Application of a Quality by Design Approach to Develop a Simple, Fast, and Sensitive UPLC-MS/MS Method for Quantification of Safinamide, an Antiparkinson’s Drug, in Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
2.3. Method
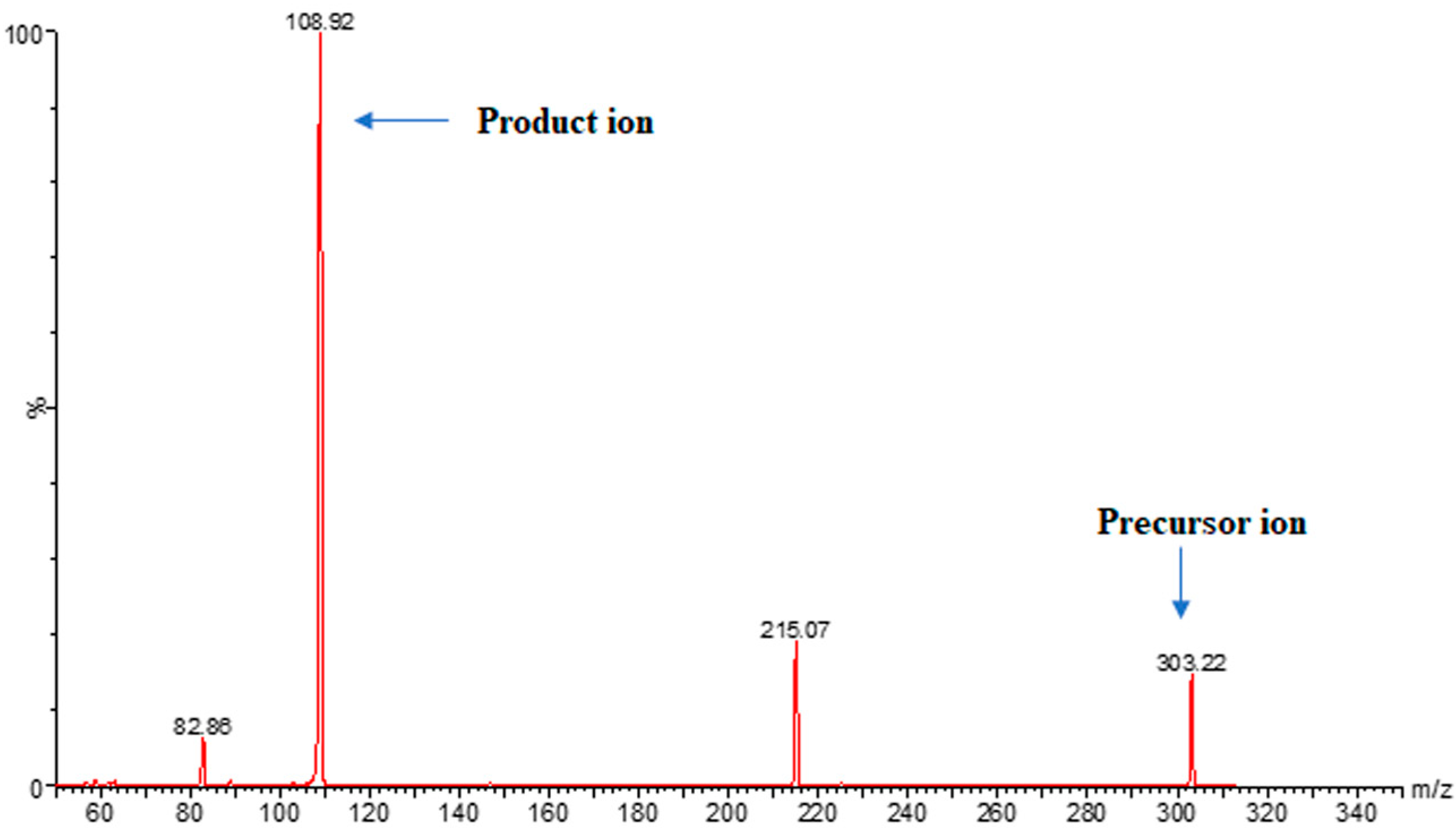
2.3.1. Optimization of MS/MS Conditions
2.3.2. Stock Solutions, Calibration Standards, and Quality Control (QC) Samples
2.3.3. Sample Preparation
2.4. Method Validation
2.4.1. Specificity and Selectivity
2.4.2. Linearity, LLOD, LLOQ
2.4.3. Extraction Recovery and Matrix Effect
2.4.4. Precision and Accuracy
2.4.5. Stability
2.5. Metabolic Stability
3. Results
3.1. Optimization of the Chromatographic Separation Conditions
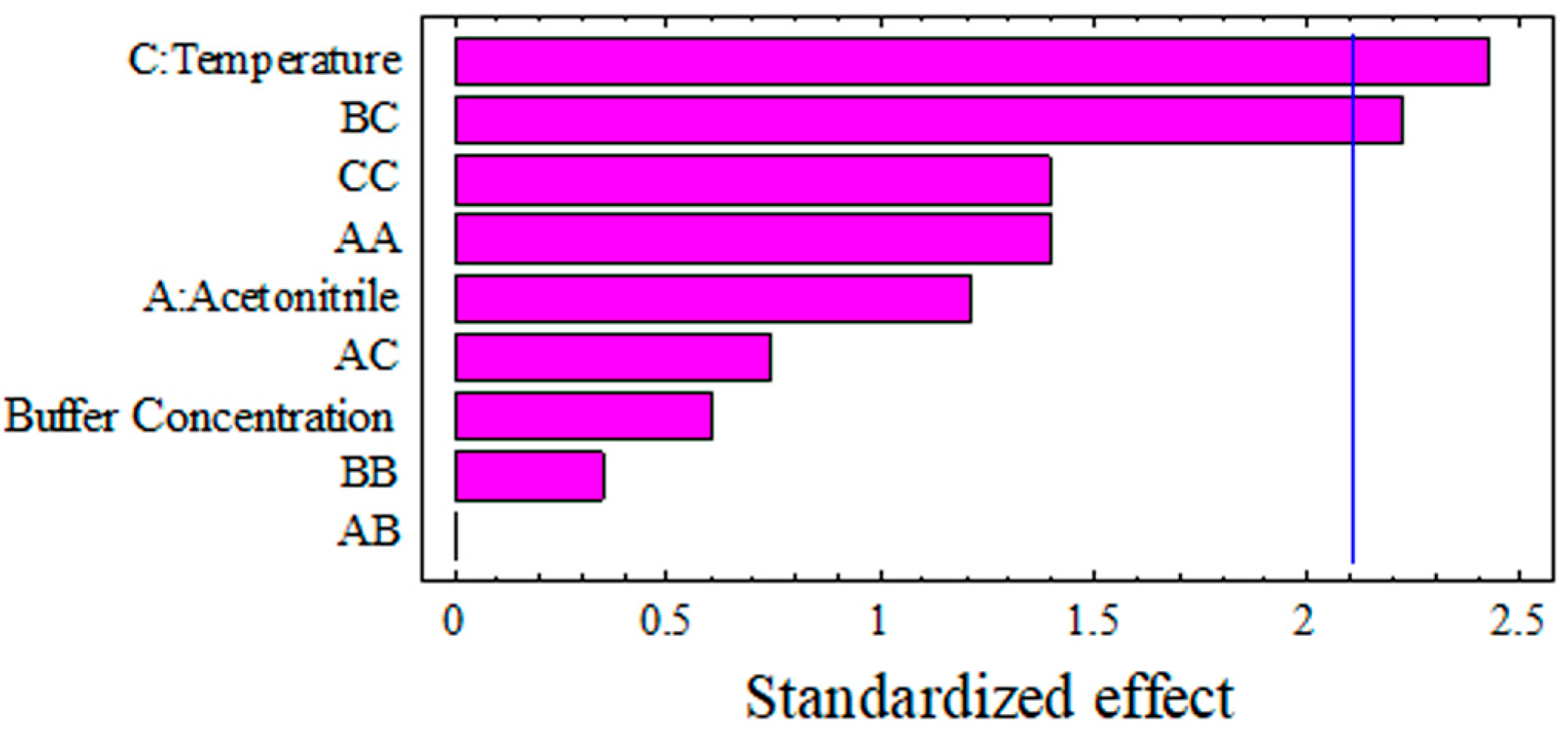
3.1.1. The Effect of the Independent Factors on the Retention Time of the Safinamide Chromatogram
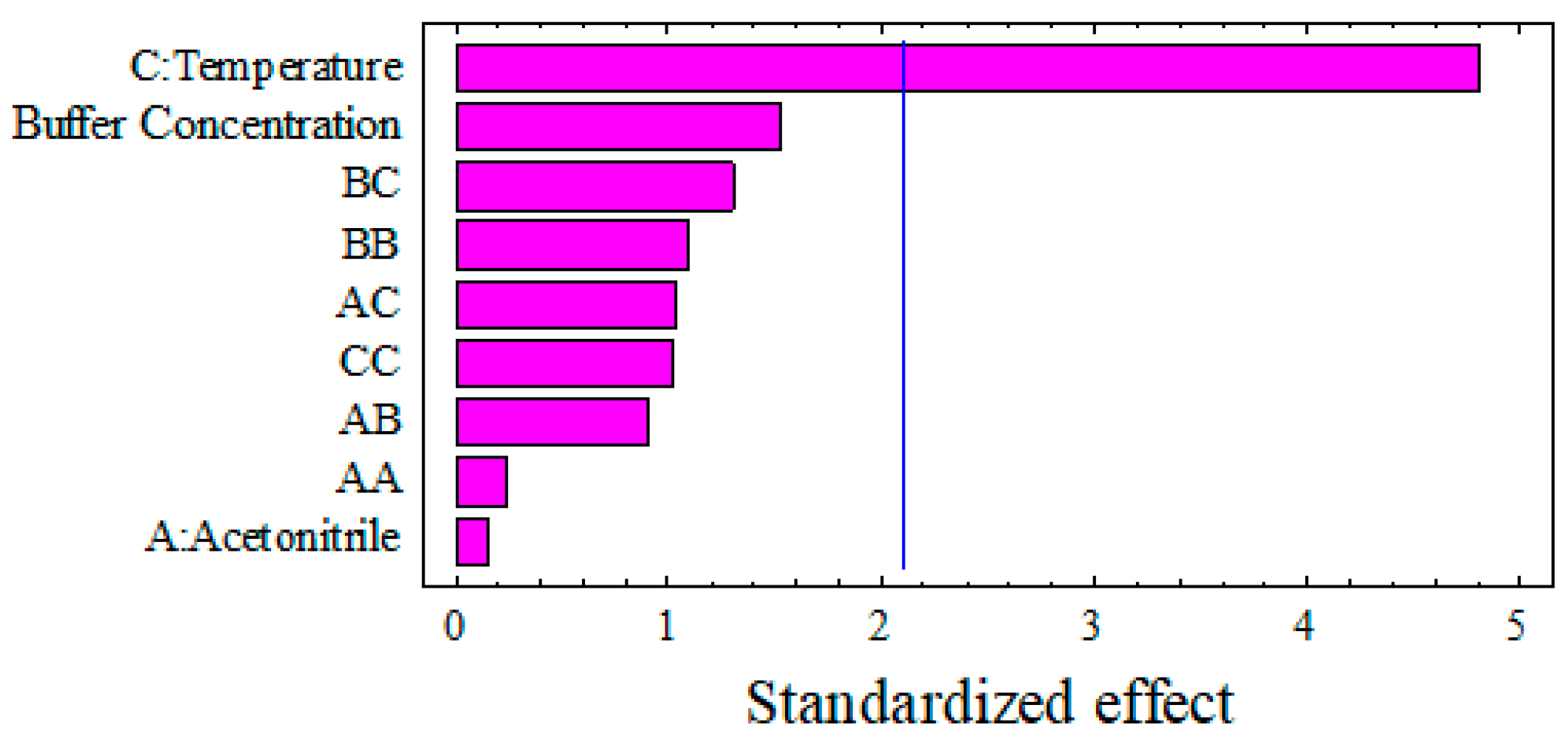
3.1.2. The Effect of the Independent Factors on the Peak Area of Safinamide Chromatogram
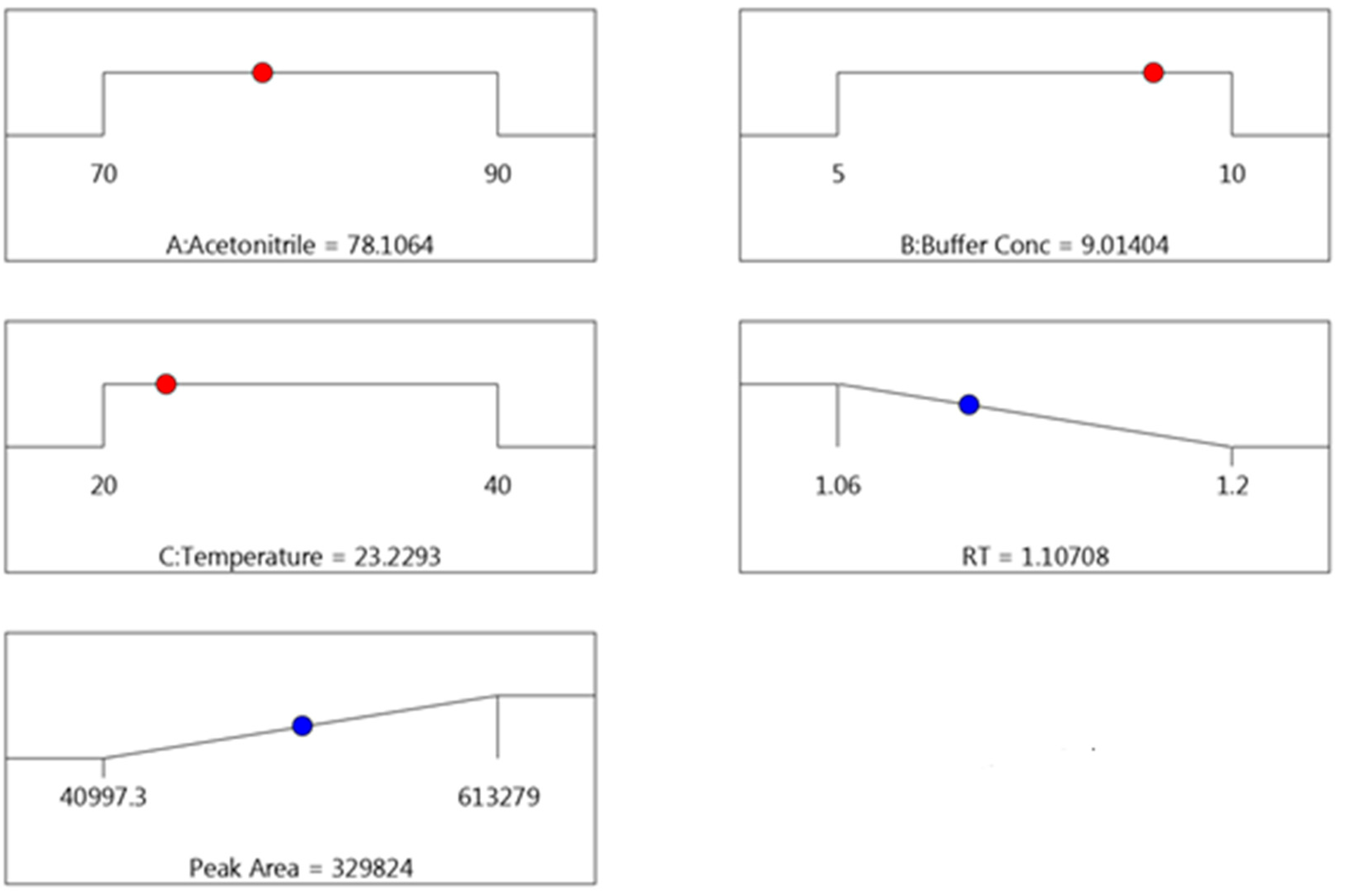
3.1.3. The Optimization of UPLC Conditions for Safinamide Analysis
3.2. Sample Preparation
3.3. Method Validation
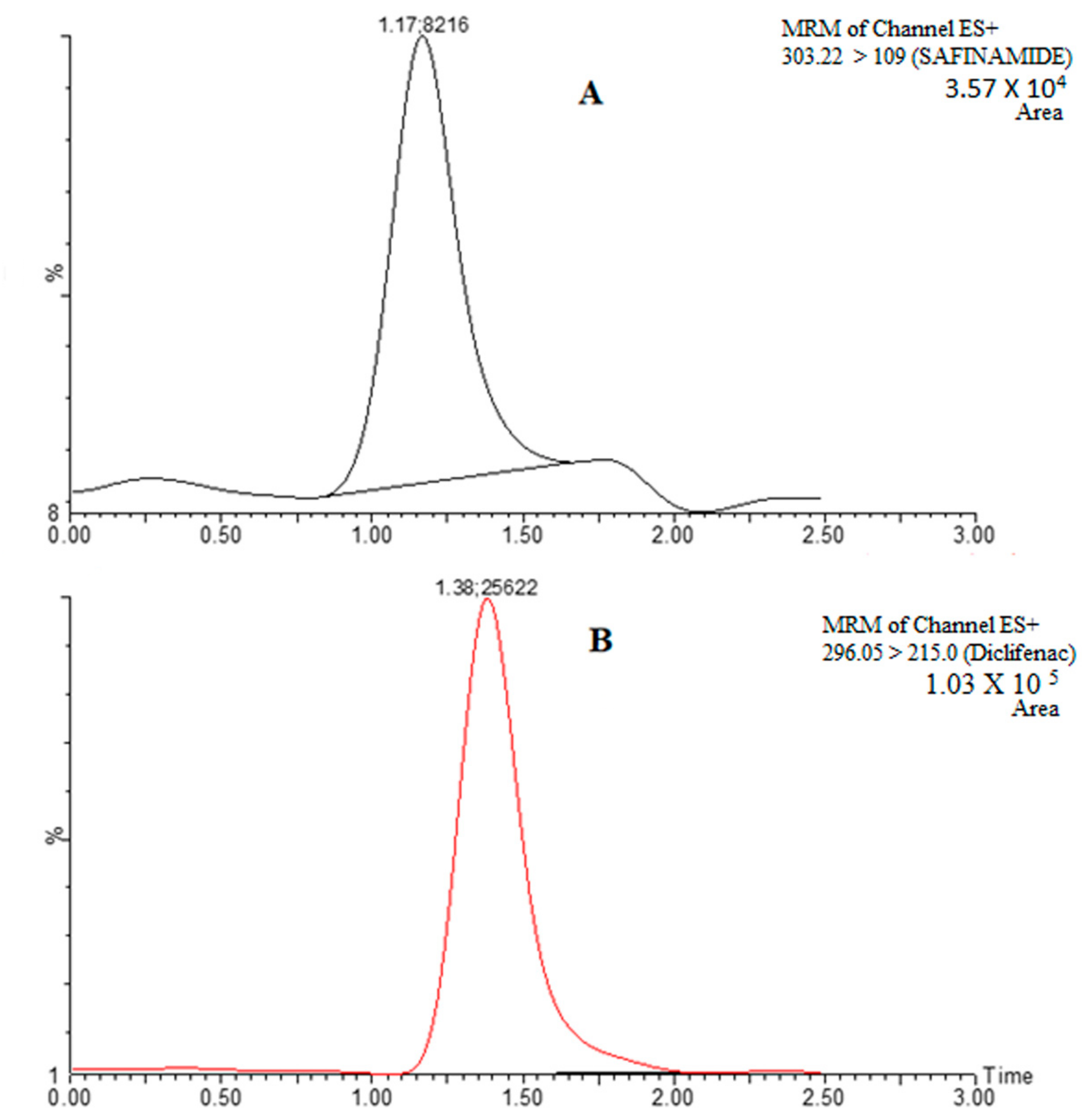
3.3.1. Selectivity and Specificity
3.3.2. Lower Limit of Quantification (LLOQ)
3.3.3. Linearity
3.3.4. Recovery and Matrix Effect
3.3.5. Precision and Accuracy
3.3.6. Stability Studies
3.4. Comparison of Current and Published Methods for Safinamide Analysis in Plasma
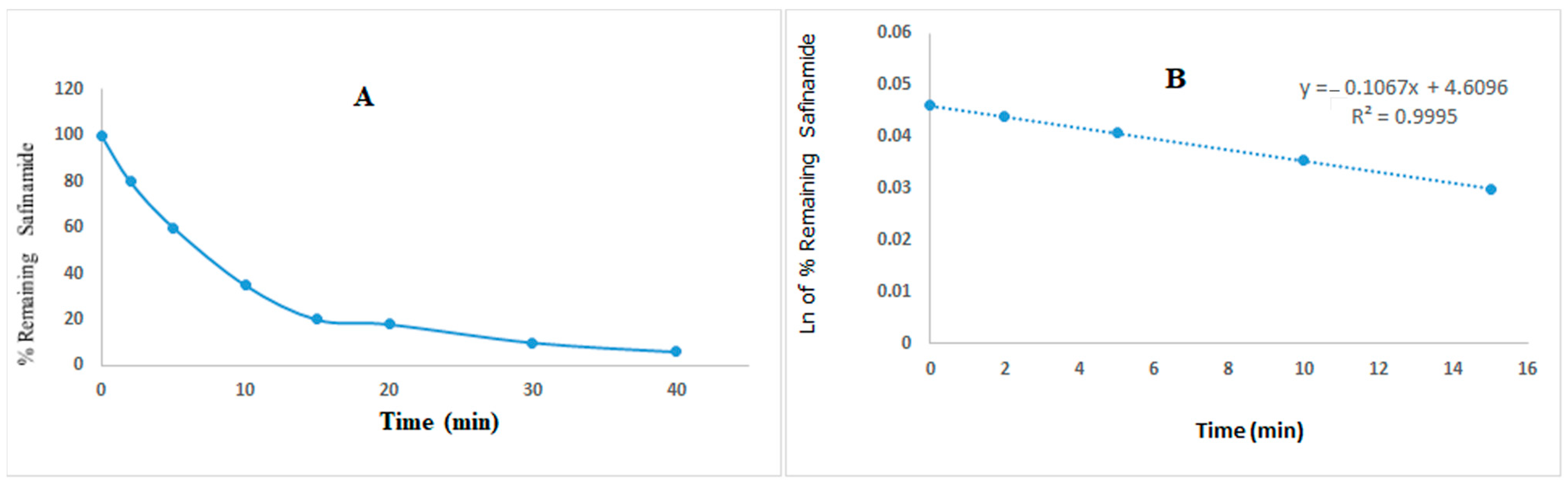
