Abstract
The increasing global reliance on pesticides for agricultural pest control has raised significant environmental concerns, particularly due to inadequate monitoring of emerging chemicals in surface waters. This study addresses the potential contamination of aquatic ecosystems by developing and validating a method for detecting trace amounts of four recently registered fungicides: three succinate dehydrogenase inhibitors (fluopyram, penthiopyrad, pydiflumetofen) and fluopicolide, a structurally related fungicide. Employing QuEChERS-based sample extraction combined with ultra-high-performance liquid chromatography (UHPLC-MS-MS), this method achieves detection limits of 0.1 to 0.2 μg/L, with recovery rates between 90% and 110%, and intra-day relative standard deviation values well within the acceptable range of less than 20%. Applied to surface grab water samples from the greater Melbourne area, Australia, the method successfully identified all four fungicides at trace levels, including a notable high concentration of fluopyram (7.3 μg/L) during autumn, with the others intermittently detected at lower concentrations. This study represents the first documented instance of quantifiable detections of these four fungicides in Australian surface water systems. Given their high toxicity to several organisms and the limited global data on these substances, our findings underscore the critical need for continuous monitoring to inform strategies to safeguard aquatic ecosystems from these chemicals.
1. Introduction
In the last decade, global pesticide usage has significantly increased. Pesticides are commonly used for controlling pests like rodents, weeds, fungi, parasites, and disease vectors, thus safeguarding global agriculture [1]. Pesticides can help maintain and increase commercial agricultural production [2]. However, increased usage, coupled with the broad range of pesticide chemicals and classes administered, has contributed to adverse effects on non-target biota and ecosystems [3].
Unlike other environmental pollutants, pesticides are developed with inherent toxicities associated with the host/pests and specified for use within a target environment. Pesticides are generally associated with long half-lives and environmental persistence and mobility within the environment leading to the possibility of off-target exposure, heightened toxicity, and bioaccumulation. In addition, pesticide contamination of surface water systems can occur in processes such as leaching, spray drift, surface runoff, and drainage through agricultural and industrial application including point source contamination [4].
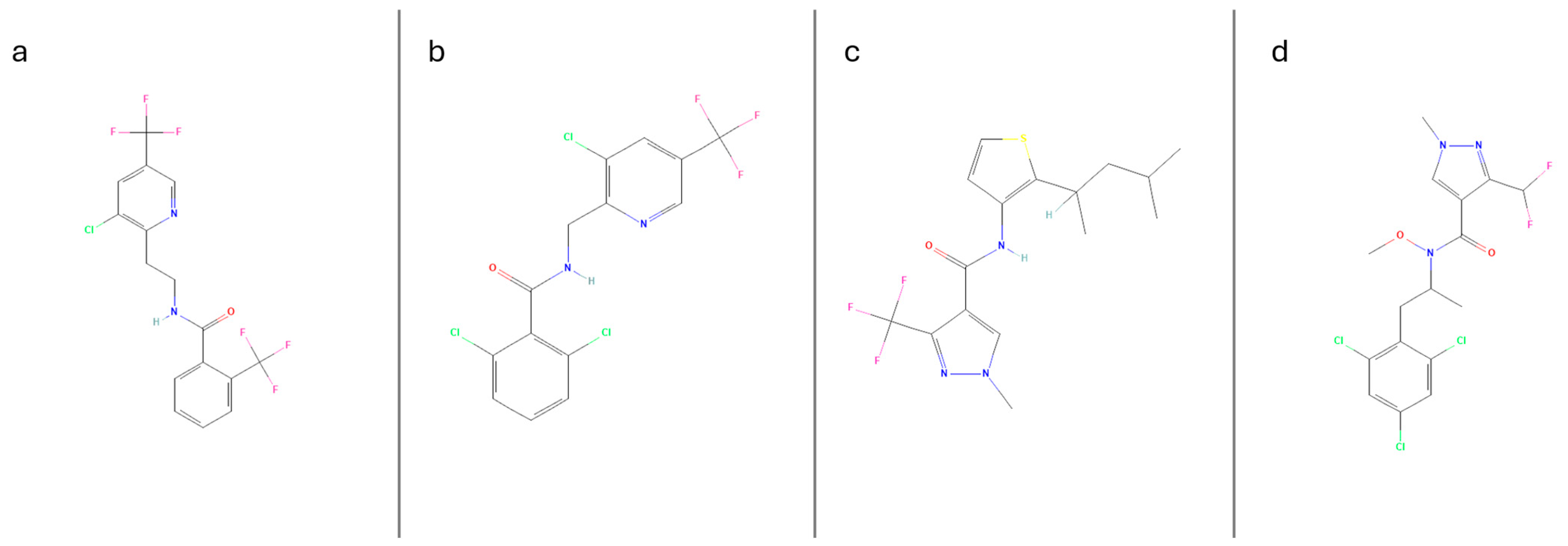
The widespread and repeated usage of pesticides with similar modes of action may contribute to emergence of resistance in pesticide hosts/pests. As such, incorporation of multiple pesticides with novel modes of action are required as part of control programs to prevent and offset the chance of pesticide resistance [5,6]. Succinate dehydrogenase inhibitors (SDHIs) are one of the most widely utilized classes of fungicides globally [7]. As such, over the years the SDHI class has seen continuous additions, including the following: fluopyram, penthiopyrad, and pydiflumetofen, which were developed within the last two decades [8,9]. The succinate dehydrogenase (SDH) complex, the molecular target of these fungicides, plays a vital role in the energy metabolism within target molds and fungi [10]. However, this complex and its significance extend to nearly all existing eukaryotes, including non-target organisms. The mode of action of these fungicides is not species-specific, and this has raised concerns regarding their potential toxicity to non-target organisms and their broader environmental impact [10,11]. Based on their physiochemical properties, SDHI fungicides are slightly polar with low-to-moderate soil and water mobilities (Figure 1). However, previous studies have demonstrated their propensity to contaminate groundwaters [12,13]. Furthermore, several reports have demonstrated the occurrence and prevalence of SDHI fungicides in surface water on a global scale across various water systems, [14,15]. Fluopicolide is another fungicide that shares similar properties to members of the SDHI category; however, its precise mode of action is not well understood (Figure 1). Similarly, residues of this fungicide and its metabolites have also been reported in several water systems across the European regions [12,16]. To our knowledge, only one published study is available confirming the detection of SDHI-class and similar fungicides, such as fluopicolide, in surface water systems in Australia [17].
