Selective Aqueous Extraction and Green Spectral Analysis of Furfural as an Aging Indicator in Power Transformer Insulating Fluid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
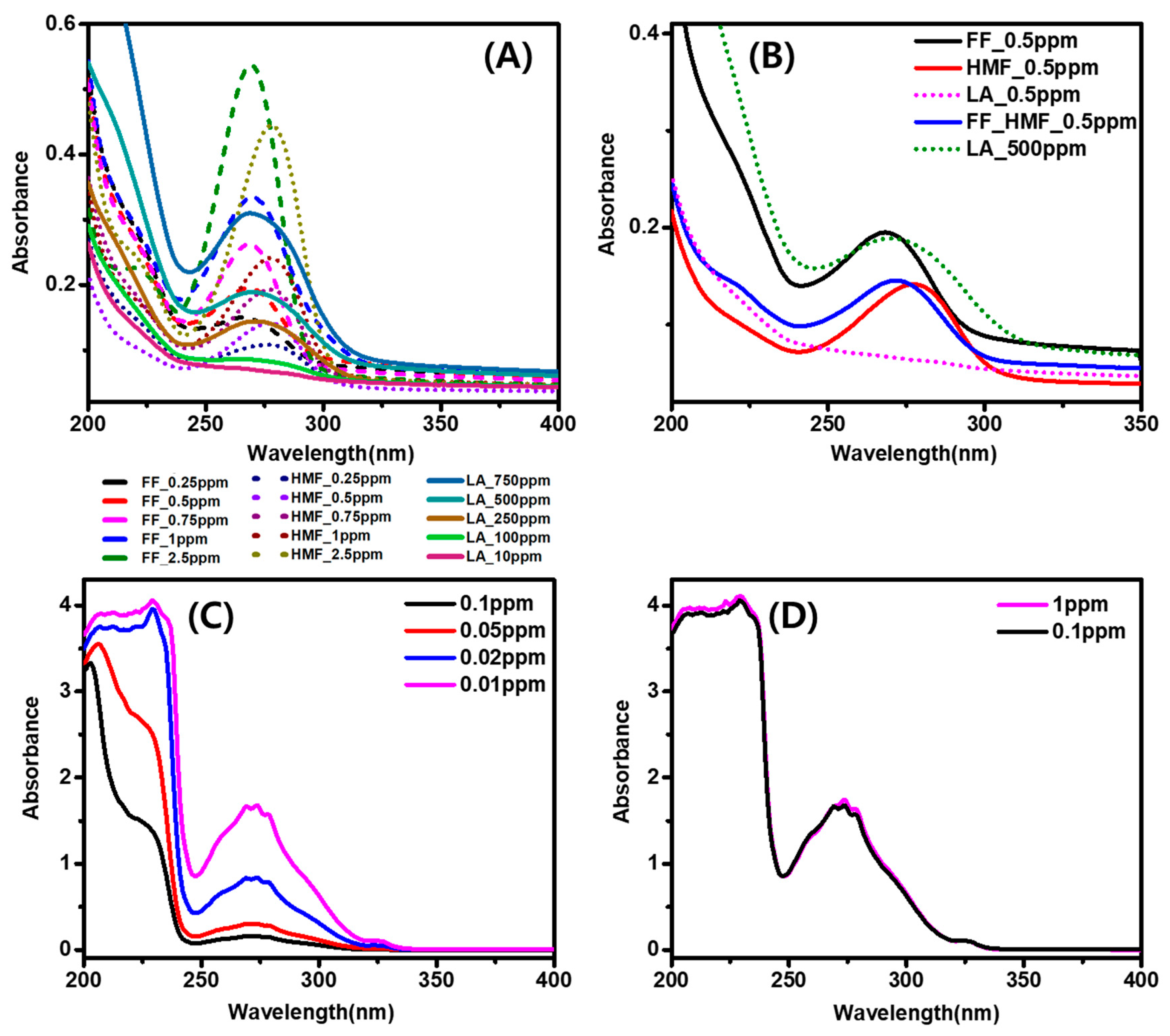
2.2. UV Absorption Features of Furfural, 5-Hydroxyfurfural (HMF), and Levulinic Acid (LA)
2.3. Preparation, Extraction, and Analysis of Furfural Standard Samples in Model Transformer Insulating Fluid
2.4. Aqueous Extraction and Analysis of Furfural in Insulating Fluids Collected from Operating Power Transformers
2.5. Comparison and Validation of Developed Analysis Methods with (1) Conventional HPLC (ASTM), (2) Aqueous Extraction and HPLC, and (3) Aqueous Extraction and Aniline-Acetate Colorimetric Analysis
3. Results
3.1. General Physical and UV Absorption Features of Furfural, 5-Hydroxyfurfural (HMF), and Levulinic Acid (LA)
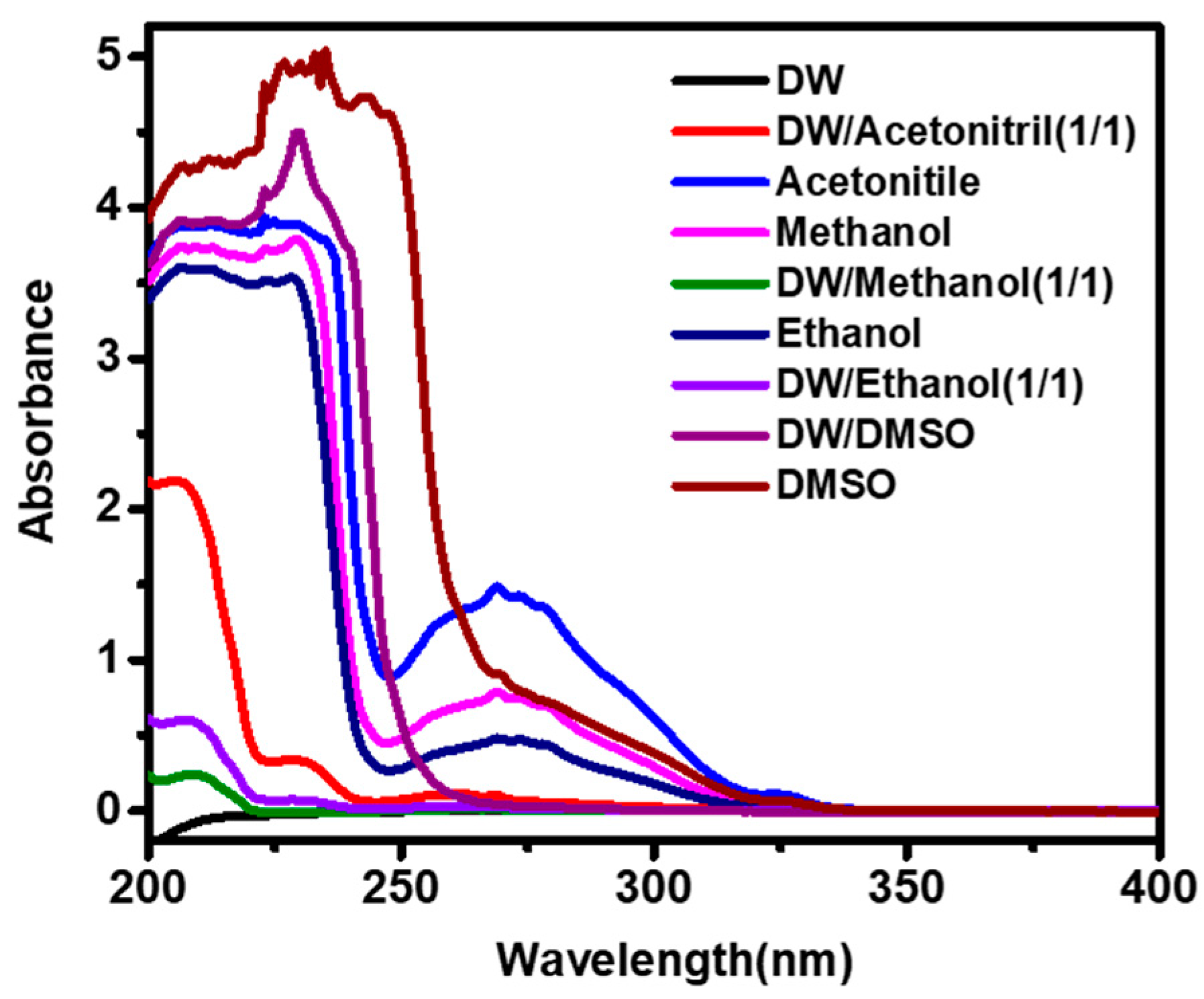
3.2. Effect and Selection of Solvent on the Extraction of Furfural from Transformer Insulating Fluid for UV–Vis Spectroscopy
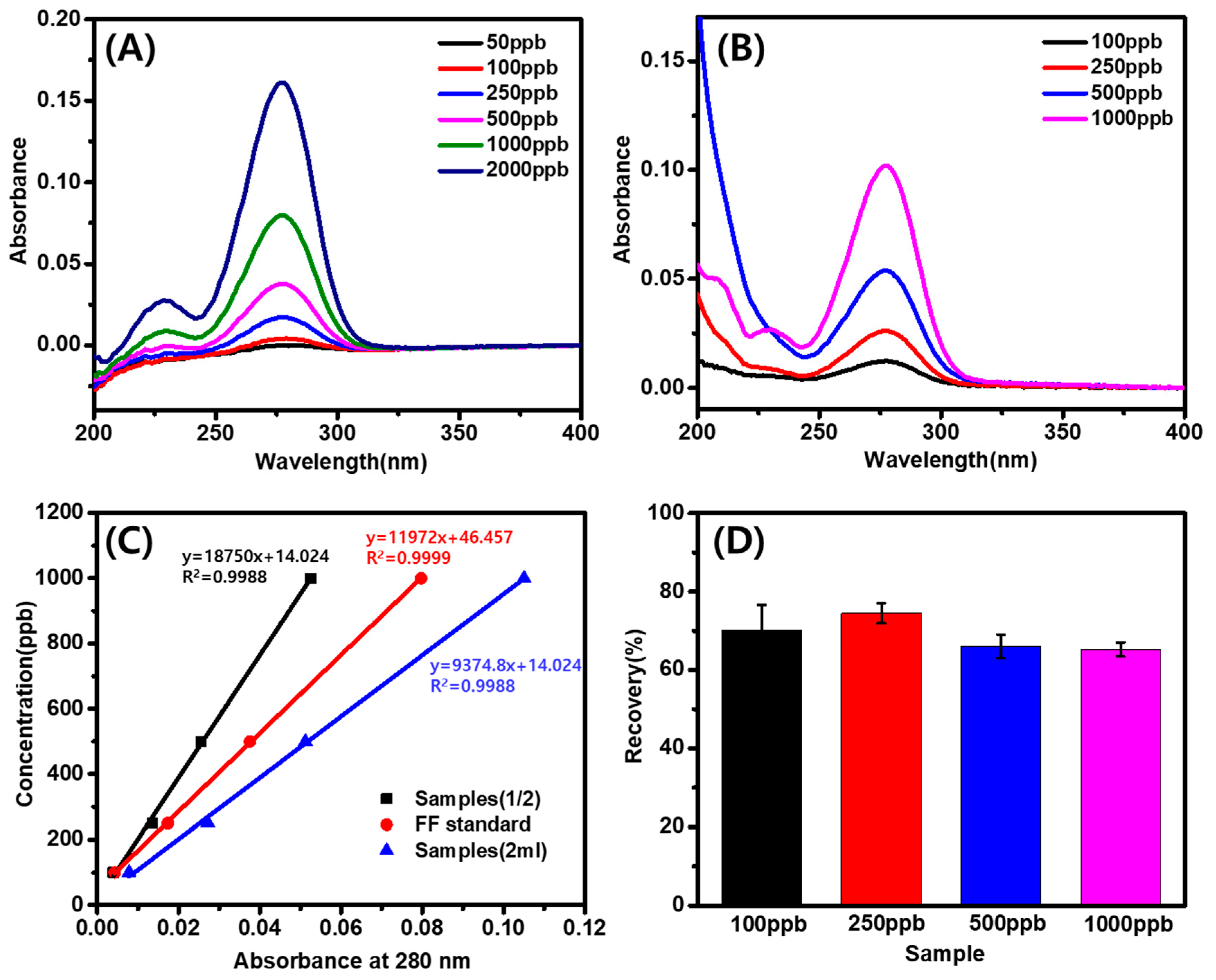