3.5. In Vitro Metabolic Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bette, S.; Shpiner, D.S.; Singer, C.; Moore, H. Safinamide in the management of patients with Parkinson’s disease not stabilized on levodopa: A review of the current clinical evidence. Ther. Clin. Risk Manag. 2018, 14, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Rascol, O. The safety and efficacy of safinamide mesylate for the treatment of Parkinson’s disease. Expert Rev. Neurother. 2016, 16, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.G.; Gago, M.F.; Marques, P.; Moreira, P.S.; Magalhães, R.; Sousa, N.; Salgado, A.J. Safinamide: A new hope for Parkinson’s disease? Drug Discov. Today 2018, 23, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Morari, M.; Brugnoli, A.; Pisanò, C.A.; Novello, S.; Caccia, C.; Melloni, E.; Padoani, G.; Vailati, S.; Sardina, M. Safinamide Differentially Modulates In Vivo Glutamate and GABA Release in the Rat Hippocampus and Basal Ganglia. J. Pharmacol. Exp. Ther. 2018, 364, 198–206. [Google Scholar] [CrossRef]
- Gardoni, F.; Morari, M.; Kulisevsky, J.; Brugnoli, A.; Novello, S.; Pisanò, C.A.; Caccia, C.; Mellone, M.; Melloni, E.; Padoani, G.; et al. Safinamide Modulates Striatal Glutamatergic Signaling in a Rat Model of Levodopa-Induced Dyskinesia. J. Pharmacol. Exp. Ther. 2018, 367, 442–451. [Google Scholar] [CrossRef]
- Bianchi, M.; Riboldazzi, G.; Mauri, M.; Versino, M. Efficacy of safinamide on non-motor symptoms in a cohort of patients affected by idiopathic Parkinson’s disease. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2019, 40, 275–279. [Google Scholar] [CrossRef]
- Fabbri, M.; Rosa, M.M.; Abreu, D.; Ferreira, J.J. Clinical pharmacology review of safinamide for the treatment of Parkinson’s disease. Neurodegener. Dis. Manag. 2015, 5, 481–496. [Google Scholar] [CrossRef]
- Fariello, R.G.; McArthur, R.A.; Bonsignori, A.; Cervini, M.A.; Maj, R.; Marrari, P.; Pevarello, P.; Wolf, H.H.; Woodhead, J.W.; White, H.S.; et al. Preclinical evaluation of PNU-151774E as a novel anticonvulsant. J. Pharmacol. Exp. Ther. 1998, 285, 397–403. [Google Scholar]