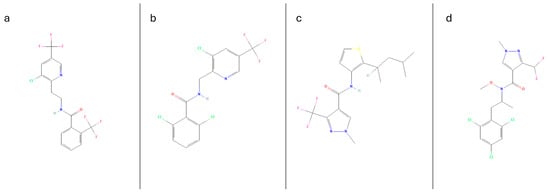
Figure 1.
Chemical structures of the 4 analytes under investigation, fluopyram (a), fluopicolide (b), penthiopyrad (c), pydiflumetofen (d), structure data adapted from the PubChem database [18].
Fungicides classified as SDHI can effectively inhibit SDH activity in various non-target species, including earthworms and humans [10]. Importantly, non-target aquatic and marine organisms may display a heightened sensitivity to these fungicides, thereby posing a potential risk due to their documented high toxicity [10,19].
Given these findings and the widespread use of SDHI fungicides, with the potential to contaminate aquatic environments, there is a distinct probability for adverse effects on non-target organisms. Adverse effects have been observed in cases of acute exposure, including developmental malformations, oxidative stress, and endocrine disruption within fish [20,21]. Additionally, chronic and sublethal exposure to multiple SDHI fungicides has been linked to issues such as mitochondrial dysfunction, metabolic disorders, visual impairments, and motor impairments within non-target aquatic organisms, such as fish and aquatic invertebrates [22,23].
Over the past decade, numerous fungicides have gained registration and approval for use in Victoria, Australia. Among them are several belonging to the novel SDHI class, along with others that share similar properties. These fungicides have collectively been approved for diverse applications across a broad spectrum of crops, including brassica and bulb vegetables, citrus fruits, berries, and nuts [24]. Additionally, these have been approved for use in commercial and industrial settings, such as on turfs and lawns. Given the limited availability of detection and toxicity studies, it is important to understand the presence of these fungicides in surface systems and potential impacts on non-target aquatic organisms.
Chromatographic separation used in tandem with mass spectrometric detection has enabled the analysis of trace level organic or chemical contaminants in several complex matrices including water, soils, and biota. The current study explores the use of liquid chromatography coupled to targeted multiple reaction monitoring (MRM) mass spectroscopy in the detection of polar molecules where the monitoring of select transitions can increase specificity, remove matrix interferences, and afford improved sensitivity and detection limits [25].
Prior to chromatographic analysis, sample extraction and pre-treatment are necessary steps which may directly impact the overall sensitivity and selectivity of the analytical method for compound analysis within a sample matrix. Some of the most established and commonly used sample extraction techniques include solid-phase extraction (SPE), liquid–liquid extraction (LLE) and solid-phase microextraction (SPME). Solid-phase extraction remains a widely used and accepted technique for residue extraction from across multiple matrices including water [26]. Offline SPE techniques are often linked to lengthy and laborious procedures, while online methods, although more accurate and efficient, tend to be considerably expensive and restricted to small sample batches per instrumental run [27,28]. The QuEChERS (quick, easy, cheap, effective, rugged, and safe) method offers a simple, rapid, and efficient technique for aiding liquid–liquid extraction in a variety of difficult matrices such as food in suspect and non-target residue screening. With various modifications to its methodology over the years, its applicability in pesticide residue analysis has expanded to different matrices, including environmental soil [29], sediment [30], and agricultural samples [31]. However, its application in the extraction of pesticides from surface water systems is limited [26], even more so for novel and emerging pesticides such as those from the SDHI class. Only a limited number of studies have developed and applied QuEChERS-based methodologies to quantify SDHI fungicides within aquatic matrices such as agricultural water [31,32].
This study has a dual purpose: firstly, to create and validate an optimized analytical method for detecting four recently registered fungicides in surface water systems within Australia; and secondly, to utilize the validated method for analyzing surface water samples from waterways in the Greater Melbourne area (GMA). The objective is to quantify the presence of the four SDHI fungicides, thereby enhancing our understanding of their occurrence in local waterways.
