1. Introduction
The capacity to overcome medication resistance and the unpleasant side effects associated with antibiotics has led to a rise in the popularity of medicinal plants and other natural products in the last few years for treating a wide range of pathogenic diseases. Therefore, scientists are investigating new antimicrobial substances that have been used for centuries in alternative medicine. The phytochemical components included in plant extracts make them a less risky and more cost-effective choice. The identification of chemical compounds derived from herbal plants has sparked research into the phytocompounds [
1,
2,
3] responsible for their biological properties.
The
Swietenia mahagoni (L.) belongs to the Meliaceae family, which consists mainly of evergreen blooming plants. This family, which has around 50 genera and 550 species, has been widely farmed in South Asia and the Pacific region [
4]. A broad variety of diseases are treated using various species from this family’s usage in traditional medicine. The bark of
S. mahagoni is used as a spice and in spirits, while its seeds are employed for the treatment of malaria, diabetic complications, autoimmune disorders, viral infections, eating disorders, and hypertension [
5]. The therapeutic properties of plants are attributed to their secondary metabolites, which can be used to produce natural antibiotics. The Meliaceae family contains various chemical compounds, including triterpenoids (limonoids), which are more abundant in this family than in others [
6]. Over 300 limonoids have been extracted from this family, including seven from the extract of
S. mahagoni seeds [
7]. Different species of
Swietenia contain in their various parts phenolic compounds, triterpenoids (limonoids), flavonoids, swiemahogins A and BC, and alkaloids [
8,
9].
According to the data from the United States Department of Agriculture (USDA) for the 2021–2022 marketing year, Egypt ranks as the 20th largest producer of rice in the world, with an estimated production of 4.2 million metric tons, out of a worldwide production of 513.85 million metric tons [
10]. Sheath blight is an economically significant rice disease worldwide caused by the fungus
Rhizoctonia solani and was reported in Egypt early in 2013 [
11,
12]. A mild pathogen in cereals,
Fusarium equiseti is occasionally detected in association with kernels affected by
Fusarium head blight [
13]. However, recent research has suggested that the
F. incarnatum-
equiseti species complex may also be associated with the rice sheath rot disease [
14]. Meanwhile, Tralamazza et al. [
15] have reported that this species complex is associated with panicle infection in wild rice in Brazil. Numerous research studies have explored the effectiveness of different extracts against
R. solani, revealing encouraging outcomes regarding their antifungal properties [
16,
17,
18]. Antifungal properties towards the fungi
Aspergillus flavus,
Candida spp., and
A. niger were detected using water, alcohol, ether, and chloroform-derived extracts of
S. macrophylla leaves [
19]. The methanolic extract of
S. mahagoni leaves was shown to be more potent against
C. albicans and
A. niger when compared to a water-based extract and other solvent extracts [
20]. Additionally, the antifungal properties of triterpenoids from
Khaya senegalensis and
S. mahagoni were investigated by Govindachari et al. [
7].
One of the wide-ranging polyphagous insects that exhibit several sophisticated biotypes and plausible distinctions in host adaptation is the cotton aphid,
Aphis gossypii Glover (Hemiptera: Aphididae). Its rapid reproduction and high insecticide resistance give rise to economic and ecological problems [
21]. Moreover, the susceptibility to secondary infections like sooty mold and virus diseases would be able to accrete in the infected host plants [
22]. Oil of mahogany from
S. mahagoni showed the potent toxic and repellent effects on the nymphs of the bean aphid,
Aphis craccivora Koch (Hemiptera: Aphididae). Therefore, mahogany oil was suggested for the management of bean aphid [
23]. One of the commonly used insect growth regulators in the control of piercing-sucking insects is the pyriproxyfen, which proved a superior efficacy in reducing the population of cotton aphid,
A. gossypii Glover [
24]. This study aimed to isolate and molecularly identify the pathogens responsible for rice sheath blight and rot, examine the antifungal and insecticidal properties of the acetone extract obtained from
Swietenia mahagoni leaves (SMAL) against fungal pathogens and the cotton aphid,
Aphis gossypii Glover, and determine the primary phytochemical constituents present in the extract through HPLC and a GC–MS analysis.
4. Discussion
In recognition of their reliability, strength, ecological nature, and sustainable impact on the ecosystem, plant-based substances, particularly medicinal plants, have been introduced in the environmental field to treat various plant diseases. In addition, such substances may mitigate the negative effects of synthetic antimicrobials [
41]. In the present investigation, qualitative evaluations of the bioactive substances in the acetone extract of
Swietenia mahagoni leaves (SMAL) were performed using a variety of conventional assays, displaying the presence of a vast array of phytochemical components. The most significant biologically active substances were rutin, gallic acid, catechin, hexadecanionic acid, oleic acid, and triterpenes. These results are consistent with those of other investigators [
42,
43], and these molecules play a major role in the treatment of various plant pathogens. Chen et al. [
9] explored the association among the botanical compound substances in the plant extract and its biological capability.
