Simple Preparation of a Unique Ionic Liquid/Deep Eutectic Solvent and β-Cyclodextrin Composite Discs and Its Use to Capture Hazardous Substances from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments
2.3. Preparation of IL and DES
2.4. Preparation of IL/DES@β-CD
2.5. Preparation of IL/DES@β-CD Discs
2.6. Removal of Hazardous Substances from Water
3. Results and Discussion
3.1. Structural Identification of the IL and DES
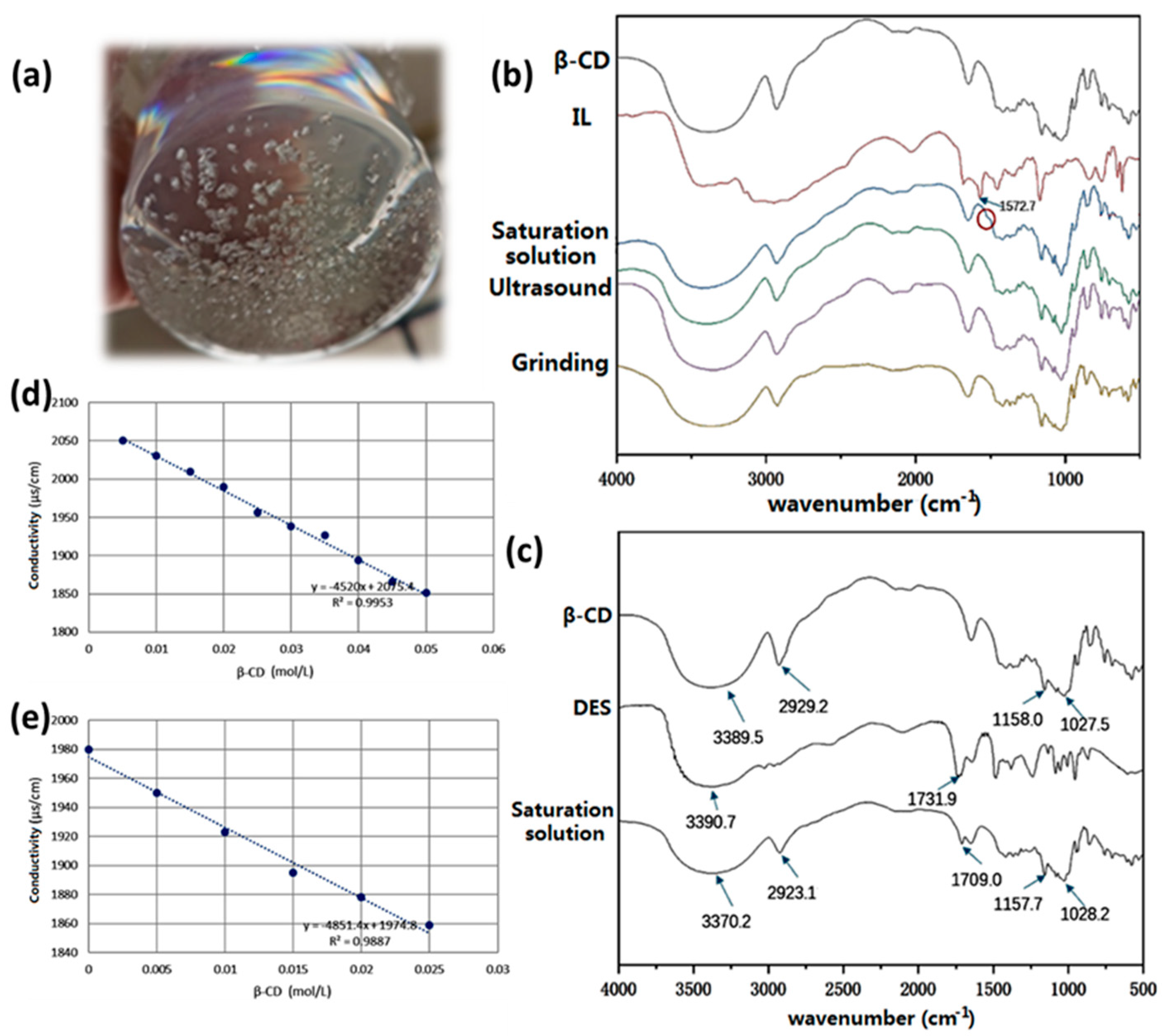
3.2. Loading of IL/DES on β-CD
3.3. Preparation of IL/DES@β-CD Sorbent Discs
3.4. Performance Validation of the Sorbent Discs to Capture Hazardous Substances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juni, F.; Bashir, M.J.K.; Haider Jaffari, Z.; Sethupathi, S.; Wong, J.W.C.; Zhao, J. Recent advancements in the treatment of emerging contaminants using activated persulfate oxidation process. Separations 2023, 10, 154. [Google Scholar] [CrossRef]
- Liu, X.; Lu, D.; Zhang, A.; Liu, Q.; Jiang, G. Data-driven machine learning in environmental pollution: Gains and problems. Environ. Sci. Technol. 2022, 56, 2124–2133. [Google Scholar] [CrossRef]
- Nitin, R.; Prabhat, K.S.; Nityanand, S.M. Pharmaceuticals in water as emerging pollutants for river health: A critical review under Indian conditions. Ecotoxicol. Environ. Saf. 2022, 247, 114220. [Google Scholar]
- Jaqueline, B.; João, H.Z.D.S. Chapter 3—Green Solvents for Remediation Technologies. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Rajender, B.I., Abdullah, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 23–30. [Google Scholar]
- Feng, X.; Song, H.; Zhang, T.; Yao, S.; Wang, Y. Magnetic Technologies and green solvents in extraction and separation of bioactive molecules together with biochemical objects: Current opportunities and challenges. Separations 2022, 9, 346. [Google Scholar] [CrossRef]
- Abu, H.N.; Abdul, S.N.; Siti, F.M.N.; Syafikah, H.P.; Muhammad, L.N.; Siti, M.N.H.; Rushdan, A.I.; Norzita, N.; Aznizam, A.B.; Zuliahani, A.; et al. Recent Advances in using adsorbent derived from agricultural waste for antibiotics and non-steroidal anti-inflammatory wastewater treatment: A review. Separations 2023, 10, 300. [Google Scholar]
- Kopac, T. Research progress on process-intensified water treatment applications. Separations 2022, 9, 353. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Luo, S.; Liu, S.; Li, J. Research advances on the applications of immobilized ionic liquids functional materials. Acta Chim. Sin. Chin. Ed. 2018, 76, 85–94. [Google Scholar] [CrossRef]
- Dowlatshah, S.; Saraji, M.; Pedersen-Bjergaard, S.; Ramos-Payán, M. Microfluidic liquid-phase microextraction based on natural deep eutectic solvents immobilized in agarose membranes. J. Chromatogr. A 2021, 1657, 462580. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, S.; Khameneh, E.S.; Hosseini, M.H.; Moharreri, M.M. A modified ionic liquid clay to remove heavy metals from water: Investigating its catalytic activity. Int. J. Environ. Sci. Technol. 2019, 17, 2043–2058. [Google Scholar] [CrossRef] [Green Version]
- Yohannes, A.; Li, J.; Yao, S. Various metal organic frameworks combined with imidazolium, quinolinum and benzothiazolium ionic liquids for removal of three antibiotics from water. J. Mol. Liq. 2020, 318, 114304. [Google Scholar] [CrossRef]
