Impact of Cumin (Cuminum cyminum) Incorporation on the Generation of Heterocyclic Aromatic Amines in Meatballs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Method
2.2.1. Cooking Conditions
2.2.2. Determination of the Water Content
2.2.3. Determination of Cooking Loss
2.2.4. Determination of pH Value
2.2.5. Determination of TBARS
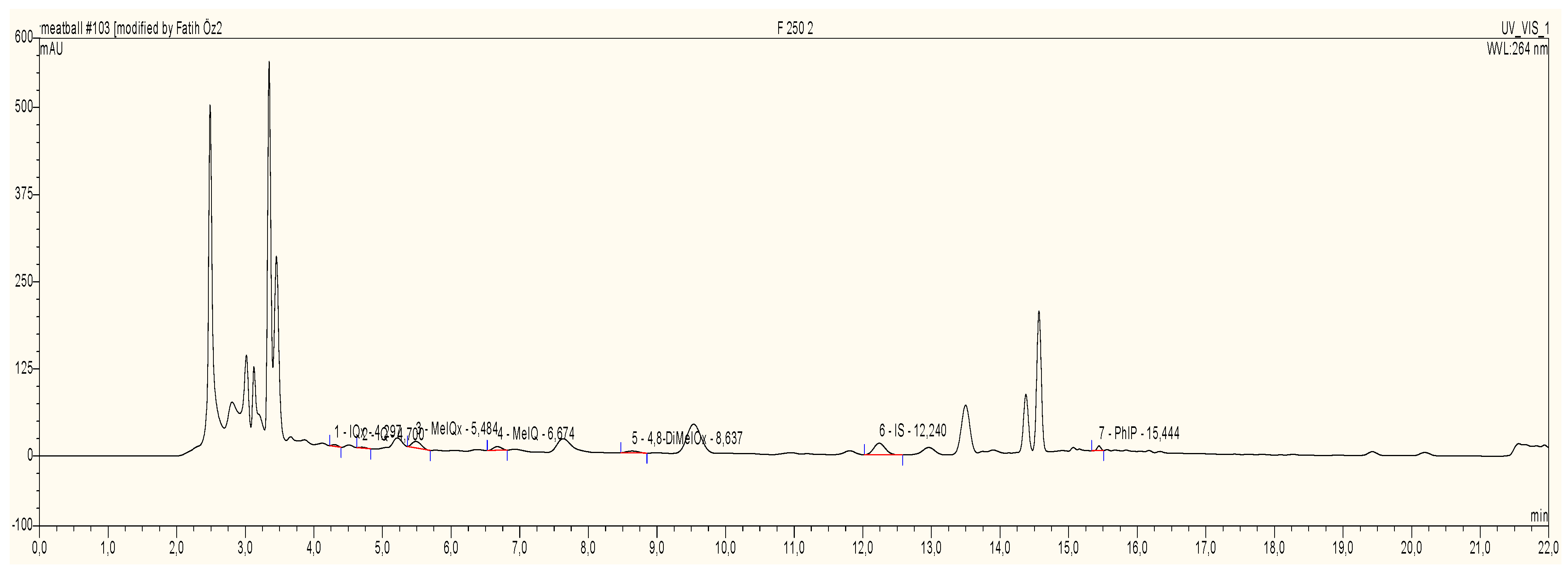
2.2.6. Determination of HAAs
2.2.7. Statistical Analysis
3. Results
3.1. Water Content, pH, and TBARS Results of Meat Muscle, Intermuscular Fat, and Meatball Dough
3.2. Water Percentage, pH, TBARS, Cooking Loss, and HAA Results of All Meatball Samples
3.2.1. Water Percentage in Meatballs
3.2.2. Cooking Loss Results of the Meatballs
3.2.3. pH Values of the Meatballs
3.2.4. TBARS Values of the Meatballs
3.2.5. Method Validation of HAAs
3.2.6. Total HAAs of the Meatballs
3.2.7. HAA Content of Meatballs
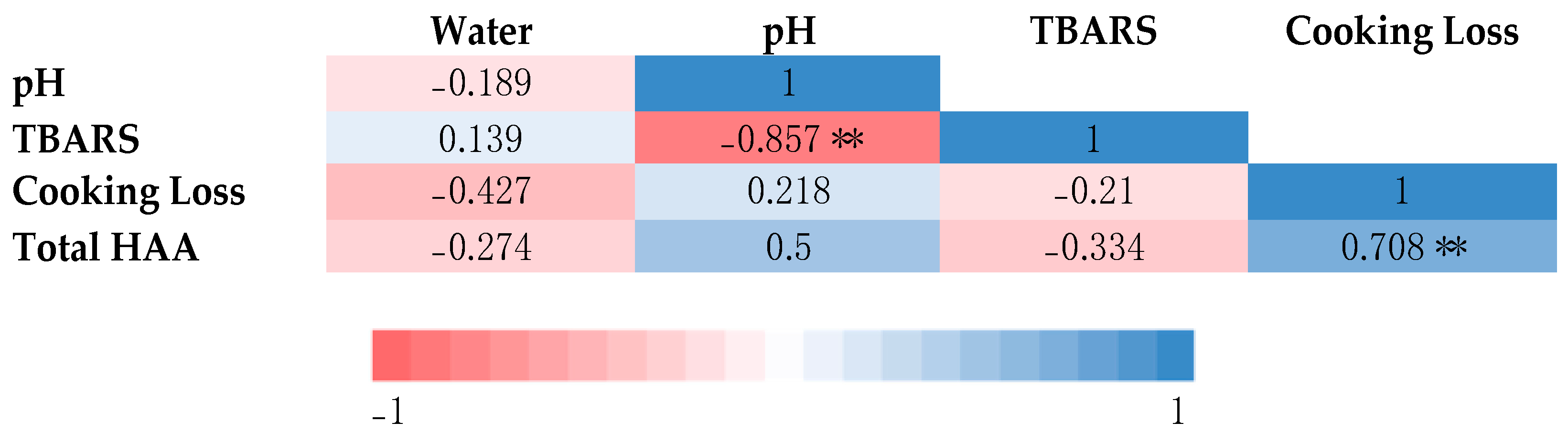
3.2.8. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oz, E. The presence of polycyclic aromatic hydrocarbons and heterocyclic aromatic amines in barbecued meatballs formulated with different animal fats. Food Chem. 2021, 352, 129378. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Alam, M.S.; Monir, M.M.; Ahmed, K. Comprehensive effects of black cumin (Nigella sativa) and synthetic antioxidant on sensory and physicochemical quality of beef patties during refrigerant storage. J. Agric. Food Res. 2021, 4, 100145. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Sultana, S.; Ripa, F.A.; Hamid, K. Comparative antioxidant activity study of some commonly used spices in Bangladesh. Pak. J. Biol. Sci. 2010, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Dua, A.; Gupta, S.K.; Mittal, A.; Mahajan, R. A study of antioxidant properties and antioxidant compounds of cumin (Cuminum cyminum). Int. J. Pharm. Biol. Arch. 2012, 3, 1110–1116. [Google Scholar]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants–A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Ani, V.; Varadaraj, M.C.; Naidu, K.A. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). Eur. Food Res. Technol. 2006, 224, 109–115. [Google Scholar] [CrossRef]
- Allahghadri, T.; Rasooli, I.; Owlia, P.; Nadooshan, M.J.; Ghazanfari, T.; Taghizadeh, M.; Astaneh, S.D.A. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J. Food Sci. 2010, 75, 54–61. [Google Scholar] [CrossRef]
- Devranbay, Ş. Kekikli Kimyonlu Beyaz Peynir Üretimi. Master’s Thesis, Kahramanmaraş Sütçü İmam University, Kahramanmaraş, Türkiye, 2016. [Google Scholar]
- Berktaş, Ö.A. Kimyon, Tarçın ve Sumak Gibi Baharatların Ratlarda Antioksidan ve Antiülserojenik Özelliklerinin Belirlenmesi ve Biyokimyasal Olarak Incelenmesi. Ph.D. Thesis, Atatürk University, Erzurum, Turkey, 2017. [Google Scholar]