3.3. Recovery and Correlation of Furfural at Various Concentrations in Aqueous Extraction
3.4. Employment of Analysis Method on Samples from Running Power Transformer Fluid
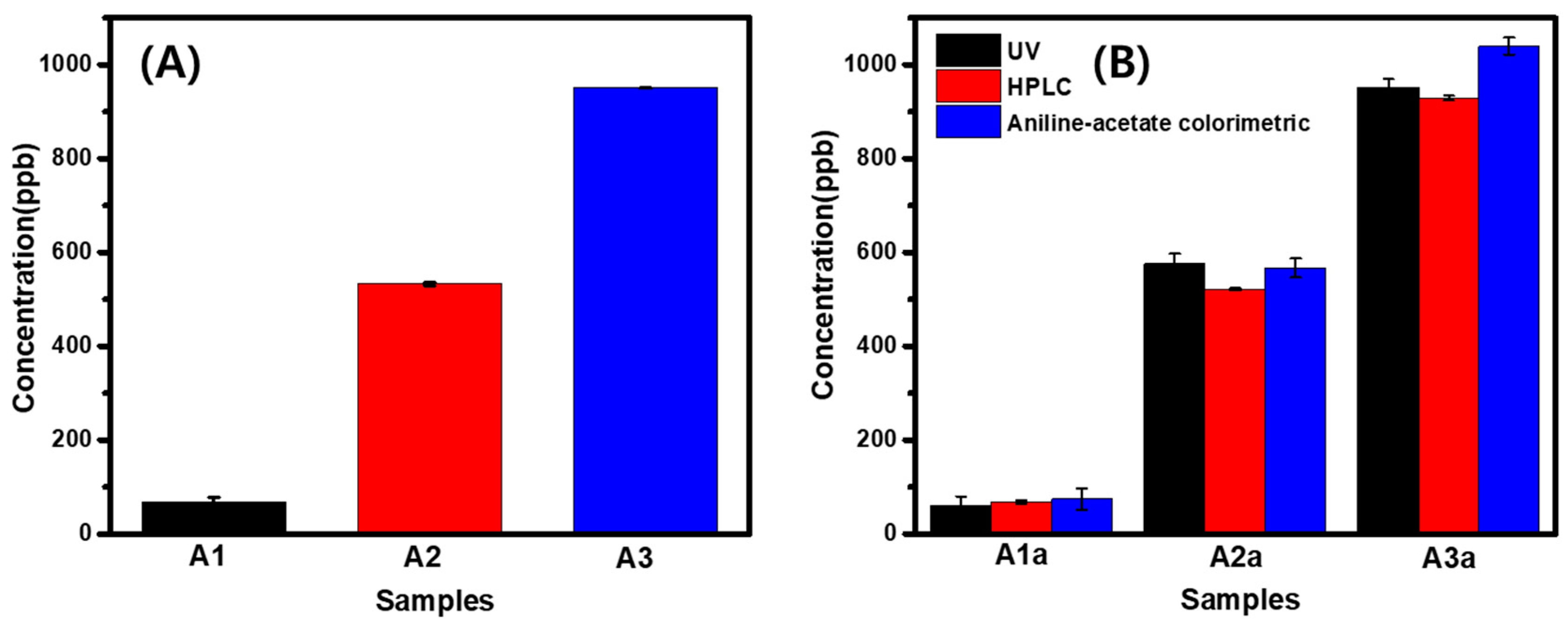
3.5. Verification of Developed Analysis Method by Comparative Investigation Using HPLC and Colorimetric Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghoneim, S.S.M. Determination of Transformers’ Insulating Paper State Based on Classification Techniques. Processes 2021, 9, 427. [Google Scholar] [CrossRef]
- Kaliappan, G.; Rengaraj, M. Aging Assessment of Transformer Solid Insulation: A Review. Mater. Today Proc. 2021, 47, 272–277. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Oparanti, S.O.; Fofana, I. Performance Assessment of Cellulose Paper Impregnated in Nanofluid for Power Transformer Insulation Application: A Review. Energies 2023, 16, 2002. [Google Scholar] [CrossRef]