2. Materials and Methods
2.1. Reagents and Chemicals
Four high-purity fungicide standards were obtained for this experiment: fluopyram (LGC Standards, Wesel, Germany), fluopicolide, penthiopyrad (AccuStandard, New Haven, CT, USA), and pydiflumetofen (HPC Standards GmbH, Cunnersdorf, Germany). All standards were supplied through Novachem (Novachem Pty Ltd., Heidelberg West, Australia). Each standard was purchased as 100 μg/mL in acetonitrile derived from the neat product at a purity of 99.90% or greater. High-performance liquid chromatography (HPLC)-grade reagents included acetonitrile, methanol, formic acid, and ammonium formate (Merck, Mountain Highway, Australia).
2.2. Preparation of Standard Solution
A working standard mixture of 10 μg/mL consisting of all four fungicides from their respective stock solutions was prepared via serial dilution in HPLC-grade acetonitrile and stored at −20 °C until analysis within amber glass ampoules. This solution was used for sample spiking and preparation of calibration curves.
2.3. Blank Matrices
For the method development work, blank surface water samples were collected from a catchment located within the GMA. Water samples were collected from a clean site where residues of fungicides to be tested were unlikely to be present. The site water samples were collected in 1 L amber glass bottles with screw caps. The water samples were stored at −18 to −22 °C upon arrival at the laboratory. Sample collection and storage conditions were based on US EPA Method 1699 [33].
2.4. Sample Preparation and Extraction
A 10 mL sample was added into a 50 mL centrifuge tube followed by 10 mL of the extraction solvent, 1% formic acid (v/v) acetonitrile. Sample extraction was performed using a QuEChERS extraction pouch—EN method (Part No. 5982-0650) containing the following: 4 g MgSO4, 1 g NaCl, 1 g sodium citrate dihydrate, and 0.5 g sodium hydrogen citrate sesquihydrate. Then, the sample tubes were vortexed (Vortex-Genie 2, Scientific Industries incorporated, New York, NY, USA) and placed on a horizontal shaker (IKA-KS260 Basic, IKA, San Diego, CA, USA) for 2 min. The samples were then centrifuged (Thermo Scientific, Heraeus, Hanau, Germany, Megafuge 40) at 5100 rpm at 2 °C for 5 min. Then, 1 mL of the supernatant was transferred into a 4 mL microcentrifuge tube (prepacked with 150 mg MgSO4, 50 mg primary secondary amine (PSA), 50 mg octadecyl (CEC18)). The tubes were vortexed for 1 min and centrifuged at 5100 rpm at 2 °C for 5 min. A 650 µL aliquot was then filtered through 0.2 µm filtration tube, and a 450 µL aliquot of this filtrate was combined with the internal standard—triphenyl phosphate (TPP) (1 µg/L in acetonitrile). Finally, 300 µL of sample was transferred into 2 mL amber LC-MS vials fitted with glass inserts for subsequent analysis.
2.5. Quality Control
Quality control was established and maintained throughout all experiments by incorporating internal standard-spiked blanks for each batch of samples to be analyzed. To further ensure accuracy, a quality control process involved running a low-level spike (20 µg/L) and a reagent blank (acetonitrile) was incorporated after every 5 samples within every instrumental run. These measures were implemented in every batch to monitor for carry-over and instrument performance during the analysis. In addition, blank surface water samples without internal standards were introduced at the beginning of each run to confirm the absence of any residual target analytes in the sample matrix and prevent potential carry-over from highly concentrated spiked samples.
2.6. Instrumentation and Software
Chromatographic analysis of the pesticides was performed using a Waters ACQUITY UPLC H-class system (Waters, Australia), consisting of a Waters Xevo TQ-S mass spectrometer utilizing multiple reaction monitoring. An ACQUITY UPLC BEH C18 column (2.1 × 100 mm: 1.7 μm) (Waters Corporation) was utilized for chromatographic separation. Several combinations of mobile phases A and B were tested for this analysis. Peaks of each target analyte were observed under varied mobile phase combinations. The following composition was chosen as the optimal mobile phase composition yielding the highest peak intensities and relative abundance. It consisted of 10 mM ammonium formate in Milli-Q water containing 0.1% formic acid (v/v), while mobile phase B comprised pure methanol. The injection was performed at a flow rate 0.4 mL/min, with an injection volume of 2.0 µL. The instrumental run time for each sample was 5.50 min. The mobile phase gradient profile was as follows: 0–0.10 min 95% A, 5% B; 0.10–0.30 min 30% A, 70% B; 0.30–3.0 min 2% A, 98% B; 3.0–5.0 min 95% A, 5% B. The instrument was run using a positive electrospray ionization (ESI) mode for all compounds with scheduled multiple reaction monitoring (MRM) used for all analyses with the following conditions: capillary voltage (3 kV), cone voltage (30 V), source temperature (150 °C), desolvation temperature (400 °C), cone gas flow (150 L/h), desolvation gas flow (1000 L/h). The UHPLC-MS/MS conditions for the four target fungicides are shown in Table 1. The conditions were provided by the Quanpedia database (Waters Corporation, Milford, MA, USA) and used without further optimization. Data acquisition and processing were conducted using MassLynx 4.2 and Target Lynx XS 4.2 software (Waters Corporation, Milford, MA, USA).

