Swietenia mahagoni Leaves Extract: Antifungal, Insecticidal, and Phytochemical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Phytopathogens
2.2. Preparation of Swietenia Mahagoni Extract
2.3. Swietenia Mahagoni Extract (SMAL) Antifungal Activity
2.4. Swietenia Mahagoni Extract (SMAL) Insecticidal Activity
2.4.1. Sampling and Rearing of Cotton Aphid
2.4.2. Toxicity Studies
2.4.3. Biological Aspects
2.4.4. Olfactory Response in Triplicate Choice Test
2.5. HPLC Analysis
2.6. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.7. Statistical Analyses
3. Results
3.1. The Process of Isolating and Identifying Fungal Strains
3.2. Effect of the SMAL Extract on the Fungal Pathogens
3.3. Toxicity of the SMAL Extract on the Adults of Cotton Aphids
3.4. Biological Aspects of Adult Females and Nymphal Stages of Aphis Gossypii Glover
3.5. Longevity of Developmental Stages and Total Life Duration of Aphis Gossypii Glover (LS)
3.6. Olfactory Response on the Adult Stage of Aphis Gossypii Glover
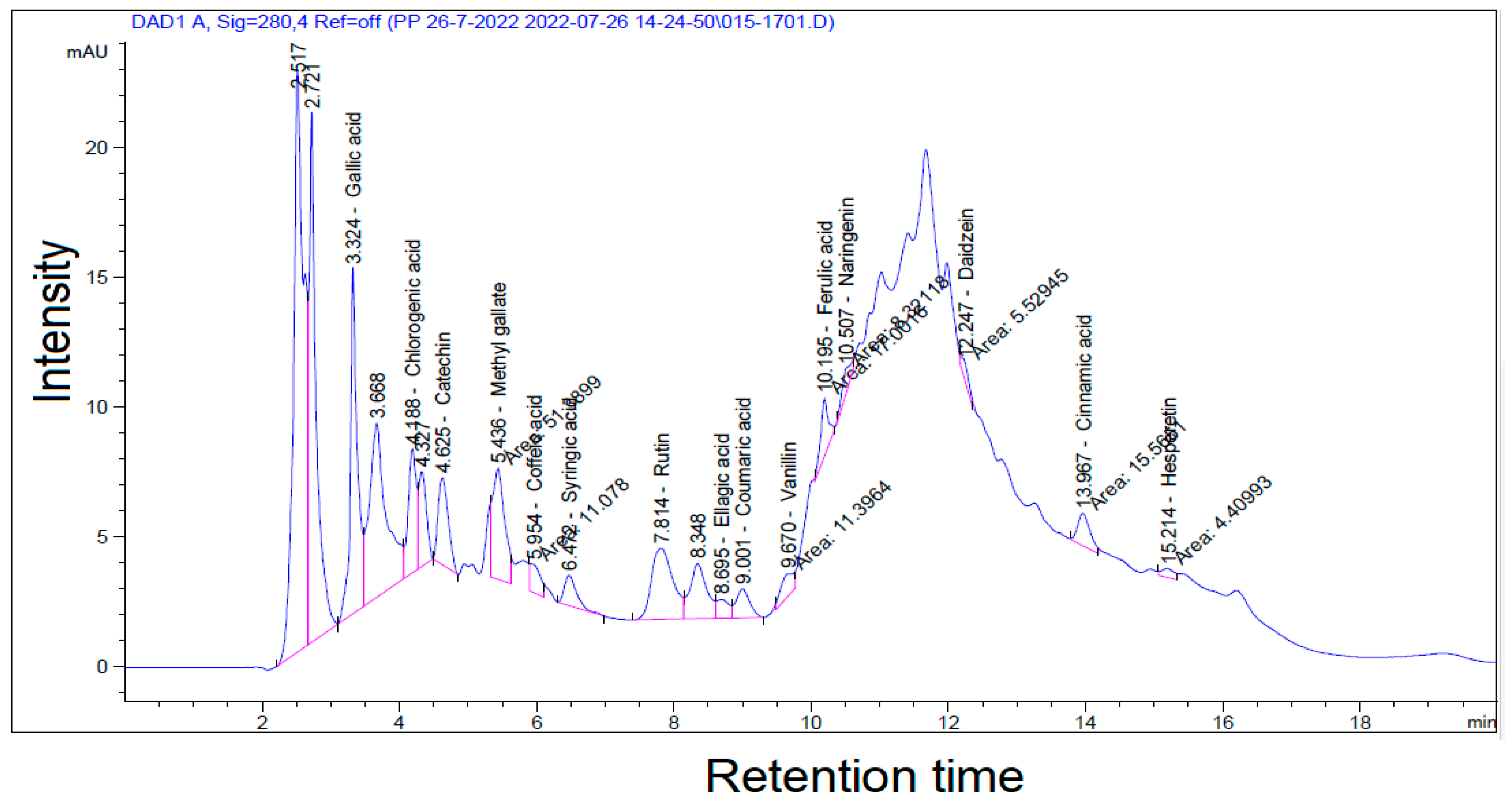
3.7. Swietenia Mahagoni Extract’s Polyphenolic Content
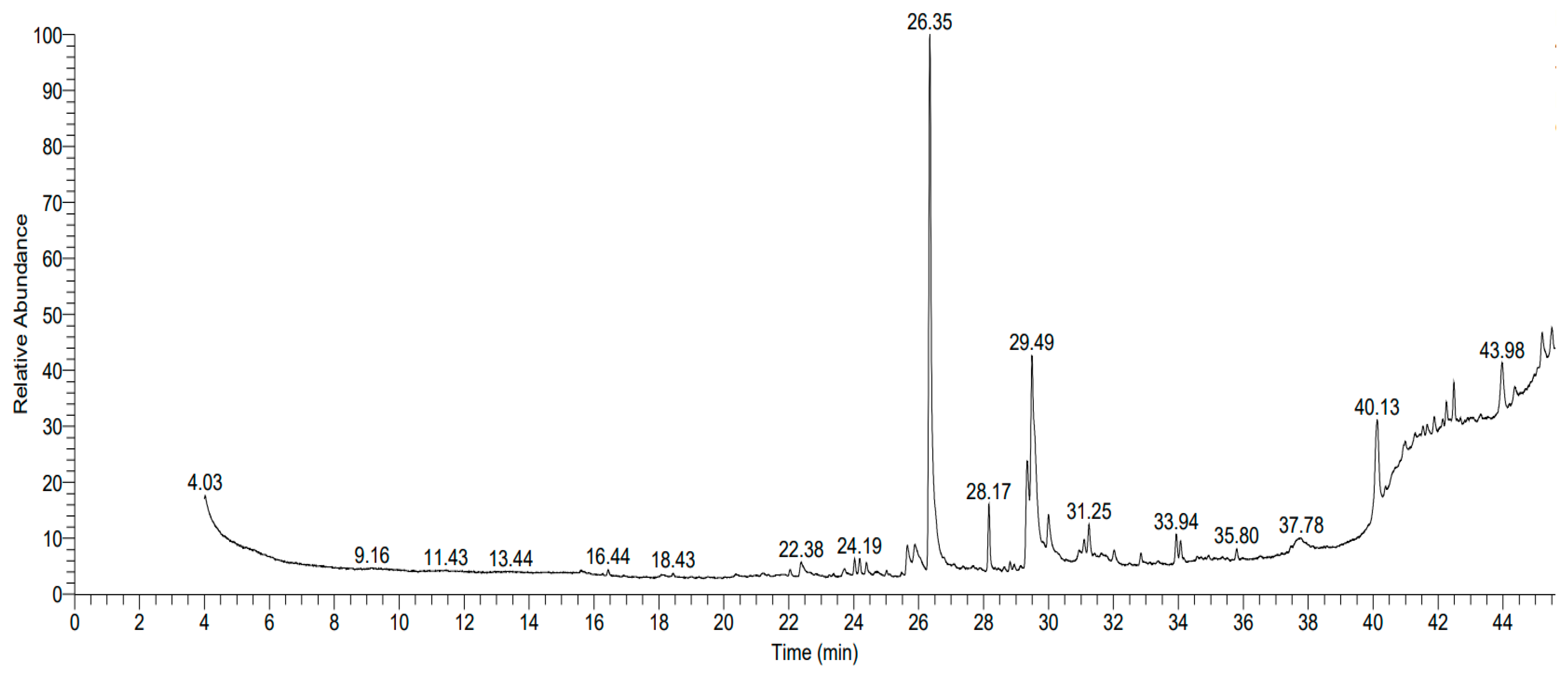
3.8. Swietenia Mahagoni (SMAL) Extract’s GC–MS Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Jothy, S.L.; Torey, A.; Darah, I.; Choong, Y.S.; Saravanan, D.; Chen, Y.; Latha, L.Y.; Deivanai, S.; Sasidharan, S. Cassia spectabilis (DC) Irwin et Barn: A promising traditional herb in health improvement. Molecules 2012, 17, 10292–10305. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, P.M.; Percha, V.; Farswan, M.; Upaganlawar, A. Cassia: A wonder gift to medical sciences. Int. J. Clin. Pharm. 2008, 1, 16–38. [Google Scholar]
- Arora, D.S.; Kaur, G.J. Antibacterial activity of some Indian medicinal plants. J. Nat. Med. 2007, 61, 313–317. [Google Scholar] [CrossRef]