- Farhan Naeem, M.; Hashmi, K.; Abdul Rahman Kashif, S.; Shahzeb Khan, M.; Alghaythi, M.L.; Aymen, F.; Ali, S.G.; AboRas, K.M.; Ben Dhaou, I. A Novel Method for Life Estimation of Power Transformers Using Fuzzy Logic Systems: An Intelligent Predictive Maintenance Approach. Front. Energy Res. 2022, 10, 977665. [Google Scholar] [CrossRef]
- Chandran, L.R.; Babu, G.A.; Nair, M.G.; Ilango, K. A Review on Status Monitoring Techniques of Transformer and a Case Study on Loss of Life Calculation of Distribution Transformers. Mater. Today Proc. 2021, 46, 4659–4666. [Google Scholar] [CrossRef]
- Li, S.; Ge, Z.; Abu-Siada, A.; Yang, L.; Li, S.; Wakimoto, K. A New Technique to Estimate the Degree of Polymerization of Insulation Paper Using Multiple Aging Parameters of Transformer Oil. IEEE Access 2019, 7, 157471–157479. [Google Scholar] [CrossRef]
- Ghoneim, S.S.M. The Degree of Polymerization in a Prediction Model of Insulating Paper and the Remaining Life of Power Transformers. Energies 2021, 14, 670. [Google Scholar] [CrossRef]
- Zhang, E.; Liu, J.; Zhang, C.; Zheng, P.; Nakanishi, Y.; Wu, T. State-of-Art Review on Chemical Indicators for Monitoring the Aging Status of Oil-Immersed Transformer Paper Insulation. Energies 2023, 16, 1396. [Google Scholar] [CrossRef]
