Distributions of OCPs and CUPs in the Sediment and Surface Water Close to a Drinking Water Reservoir in Northeastern China
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Sample Collection
2.2. Chemical
2.3. Extraction and Clean-Up
2.4. Analytical Procedure
2.5. Quality Assurance and Quality Control
3. Results
3.1. OCPs
3.2. CUPs
4. Discussion
4.1. OCPs
4.2. CUPs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, S.; Cheng, H.X.; Liu, Y.H.; Xu, X.B. Levels and distribution of organochlorine pesticides in various media in a mega-city, China. Chemosphere 2009, 75, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.Y.; Lu, Y.L.; Wang, G.; Wang, T.Y.; Luo, W.; Shi, Y.J.; Zhang, X.; Jiao, W.T. Organochlorine Pesticides in Soils Around Watersheds of Beijing Reservoirs: A Case Study in Guanting and Miyun Reservoirs. Bull. Environ. Contam. Toxicol. 2009, 82, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, S.C.; Kim, K.H.; Shim, W.J.; Hong, S.H.; Choi, K.H.; Yoon, J.H.; Kim, J.G. Survey on organochlorine pesticides, PCDD/Fs, dioxin-like PCBs, and HCB in sediments from the Han river, Korea. Chemosphere 2009, 75, 580–587. [Google Scholar] [CrossRef]
- Zhang, P.; Song, J.M.; Yuan, H.M. Persistent organic pollutant residues in the sediments and mollusks from the Bohai Sea coastal areas, North China: An overview. Environ. Int. 2009, 35, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Iturburu, F.G.; Calderon, G.; Amé, M.V.; Menone, M.L. Ecological Risk Assessment (ERA) of pesticides from freshwater ecosystems in the Pampas region of Argentina: Legacy and current use chemicals contribution. Sci. Total Environ. 2019, 691, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Cortes, S.; Pozo, K.; Llanos, Y.; Martinez, N.; Foerster, C.; Leiva, C.; Ustáriz, J.; Přibylová, P.; Klánová, J.; Jorquera, H. First measurement of human exposure to current use pesticides (CUPs) in the atmosphere of central Chile: The case study of Mauco cohort. Atmos. Pollut. Res. 2020, 11, 776–784. [Google Scholar] [CrossRef]
- Li, H.; Sun, B.; Lydy, M.J.; You, J. Sediment-associated pesticides in an urban stream in Guangzhou, China: Implication of a shift in pesticide use patterns. Environ. Toxicol. Chem. 2013, 32, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Matthies, M.; Klasmeier, J.; Beyer, A.; Ehling, C. Assessing Persistence and Long-Range Transport Potential of Current-Use Pesticides. Environ. Sci. Technol. 2009, 43, 9223–9229. [Google Scholar] [CrossRef] [PubMed]