- Başer, K.H.C. Kimyon (Cuminum cyminum L.). Bağbahçe Derg. 2014, 55, 26–27. [Google Scholar]
- Oz, F.; Kızıl, M.; Zaman, A.; Turhan, S. The effects of direct addition of low and medium molecular weight chitosan on the formation of heterocyclic aromatic amines in beef chop. LWT-Food Sci. Technol. 2016, 65, 861–867. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Formation of heterocyclic aromatic amines with the structure of aminoimidazoazarenes in food products. Food Chem. 2020, 313, 126128. [Google Scholar] [CrossRef] [PubMed]
- Chevolleau, S.; Bouville, A.; Debrauwer, L. Development and validation of a modified QuEChERS protocol coupled to UHPLC-APCI-MS/MS for the simple and rapid quantification of 16 heterocyclic aromatic amines in cooked beef. Food Chem. 2020, 316, 126327. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yim, D.G.; Kim, O.Y.; Kang, H.J.; Kim, H.S.; Jang, A.; Park, T.S.; Jin, S.K.; Hur, S.J. Overview of the effect of natural products on reduction of potential carcinogenic substances in meat products. Trends Food Sci. Technol. 2020, 99, 568–579. [Google Scholar] [CrossRef]
- Oz, E.; Oz, F. Mutagenic and/or carcinogenic compounds in meat and meat products: Heterocyclic aromatic amines perspective. Theory Pract. Meat Process. 2022, 7, 112–117. [Google Scholar] [CrossRef]
- Sugimura, T.; Adamson, R.H. Introduction. In Food Borne Carcinogens; Nagao, M., Sugimura, T., Eds.; JohnWiley & Sons Ltd.: West Sussex, UK, 2000; pp. 1–5. [Google Scholar]
- Zamora, R.; Hidalgo, F.J. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]-pyridine (PhIP) formation and fate: An example of the coordinate contribution of lipid oxidation and Maillard reaction to the production and elimination of processing-related food toxicants. R. Soc. Chem. 2015, 5, 9709–9721. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, S.Y.; Kang, J.H.; Kim, J.H.; Kim, H.W.; Oh, D.H.; Jeong, J.W.; Hur, S.J. Main mechanisms for carcinogenic heterocyclic amine reduction in cooked meat by natural materials. Meat Sci. 2022, 183, 108663. [Google Scholar] [CrossRef] [PubMed]
- Skog, K.I.; Johansson, M.A.E.; Jägerstad, M.I. Carcinogenic heterocyclic amines in model systems and cooked foods: A Review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Khan, I.A.; Khan, A.; Zou, Y.; Zongshuai, Z.; Xu, W.; Wang, D.; Huang, M. Heterocyclic amines in cooked meat products, shortcomings during evaluation, factors influencing formation, risk assessment and mitigation strategies. Meat Sci. 2022, 184, 108693. [Google Scholar] [CrossRef]