- Rana, A.Q.; Saleh, M.; Yousuf, M.S.; Mansoor, W.; Hussaini, S.; Rahman, M.; Iqbal, Z. DOPA-sparing strategy in the treatment of young onset Parkinson’s disease. J. Neurosci. Rural. Pract. 2016, 7, 67–69. [Google Scholar] [CrossRef]
- Singer, C. Managing the patient with newly diagnosed Parkinson disease. Clevel. Clin. J. Med. 2012, 79 (Suppl. 2), S3–S7. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Park. Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, L.; Jourdain, V.A.; Townsend, M.; Roach, A.; Di Paolo, T. Safinamide reduces dyskinesias and prolongs L-DOPA antiparkinsonian effect in parkinsonian monkeys. Park. Relat. Disord. 2013, 19, 508–514. [Google Scholar] [CrossRef]
- Borgohain, R.; Szasz, J.; Stanzione, P.; Meshram, C.; Bhatt, M.; Chirilineau, D.; Stocchi, F.; Lucini, V.; Giuliani, R.; Forrest, E.; et al. Study 016 Investigators Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.; Fox, S.H.; Hauser, R.A.; Jankovic, J.; Jost, W.H.; Kenney, C.; Kulisevsky, J.; Pahwa, R.; Poewe, W.; Anand, R. Assessment of Safety and Efficacy of Safinamide as a Levodopa Adjunct in Patients with Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA Neurol. 2017, 74, 216–224. [Google Scholar] [CrossRef]
- Cattaneo, C.; Ferla, R.L.; Bonizzoni, E.; Sardina, M. Long-Term Effects of Safinamide on Dyskinesia in Mid- to Late-Stage Parkinson’s Disease: A Post-Hoc Analysis. J. Park. Dis. 2015, 5, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Pharmacokinetic drug evaluation of safinamide mesylate for the treatment of mid-to-late stage Parkinson’s disease. Expert Opin. Drug Metab. Toxicol. 2017, 13, 693–699. [Google Scholar] [CrossRef]