- Thiviyanathan, V.A.; Ker, P.J.; Leong, Y.S.; Abdullah, F.; Ismail, A.; Jamaludin, Z. Power Transformer Insulation System: A Review on the Reactions, Fault Detection, Challenges and Future Prospects. Alex. Eng. J. 2022, 61, 7697–7713. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Le, T.T.; Darveniza, M.; Saha, T. A Study of Degradation of Cellulosic Insulation Materials in a Power Transformer, Part 1. Molecular Weight Study of Cellulose Insulation Paper. Polym. Degrad. Stab. 1995, 48, 79–87. [Google Scholar] [CrossRef]
- Teymouri, A.; Vahidi, B. Power Transformer Cellulosic Insulation Destruction Assessment Using a Calculated Index Composed of CO, CO2, 2-Furfural, and Acetylene. Cellulose 2020, 28, 489–502. [Google Scholar] [CrossRef]
- Łojewski, T.; Sawoszczuk, T.; Łagan, J.M.; Zięba, K.; Łojewska, J. Furfural as a Marker of Cellulose Degradation. A Quantitative Approach. Appl. Phys. A Mater. Sci. Process. 2010, 100, 873–884. [Google Scholar] [CrossRef]
- Srivastava, R.; Bousquières, J.; Cepeda-Vázquez, M.; Roux, S.; Bonazzi, C.; Rega, B. Kinetic Study of Furan and Furfural Generation during Baking of Cake Models. Food Chem. 2018, 267, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Rannou, C.; Laroque, D.; Renault, E.; Prost, C.; Sérot, T. Mitigation Strategies of Acrylamide, Furans, Heterocyclic Amines and Browning during the Maillard Reaction in Foods. Food Res. Int. 2016, 90, 154–176. [Google Scholar] [CrossRef]
- Rigling, M.; Höckmeier, L.; Leible, M.; Herrmann, K.; Gibis, M.; Weiss, J.; Zhang, Y. Characterization of the Aroma Profile of Food Smoke at Controllable Pyrolysis Temperatures. Separations 2023, 10, 176. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, N.H.; Nam, C.H. Simple Test Method for Furan in Aged Transformer Oil and its kit. KR patent KR101231586, 4 February 2013. [Google Scholar]
- ASTM D5837-99 (reapproved2005); Standard Test Method for Furanic Compounds in Electrical Insulating Liquids by High-Performance Liquid Chromatography (HPLC). ASTM International: Conshohocken, PA, USA, 2005.