Table 1.
Ultra-high-performance liquid chromatography parameters for the detection of four fungicides in positive ESI mode.
2.7. Method Validation and Matrix Effects
The method validation criteria in the European Commission SANTE/12682/2019 guidelines [34] for method linearity, matrix effects, limit of detection (LOD) and quantification (LOQ), accuracy (percentage recovery), and precision (relative standard deviation %) were followed.
Method linearity was determined using matrix-matched calibration curves produced using spiked blank samples at 0, 0.5, 5, 10, 20, 50 (for fluopyram and fluopicolide), and up to 100 µg/L (penthiopyrad and pydiflumetofen).
Method recovery was determined using seven replicate extractions and analysis at five (fluopyram, fluopicolide) and six (penthiopyrad, pydiflumetofen) spiked concentrations, respectively. Calculations were performed using peak areas according to Equation (1), where C1 is the concentration of analyte in the spiked sample, C2 is the concentration of analyte within the blank sample, and C3 is the known concentration added to the sample [35,36,37]. The acceptable recovery range is between 70 and 120% with relative standard deviation (% RSD) less than 20% [34]. Intra-day accuracy and precision were evaluated using seven replicates at n = 5 (fluopyram, fluopicolide) and 6 (penthiopyrad, pydiflumetofen) spiked concentrations, respectively.
The lowest spike level meeting the method’s performance and acceptability requirements throughout the validation process is defined as the limit of quantification (LOQ) [34]. As such, the LOQ for method sensitivity was established using the lowest calibration level spike for each analyte. The LOD and LOQ were calculated according to equations as specified by Magnusson [38]. In Equations (2) and (3), S is the standard deviation of the average (n = 7) replicates of a spiked low-level blank which undergoes extraction and analysis.
The LOD and LOQ were calculated as the 3- and 10-times corresponding standard deviation (SD) of seven replicate analyses of a low-level spike which meet acceptable recoveries (70–120%) and precision (RSD ≤ 20%). The determination of the measurement uncertainty (MU) is a requirement under the ISO/IEC 17025 [34] guidelines stated within the SANTE/12682/2019 [39] guidelines. Utilizing validation/QC data available through intra-lab experiments, an estimation of MU ( and expanded uncertainty (U) were calculated for each pesticide analyzed in this study.
The following definitions [39] were used in the above equations for the derivation of MU and its expanded uncertainty for each compound. The variable “” is the mean of the relative bias, is the population standard deviation of relative bias, is defined as the within-laboratory reproducibility, and is the uncertainty associated with method and laboratory bias estimated through proficiency testing data [39].