- Naveen, Y.P.; Rupini, G.D.; Ahmed, F.; Urooj, A. Pharmacological effects and active phytoconstituents of Swietenia mahagoni: A review. J. Integr. Med. 2014, 12, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Nweze, E.I.; Okafor, J.I.; Njoku, O. Antimicrobial activities of methanolic extracts of Trema guineensis (Schumm and Thorn) and Morinda lucida benth used in Nigerian. Bio-Research 2004, 2, 39–46. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Suresh, G.; Banumathy, B.; Masilamani, S.; Gopalakrishnan, G.; Krishna Kumari, G.N. Antifungal activity of some B, D-seco limonoids from two meliaceous plants. J. Chem. Ecol. 1999, 25, 923–933. [Google Scholar] [CrossRef]
- Ervina, M. The recent use of Swietenia mahagoni (L.) Jacq. as antidiabetes type 2 phytomedicine: A systematic review. Heliyon 2020, 6, e03536. [Google Scholar]
- Chen, J.-J.; Huang, S.-S.; Liao, C.-H.; Wei, D.-C.; Sung, P.-J.; Wang, T.-C.; Cheng, M.-J. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010, 120, 379–384. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture The Agricultural Research Service. USDA Composition Database; U.S. Department of Agriculture The Agricultural Research Service: Washington, DC, USA, 2019. [Google Scholar]
- Singh, R.; Sunder, S.; Kumar, P. Sheath blight of rice: Current status and perspectives. Indian Phytopathol. 2016, 69, 340–351. [Google Scholar]
- El-Shafey, R.A.S.; Elamawi, R.M.; Saleh, M.M.; Tahoon, A.M.; Emeran, A.A. Morphological, pathological and molecular characterisation of rice sheath blight disease causal organism Rhizoctonia solani AG-1 IA in Egypt. Arch. Phytopathol. Plant Prot. 2019, 52, 507–529. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khanal, S.; Zhou, X.-G.; Antony-Babu, S.; Atiq, M. First Report of Fusarium Sheath Rot of Rice Caused by Fusarium incarnatum-equiseti Species Complex in the United States. Plant Dis. 2022, 106, 3206. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Piacentini, K.C.; Savi, G.D.; Carnielli-Queiroz, L.; de Carvalho Fontes, L.; Martins, C.S.; Corrêa, B.; Rocha, L.O. Wild rice (O. latifolia) from natural ecosystems in the Pantanal region of Brazil: Host to Fusarium incarnatum-equiseti species complex and highly contaminated by zearalenone. Int. J. Food Microbiol. 2021, 345, 109127. [Google Scholar] [CrossRef] [PubMed]
- Behiry, S.I.; Soliman, S.A.; Al-Mansori, A.-N.A.; Al-Askar, A.A.; Arishi, A.A.; Elsharkawy, M.M.; Abdelkhalek, A.; Heflish, A.A. Chorisia speciosa Extract Induces Systemic Resistance against Tomato Root Rot Disease Caused by Rhizoctonia solani. Agronomy 2022, 12, 2309. [Google Scholar] [CrossRef]
- Behiry, S.I.; Soliman, S.A.; Al-Askar, A.A.; Alotibi, F.O.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M.; Hafez, E.E.; Heflish, A.A. Plantago lagopus extract as a green fungicide induces systemic resistance against Rhizoctonia root rot disease in tomato plants. Front. Plant Sci. 2022, 13, 2818. [Google Scholar] [CrossRef]
- Derbalah, A.; Shebl, A.M.; Elgobashy, S.F.; Ahmad, A.A.; Ramadan, N.E.; Behiry, S.I.; Abdelkhalek, A.; Saleem, M.H.; Al-Askar, A.A.; Kamran, M. Resistance Induction and Direct Antifungal Activity of Some Monoterpenes against Rhizoctonia solani, the Causal of Root Rot in Common Bean. Life 2022, 12, 1040. [Google Scholar] [CrossRef]
- Ayyappadhas, R.; Jestin, C.; Kenneth, N.; Dayana, N.; Dhanalekshmi, U.M. Preliminary studies on antimicrobial activity of Swietenia macrophylla leaf extract. Int. J. Pharm. Sci. Rev. Res 2012, 16, 1–4. [Google Scholar]
- Chiranjib, B.; Laxmaiah, C.; Srikanth, V.; Gouda, T.S.; Kumar, C.S.; Debnath, S. Antimicrobial activity of Swietenia mahagoni L. (leaf) against various human pathogenic microbes. Indo Am. J. Pharm. Res 2011, 1, 257–261. [Google Scholar]
- Quan, Q.; Hu, X.; Pan, B.; Zeng, B.; Wu, N.; Fang, G.; Cao, Y.; Chen, X.; Li, X.; Huang, Y. Draft genome of the cotton aphid Aphis gossypii. Insect Biochem. Mol. Biol. 2019, 105, 25–32. [Google Scholar] [CrossRef]