- Marzo, A.; Dal Bo, L.; Monti, N.C.; Crivelli, F.; Ismaili, S.; Caccia, C.; Cattaneo, C.; Fariello, R.G. Pharmacokinetics and pharmacodynamics of safinamide, a neuroprotectant with antiparkinsonian and anticonvulsant activity. Pharmacol. Res. 2004, 50, 77–85. [Google Scholar] [CrossRef]
- Alborghetti, M.; Nicoletti, F. Different Generations of Type-B Monoamine Oxidase Inhibitors in Parkinson’s Disease: From Bench to Bedside. Curr. Neuropharmacol. 2019, 17, 861–873. [Google Scholar] [CrossRef]
- Marquet, A.; Kupas, K.; Johne, A.; Astruc, B.; Patat, A.; Krösser, S.; Kovar, A. The effect of safinamide, a novel drug for Parkinson’s disease, on pressor response to oral tyramine: A randomized, double-blind, clinical trial. Clin. Pharmacol. Ther. 2012, 92, 450–457. [Google Scholar] [CrossRef]
- Cattaneo, C.; Barone, P.; Bonizzoni, E.; Sardina, M. Effects of Safinamide on Pain in Fluctuating Parkinson’s Disease Patients: A Post-Hoc Analysis. J. Park. Dis. 2017, 7, 95–101. [Google Scholar] [CrossRef]
- Ermer, J.; Aguiar, D.; Boden, A.; Ding, B.; Obeng, D.; Rose, M.; Vokrot, J. Lifecycle management in pharmaceutical analysis: How to establish an efficient and relevant continued performance monitoring program. J. Pharm. Biomed. Anal. 2020, 181, 113051. [Google Scholar] [CrossRef]
- Tsuboi, T.; Satake, Y.; Hiraga, K.; Yokoi, K.; Hattori, M.; Suzuki, M.; Hara, K.; Ramirez-Zamora, A.; Okun, M.S.; Katsuno, M. Effects of MAO-B inhibitors on non-motor symptoms and quality of life in Parkinson’s disease: A systematic review. NPJ Park. Dis. 2022, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, K.; Leta, V.; Sportelli, C.; Buhidma, Y.; Duty, S.; Malcangio, M.; Ray Chaudhuri, K. Pain in Parkinson’s disease: New concepts in pathogenesis and treatment. Curr. Opin. Neurol. 2019, 32, 579–588. [Google Scholar] [CrossRef]