- Nezami, M.; Wani, S.A.; Khan, S.A.; Khera, N.; Sohail, S. An MIP-Based Novel Capacitive Sensor to Detect 2-FAL Concentration in Transformer Oil. IEEE Sens. J. 2018, 18, 7924–7931. [Google Scholar] [CrossRef]
- Rico-Yuste, A.; González-Vallejo, V.; Benito-Peña, E.; de las Casas Engel, T.; Orellana, G.; Moreno-Bondi, M.C. Furfural Determination with Disposable Polymer Films and Smartphone-Based Colorimetry for Beer Freshness Assessment. Anal. Chem. 2016, 88, 3959–3966. [Google Scholar] [CrossRef]
- Xing, Q.; Ma, Y.; Fu, X.; Cao, Q.; Zhang, Y.; You, C. Effects of heat treatment, homogenization pressure, and overprocessing on the content of furfural compounds in liquid milk. J. Sci. Food Agric. 2020, 100, 5276–5282. [Google Scholar] [CrossRef]
- Kishikawa, N.; El-Maghrabey, M.H.; Kuroda, N. Chromatographic Methods and Sample Pretreatment Techniques for Aldehydes Determination in Biological, Food, and Environmental Samples. J. Pharm. Biomed. Anal. Open 2019, 175, 112782. [Google Scholar] [CrossRef]
- N’cho, J.; Fofana, I.; Hadjadj, Y.; Beroual, A. Review of Physicochemical-Based Diagnostic Techniques for Assessing Insulation Condition in Aged Transformers. Energies 2016, 9, 367. [Google Scholar] [CrossRef]
- Lin, M.; Lin, K.; Lin, A.; Gras, R.; Luong, J. Ultra-Trace Analysis of Furanic Compounds in Transformer/Rectifier Oils with Water Extraction and High-Performance Liquid Chromatography. J. Sep. Sci. 2016, 39, 2777–2784. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical Methods Used in the Quality Control of Honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.S.; Ker, P.J.; Jamaludin, M.Z.; Nomanbhay, S.M.; Ismail, A.; Abdullah, F.; Looe, H.M.; Lo, C.K. UV-Vis Spectroscopy: A New Approach for Assessing the Color Index of Transformer Insulating Oil. Sensors 2018, 18, 2175. [Google Scholar] [CrossRef]
- Alshehawy, A.M.; Mansour, D.-E.A.; Ghali, M.; Lehtonen, M.; Darwish, M.M.F. Photoluminescence Spectroscopy Measurements for Effective Condition Assessment of Transformer Insulating Oil. Processes 2021, 9, 732. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Wang, J.; Yu, H.; He, Y. Cascade Chromogenic System with Exponential Signal Amplification for Visual Colorimetric Detection of Acetone. Anal. Chem. 2020, 92, 6548–6554. [Google Scholar] [CrossRef]
- Li, Z.; Fang, M.; LaGasse, M.K.; Askim, J.R.; Suslick, K.S. Colorimetric Recognition of Aldehydes and Ketones. Angew. Chem. Int. Ed. 2017, 56, 9860–9863. [Google Scholar] [CrossRef]