- Morando, R.; da Silva, I.F.; da Silva Santana, A.; Sampaio, G.S.L.; Lourenção, A.L.; Baldin, E.L.L. Assessing cotton genotypes for resistance to Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2021, 114, 387–396. [Google Scholar] [CrossRef]
- Hossain, M.A.; Yasmin, M.S.; Bachchu, M.A.A.; Alim, M.A. Potency of Three Botanical Oils Against the Aphis Craccivora Koch (Homoptera: Aphididae) Nymphs Under Laboratory Conditions. SAARC J. Agric. 2021, 19, 139–154. [Google Scholar] [CrossRef]
- Kamble, B.; Bhalkare, S.K.; Bhonde, A.; Dabhade, P.; Undirwade, D.B. Efficacy of Insect Growth Regulators against Aphids and Thrips on BT Cotton. Indian J. Entomol. 2022, 84, 920–922. [Google Scholar] [CrossRef]
- Dugan, F.M.; Dugan, F.M. The Identification of Fungi: An Illustrated Introduction with Keys, Glossary, and Guide to Literature; The American Phytopathological Society: St. Paul, MN, USA, 2006. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Dissanayake, M. Inhibitory effect of selected medicinal plant extracts on phytopathogenic fungus Fusarium oxysporum (Nectriaceae) Schlecht. Emend. Snyder and Hansen. Annu. Res. Rev. Biol. 2014, 4, 133–142. [Google Scholar] [CrossRef]
- Gaimari, S.D.; Turner, W.J. Methods for rearing Aphidophagous Leucopis spp.(Diptera: Chamaemyiidae). J. Kansas Entomol. Soc. 1996, 69, 363–369. [Google Scholar]
- Stribley, M.F.; Moores, G.D.; Devonshire, A.L.; Sawicki, R.M. Application of the FAO-recommended method for detecting insecticide resistance in Aphis jabae Scopoli, Sitobion avenae (F.), Metopolophium dirhodum (Walker) and Rhopalosiphum padi (L.) (Hemiptera: Aphididae). Bull. Entomol. Res. 1983, 73, 107–115. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Insecticide Resistance Action Committee (IRAC). Susceptibility Test Methods Series: Method 026. 2011. Available online: https://irac-online.org/methods/musca-domestica-adults/ (accessed on 23 July 2020).