- Ruggirello, R.M.; Hermanson, M.H.; Isaksson, E.; Teixeira, C.; Forsstrom, S.; Muir, D.C.G.; Pohjola, V.; van de Wal, R.; Meijer, H.A.J. Current use and legacy pesticide deposition to ice caps on Svalbard, Norway. J. Geophys. Res.-Atmos. 2010, 115, D18308. [Google Scholar] [CrossRef]
- De Wit, C.A.; Muir, D. Levels and trends of new contaminants, temporal trends of legacy contaminants and effects of contaminants in the Arctic: Preface. Sci. Total Environ. 2010, 408, 2852–2853. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, P.; Kyriakidis, N.B.; Georgitsanakou, I. Effect of storage temperature on the degradation of dimethoate in fortified orange and peach juices. J. Agric. Food Chem. 2000, 48, 4896–4899. [Google Scholar] [CrossRef] [PubMed]
- Mast, M.A.; Foreman, W.T.; Skaates, S.V. Current-use pesticides and organochlorine compounds in precipitation and lake sediment from two high-elevation national parks in the Western United States. Arch. Environ. Contam. Toxicol. 2007, 52, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.L.; Li, Y.F.; Qi, H.; Sun, D.Z.; Cheng, X. ORGN 473-Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in topsoils of Harbin, China. Abstr. Pap. Am. Chem. Soc. 2008, 235, 1053. [Google Scholar]
- Ma, W.L.; Li, Y.F.; Sun, D.Z.; Qi, H. Polycyclic Aromatic Hydrocarbons and Polychlorinated Biphenyls in Topsoils of Harbin, China. Arch. Environ. Contam. Toxicol. 2009, 57, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.Q.; Que, M.X.; Li, Y.F.; Liu, Y.F.; Wan, X.N.; Xu, D.D.; Sverko, E.; Ma, J. Polychlorinated biphenyls in Chinese surface soils. Environ. Sci. Technol. 2007, 41, 3871–3876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Hong, H.S.; Zhou, J.L.; Huang, J.; Yu, G. Fate and assessment of persistent organic pollutants in water and sediment from Minjiang River Estuary, Southeast China. Chemosphere 2003, 52, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chai, Z.F.; Sun, H.B.; Zhang, J.L. A survey of extractable persistent organochlorine pollutants in Chinese commercial yogurt. J. Dairy Sci. 2006, 89, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Moisey, J.; Fisk, A.T.; Hobson, K.A.; Norstrom, R.J. Hexachlorocyclohexane (HCH) isomers and chiral signatures of alpha-HCH in the arctic marine food web of the Northwater Polynya. Environ. Sci. Technol. 2001, 35, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, Y.L.; Han, J.Y.; Luo, W.; Shi, Y.J.; Wang, T.Y.; Sun, Y.M. Hexachlorobenzene sources, levels, and human exposure in the environment of China. Environ. Int. 2010, 36, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.F.; Liu, L.Y.; Zhu, F.J.; Ma, W.L. National-scale monitoring of historic used organochlorine pesticides (OCPs) and current used pesticides (CUPs) in Chinese surface soil: Old topic and new story. J. Hazard. Mater. 2023, 443 Pt B, 130285. [Google Scholar] [CrossRef]