- Barzegar, F.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Hamzalioglu, A.; Gokmen, V.; Kizil, M. Inhibitory effect of hawthorn extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Food Res. Int. 2017, 99, 586–595. [Google Scholar] [CrossRef]
- Savaş, A.; Oz, E.; Oz, F. Is oven bag really advantageous in terms of heterocyclic aromatic amines and bisphenol-A? Chicken meat perspective. Food Chem. 2021, 355, 129646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Xia, X.; Xu, M.; Kong, B.; Liu, Q. Comparative study on the formation of heterocyclic aromatic amines in different sugar smoking time. Food Control 2021, 124, 107905. [Google Scholar] [CrossRef]
- Elbir, Z.; Ekiz, E.; Aoudeh, E.; Oz, E.; Savaş, A.; Brennan, C.; Proestos, C.; Khan, M.R.; Elobeid, T.; Brennan, M.; et al. Enhancing effect of chia seeds on heterocyclic amine generation in meatball. Int. J. Food Sci. Technol. 2023, 58, 2560–2572. [Google Scholar] [CrossRef]
- Jinap, S.; Iqbal, S.Z.; Selvam, R.M. Effect of selected local spices marinades on the reduction of heterocyclic amines in grilled beef (satay). LWT-Food Sci. Technol. 2015, 63, 919–926. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Kilic, S.; Oz, E.; Oz, F. Effect of turmeric on the reduction of heterocyclic aromatic amines and quality of chicken meatballs. Food Control 2021, 128, 108189. [Google Scholar] [CrossRef]
- Savaş, A.; Ekiz, E.; Elbir, Z.; Savaş, B.D.; Proestos, C.; Elobeid, T.; Khan, M.R.; Oz, F. Advantageous Effects of Sumac Usage in Meatball Preparation on Various Quality Criteria and Formation of Heterocyclic Aromatic Amines. Separations 2023, 10, 29. [Google Scholar] [CrossRef]
- Puangsombat, K.; Jirapakkul, W.; Smith, J.S. Inhibitory activity of Asian spices on heterocyclic amines formation in cooked beef patties. J. Food Sci. 2011, 76, T174–T180. [Google Scholar] [CrossRef]
- Tengilimoglu-Metin, M.M.; Kizil, M. Reducing effect of artichoke extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Meat Sci. 2017, 134, 68–75. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, M.; He, Z.; Qin, F.; Tao, G.; Zhang, S.; Gao, Y.; Chen, J. Inhibitory profiles of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties. Food Chem. 2017, 221, 404–411. [Google Scholar] [CrossRef]
- Meurillon, M.; Angenieux, M.; Mercier, F.; Blinet, P.; Chaloin, L.; Chevolleau, S.; Debrauwer, L.; Engel, E. Mitigation of heterocyclic aromatic amines in cooked meat. Part I: Informed selection of antioxidants based on molecular modeling. Food Chem. 2020, 331, 127264. [Google Scholar] [CrossRef] [PubMed]
- Uzun, I.; Oz, F. Effect of basil use in meatball production on heterocyclic aromatic amine formation. J. Food Sci. Technol. 2021, 58, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Gökalp, H.Y.; Kaya, M.; Tülek, Y.; Zorba, Ö. Et ve Ürünlerinde Kalite Kontrolü ve Laboratuvar Uygulama Klavuzu. (V. Baskı); Atatürk Üniv. Yayınları, Yayın No: 751, Ziraat Fak. Yayın No: 318; Ders kitapları seri no:69; Atatürk Üniversity Ofset Tesisi: Erzurum, Turkey, 2010. [Google Scholar]