- Michelotto, M.D.; Busoli, A.C. Aspectos biológicos de Aphis gossypii Glover, 1877 (Hemiptera: Aphididae) em três cultivares de algodoeiro e em três espécies de plantas daninhas. Ciência Rural 2003, 33, 999–1004. [Google Scholar] [CrossRef]
- Jaba, J.; Haseena, B.; Tripathy, S.; Hosamani, A.C.; Amaresh, Y.S. Olfactory response of cowpea aphid, Aphis craccivora Koch, to host odours and population of conspecifics. J. Biopestic. 2010, 3, 405. [Google Scholar]
- Weeks, E.N.I.; Logan, J.G.; Gezan, S.A.; Woodcock, C.M.; Birkett, M.A.; Pickett, J.A.; Cameron, M. A bioassay for studying behavioural responses of the common bed bug, Cimex lectularius (Hemiptera: Cimicidae) to bed bug-derived volatiles. Bull. Entomol. Res. 2011, 101, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense Responses and Metabolic Changes Involving Phenylpropanoid Pathway and PR Genes in Squash (Cucurbita pepo L.) following Cucumber mosaic virus Infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. (SAS) PC-SAS User Guide, Version 8; SAS Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Ekimoto, H.; Irie, Y.; Araki, Y.; Han, G.-Q.; Kadota, S.; Kikuchi, T. Platelet aggregation inhibitors from the seeds of Swietenia mahagoni: Inhibition of in vitro and in vivo platelet-activating factor-induced effects of tetranortriterpenoids related to swietenine and swietenolide. Planta Med. 1991, 57, 56–58. [Google Scholar] [CrossRef]
- Nakatani, M.; Abdelgaleil, S.A.M.; Kurawaki, J.; Okamura, H.; Iwagawa, T.; Doe, M. Antifeedant Rings B and D Opened Limonoids from Khaya senegalensis. J. Nat. Prod. 2001, 64, 1261–1265. [Google Scholar] [CrossRef]
- Syame, S.M.; Mohamed, S.M.; Elgabry, E.A.; Darwish, Y.A.A.; Mansour, A.S. Chemical characterization, antimicrobial, antioxidant, and cytotoxic potentials of Swietenia mahagoni. AMB Express 2022, 12, 77. [Google Scholar] [CrossRef]
- Sahgal, G.; Ramanathan, S.; Sasidharan, S.; Mordi, M.N.; Ismail, S.; Mansor, S.M. Phytochemical and antimicrobial activity of Swietenia mahagoni crude methanolic seed extract. Trop. Biomed. 2009, 26, 274–279. [Google Scholar]
- Durai, M.V.; Balamuniappan, G.; Geetha, S. Phytochemical screening and antimicrobial activity of leaf, seed and central-fruit-axis crude extract of Swietenia macrophylla King. J. Pharmacogn. Phytochem. 2016, 5, 181–186. [Google Scholar]
- Behiry, S.I.; Philip, B.; Salem, M.Z.M.; Amer, M.A.; El-Samra, I.A.; Abdelkhalek, A.; Heflish, A. Urtica dioica and Dodonaea viscosa leaf extracts as eco-friendly bioagents against Alternaria alternata isolate TAA-05 from tomato plant. Sci. Rep. 2022, 12, 16468. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short-and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar]
- Wang, X.; Wei, X.; Huang, X.; Shen, L.; Tian, Y.; Xu, H. Insecticidal constructure and bioactivities of compounds from Ficus sarmentosa var. henryi. Agric. Sci. China 2011, 10, 1402–1409. [Google Scholar] [CrossRef]