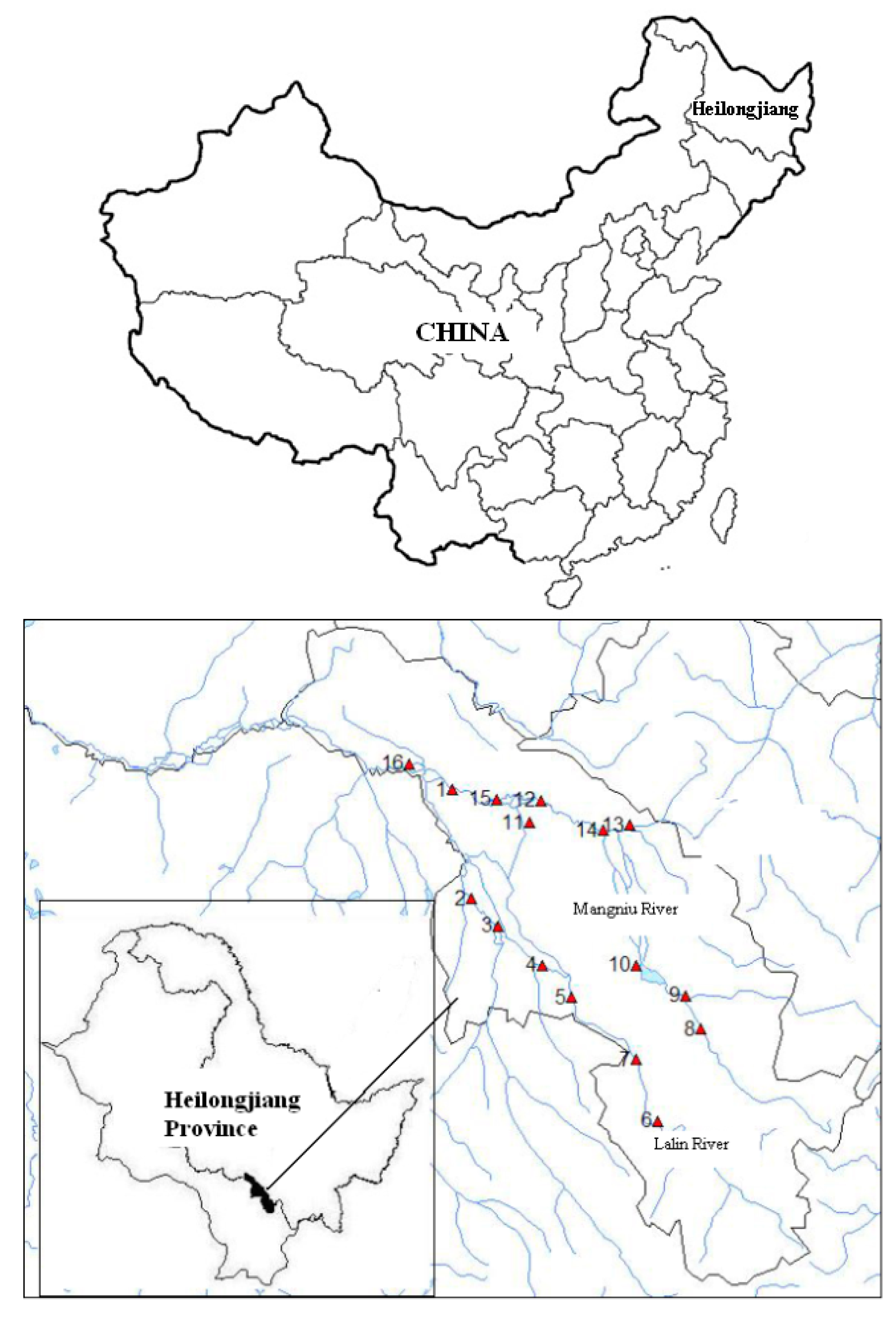




| Sampling Site | Latiude | Longitude | Sampling Site | Latiude | Longitude |
|---|---|---|---|---|---|
| 1#Lalin, Jinsan Bridge | E127°02′5 | N45°05′45 | 9#Chonghe Town, Xingguo | E127°35′1 | N44°31′47 |
| 2#Xingsheng Town, Gaojiatun | E127°06′1 | N44°51′57 | 10#Longfeng Town, Longfeng Reservoir | E127°35′1 | N44°43′48 |
| 3#Dujia Town, Shuguang | E127°10′4 | N44°48′41 | 11#Changpu Town, Zhonghua Xiaoluowezi | E127°16′3 | N45°01′42 |
| 4#Shanhe Town, Taipingchuan | E127°18′3 | N44°43′50 | 12#Erhe Town, Shuanghe | E127°18′3 | N45°04′28 |
| 5#Xiaoyang Town, Qichuankou | E127°23′5 | N44°39′46 | 13#Zhiguang Town, Songjiajie | E127°34′0 | N45°01′20 |
| 6#Shahezi Town, Mopanshan Reservoir | E127°38′5 | N44°24′17 | 14#Zhiguang Town, Wuxing | E127°29′2 | N45°00′43 |
| 7#Shahezi Town, Gali | E127°35′1 | N44°31′56 | 15#Changpu Town, Xingzhuang | E127°10′4 | N45°04′36 |
| 8#Chonghe Town, Changcuizi | E127°46′3 | N44°35′46 | 16#Yingchangzi Town, Xingguang | E126°55′0 | N45°09′00 |
| Sampling Location | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-HCH/γ-HCH (water) | 1.1 | 2.5 | 2.1 | 6.8 | 6.5 | 1.9 | 3.5 | 1.6 | 1.6 | 1.8 | 2.1 | 1.4 | 1.9 | 2.1 | 2.7 | 2.2 |
| α-HCH/γ-HCH (sediment) | 1.1 | 2.4 | 2.4 | 6.0 | 7.2 | 1.7 | 4.6 | 4.2 | 2.7 | 2.5 | 3.7 | 4.6 | 2.0 | 2.7 | 5.0 | 2.7 |
| Compounds | Sediment (ng/g Dry wt) | Surface Water (ng/dm3) | ||
|---|---|---|---|---|
| Range | Mean | Range | Mean | |
| Butylate | <LoD | 0 | LoD-5.6 | 0.41 |
| Ethalfluralin | <LoD | 0 | <LoD | 0 |
| Trifluralin | <LoD | 0 | <LoD | 0 |
| Demeton-o | LoD-19.0 | 2.87 | LoD-17.8 | 3.46 |
| Diallate | LoD-4.9 | 0.26 | LoD-2.4 | 0.30 |
| Atrazine desethyl | LoD-13.9 | 0.65 | <LoD | 0 |
| Atrazina desisopropyl | LoD-40.4 | 2.35 | LoD-52.3 | 2.09 |
| Demeton-s | LoD-26.9 | 2.74 | LoD-1.1 | 0.04 |
| Diazinone | LoD-606.5 | 27.65 | LoD-10.6 | 0.42 |
| Atrazine | <LoD | 0 | LoD-14.5 | 2.15 |
| Simazine | <LoD | 0 | LoD-1.2 | 0.08 |
| Triallate | LoD-2.1 | 0.11 | <LoD | 0 |
| Disulfoton | LoD-2.0 | 0.18 | LoD-42.2 | 4.03 |
| Carbofuran | <LoD | 0 | <LoD | 0 |