- Fan, T.; Yan, Z.; Yang, C.; Qiu, S.; Peng, X.; Zhang, J.; Hu, L.; Chen, L. Preparation of menthol-based hydrophobic deep eutectic solvents for the extraction of triphenylmethane dyes: Quantitative properties and extraction mechanism. Analyst 2021, 146, 1996–2008. [Google Scholar] [CrossRef]
- Phosiri, P.; Burakham, R. Deep eutectic solvent-modified mixed iron hydroxide–silica: Application in magnetic solid-phase extraction for enrichment of organochlorine pesticides prior to GC-MS analysis. J. Sep. Sci. 2022, 44, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Liu, Y.; Chen, D.; Chen, Z.; Chunan, M.A. Electrochemical reduction of CO2 in [NH2-emim]Br/[Bmim]BF4 ionic liquid composite. CIESC J. 2017, 68, 2027–2034. [Google Scholar]
- Sethi, O.; Singh, M.; Kang, T.S.; Sood, A.K. Volumetric and compressibility studies on aqueous mixtures of deep eutectic solvents based on choline chloride and carboxylic acids at different temperatures: Experimental, theoretical and computational approach. J. Mol. Liq. 2021, 340, 117212. [Google Scholar] [CrossRef]
- Das, K.; Datta, B.; Rajbanshi, B.; Roy, M.N. Evidences for inclusion and encapsulation of an ionic liquid with β-CD and 18-C-6 in aqueous environments by physicochemical investigation. J. Phys. Chem. B 2018, 122, 1679. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lee, Y.R.; Row, K.H. Synthesis of mesoporous siliceous materials in choline chloride deep eutectic solvents and the application of these materials to high-performance size exclusion chromatography. Chromatographia 2016, 79, 375–382. [Google Scholar] [CrossRef]
- Pedro, A.Q.; Coutinho, J.A.P.; Freire, M.G. Immobilization of Ionic Liquids, Types of Materials, and Applications. In Encyclopedia of Ionic Liquids; Zhang, S., Ed.; Springer: Singapore, 2022. [Google Scholar]
- Shah, R.V.; Pandey, A.K.; Kumar, S.J.; Paul, S.; Rao, R.M.; Jaison, P.G. One step sample treatment and loading using a deep eutectic solvent immobilized in a porous substrate for thermal ionization mass spectrometry of Pu(IV) ions. J. Anal. Atom. Spectrom. 2020, 35, 2315–2321. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, Y.; Luo, X.; Qin, H.; Cao, S. FTIR spectral analysis of ionic liquids 1-buty 1-3-methylimidazolium bromine synthesis. Adv. Meas. Lab. Manag. 2007, 2, 13–14. [Google Scholar]
- Bratu, I.; Veiga, F.; Fernandes, C.; Hernanz, A.; Gavira, J.M. Infrared spectroscopic study of triacetyl β-cyclodextrin and its inclusion complex with nicardipine. Spectroscopy 2015, 18, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Feng, X.; Yao, S. Ionic liquid-multi walled carbon nanotubes composite tablet for continuous adsorption of tetracyclines and heavy metals. J. Clean. Prod. 2020, 286, 124937. [Google Scholar] [CrossRef]
- Marten, K.; Jian, X.W.; Jukka, R.; Jens, M.C.; Thomas, R.; Claudia, S.L. Multispectral UV imaging for fast and non-destructive quality control of chemical and physical tablet attributes. Eur. J. Pharm. Sci. 2016, 90, 85–95. [Google Scholar]
- Park, G.; Lee, J.H.; Kim, I.S.; Cho, J. Pharmaceutical rejection by membranes for wastewater reclamation and reuse. Water Sci. Technol. 2004, 502, 239–244. [Google Scholar] [CrossRef]
- Dietzel, B.M. Novel green technology for wastewater treatment: Geo-material/geopolymer applications for heavy metal removal from aquatic media. Int. J. Sediment Res. 2023, 38, 33–48. [Google Scholar]