- Huang, G.-Y.; Cui, C.; Wang, Z.-P.; Li, Y.-Q.; Xiong, L.-X.; Wang, L.-Z.; Yu, S.-J.; Li, Z.-M.; Zhao, W.-G. Synthesis and characteristics of (Hydrogenated) ferulic acid derivatives as potential antiviral agents with insecticidal activity. Chem. Cent. J. 2013, 7, 33. [Google Scholar] [CrossRef]
- Devi, T.B.; Jena, S.; Patra, B.; Singh, K.D.; Chawla, S.; Raina, V.; Koijam, A.S.; Parida, A.; Rajashekar, Y. Acute and sub-acute toxicity evaluation of dihydro-p-coumaric acid isolated from leaves of Tithonia diversifolia Hemsl. A. Gray in BALB/c mice. Front. Pharmacol. 2022, 13, 1055765. [Google Scholar] [CrossRef]
- Riddick, E.W. Potential of quercetin to reduce herbivory without disrupting natural enemies and pollinators. Agriculture 2021, 11, 476. [Google Scholar] [CrossRef]
- Herrera-Mayorga, V.; Guerrero-Sánchez, J.A.; Méndez-Álvarez, D.; Paredes-Sánchez, F.A.; Rodríguez-Duran, L.V.; Niño-García, N.; Paz-González, A.D.; Rivera, G. Insecticidal Activity of Organic Extracts of Solidago graminifolia and Its Main Metabolites (Quercetin and Chlorogenic Acid) against Spodoptera frugiperda: An In Vitro and In Silico Approach. Molecules 2022, 27, 3325. [Google Scholar] [CrossRef]
- Joshi, R.S.; Wagh, T.P.; Sharma, N.; Mulani, F.A.; Sonavane, U.; Thulasiram, H.V.; Joshi, R.; Gupta, V.S.; Giri, A.P. Way toward “dietary pesticides”: Molecular investigation of insecticidal action of caffeic acid against Helicoverpa armigera. J. Agric. Food Chem. 2014, 62, 10847–10854. [Google Scholar] [CrossRef] [PubMed]
- Kletskov, A.V.; Potkin, V.I.; Dikusar, E.A.; Zolotar, R.M. New Data on Vanillin-Based Isothiazolic Insecticide Synergists. Nat. Prod. Commun. 2017, 12, 105–106. [Google Scholar] [CrossRef]
- Hay, W.T.; Behle, R.W.; Berhow, M.A.; Miller, A.C.; Selling, G.W. Biopesticide synergy when combining plant flavonoids and entomopathogenic baculovirus. Sci. Rep. 2020, 10, 6806. [Google Scholar] [CrossRef]
- Da Silva, D.F.; Bomfim, J.; Marchi, R.C.; Amaral, J.C.; Pinto, L.S.; Carlos, R.M.; Ferreira, A.G.; Forim, M.R.; Fernandes, J.B.; da Silva, M.F.G.F. Valorization of hesperidin from citrus residues: Evaluation of microwave-assisted synthesis of hesperidin-Mg complex and their insecticidal activity. J. Braz. Chem. Soc. 2022, 33, 772–782. [Google Scholar]
- Eldesouky, S.E.; Khamis, W.M.; Hassan, S.M. Joint action of certain fatty acids with selected insecticides against cotton leafworm, Spodoptera littoralis and their effects on biological aspects. J. Basic Environ. Sci. 2019, 6, 23–32. [Google Scholar]
- Khamis, W.M.; El-Desouky, S.E.; Gad, A.A. Toxicity and Antifeedant Effects of Apricot Kernel Extract and Its Main Components against Cotton Leaf Worm, Spodoptera littoralis (Lepidoptera: Noctuidae) Larvae With Reference To Some Physiological Effects. Alex. Sci. Exch. J. 2016, 37, 637–646. [Google Scholar]
