Categorization of Marijuana Suspected Policies’ Seizures in Southeast Serbia According to Cannabinoids Content
Abstract
:1. Introduction
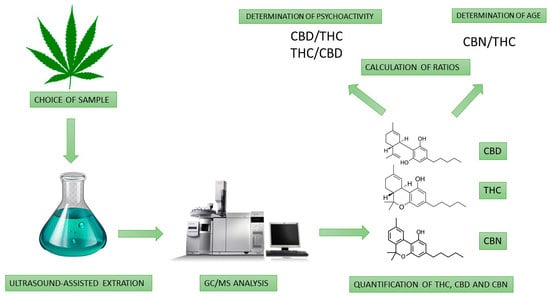
2. Materials and Methods
2.1. Marijuana Suspected Policies’ Seizures
2.2. Analytical Methodology
2.3. Regents
2.4. Instrument and Instrument Parameters
2.5. Stock and Working Solutions and Calibration
2.6. Sample Preparation
2.7. Qualitative and Quantitative Analysis
2.8. Statistical Analysis
3. Results
3.1. Weights of MSPS
3.2. Cannabinoid Content
3.3. Classification of Seized Plant Material
3.4. Changes in the Ratio CBN/THC in MSPS with the Time-Determination of MSPS Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hillig, K.W.; Mahlberg, P.G. A Chemotaxonomic Analysis of Cannabinoid Variation in Cannabis (Cannabaceae). Am. J. Bot. 2004, 91, 966–975. [Google Scholar] [CrossRef]
- Russo, E.B. Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential; Routledge: London, UK, 2013. [Google Scholar]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A New ESI-LC/MS Approach for Comprehensive Metabolic Profiling of Phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef]
- Russo, E.B.; Guy, G.W.; Robson, P.J. Cannabis, Pain, and Sleep: Lessons from Therapeutic Clinical Trials of Sativex, a Cannabis-Based Medicine. Chem. Biodivers. 2007, 4, 1729–1743. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of Cannabis as a Medicine: A Review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Knopf, A. NIDA: Daily Marijuana Use at 13% in Noncollege Young Adults. Alcohol. Drug Abuse Wkly. 2018, 30, 7. [Google Scholar] [CrossRef]
- National Institute on Drug Abuse (NIDA). Marijuana. Available online: https://www.drugabuse.gov/drugs-abuse/marijuana (accessed on 12 May 2020).
- Spetz, J.; Chapman, S.A.; Bates, T.; Jura, M.; Schmidt, L.A. Social and Political Factors Associated with State-Level Legalization of Cannabis in the United States. Contemp. Drug Probl. 2019, 46, 165–179. [Google Scholar] [CrossRef]
- Mounteney, J.; Griffiths, P. Increasing Complexity in European Drug Use: Highlights from the EMCDDA’s 2014 European Drug Report. Drugs Educ. Prev. Policy 2014, 21, 482–483. [Google Scholar] [CrossRef]
- Mounteney, J.; Griffiths, P.; Cunningham, A.; Evans-Brown, M.; Ferri, M.; Hedrich, D.; Noor, A. Continued Signs of Resilience in the European Drug Market: Highlights from the EMCDDA’s 2016 European Drug Report. Drugs Educ. Prev. Policy 2016, 23, 492–495. [Google Scholar] [CrossRef]
- Aiello, G.; Fasoli, E.; Boschin, G.; Lammi, C.; Zanoni, C.; Citterio, A.; Arnoldi, A. Proteomic Characterization of Hempseed (Cannabis sativa L.). J. Proteom. 2016, 147, 187–196. [Google Scholar] [CrossRef]
- D’Souza, D.C.; Sewell, R.A.; Ranganathan, M. Cannabis and Psychosis/schizophrenia: Human Studies. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 259, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.V.; Valerie Curran, H.; Freeman, T.P.; Mokrysz, C.; Lewis, D.A.; Morgan, C.J.A.; Parsons, L.H. Keep off the Grass? Cannabis, Cognition and Addiction. Nat. Rev. Neurosci. 2016, 17, 293–306. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Morrison, P.D.; Fusar-Poli, P.; Martin-Santos, R.; Borgwardt, S.; Winton-Brown, T.; Nosarti, C.; O’ Carroll, C.M.; Seal, M.; Allen, P.; et al. Opposite Effects of Δ-9-Tetrahydrocannabinol and Cannabidiol on Human Brain Function and Psychopathology. Neuropsychopharmacology 2010, 35, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.; Freeman, T.P.; Kilian, C.; López-Pelayo, H.; Rehm, J. Public Health Monitoring of Cannabis Use in Europe: Prevalence of Use, Cannabis Potency, and Treatment Rates. Lancet Reg. Health-Europe 2021, 10, 100227. [Google Scholar] [CrossRef] [PubMed]