The uncertainty associated with spiked concentrations, is assumed negligible when certified analytical standards and calibrated/verified volumetric balances are used in the preparation of spiked samples [39]. As such, only the and were used for calculating within this study.
Finally, the expanded uncertainty of the method, expressed as (U), was obtained by multiplying combined uncertainty ( with a coverage factor k = 2 at a 95% confidence level.
2.8. Applications to Surface Water Samples
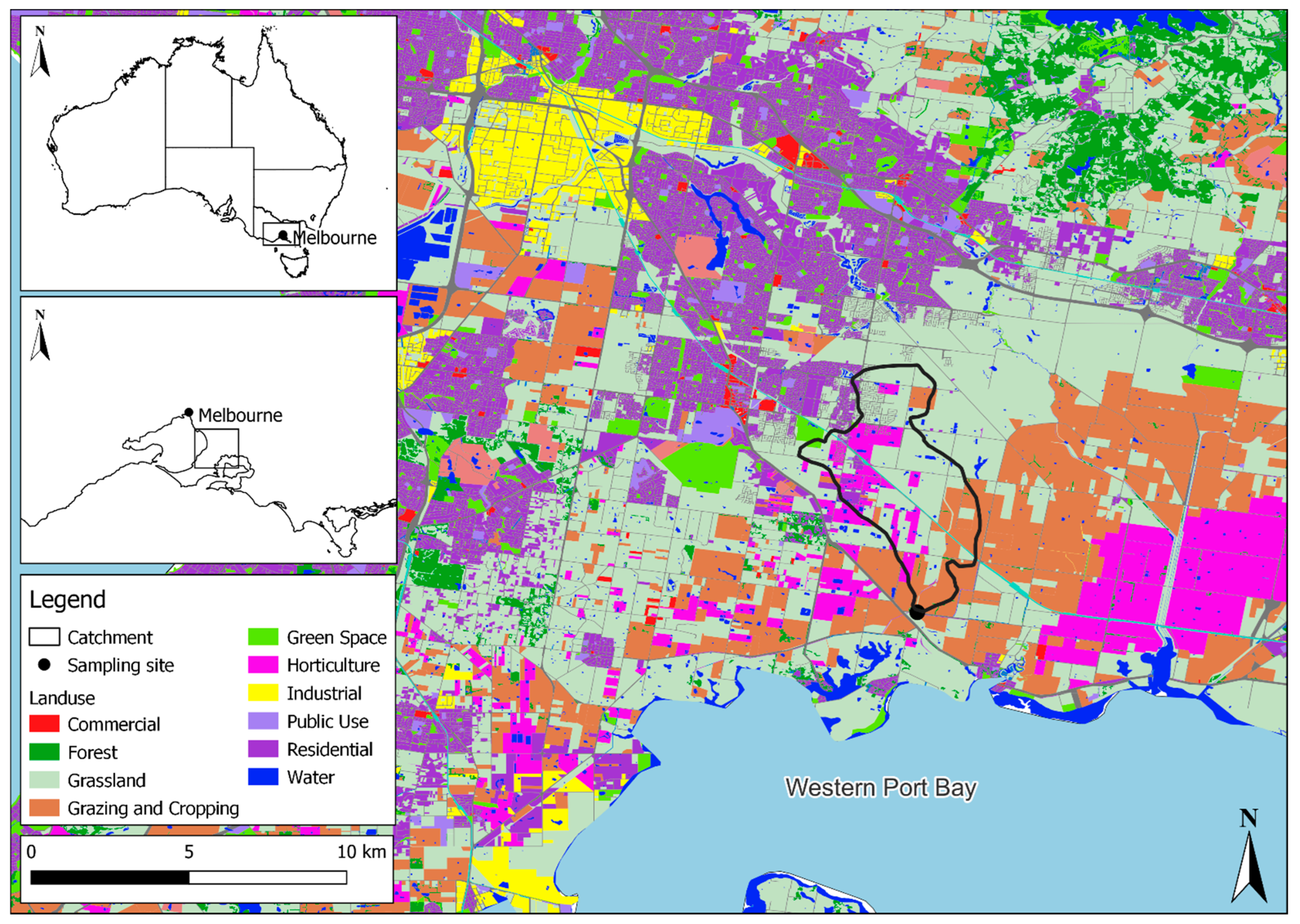
After the optimization and validation, this method was applied to surface water samples from one site within the GMA (Figure 2) collected during the periods of May, June, and September 2023. This site was previously linked to the detection of all four fungicides in a recent semi-quantitative passive sampling study [17]. The catchment sampled was in a location with intensive agricultural and residential land uses (Figure 2). Grab water samples of 1.0 L were taken in triplicate during each sampling month. These samples were stored at 2 °C overnight prior to analysis.
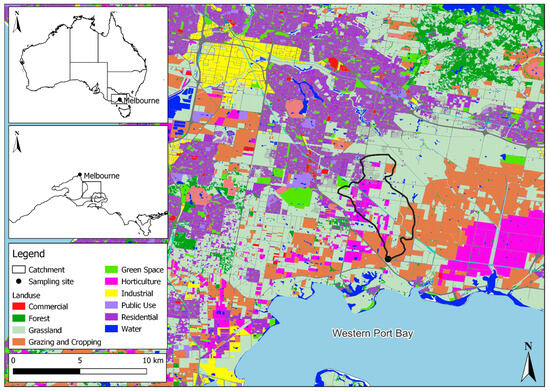
Figure 2.
The location of surface water sampling site (n = 1) for method application within the Greater Melbourne area, Victoria, Australia. The land use layers showcased in this image were provided by Melbourne Water and the Victorian Department of Environment, Land, Water, and Planning (DELWP) through Spatial Economics [40].
3. Results and Discussion
3.1. Instrumental Method Optimization
Several adjustments were made to optimize the instrumental method to yield satisfactory results for key parameters such as chromatographic peak shapes, separations, and recoveries for all four target compounds.
The utilization of formic acid and ammonium formate as additives in the mobile phase has been widely explored in various studies, with reported enhancements in peak intensity, separation of target compounds, and sensitivity of LC-ESI-MS detection [36,38,41]. Consequently, a mobile phase composition of 0.1% formic acid (v/v) and 10 mM ammonium formate in Milli-Q water (mobile phase A) was chosen. During the initial optimization studies, it was observed that methanol as the second mobile phase yielded higher relative abundance and peak intensities compared to solvents such as pure acetonitrile. Similar findings were reported in a multi-residue analysis study of pesticides employing QuEChERS for water samples [36]. Therefore, pure methanol (mobile phase B) was selected as the final optimized configuration for mobile phase B.