| Dimethoate | LoD-15.2 | 0.70 | LoD-31.5 | 4.44 |
| 2.4.-D butylate | LoD-376.3 | 17.79 | LoD-16.2 | 2.95 |
| Acetochlor | LoD-28.1 | 2.53 | LoD-32.3 | 4.76 |
| Alachlor | LoD-64.9 | 7.72 | LoD-36.5 | 4.60 |
| Chlorpyrifos methyl | <LoD | 0 | LoD-5.9 | 0.39 |
| Paraoxon methyl | LoD-50.7 | 4.23 | LoD-19.7 | 0.79 |
| Chlorothalonil | LoD-6.3 | 0.58 | LoD-74 | 5.80 |
| Plifenate | <LoD | 0 | LoD-20.5 | 0.82 |
| Parathion methyl | <LoD | 0 | <LoD | 0 |
| Metribuzin | LoD-9.42 | 1.26 | LoD-1.2 | 0.10 |
| Metolachlor | LoD-67.1 | 3.05 | LoD-168 | 13.01 |
| Malathion | LoD-57.29 | 3.39 | LoD-26.7 | 5.74 |
| Chloropyrifos | LoD-30.7 | 5.01 | LoD-23.9 | 2.08 |
| Thiobencarb | LoD-8.3 | 0.72 | <LoD | 0.00 |
| Parathion | <LoD | 0 | <LoD | 0 |
| Butachlor | LoD-97.5 | 6.95 | LoD-7 | 1.60 |
| Endosulfan I | LoD-15.1 | 1.27 | LoD-187 | 70.64 |
| Isoprothiolane | LoD-59.2 | 5.26 | LoD-682 | 28.13 |
| Nitrofene | <LoD | 0 | LoD-214 | 15.11 |
| Ethion | LoD-16.6 | 2.48 | LoD-12.6 | 1.41 |
| Endosulfan II | LoD-118.7 | 10.17 | LoD-7.4 | 0.29 |
| Hoegrass | <LoD | 0 | LoD-104 | 22.43 |
| Endaven | <LoD | 0 | LoD-131 | 34.12 |
| Dicofol | LoD-158.6 | 18.07 | LoD-33.5 | 3.86 |
| Fenoxaprop-ethyl | LoD-2 | 0.09 | LoD-1.2 | 0.01 |
| Azinphos-methyl | LoD-27.7 | 1.25 | LoD-31.3 | 4.13 |
| Decamethrin | <LoD | 0 | LoD-30 | 1.20 |
| ∑CUPs | LoD-827.63 | 129.33 | 18.3–948.9 | 238.1 |
| ∑11OPPs | LoD-96.91 | 24.66 | LoD-184.1 | 35.3 |
| ∑13NHPs | LoD-203.03 | 27.60 | 6.3–735.7 | 128.7 |
| The LoD of CUPs is 1.01–61.5 ng/dm3 (water) and 47–734 pg/g (sediment), respectively. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Chi, J. Distributions of OCPs and CUPs in the Sediment and Surface Water Close to a Drinking Water Reservoir in Northeastern China. Separations 2023, 10, 312. https://doi.org/10.3390/separations10050312
Yu Y, Chi J. Distributions of OCPs and CUPs in the Sediment and Surface Water Close to a Drinking Water Reservoir in Northeastern China. Separations. 2023; 10(5):312. https://doi.org/10.3390/separations10050312
Chicago/Turabian StyleYu, Yingying, and Jialong Chi. 2023. "Distributions of OCPs and CUPs in the Sediment and Surface Water Close to a Drinking Water Reservoir in Northeastern China" Separations 10, no. 5: 312. https://doi.org/10.3390/separations10050312
APA StyleYu, Y., & Chi, J. (2023). Distributions of OCPs and CUPs in the Sediment and Surface Water Close to a Drinking Water Reservoir in Northeastern China. Separations, 10(5), 312. https://doi.org/10.3390/separations10050312