- Pourseyed Lazarjani, M.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for Quantification of Cannabinoids: A Narrative Review. J. Cannabis Res. 2020, 2, 35. [Google Scholar] [CrossRef]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and Biomedical Analysis of Cannabinoids: A Critical Review. J. Pharm. Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbrand, Z.L.; Schug, K.A. A Review of Methods for the Chemical Characterization of Cannabis Natural Products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Δ9-THCA-A from Hemp and Analytical Aspects Concerning the Determination of Δ9-THC in Cannabis Products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef]
- Hazekamp, A.; Simons, R.; Peltenburg-Looman, A.; Sengers, M.; van Zweden, R.; Verpoorte, R. Preparative Isolation of Cannabinoids from Cannabis sativa by Centrifugal Partition Chromatography. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 2421–2439. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Omar, J.; Navarro, P.; Olivares, M.; Etxebarria, N.; Usobiaga, A. Identification and Quantification of Cannabinoids in Cannabis sativa L. Plants by High Performance Liquid Chromatography-Mass Spectrometry. Anal. Bioanal. Chem. 2014, 406, 7549–7560. [Google Scholar] [CrossRef]
- Grauwiler, S.B.; Scholer, A.; Drewe, J. Development of a LC/MS/MS Method for the Analysis of Cannabinoids in Human EDTA-Plasma and Urine after Small Doses of Cannabis sativa Extracts. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 850, 515–522. [Google Scholar] [CrossRef] [PubMed]
- United Nations Office on Drugs and Crime (UNODC). Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products; United Nations: New York, NY, USA, 2009.
- Freeman, T.P.; Craft, S.; Wilson, J.; Stylianou, S.; ElSohly, M.; Di Forti, M.; Lynskey, M.T. Changes in Delta-9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) Concentrations in Cannabis over Time: Systematic Review and Meta-Analysis. Addiction 2021, 116, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Boggs, D.L.; Nguyen, J.D.; Morgenson, D.; Taffe, M.A.; Ranganathan, M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol. Neuropsychopharmacology 2018, 43, 142–154. [Google Scholar] [CrossRef]
- Stuyt, E. The Problem with the Current High Potency THC Marijuana from the Perspective of an Addiction Psychiatrist. Mo. Med. 2018, 115, 482–486. [Google Scholar] [PubMed]
- Morgan, C.J.A.; Freeman, T.P.; Schafer, G.L.; Valerie Curran, H. Cannabidiol Attenuates the Appetitive Effects of Δ9-Tetrahydrocannabinol in Humans Smoking Their Chosen Cannabis. Neuropsychopharmacology 2010, 35, 1879–1885. [Google Scholar] [CrossRef]
- Freeman, T.P.; Morgan, C.J.A.; Hindocha, C.; Schafer, G.; Das, R.K.; Valerie Curran, H. Just Say “know”: How Do Cannabinoid Concentrations Influence Users’ Estimates of Cannabis Potency and the Amount They Roll in Joints? Addiction 2014, 109, 1686–1694. [Google Scholar] [CrossRef]
- De Meijer, E.P.M.; van der Kamp, H.J.; van Eeuwijk, F.A. Characterisation of Cannabis Accessions with Regard to Cannabinoid Content in Relation to Other Plant Characters. Euphytica 1992, 62, 187–200. [Google Scholar] [CrossRef]
- Fetterman, P.S.; Keith, E.S.; Waller, C.W.; Guerrero, O.; Doorenbos, N.J.; Quimby, M.W. Mississippi-Grown Cannabis sativa L.: Preliminary Observation on Chemical Definition of Phenotype and Variations in Tetrahydrocannabinol Content versus Age, Sex, and Plant Part. J. Pharm. Sci. 1971, 60, 1246–1249. [Google Scholar] [CrossRef]