- Oz, F.; Kızıl, M. Determination of heterocyclic aromatic amines in cooked commercial frozen meat products by ultrafast liquid chromatography. Food Anal. Methods 2013, 6, 1370–1378. [Google Scholar] [CrossRef]
- Kilic, B.; Richards, M.P. Lipid oxidation in poultry döner kebab: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- Messner, C.; Murkovic, M. Evaluation of a new model system for studying the formation of heterocyclic amines. J. Chromatogr. B 2004, 802, 19–26. [Google Scholar] [CrossRef]
- Oz, F.; Kizil, M.; Cakmak, I.H.; Aksu, M.I. The effect of direct addition of conjugated linoleic acid on the formation of heterocyclic aromatic amines in beef chops. J. Food Process. Preserv. 2015, 39, 2820–2833. [Google Scholar] [CrossRef]
- Aksoy, A. Gıdalarda pH ölçümünün önemi. Haliç Üniv. Fen Bil. Derg. 2021, 4, 193–216. [Google Scholar] [CrossRef]
- Sabuncular, G.; Akbulut, G.; Yaman, M. Ette lipit oksidasyonu ve etkileyen faktörler. EJOSAT 2021, 27, 362–369. [Google Scholar] [CrossRef]
- Sánchez del Pulgar, J.; Gázquez, A.; Ruiz-Carrascal, J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef]
- Korkmaz, A.; Oz, F. Effect of the use of dry breadcrumb in meatball production on the formation of heterocyclic aromatic amines. Br. Food J. 2020, 122, 2105–2119. [Google Scholar] [CrossRef]
- Kondjoyan, A.; Oillic, S.; Portanguen, S.; Gros, J.B. Combined heat transfer and kinetic models to predict cooking loss during heat treatment of beef meat. Meat Sci. 2013, 95, 336–344. [Google Scholar] [CrossRef]
- Serrano, A.; Librelotto, J.; Cofrades, S.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Composition and physicochemical characteristics of restructured beef steaks containing walnuts as affected by cooking method. Meat Sci. 2007, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Gerber, N.; Scheeder, M.R.L.; Wenk, C. The influence of cooking and fat trimming on the actual nutrient intake from meat. Meat Sci. 2009, 81, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gómez, M.; Fonseca, S.; Lorenzo, J.M. Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT-Food Sci. Technol. 2014, 58, 439–445. [Google Scholar] [CrossRef]
- Oz, E. Inhibitory effects of black cumin on the formation of heterocyclic aromatic amines in meatball. PLoS ONE 2019, 14, e0221680. [Google Scholar] [CrossRef] [PubMed]