A study was conducted to investigate the antifungal properties of the methanolic extract obtained from the leaves of
S. mahagoni (methSM). The study showed that fungal strains such as
Candida albicans,
Aspergillus flavus, and
A. niger were highly susceptible to the extract at concentrations of 25 and 50 mg·mL
−1, with inhibition zones (IZ) ranging from 12.0 to 22.1 mm. However, less activity was observed at a concentration of 15 mg·mL
−1, with IZ ranging from 9.5 to 13.1 mm. Conversely, no antifungal activity was observed against
A. fumigatus and
C. glabrata [
44]. However, our promising antifungal results are consistent with those obtained by previous studies, which showed that the methSM extract was effective against
C. albicans and
A. niger at a concentration of 100 mg·mL
−1, with IZ ranging from 16 to 20 mm [
20]. Other studies have also reported the antifungal properties of
S. mahagoni and
macrophylla methanol seed extract against
C. albicans, Rhizopus spp.,
Fusarium, and
Alternaria species [
45,
46].
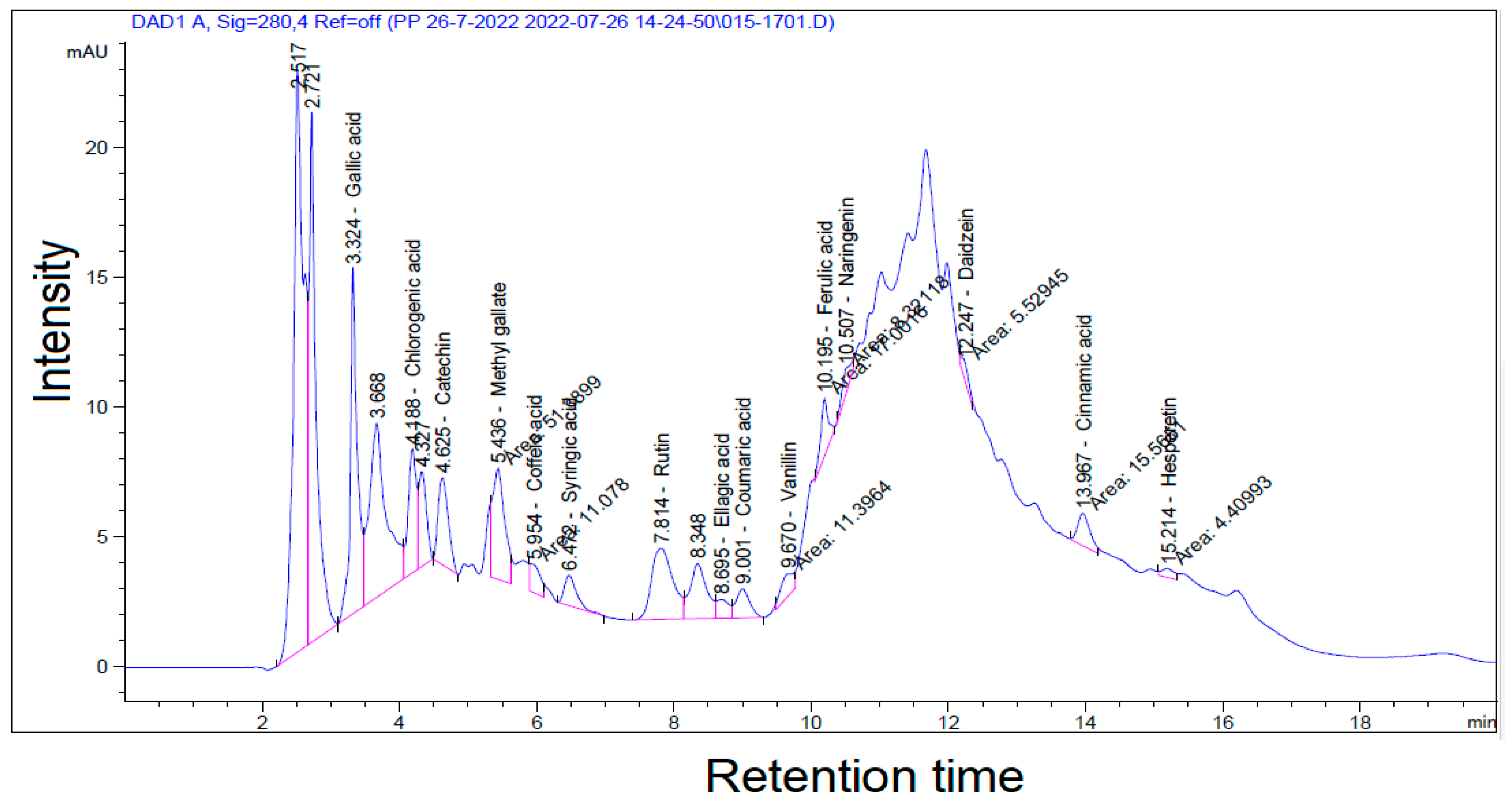
The most potent molecules found in our SMAL-HPLC and GC–MS analyses, such as catechin, gallic acid, ferulic acid, and hexadecanoic acid have been studied for their antibacterial and antifungal properties [
47,
48,
49]. The SMAL extract’s detected molecules (e.g., coumaric, caffeic, and ferulic acids) were found to have antifungal activity against
Alternaria alternata when applied alone or synergistically [
47]. Moreover, in contrast, the second constituent in SMAL’s GC–MS, oleic acid (monoene fatty acid), did not have a significant effect on the growth of
R. solani or
Pythium avenae but did reduce the mycelial growth of
P. ultimum [
50].