- Potter, D.J.; Hammond, K.; Tuffnell, S.; Walker, C.; Di Forti, M. Potency of Δ9-Tetrahydrocannabinol and Other Cannabinoids in Cannabis in England in 2016: Implications for Public Health and Pharmacology. Drug Test. Anal. 2018, 10, 628–635. [Google Scholar] [CrossRef]
- Ross, S.A.; ElSohly, M.A. CBN And∆ 9-THC Concentration Ratio as an Indicator of the Age of Stored Marijuana Samples. Bull. Narc. 1997, 49, 139. [Google Scholar]
- Dragoljic, M.; Rodic-Grabovac, B.; Vasiljevic, L.; Matic, V.; Simurdic, L. The Content of Basic Cannabinoids and Their Mutual Ratios in Cannabis sativa L. Plant. Acta Periodica Technol. 2019, 59–68. [Google Scholar] [CrossRef]
- Chandra, S.; Radwan, M.M.; Majumdar, C.G.; Church, J.C.; Freeman, T.P.; ElSohly, M.A. New Trends in Cannabis Potency in USA and Europe during the Last Decade (2008–2017). Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Swift, W.; Wong, A.; Li, K.M.; Arnold, J.C.; McGregor, I.S. Analysis of Cannabis Seizures in NSW, Australia: Cannabis Potency and Cannabinoid Profile. PLoS ONE 2013, 8, e70052. [Google Scholar] [CrossRef]
- Dujourdy, L.; Besacier, F. A Study of Cannabis Potency in France over a 25 Years Period (1992–2016). Forensic Sci. Int. 2017, 272, 72–80. [Google Scholar] [CrossRef]
- Niesink, R.J.M.; Rigter, S.; Koeter, M.W.; Brunt, T.M. Potency Trends of Δ9-Tetrahydrocannabinol, Cannabidiol and Cannabinol in Cannabis in the Netherlands: 2005–2015. Addiction 2015, 110, 1941–1950. [Google Scholar] [CrossRef]
- Cascini, F.; Aiello, C.; Di Tanna, G. Increasing Delta-9-Tetrahydrocannabinol (Δ-9-THC) Content in Herbal Cannabis Over Time: Systematic Review and Meta-Analysis. Curr. Drug Abuse Rev. 2012, 5, 32–40. [Google Scholar] [CrossRef]
- Pijlman, F.T.A.; Rigter, S.M.; Hoek, J.; Goldschmidt, H.M.J.; Niesink, R.J.M. Strong Increase in Total Delta-THC in Cannabis Preparations Sold in Dutch Coffee Shops. Addict. Biol. 2005, 10, 171–180. [Google Scholar] [CrossRef]
- Zamengo, L.; Frison, G.; Bettin, C.; Sciarrone, R. Cannabis Potency in the Venice Area (Italy): Update 2013. Drug Test. Anal. 2015, 7, 255–258. [Google Scholar] [CrossRef]
- Freeman, T.P.; Groshkova, T.; Cunningham, A.; Sedefov, R.; Griffiths, P.; Lynskey, M.T. Increasing Potency and Price of Cannabis in Europe, 2006–2016. Addiction 2019, 114, 1015–1023. [Google Scholar] [CrossRef]
- Viviers, H.J.; Petzer, A.; Gordon, R. An Assessment of Heavy Metal Contaminants Related to Cannabis-Based Products in the South African Market. Forensic Sci. Int. Rep. 2021, 4, 100224. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Machera, K. Macro and Trace Elements in Hemp (Cannabis sativa L.) Culti-vated in Greece: Risk Assessment of Toxic Elements. Front. Chem. 2021, 9, 654308. [Google Scholar] [CrossRef] [PubMed]
- Nava, V.; Albergamo, A.; Bartolomeo, G.; Rando, R.; Litrenta, F.; Lo Vecchio, G.; Giorgianni, M.C.; Cicero, N. Monitoring Cannabinoids and the Safety of the Trace Element Profile of Light Cannabis sativa L. from Different Varieties and Geo-graphical Origin. Toxics 2022, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.; Elzinga, S.; Raber, J.C. Determination of Pesticide Residues in Cannabis Smoke. J. Toxicol. 2013, 2013, 378168. [Google Scholar] [CrossRef] [PubMed]
- Douvris, C.; Bentil, E.; Ayensu, I.; Osei Akoto, C.; Amponsah, I.K.; Adu, J.; Bussan, D. Trace Metals in Cannabis Seized by Law Enforcement in Ghana and Multivariate Analysis to Distinguish among Different Cannabis Farms. Toxics 2022, 10, 567. [Google Scholar] [CrossRef]