- Peraman, R.; Bhadraya, K.; Padmanabha Reddy, Y. Analytical quality by design: A tool for regulatory flexibility and robust analytics. Int. J. Anal. Chem. 2015, 2015, 868727. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.; Pohl, M.; Hanna-Brown, M.; Nethercote, P.; Borman, P.; Hansen, G.; Smith, K.; Larew, J. Implications and opportunities of applying QbD principles to analytical measurements. Pharm. Technol. 2010, 34, 52–59. [Google Scholar]
- Elder, D.P.; Borman, P. Improving analytical method reliability across the entire product lifecycle using QbD approaches. Pharm. Outsourcing 2013, 14, 14–19. [Google Scholar]
- Borman, P.; Chatfield, M.; Nethercote, P.; Thompson, D.; Truman, K. The application of quality by design to analytical methods. Pharm. Technol. 2007, 31, 142–152. [Google Scholar]
- Schmidt, A.H.; Wess, C. A Qbd with Design-Of-Experiments Approach for Development of a State-Of-The-Art Uplc Purity Method for Carbamazepine. J. Liq. Chromatogr. Rel. Technol. 2014, 37, 2653–2666. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Guideline Q14 on Analytical Procedure Development Step 2b. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q14-analytical-procedure-development-step-2b_en.pdf (accessed on 5 February 2023).
- Dal Bo, L.; Mazzucchelli, P.; Fibbioli, M.; Marzo, A. Bioassay of safinamide in biological fluids of humans and various animal species. Arzneimittelforschung 2006, 56, 814–819. [Google Scholar] [CrossRef]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta. 2009, 652, 161–172. [Google Scholar] [CrossRef]