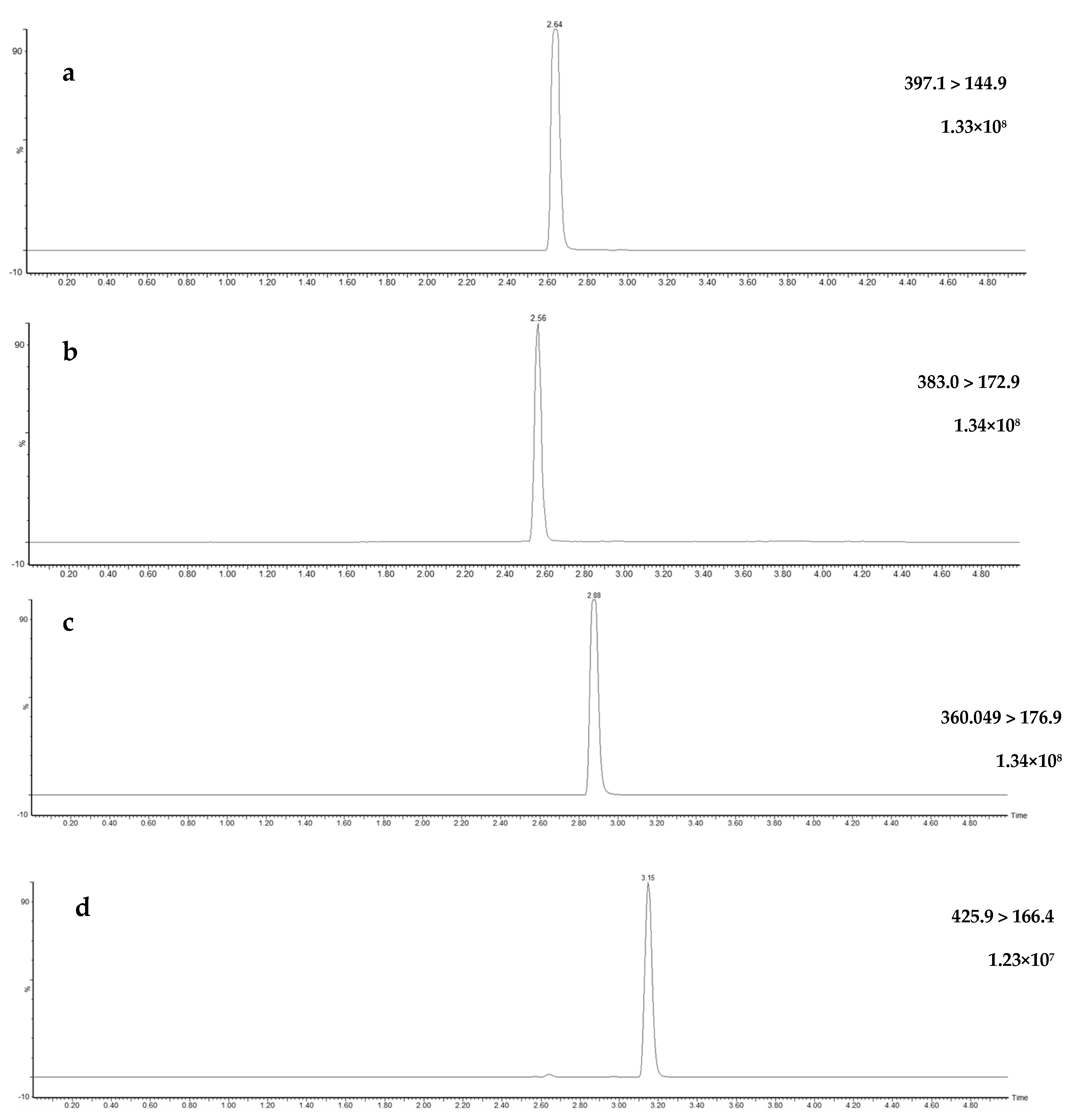
UHPLC instruments exhibit superior peak separations, lower flow rates, and faster run times compared to their more conventionally used predecessor, high-performance liquid chromatography (HPLC) instruments [42,43,44,45]. Method optimization and validation experiments for this study were conducted using a UHPLC system, with the flow rate adjusted to 0.4 mL/min, resulting in optimal separation of all target pesticides through gradient elution during a 5.5-min run (Figure 3). Additional MRM chromatograms, displaying the qualifier transitions for each compound at 50 µg/L and the quantification transitions at 5.0 µg/L, are available in Supplementary Figures S1 and S2, respectively. In this study, a variety of injection volumes were tested on the UHPLC instrument, which has the advantage of superior resolution and sensitivity relative to HPLC instrumentation [46,47]. The injection volumes of 0.5, 1, 2, and 5 µL were trialed and although larger injection volumes are associated with increased sensitivity and lower limits of detection [48,49], 2 µL was selected as providing adequate sensitivity without impairing analyte resolution when used as the injection solvent in acetonitrile.
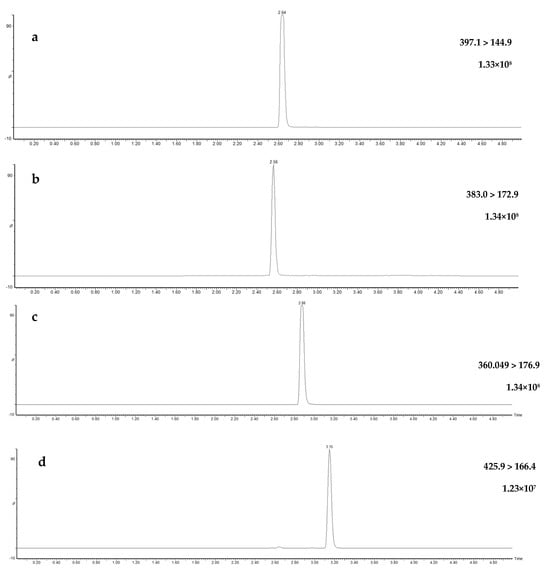
Figure 3.