- Nuray, M.; Oz, F. The effect of using different types and rates of onion-water extract in meatball production on the formation of heterocyclic aromatic amines. J. Sci. Food Agric. 2019, 99, 3538–3547. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Abreu, V.K.G. Lipid Peroxidation in Meat and Meat Products. In Lipid Peroxidation Research; Ahmed Mansour, M., Ed.; IntechOpen: London, UK, 2018; p. 29. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, I.; Khare, A.; Kumar, V.; Singh, S.; Gautam, P.; Upadhyay, S.; Anand, J.; Singh, A.; Goswami, U. Numerical optimization of microwave treatment and impact of storage period on bioactive components of cumin, black pepper and mustard oil incorporated coriander leave paste. J. Food Meas. Charact. 2022, 16, 2071–2085. [Google Scholar] [CrossRef]
- Gunasena, B.V.A.P.; Rajapakse, R.P.N.P. Effect of Cooking Time and Cooking Temperature on Antioxidant Activity and Antimicrobial Activity of Cinnamon, Garlic, Ginger and Turmeric. In Proceedings of the Extended Abstracts of the 1st IFSTSL Research Session, Colombo, Sri Lanka, 15 December 2015; pp. 26–30. [Google Scholar]
- Lepper-Blilie, A.N.; Berg, E.P.; Buchanan, D.S.; Keller, W.L.; Maddock-Carlin, K.R.; Berg, P.T. Effectiveness of oxygen barrier oven bags in low temperature cooking on reduction of warmed-over flavor in beef roasts. Meat Sci. 2014, 96, 1361–1364. [Google Scholar] [CrossRef]
- Shan, S.; Ma, Y.; Sun, C.; Guo, X.; Zheng, H.; Xu, X.; Qin, L.; Hu, J. A novel magnetic solid-phase extraction method for detection of 14 heterocyclic aromatic amines by UPLC-MS/MS in meat products. Food Chem. 2021, 337, 127630. [Google Scholar] [CrossRef]
- Ekiz, E.; Oz, F. The effects of different frying oils on the formation of heterocyclic aromatic amines in meatballs and the changes in fatty acid compositions of meatballs and frying oils. J. Sci. Food Agric. 2019, 99, 1509–1518. [Google Scholar] [CrossRef]
- Weber, J.; Bochi, V.C.; Ribeiro, C.P.; Victório, A.D.M.; Emanuelli, T. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 2008, 106, 140–146. [Google Scholar] [CrossRef]
- Johansson, M.A.E.; Jägerstad, M. Influence of pro- and antioxidants on the formation of mutagenic-carcinogenic heterocyclic amines in a model system. Food Chem. 1996, 56, 69–75. [Google Scholar] [CrossRef]
- Damašius, J.; Venskutonis, P.R.; Ferracane, R.; Fogliano, V. Assessment of the influence of some spice extracts on the formation of heterocyclic amines in meat. Food Chem. 2011, 126, 149–156. [Google Scholar] [CrossRef]