- Zhang, T.; Jiang, W.; Cao, Y.; Zhu, C.; Toukouki, S.; Yao, S. A facile one-pot synthesis of ionic liquid@porous organic frameworks for rapid high-capacity removal of heavy metal ions, pesticides and aflatoxin from two non-food bioactive products. Ind. Crops Prod. 2022, 181, 114859. [Google Scholar] [CrossRef]
- Lee, S.P.; Ali, G.; Algarni, H.; Chong, K.F. Flake size-dependent adsorption of graphene oxide aerogel. J. Mol. Liq. 2019, 277, 175–180. [Google Scholar] [CrossRef]
- Wang, X.; Ying, C. UV-Vis detection-reversed- phase high performance liquid chromatography determination of basic red 1:1. Text. Aux. 1998, 5, 35–37. [Google Scholar]
- Kopsidas, O. Pigment adsorption optimization in various low cost adsorbents. J. Environ. Sci. Eng. A 2021, 10, 20. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Kozonoi, N.; Ikeda, Y. Extraction mechanism of metal ion from aqueous solution to the hydrophobic ionic liquid, 1-butyl-3-methylimidazolium nonafluorobutanesulfonate. Monatshefte Chemie-Chem. Mon. 2007, 138, 1145–1151. [Google Scholar] [CrossRef]
- Li, Z.; Tang, C.; Hu, S.; Wang, C. Preparation and dye-adsorption of ionic liquid loading molecular sieve. Chin. J. Environ. Eng. 2014, 8, 3848–3852. [Google Scholar]
- Lakshmi, D.S.; Santoro, S.; Avruscio, E.; Tagarelli, A.; Figoli, A. Preparation of polymer inclusion membranes (PIMs) with ionic liquid and its application in dye adsorption process supported by statistical analysis. Int. J. Membr. Sci. Technol. 2015, 2, 65–77. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Zhai, X.; Liu, Q.; Sun, L.; Liu, M.; An, L.; Xian, L.; Zhang, P.; Chen, L. Study on adsorption performance of benzoic acid in cyclocarya paliurus extract by ethyl cellulose microspheres. Chemistry 2021, 3, 1113–1125. [Google Scholar] [CrossRef]
- Wang, T.; Li, B.; Feng, Y.; Guo, Q. Preparation, quantitive analysis and bacteriostasis of solid state iodine inclusion complex with β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 255–262. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, N.; Chen, H.; Ali, A.; Toufouki, S.; Yao, S. Simple Preparation of a Unique Ionic Liquid/Deep Eutectic Solvent and β-Cyclodextrin Composite Discs and Its Use to Capture Hazardous Substances from Water. Separations 2023, 10, 357. https://doi.org/10.3390/separations10060357
Guo Y, Liu N, Chen H, Ali A, Toufouki S, Yao S. Simple Preparation of a Unique Ionic Liquid/Deep Eutectic Solvent and β-Cyclodextrin Composite Discs and Its Use to Capture Hazardous Substances from Water. Separations. 2023; 10(6):357. https://doi.org/10.3390/separations10060357
Chicago/Turabian StyleGuo, Yingying, Na Liu, Hangping Chen, Ahmad Ali, Sara Toufouki, and Shun Yao. 2023. "Simple Preparation of a Unique Ionic Liquid/Deep Eutectic Solvent and β-Cyclodextrin Composite Discs and Its Use to Capture Hazardous Substances from Water" Separations 10, no. 6: 357. https://doi.org/10.3390/separations10060357
APA StyleGuo, Y., Liu, N., Chen, H., Ali, A., Toufouki, S., & Yao, S. (2023). Simple Preparation of a Unique Ionic Liquid/Deep Eutectic Solvent and β-Cyclodextrin Composite Discs and Its Use to Capture Hazardous Substances from Water. Separations, 10(6), 357. https://doi.org/10.3390/separations10060357