However, a sublethal concentration (LC
20) of SMAL extract significantly decreased the survival percentages of
A. gossypii Glover nymphs, with no discernible influence on the adult survival percentages. Certain HPLC–MS phytochemical components of
Swietenia mahagoni extract, such as derivatives of dihydro-p-coumaric, cinnamic acid, and 7-hydroxycoumarin, may be one of the primary sources of toxic effects and viable insecticidal activities [
51,
52,
53]. In addition, the SMAL extract was distinguished by derivatives of quercetin, in which it attained a good affinity on the active site of acetylcholinesterase but with a lower binding energy compared to chlorpyrifos against insect pests of the Hemiptera, Diptera, and Lepidoptera. On the contrary, quercetin was almost safe for natural enemies of Coleoptera and pollinators of Hymenoptera [
54,
55]. Moreover, the SMAL extract in this study featured derivatives of caffeic acid. Sequential binding of multiple molecules of caffeic acid was shown to possess a potent inhibitor of
Helicoverpa armigera Hubner gut proteases’ activity and gene expression [
56]. Ultimately, some of the moieties of the SMAL extract might share only a synergistic action, for instance, the synergistic effects of the derivatives of chlorogenic acid on quercetin [
55], vanillin mixtures with imidacloprid or α-cypermethrin [
57], and daidzein on the baculovirus biopesticide [
58]. Moreover, the hesperidin–Mg complex was found to be a trigger for the insecticide’s potential against the adults of
Bemisia tabaci Gennadius and
Spodoptera frugiperda (J.E. Smith) [
59]. On the other hand, the unsaturated free carboxylic fatty acids detected by GC–MS in the SMAL extract, exemplified in oleic acid, n-hexadecanoic acid, 9,12-octadecadienoic acid (
Z,
Z), and
trans-13-octadecenoic acid, were thought to attain an insecticidal activity against
A. gossypii Glover. These findings are in accordance with the investigations on the insecticidal activity of corresponding fatty acids on lepidopteran pests, such as
S. littoralis Boisd. [
60,
61],
Earias insulana Boisd. [
62], and the hemipteran,
A. gossypii Glover [
63]. This phenomenon could be elucidated by the inferences of Eldesouky et al. [
60] and Khamis et al. [
61] stating that the ascendancy of the larvicidal activity against
S. littoralis Boisd was correlated with the increases in the double-bond quantities in the fatty acid chains. Furthermore, the free carboxylic fatty acids could accomplish more toxicity than their methylated ones, as the free carboxyl group had a high tendency to obstruct the systemic constancy of target cells [
61,
64].
In this study, both the SMAL extract and pyriproxyfen realized equally prolonging effects on the longevity of the nymphal stages of
A. gossypii Glover and its whole life duration. The LC
20 of the SMAL extract and pyriproxyfen had equipollent effects on reducing the reproductive activity of the adult female more than the control. The biological aspects of the SMAL extract on cotton aphids may be attributed to particular HPLC–MS components that were investigated in several research studies. For instance, in polyphenols, gallic acid was found to exhibit antifeeding effects via jasmonic acid signals, which induced astragalin, naringenin, and epigallocatechin-3-gallate [
65]. In addition, ellagic acid adversely increased larval mortality, reduced pupation, and prolonged the development of the immature stages of
S. litura Fabricius [
66]. Moreover, naringenin and quercetin increased the developmental duration, the pre-reproductive period, and mortality, while decreasing fecundity and the reproduction rate of the pea aphid,
Acyrthosiphon pisum Harris.
In our study, the SMAL extract possessed a repellent response on the adult stage of
A. gossypii compared to pyriproxyfen and the control treatment. The explication of these findings may be associated with the presence of specific HPLC–MS phytochemical moieties in the SMAL extract represented in pyrocatechol, catechin, and gallic acid [
65,
67,
68]. The repellency and insecticidal activity of 1,2-benzenediol (pyrocatechol) were observed on insects due to the presence of two hydroxyl groups in the
ortho-position [
68]. Flavonoid derivatives, (+)-catechin, showed no fatal effects but potent repellency and antifeedant activity against
Coptotermes curvignathus Holmgren (Isoptera: Rhinotermitidae) [
67]. Gallic acid could induce the direct defense of
Camellia sinensis against the larvae of
Ectropis oblique, via stimulating the signals of jasmonic acid and the pathways of phenylpropanoid [
65].
Overall, our data suggest that the tested compound has potential as a natural fungicide against R. solani and F. equiseti. Moreover, an insecticide could be used to kill cotton aphids. However, further studies are needed to determine the mechanism of action and potential applications of the tested extract in agriculture.