- Feng, L.; Musto, C.J.; Suslick, K.S. A Simple and Highly Sensitive Colorimetric Detection Method for Gaseous Formaldehyde. J. Am. Chem. Soc. 2010, 132, 4046–4047. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, Properties and Catalytic Hydrogenation of Furfural to Fuel Additives and Value-Added Chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Jakob, A.; Grilc, M.; Teržan, J.; Likozar, B. Solubility Temperature Dependence of Bio-Based Levulinic Acid, Furfural, and Hydroxymethylfurfural in Water, Nonpolar, Polar Aprotic and Protic Solvents. Processes 2021, 9, 924. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, R.; Tao, F.; Wei, C. Effects of Moisture on Furfural Partitioning in Oil-Paper Insulation System and Aging Assessment of Power Transformers. Electr. Power Compon. Syst. 2019, 47, 192–199. [Google Scholar] [CrossRef]
- Feng, D.; Yang, L.; Zhou, L.; Liao, R.; Chen, X. Effect of Oil–Paper–Pressboard Mass Ratio on Furfural Content in Transformer Oil. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1308–1315. [Google Scholar] [CrossRef]
- Cepeda-Vázquez, M.; Blumenthal, D.; Camel, V.; Rega, B. Multivariate Optimization of Headspace Trap for Furan and Furfural Simultaneous Determination in Sponge Cake. Talanta 2017, 164, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.I.; Rossini, E.L.; Castoldi, K.; Pezza, L.; Pezza, H.R. Paper Platform for Reflectometric Determination of Furfural and Hydroxymethylfurfural in Sugarcane Liquor. Microchem. J. 2017, 133, 286–292. [Google Scholar] [CrossRef]



| UV–Vis Spectral Analysis | HPLC Method | Aniline-Acetate Colorimetric Analysis | ||||
|---|---|---|---|---|---|---|
| UV Absorbance | Furfural Concentration (ppb) | HPLC Intensity | Furfural Concentration (ppb) | UV Absorbance | Furfural Concentration (ppb) | |
| A1a | 0.005 | 60.9 | 0.0022 | 67.7 | 0.084 | 74.2 |
| A2a | 0.060 | 576.5 | 0.0164 | 521.0 | 0.220 | 566.8 |
| A3a | 0.101 | 951.5 | 0.0286 | 929.5 | 0.350 | 1039.8 |
| Peak Area | Furfural Concentration (ppb) | |
|---|---|---|
| A1 | 4041 | 69.2 |
| A2 | 31,107 | 532.6 |
| A3 | 55,497 | 950.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kim, E.; Kwak, B.S.; Jun, T.; Kawano, R.; Pyo, S.-H. Selective Aqueous Extraction and Green Spectral Analysis of Furfural as an Aging Indicator in Power Transformer Insulating Fluid. Separations 2023, 10, 381. https://doi.org/10.3390/separations10070381
Park H, Kim E, Kwak BS, Jun T, Kawano R, Pyo S-H. Selective Aqueous Extraction and Green Spectral Analysis of Furfural as an Aging Indicator in Power Transformer Insulating Fluid. Separations. 2023; 10(7):381. https://doi.org/10.3390/separations10070381
Chicago/Turabian StylePark, Hyunjoo, Eunyoung Kim, Byeong Sub Kwak, Taehyun Jun, Riko Kawano, and Sang-Hyun Pyo. 2023. "Selective Aqueous Extraction and Green Spectral Analysis of Furfural as an Aging Indicator in Power Transformer Insulating Fluid" Separations 10, no. 7: 381. https://doi.org/10.3390/separations10070381
APA StylePark, H., Kim, E., Kwak, B. S., Jun, T., Kawano, R., & Pyo, S.-H. (2023). Selective Aqueous Extraction and Green Spectral Analysis of Furfural as an Aging Indicator in Power Transformer Insulating Fluid. Separations, 10(7), 381. https://doi.org/10.3390/separations10070381