- Guidance for Industry on Bioanalytical Method Validation, Center for Drug Evaluation and Research, US Food and Drug Administration, Rockville, MD. 2001. Available online: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (accessed on 1 March 2018).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Bioanalytical Method Validation. 2019. Available online: https://database.ich.org/sites/default/files/M10_Step4_Guideline_2019_1204.pdf (accessed on 14 April 2023).
- Chao, P.; Uss, A.S.; Cheng, K.C. Use of intrinsic clearance for prediction of human hepatic clearance. Expert Opin. Drug Metab. Toxicol. 2010, 6, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Obach, R.S. The prediction of human clearance from hepatic microsomal metabolism data. Curr. Opin. Drug Discov. Dev. 2001, 4, 36–44. [Google Scholar]
- Yang, Y.; Lamm, L.J.; He, P.; Kondo, T. Temperature effect on peak width and column efficiency in subcritical water chromatography. J. Chromatogr. Sci. 2002, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]


| Independent Factors | Dependent Factors (Response) | ||
|---|---|---|---|
| Acetonitrile % (A) | Ammonium Acetate mM (B) | Temperature °C | |
| 90 | 15 | 40 | Peak area (mAU/min) |
| 80 | 10 | 30 | Retention time (min) |
| 70 | 5 | 20 | |
| Source | Sum of Square | Df | Mean Square | R-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Acetonitrile | 0.00222222 | 1 | 0.022222 | 1.47 | 0.2423 |
| B: Acetate buffer Con. | 0.55555556 | 1 | 0.00555556 | 0.37 | 0.5527 |
| C: Temperature | 0.0088889 | 1 | 0.00888889 | 5.87 | 0.0265 |
| AA | 0.00296296 | 1 | 0.00296296 | 1.96 | 0.1798 |
| AB | 0.0 | 1 | 0.0 | 0.00 | 1.000 |
| AC | 0.00083333 | 1 | 0.000833333 | 0.55 | 0.4683 |