| Sample Weight of the Cannabis Plant and Products | Number of Samples | Percentage of Samples |
|---|---|---|
| >900 g | 45 | 35.43 |
| <50 mg | 45 | 35.43 |
| 50 mg–900 g | 37 | 29.14 |
| Cannabinoids | % THC | % CBD | % CBN |
|---|---|---|---|
| Min | 4.9 | 0.046 | 0.03 |
| Max | 16.1 | 2.54 | 2.59 |
| Average | 8.91 | 0.51 | 0.64 |
| STV DEV | 2.42 | 0.55 | 0.61 |
| Median | 8.8 | 0.33 | 0.44 |
| Types of MSPS | Number of Samples | % THC | % CBD | % CBN | THC/CBD | CBD/THC |
|---|---|---|---|---|---|---|
| Joint | 29 | 5.94 | 0.37 | 0.58 | 36.4 | 0.062 |
| Mixture of buds and leaves | 72 | 8.89 | 0.55 | 0.62 | 36.80 | 0.062 |
| Buds | 26 | 12.39 | 0.50 | 0.75 | 47.14 | 0.040 |
| Criteria for Classification | |||
|---|---|---|---|
| Hilih at al. 2004 [1] | |||
| Type of cannabis | Chemotype I Drug type: THC/CBD > 10 | Chemotype II Intermediate type: THC/CBD 0.25–10 | Chemotype III Fiber-type: THC/CBD < 0.2 |
| Percentage of samples | 85.59 | 14.41 | / |
| Note | Approximately 6.78% of the samples have a ratio of more than 100, which makes these cannabis samples extremely psychoactive. | ||
| De Meier et al. 1992 [29] | |||
| Type of cannabis | Drug type: THC+CBD/CBN > 1 | Fiber type: THC+CBD/CBN < 1 | |
| Percentage of samples | 100 | / | |
| Note | All of the confiscated samples analyzed have a ratio of more than 1. The highest obtained ratio is 150. | ||
| Fetterman et al. 1971 [30] | |||
| Type of cannabis | Drug type: THC/CBD > 1 | Fiber type: THC/CBD < 1 | |
| Percentage of samples | 100 | / | |
| Serbian Law on psychoactive controlled substances | |||
| Type of cannabis | Drug type THC > 0.3% | Fiber type THC < 0.3% | |
| Percentage of samples | 100 | / | |
| Reference | Country | Note | % THC | % CBD | % CBN |
|---|---|---|---|---|---|
| [39] | The Netherlands | domestic | 15.3 | / | / |
| imported | 4.8 | / | / | ||
| [42] | Italy | 9.8 | / | / | |
| [31] | The United Kingdom | 14.2 | / | / | |
| [37] | Australia | indoor | 19.16 | 0.14 | 0.01 |
| outdoor | 15.47 | 0.03 | 0.01 | ||
| rural | 18.66 | 0.05 | 0.09 | ||
| urban | 12.38 | 0.03 | 0.02 | ||
| average | 14.88 | 0.14 | 0.09 | ||
| [35] | The Republic of Srpska | 4.95 | 0.26 | 0.48 | |
| [43] | EU, Norway, and Turkey | 10.22 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostic, E.; Djukic, M.; Antovic, A.; Zdravkovic, M.; Milic, M.; Stojanovic, I.; Todorovic, S.; Vujovic, M. Categorization of Marijuana Suspected Policies’ Seizures in Southeast Serbia According to Cannabinoids Content. Separations 2023, 10, 307. https://doi.org/10.3390/separations10050307
Kostic E, Djukic M, Antovic A, Zdravkovic M, Milic M, Stojanovic I, Todorovic S, Vujovic M. Categorization of Marijuana Suspected Policies’ Seizures in Southeast Serbia According to Cannabinoids Content. Separations. 2023; 10(5):307. https://doi.org/10.3390/separations10050307
Chicago/Turabian StyleKostic, Emilija, Mirjana Djukic, Aleksandra Antovic, Miodrag Zdravkovic, Miroslav Milic, Ivan Stojanovic, Stevan Todorovic, and Maja Vujovic. 2023. "Categorization of Marijuana Suspected Policies’ Seizures in Southeast Serbia According to Cannabinoids Content" Separations 10, no. 5: 307. https://doi.org/10.3390/separations10050307
APA StyleKostic, E., Djukic, M., Antovic, A., Zdravkovic, M., Milic, M., Stojanovic, I., Todorovic, S., & Vujovic, M. (2023). Categorization of Marijuana Suspected Policies’ Seizures in Southeast Serbia According to Cannabinoids Content. Separations, 10(5), 307. https://doi.org/10.3390/separations10050307