- Bouhenni, H.; Doukani, K.; Şekeroğlu, N.; Gezici, S.; Tabak, S. Comparative study on chemical composition and antibacterial activity of fenugreek (Trigonella foenum graecum L.) and cumin (Cuminum cyminum L.) seeds. Ukr. Food J. 2019, 8, 755–767. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Li, Y.; Liu, Q.; Chen, Q.; Kong, B. Understanding the Development of heterocyclic aromatic amines in fried bacon and in the remaining oil after pan-frying in five different vegetable oils. Foods 2022, 11, 3491. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Özbek, H.; Ağaoğlu, Z. Cuminum cyminum L. (kimyon) meyvesi uçucu yağının median lethal doz (LD50) düzeyi ve sağlıklı ve diyabetli farelerde hipoglisemik etkisinin araştırılması. Van Tıp Derg. 2003, 10, 29–35. [Google Scholar]
- Sabally, K.; Sleno, L.; Jauffrit, J.A.; Iskandar, M.M.; Kubow, S. Inhibitory effects of apple peel polyphenol extract on the formation of heterocyclic amines in pan fried beef patties. Meat Sci. 2016, 117, 57–62. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, S.; Wang, M.F.; Chen, J.; Zheng, Z.P. Inhibitory effects of selected dietary flavonoids on the formation of total heterocyclic amines and 2-amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) in roast beef patties and in chemical models. Food Funct. 2016, 7, 1057–1066. [Google Scholar] [CrossRef]
- Turesky, R.J.; Taylor, J.; Schnackenberg, L.; Freeman, J.P.; Holland, R.D. Quantitation of carcinogenic heterocyclic aromatic amines and detection of novel heterocyclic aromatic amines in cooked meats and grill scrapings by HPLC/ESI-MS. J. Agric. Food Chem. 2005, 53, 3248–3258. [Google Scholar] [CrossRef]
- Pais, P.; Salmon, C.P.; Knize, M.G.; Felton, J.S. Formation of Mutagenic/carcinogenic heterocyclic amines in dry-heated model systems, meats, and meat drippings. J. Agric. Food Chem. 1999, 47, 1098–1108. [Google Scholar] [CrossRef]
- Oz, F.; Zaman, A.; Kaya, M. Effect of chitosan on the formation of heterocyclic aromatic amines and some quality properties of meatball. J. Food Process. Preserv. 2017, 41, e13065. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, S.; Tao, G.J.; You, F.H.; Chen, J.; Zeng, M.M. Acetonitrile extraction coupled with UHPLC–MS/MS for the accurate quantification of 17 heterocyclic aromatic amines in meat products. J. Chromatogr. B 2017, 1068, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Öz, F.; Kaban, G.; Kaya, M. Heterocyclic aromatic amine contents of beef and lamb chops cooked by different methods to varying levels. J. Anim. Vet. Adv. 2010, 9, 1436–1440. [Google Scholar] [CrossRef]
- Shin, H.S.; Rodgers, W.J.; Gomaa, E.A.; Strasburg, G.M.; Gray, J.I. Inhibition of heterocyclic aromatic amine formation in fried ground beef patties by garlic and selected garlic-related sulfur compounds. J. Food Prot. 2002, 65, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Weiss, J. Inhibitory effect of marinades with hibiscus extract on formation of heterocyclic aromatic amines and sensory quality of fried beef patties. Meat Sci. 2010, 85, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Oz, F.; Kaya, M. The inhibitory effect of red pepper on heterocyclic aromatic amines in fried beef Longissimus dorsi muscle. J. Food Process. Preserv. 2011, 35, 806–812. [Google Scholar] [CrossRef]
