 | 2.60 | 3.24 |
| Parameter | SLE | MAE | SFE | SFE+10% Ethanol |
|---|---|---|---|---|
| Solvent | aq. Ethanol 90% v/v | aq. Ethanol 90% v/v | CO2 | CO2-Ethanol 90/10 w/w |
| Solvent-to-biomass ratio (mLsolv/gbiom) | 90 | 90 | 100 | 100 |
| Stirring (rpm) | 500 | 500 | n/a * | n/a * |
| Temperature (°C) | 30 | 60 | 60 | 60 |
| Pressure (bar) | 1 | 1 | 250 | 250 |
| Solvent flow rate (g/min) | n/a * | n/a * | 40 | 40 |
| Microwave power (Watt) | n/a * | 300 | n/a * | n/a * |
| Duration (h) | 24 | 0.083 | 6.67 | 6.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiopoulou, I.; Louli, V.; Magoulas, K. Comparative Study of Conventional, Microwave-Assisted and Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: The Case of Scenedesmus obliquus. Separations 2023, 10, 290. https://doi.org/10.3390/separations10050290
Georgiopoulou I, Louli V, Magoulas K. Comparative Study of Conventional, Microwave-Assisted and Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: The Case of Scenedesmus obliquus. Separations. 2023; 10(5):290. https://doi.org/10.3390/separations10050290
Chicago/Turabian StyleGeorgiopoulou, Ioulia, Vasiliki Louli, and Kostis Magoulas. 2023. "Comparative Study of Conventional, Microwave-Assisted and Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: The Case of Scenedesmus obliquus" Separations 10, no. 5: 290. https://doi.org/10.3390/separations10050290
APA StyleGeorgiopoulou, I., Louli, V., & Magoulas, K. (2023). Comparative Study of Conventional, Microwave-Assisted and Supercritical Fluid Extraction of Bioactive Compounds from Microalgae: The Case of Scenedesmus obliquus. Separations, 10(5), 290. https://doi.org/10.3390/separations10050290