Ultra-high-performance liquid chromatography multiple reaction monitoring chromatogram (quantification) for the four fungicides—fluopyram (a), fluopicolide (b), penthiopyrad (c), pydiflumetofen (d)—in spiked surface water (50 µg/L), their respective quantification ion transition including raw count intensities.
3.2. Sample Preparation and Extraction
The initial method development experiments were performed using ultrapure Milli-Q water. Optimization studies and subsequent analyses, including method validation, used blank surface water samples with non-detectable residues of the compounds of interest. This assurance was attained through the analysis of matrix blanks using the optimized conditions, which exhibited low background noise across all analytes. These blank surface water samples are representative of a typical matrix within shallow surface water with low flow, influenced by agricultural land use applications in proximity.
The QuEChERS sample preparation and extraction procedure can be modified and optimized based on the compounds of interest, matrix, and instrumental conditions. Various configurations of buffering salts and sorbents for QuEChERS clean-up are available; however, most of the literature on QuEChERS methods is focused on the analysis of pesticide residues with food, sediment, and soil matrices [50,51]. Only a limited number of examples of QuEChERS extractions from water matrices, particularly in the context of micropollutant analysis, are available [26,52].
As such, experiments were carried out to trial this clean-up technique for application within the surface water matrices for pesticide residue analysis. In this study, 1 g of NaCl was added along with 4 g MgSO4 during sample extraction phase for the blank surface water. This combination is widely employed in QuEChERS extraction for various contaminants, including fungicides, and is expected to enhance the selectivity of the analyzed compounds [32,53,54].
This study utilized sorbent conditions similar to those optimized by a recent study which focused on determining pydiflumetofen residues in multiple matrices, including paddy field water [32]. The researchers found the addition of PSA (150 mg), MgSO4, and C18 (50 mg) exhibited the highest efficiency in purification. Notably, the addition of 150 mg of PSA resulted in high recoveries in their sorbent comparison study. A similar result in the use of PSA was observed in this study, where 50 mg PSA achieved satisfactory recoveries (>90%). It is recommended that future optimization studies include an addition of 150 mg of PSA under the same experimental conditions to assess its impact on overall fungicide recoveries.
Other refinements reported in the literature, such as utilizing graphite carbon black (GCB) instead of the C18 QuEChERS additive, have yielded modest improvements in recoveries across a diverse range of pesticide classes [31]. However, this approach was not trialed in the current study. Acetonitrile with 0.1% formic acid (v/v) was used as the extraction solvent, as it was also found to be suitable for pydiflumetofen in a study analyzing this compound in paddy field water [32].
3.3. Method Validation
3.3.1. Linearity and Limit of Quantification
As advised within the EU guidelines [39], validation experiments were carried out using matrix-matched calibration curves for more accurate results, excluding influences of matrix effects. Method performances for the four fungicides in blank surface water are summarized in Table 2 and Table 3. Internal standard, triphenyl phosphate, was used to normalize data obtained during method development and validation experiments. Satisfactory linearities were observed for each pesticide with coefficients of determination: R2 > 0.99. During the development process using matrix-matched calibration curves, we found that the high sensitivity of the instrument affected the linearity of results. For fluopicolide and fluopyram, the linearity was maintained only up to 50 µg/L. In contrast, penthiopyrad and pydiflumetofen showed linear results up to 100 µg/L, which was the maximum concentration of the calibration curve. Method LOQ values for each of the fungicides are within the range of 0.2–0.6 µg/L (Table 2).

Table 2.
Limit of detection, limit of quantification, and method uncertainty parameters of the four target fungicides within the blank surface water samples.

Table 3.
Linearity, precision, mean recovery, and intra-day relative standard deviation values of the four target fungicides within the blank surface water samples.
3.3.2. Precision and Accuracy
The determined LOD values for individual pesticides were within the range of 0.1–0.2 (Table 2). All compounds had excellent recoveries at tested spike levels within blank surface water samples (Table 3) and were within the acceptable range of 70–120%. Method repeatability was determined through seven parallel analyses and results were expressed as intra-day RSD % values, with all values at ≤20%, which is within the acceptable range for environmental analysis [36,55].