- Balogh, Z.; Gray, J.I.; Gomaa, E.A.; Booren, A.M. Formation and inhibition of heterocyclic aromatic amines in fried ground beef patties. Food Chem. Toxicol. 2000, 38, 395–401. [Google Scholar] [CrossRef]
- Khan, I.A.; Liu, D.; Yao, M.; Memon, A.; Huang, J.; Huang, M. Inhibitory effect of Chrysanthemum morifolium flower extract on the formation of heterocyclic amines in goat meat patties cooked by various cooking methods and temperatures. Meat Sci. 2019, 147, 70–81. [Google Scholar] [CrossRef]
- Skog, K. Problems associated with the determination of heterocyclic amines in cooked foods and human exposure. Food Chem. Toxicol. 2002, 40, 1197–1203. [Google Scholar] [CrossRef]

| HPLC | Thermo Ultimate 3000, Thermo Scientific, USA (Germering, Germany) |
|---|---|
| Diode Array Detector | DAD-3000 |
| Autosampler | WPS-3000 |
| Column Oven | TCC-3000 |
| Pump | LPG-3400SD |
| Flow Rate | 0.7 mL/min |
| Column | AcclaimTM 120 C18, 3 μm (4.6 × 150 mm) |
| Solvent A | Methanol/Acetonitrile/Water/Acetic acid (8/14/76/2, v/v/v/v) (pH 5.0) |
| Solvent B | Acetonitrile |
| Gradient Program | 0% B, 0–10 min; 0–23% B, 11–20 min; 23% B, 21–30 min; 0% B, 31–45 min |
| n | Water (%) | pH | TBARS (mg MDA/kg) | |
|---|---|---|---|---|
| Meat | 2 | 74.70 ± 0.18 a | 5.46 ± 0.01 b | 0.31 ± 0.04 a |
| Intermuscular fat | 2 | 13.89 ± 0.16 c | 6.47 ± 0.37 a | 0.38 ± 0.13 a |
| Meatball dough | 2 | 65.62 ± 0.8 b | 5.63 ± 0.08 b | 0.34 ± 0.07 a |
| Sign. | ** | * | ns |
| Water (%) | pH | TBARS (mg MDA/kg) | Cooking Loss (%) | Total HAA (ng/g) | |
|---|---|---|---|---|---|
| Usage rate (UR, %) | |||||
| 0 | 57.05 ± 6.44 a | 5.73 ± 0.13 b | 1.03 ± 0.27 a | 43.81 ± 3.23 a | 0.33 ± 0.43 b |
| 0.5 | 58.45 ± 7.94 a | 5.92 ± 0.06 a | 0.63 ± 0.15 b | 45.74 ± 0.79 a | 1.20 ± 0.71 a |
| 1 | 58.45 ± 7.67 a | 5.95 ± 0.06 a | 0.67 ± 0.19 b | 44.29 ± 1.78 a | 1.11 ± 1.00 a |
| Sign. | ns | ** | ** | ns | ** |
| Cooking Process (CP) | |||||
| Raw | 64.51 ± 2.26 a | 5.81 ± 0.14 b | 0.60 ± 0.14 b | - | - |
| Cooked | 51.45 ± 2.60 b | 5.93 ± 0.08 a | 0.96 ± 0.26 a | - | - |
| Sign. | ** | ** | ** | ||
| Cooking Temperature (CT, °C) | |||||
| 150 | 58.56 ± 6.49 a | 5.86 ± 0.13 a | 0.77 ± 0.29 a | 43.05 ± 1.96 b | 0.30 ± 0.29 b |
| 250 | 57.40 ± 7.88 a | 5.87 ± 0.13 a | 0.78 ± 0.27 a | 46.18 ± 0.66 a | 1.46 ± 0.69 a |
| Sign. | ns | ns | ns | ** | ** |
| HAA | Recovery (%) | LOD (ng/g) | LOQ (ng/g) | Linear Equation | R2 |
|---|---|---|---|---|---|