| BB | 0.000185185 | 1 | 0.000185185 | 0.12 | 0.7308 |
| BC | 0.0075 | 1 | 0.0075 | 4.95 | 0.0399 |
| CC | 0.00296296 | 1 | 0.00296296 | 1.96 | 0.1798 |
| Total error | 0.25747 | 17 | 0.00151416 |
| Source | Sum of Square | Df | Mean Square | R-Ratio | p-Value |
|---|---|---|---|---|---|
| A: Acetonitrile | 2.6295 × 108 | 1 | 2.62995 × 108 | 0.02 | 0.8803 |
| B: Acetate buffer Con. | 2.63246 × 1010 | 1 | 2.63246 × 1010 | 2.34 | 0.1446 |
| C: Temperature | 2.59232 × 1011 | 1 | 2.59232 × 1011 | 23.03 | 0.0002 |
| AA | 6.49689 × 109 | 1 | 6.49689 × 108 | 0.06 | 0.8130 |
| AB | 9.16371 × 109 | 1 | 9.16371 × 109 | 0.81 | 0.3795 |
| AC | 1.21055 × 1010 | 1 | 1.21055 × 1010 | 1.08 | 0.3142 |
| BB | 1.34664 × 1010 | 1 | 1.34664 × 1010 | 1.20 | 0.2893 |
| BC | 1.91826 × 1010 | 1 | 1.91826 × 1010 | 1.7 | 02091 |
| CC | 1.17761 × 101 | 1 | 1.17761 × 1010 | 1.05 | 0.2207 |
| Total error | 1.9133 × 1011 | 17 | 1.12547 × 1010 |
| Compound | Nominal Conc. | Extraction Recovery | Matrix Effect | ||
|---|---|---|---|---|---|
| Mean | CV | Mean | CV | ||
| (ng/mL) | (%) | (%) | (%) | (%) | |
| SAFINAMIDE | 0.3 | 93.33 | 5.35 | 86.66 | 5.20 |
| 15 | 78.18 | 12.12 | 90.90 | 12.61 | |
| 300 | 91.98 | 9.82 | 81.22 | 10.34 | |
| 750 | 77.96 | 12.36 | 85.47 | 7.49 | |
| IS | 100 | 81.26 | 7.4 | 91.26 | 7.46 |
| Nominal | Inter-Day | Intra-Day | ||||
|---|---|---|---|---|---|---|
| Actual | Precision | Accuracy | Actual | Precision | Accuracy | |
| Con. (ng/mL) | Con. (ng/mL) | (%) | (%) | Con. (ng/mL) | (%) | (%) |
| 0.1 | 0.08 ± 0.004 | 5.60 | 87.25 | 0.08 ± 0.004 | 5.50 | 82.50 |
| 0.3 | 0.27 ± 0.015 | 5.50 | 89.16 | 0.25 ± 0.014 | 5.40 | 86.66 |
| 15 | 13.03 ± 1.57 | 12.10 | 86.87 | 13.60 ± 3.76 | 2.74 | 86.26 |