- Moustafa, H.Z.; Yousef, H.; EL-lakwah, S.F. Toxicological and biochemical activities of fatty acids against Earias insulana (boisd.)(lepidoptera: Noctuidae). Egypt. J. Agric. Res. 2018, 96, 503–515. [Google Scholar] [CrossRef]
- Abdullah, R.R. Insecticidal activity of secondary metabolites of locally isolated fungal strains against some cotton insect pests. J. Plant Prot. Pathol. 2019, 10, 647–653. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef]
- Zhang, X.; Ran, W.; Li, X.; Zhang, J.; Ye, M.; Lin, S.; Liu, M.; Sun, X. Exogenous Application of Gallic Acid Induces the Direct Defense of Tea Plant Against Ectropis obliqua Caterpillars. Front. Plant Sci. 2022, 13, 833489. [Google Scholar] [CrossRef]
- Punia, A.; Chauhan, N.S.; Kaur, S.; Sohal, S.K. Effect of ellagic acid on the larvae of Spodoptera litura (Lepidoptera: Noctuidae) and its parasitoid Bracon hebetor (Hymenoptera: Braconidae). J. Asia. Pac. Entomol. 2020, 23, 660–665. [Google Scholar] [CrossRef]
- Adfa, M.; Kusnanda, A.J.; Livandri, F.; Rahmad, R.; Darwis, W.; Efdi, M.; Ninomiya, M.; Koketsu, M. Insecticidal activity of Toona sinensis against Coptotermes curvignathus Holmgren. Rasayan J. Chem 2017, 10, 153–159. [Google Scholar]
- Chuang, K.-J.; Chen, Z.-J.; Cheng, C.-L.; Hong, G.-B. Investigation of the antioxidant capacity, insecticidal ability and oxidation stability of Chenopodium formosanum seed extract. Int. J. Mol. Sci. 2018, 19, 2726. [Google Scholar] [CrossRef] [PubMed]

| Treatments of Swietenia mahagoni Acetone Extract Conc. (μg·mL−1) | Inhibition Percentage (%) | |
|---|---|---|
| Rhizoctonia solani | Fusarium equiseti | |
| 1000 | 18.21 c | 37.86 b |
| 2000 | 53.22 b | 36.07 bc |
| 3000 | 77.50 a | 82.50 a |
| * Nc | 0.00 d | 0.00 c |
| Tested Compound | Lethal Concentration (mg·L−1) | Confidence Limits (mg·L−1) | Slope ± SE ** | χ2 *** | df | N **** | |
|---|---|---|---|---|---|---|---|
| Swietenia mahagoni acetone extract | LC20 | 49.86 | (30.57–72.21) | 0.42 ± 0.05 | 9.79 | 4 | 360 |
| LC50 | 197.70 | (155.12–251.96) | |||||
| Pyriproxyfen10% EC | LC20 | 1.96 | (1.62–2.38) | 0.71 ± 0.11 | 7.94 | 4 | 360 |
| LC50 | 4.18 | (3.67–4.77) | |||||
| Treatments | Nymph Production/Female (Days) ± SD 1 | Reproductive Period (Days) ± SD | Total Mean of Survival% ± SD 1 | ||
|---|---|---|---|---|---|
| Daily | Total | Adult Stage | Nymphal Stage | ||
| Swietenia mahagoni acetone extract | 0.38 b ± 0.30 | 1.50 b ± 0.30 | 2.33 b ± 0.58 | 62.36 a ± 13.96 | 49.58 c ± 3.82 |
| Pyriproxyfen 10% EC | 0.50 b ± 0.58 | 2.01 b ± 0.58 | 3.00 ba ± 0.00 | 67.92 a ± 4.02 | 61.67 b ± 1.44 |
| Control | 3.94 a ± 1.55 | 15.77 a ± 1.55 | 3.67 a ± 0.58 | 76.33 a ± 5.93 | 98.57 a ± 1.43 |
| Treatments | Longevity (Days) ± SD 1 | Total Life Duration (Adult + Nymph) (Days) ± SD 1 | |
|---|---|---|---|
| Adult Stage | Nymphal Stage | ||
| Swietenia mahagoni acetone extract | 6.00 a ± 0.00 | 9.67 a ± 0.58 | 15.67 a ± 0.58 |