| IQx (2-amino-3-methylimidazo[4,5-ƒ]quinoxaline) | 94.44 | 0.004 | 0.013 | y = 2.3771x − 0.0111 | 0.9999 |
| IQ (2-amino-3-methylimidazo[4,5-ƒ]quinoline) | 82.17 | 0.009 | 0.029 | y = 0.9659x − 0.0132 | 0.9999 |
| MeIQx (2-amino-3,8-dimethylimidazo[4,5-ƒ]quinoxaline) | 93.07 | 0.024 | 0.081 | y = 1.5303x − 0.0103 | 0.9999 |
| MeIQ (2-amino-3,4-dimethylimidazoimidazo[4,5-ƒ]quinoline) | 81.52 | 0.014 | 0.047 | y = 0.8876x − 0.0195 | 0.9999 |
| 7,8-DiMeIQx (2-amino-3,7,8-trimethylimidazo[4,5-ƒ]quinoxaline) | 92.96 | 0.005 | 0.018 | y = 2.3739x − 0.0138 | 0.9999 |
| 4,8-DiMeIQx (2-amino-3,4, 8-trimethylimidazo[4,5-ƒ]quinoxaline) | 94.56 | 0.008 | 0.025 | y = 1.841x − 0.0366 | 0.9999 |
| PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) | 95.44 | 0.025 | 0.085 | y = 0.168x + 0.0016 | 0.9999 |
| AαC (2-amino-9H-pyrido [2,3-b]indole) | 95.64 | 0.012 | 0.039 | y = 0.4585x − 0.0036 | 0.9999 |
| MeAαC (2-amino-9H-pyrido [2,3-b]indole) | 90.53 | 0.010 | 0.035 | y = 0.332x − 0.0167 | 0.9998 |
| Cooking Temperature (°C) | Usage Rate (%) | IQx | IQ | MeIQx | MeIQ | 7,8-DiMeIQx | 4,8-DiMeIQx | PhIP | AαC | MeAαC | Total HAA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 | 0 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 0.5 | nd | nd | 0.51 | 0.08 | nd | nd | nd | nd | nd | 0.59 | |
| 1 | nd | nd | 0.24 | 0.06 | nd | nd | nd | nd | nd | 0.30 | |
| 250 | 0 | nd | nd | 0.91 | nd | nd | nd | nd | nd | nd | 0.91 |
| 0.5 | nd | nd | 0.76 | 0.23 | nd | nd | 0.82 | nd | nd | 1.81 | |
| 1 | 0.08 | nd | 0.52 | 0.20 | nd | nd | 1.21 | nd | nd | 2.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekiz, E.; Savaş, A.; Aoudeh, E.; Elbir, Z.; Oz, E.; Proestos, C.; Ahmad, N.; Oz, F. Impact of Cumin (Cuminum cyminum) Incorporation on the Generation of Heterocyclic Aromatic Amines in Meatballs. Separations 2023, 10, 458. https://doi.org/10.3390/separations10080458
Ekiz E, Savaş A, Aoudeh E, Elbir Z, Oz E, Proestos C, Ahmad N, Oz F. Impact of Cumin (Cuminum cyminum) Incorporation on the Generation of Heterocyclic Aromatic Amines in Meatballs. Separations. 2023; 10(8):458. https://doi.org/10.3390/separations10080458
Chicago/Turabian StyleEkiz, Elif, Adem Savaş, Eyad Aoudeh, Zeynep Elbir, Emel Oz, Charalampos Proestos, Naushad Ahmad, and Fatih Oz. 2023. "Impact of Cumin (Cuminum cyminum) Incorporation on the Generation of Heterocyclic Aromatic Amines in Meatballs" Separations 10, no. 8: 458. https://doi.org/10.3390/separations10080458
APA StyleEkiz, E., Savaş, A., Aoudeh, E., Elbir, Z., Oz, E., Proestos, C., Ahmad, N., & Oz, F. (2023). Impact of Cumin (Cuminum cyminum) Incorporation on the Generation of Heterocyclic Aromatic Amines in Meatballs. Separations, 10(8), 458. https://doi.org/10.3390/separations10080458