| 300 | 272.34 ± 18.4 | 9.78 | 90.20 | 259.22 ±1.67 | 6.35 | 86.4 |
| 750 | 649.67 ± 80.31 | 12.26 | 86.62 | 652.87 ± 86.21 | 13.20 | 87.05 |
| Parameters | Nominal Con | Measured Con. | Precision | Accuracy |
|---|---|---|---|---|
| (%) | (%) | (%) | (%) | |
| Short-term | 15 | 13.03 ±1.28 | 9.82 | 86.86 |
| 750 | 643.50 ± 41.79 | 6.49 | 85.8 | |
| Long-term | 15 | 13.6 ± 1.51 | 11.16 | 90.66 |
| 750 | 640.95 ± 82.10 | 12.80 | 85.46 | |
| Thaw and freeze | 15 | 12.77 ± 1.17 | 9.15 | 85.16 |
| 750 | 652.75 ± 51.62 | 7.90 | 87.03 | |
| Auto-sampler (24 h) | 15 | 12.83 ± 1.25 | 9.71 | 85.53 |
| 750 | 673.07 ± 69.92 | 10.38 | 89.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, E.A.; Ibrahim, M.A.; Iqbal, M.; Alsalahi, R.; Mostafa, G.A.; Al Jarboua, S. Application of a Quality by Design Approach to Develop a Simple, Fast, and Sensitive UPLC-MS/MS Method for Quantification of Safinamide, an Antiparkinson’s Drug, in Plasma. Separations 2023, 10, 474. https://doi.org/10.3390/separations10090474
Ali EA, Ibrahim MA, Iqbal M, Alsalahi R, Mostafa GA, Al Jarboua S. Application of a Quality by Design Approach to Develop a Simple, Fast, and Sensitive UPLC-MS/MS Method for Quantification of Safinamide, an Antiparkinson’s Drug, in Plasma. Separations. 2023; 10(9):474. https://doi.org/10.3390/separations10090474
Chicago/Turabian StyleAli, Essam A., Mohamed A. Ibrahim, Muzaffar Iqbal, Rashad Alsalahi, Gamal A. Mostafa, and Suliman Al Jarboua. 2023. "Application of a Quality by Design Approach to Develop a Simple, Fast, and Sensitive UPLC-MS/MS Method for Quantification of Safinamide, an Antiparkinson’s Drug, in Plasma" Separations 10, no. 9: 474. https://doi.org/10.3390/separations10090474