| Pyriproxyfen 10% EC | 5.67 a ± 0.58 | 8.67 b ± 0.58 | 14.33 a ± 1.15 |
| Control | 5.67 a ± 0.58 | 7.00 c ± 0.00 | 12.67 b ± 0.58 |
| Compounds | Area | Concentration (µg·g−1) |
|---|---|---|
| Gallic acid | 94.29 | 690.54 |
| Chlorogenic acid | 39.46 | 471.15 |
| Catechin | 34.57 | 752.64 |
| Methyl gallate | 51.39 | 316.17 |
| Caffeic acid | 11.08 | 89.01 |
| Syringic acid | 14.44 | 128.93 |
| Pyro catechol | * nd | nd |
| Rutin | 59.82 | 585.24 |
| Ellagic acid | 8.79 | 146.29 |
| Coumaric acid | 15.46 | 40.62 |
| Vanillin | 11.40 | 58.30 |
| Ferulic acid | 17.00 | 119.16 |
| Naringenin | 8.32 | 82.18 |
| Daidzein | 5.53 | 27.91 |
| Querectin | nd | nd |
| Cinnamic acid | 15.57 | 40.34 |
| Apigenin | nd | nd |
| Kaempferol | nd | nd |
| Hesperetin | 4.41 | 32.51 |
| * RT (min) | Relative Abundance % | Compound | Compound Class |
|---|---|---|---|
| 24.03 | 1.01 | Methyl 11,14-eicosadienoate | Fatty acid derivative |
| 25.64 | 2.35 | Oxiraneundecanoic acid, 3-pentyl-,methyl ester | Epoxy fatty acid ester |
| 26.35 | 37.1 | n-Hexadecanoic acid | Saturated fatty acid |
| 28.16 | 4.4 | Palmitic acid | Saturated fatty acid |
| 29.34 | 6.24 | 9,12-Octadecadienoic acid | Polyunsaturated fatty acid |
| 29.49 | 19.05 | Oleic acid | Monounsaturated fatty acid |
| 31.24 | 2.24 | Glycidyl oleate | Epoxy fatty acid ester |
| 33.93 | 2.31 | α-N-Normethadol | Opioid derivative |
| 34.07 | 1.94 | Oleic anhydride | Fatty acid derivative |
| 40.13 | 8.08 | Friedelan-3-one | Triterpenoid |
| 43.97 | 4.65 | Strychane 1-acetyl-20α-hydroxy-16-methylene | Alkaloid derivative |
| 45.21 | 3.22 | Trilinolein | Triacylglycerol |
| 45.51 | 2.08 | Lupeol | Triterpenoid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamis, W.M.; Heflish, A.A.; El-Messeiry, S.; Behiry, S.I.; Al-Askar, A.A.; Su, Y.; Abdelkhalek, A.; Gaber, M.K. Swietenia mahagoni Leaves Extract: Antifungal, Insecticidal, and Phytochemical Analysis. Separations 2023, 10, 301. https://doi.org/10.3390/separations10050301
Khamis WM, Heflish AA, El-Messeiry S, Behiry SI, Al-Askar AA, Su Y, Abdelkhalek A, Gaber MK. Swietenia mahagoni Leaves Extract: Antifungal, Insecticidal, and Phytochemical Analysis. Separations. 2023; 10(5):301. https://doi.org/10.3390/separations10050301
Chicago/Turabian StyleKhamis, Wael M., Ahmed A. Heflish, Sarah El-Messeiry, Said I. Behiry, Abdulaziz A. Al-Askar, Yiming Su, Ahmed Abdelkhalek, and Mohamed K. Gaber. 2023. "Swietenia mahagoni Leaves Extract: Antifungal, Insecticidal, and Phytochemical Analysis" Separations 10, no. 5: 301. https://doi.org/10.3390/separations10050301
APA StyleKhamis, W. M., Heflish, A. A., El-Messeiry, S., Behiry, S. I., Al-Askar, A. A., Su, Y., Abdelkhalek, A., & Gaber, M. K. (2023). Swietenia mahagoni Leaves Extract: Antifungal, Insecticidal, and Phytochemical Analysis. Separations, 10(5), 301. https://doi.org/10.3390/separations10050301