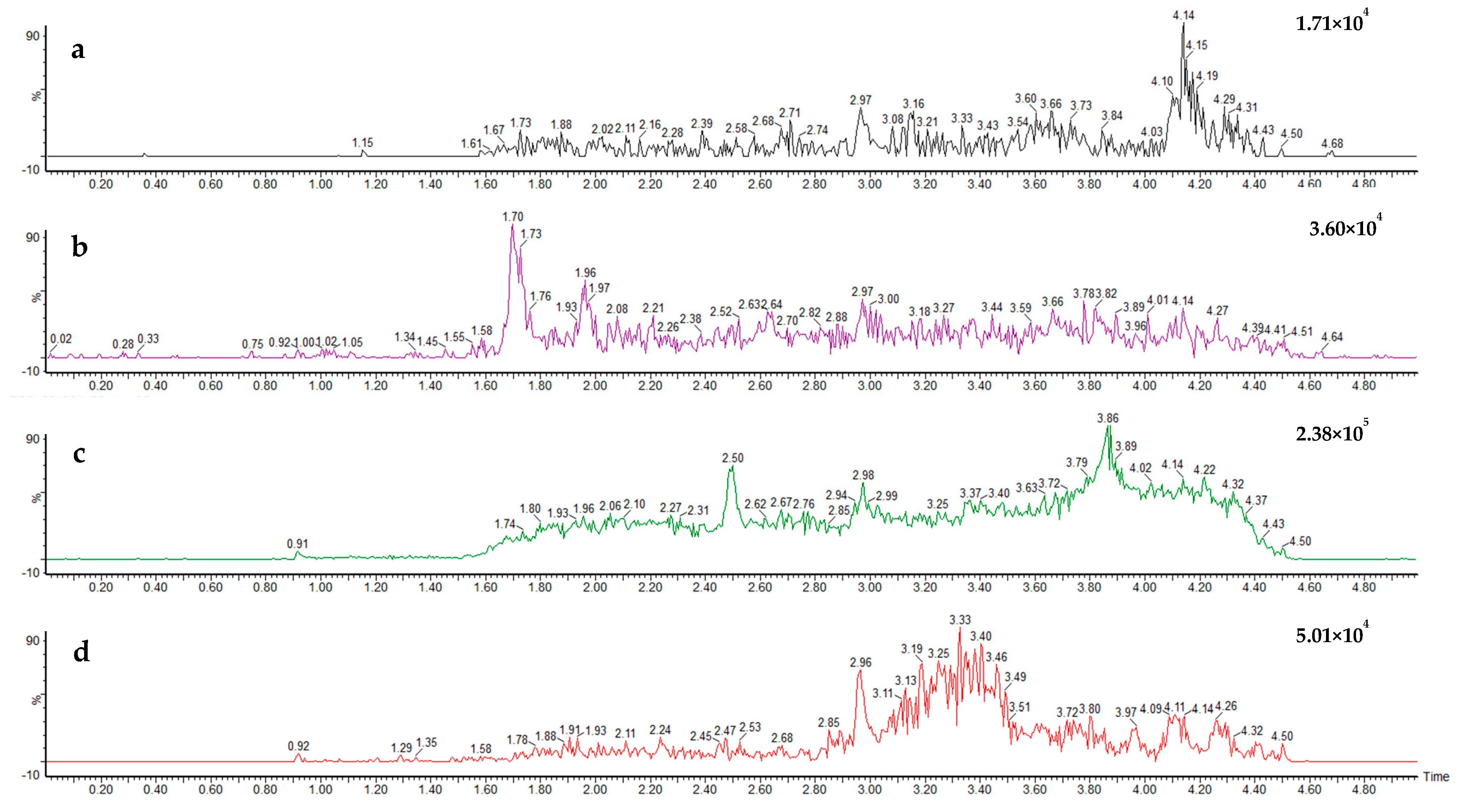
The results obtained during the validation experiments showed the developed method can successfully recover all target analytes within spiked blank environmental surface water samples at each tested range. An MRM chromatogram from the analysis of a standard mixture of the four compounds, presented in Figure 3, showcases the transitions used for quantification of each analyte. An example chromatogram for a blank surface water sample is provided (Figure 4), showing no detectable residues of any target analytes within its matrix.
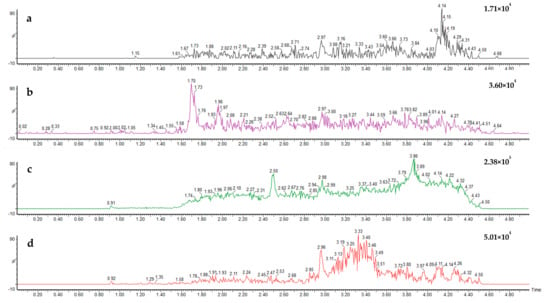
Figure 4.
Ultra-high-performance liquid chromatography multiple reaction monitoring chromatogram of a blank surface water sample showing non-detectable residues for all four target fungicides—pydiflumetofen (a), fluopyram (b), fluopicolide (c), penthiopyrad (d)—within its matrix.
3.3.3. Estimation of Measurement Uncertainty
Measurement uncertainty serves as a quantitative indicator for analytical data, prescribed by ISO/IEC 17025 [34]. In this study, we employed an empirical method to calculate uncertainty, incorporating reproducible results, including linearity, recovery, and precision, to determine a total uncertainty value. The values for combined and expanded uncertainty are presented in Table 2. Expanded uncertainty values at a 95% confidence level ranged from 20 to 35%. Pydiflumetofen exhibited the highest U value, potentially attributed to poorer ionization and sensitivity relative to other analytes, particularly at lower spiking levels (Table 2).
Despite this, all four compounds demonstrated a mean bias of less than 20% across the calibration range, with each expanded uncertainty value equal to or less than 35%. These results align with the requirements outlined in SANTE 12682/2019 guidelines [39], which stipulate that a default value of 50% should not be exceeded.
The notable variation in uncertainty associated with pydiflumetofen, in comparison to the other three fungicides, may be linked to differences in its chromatographic profile, interactions with the matrix, and stability. A comparable study utilized a QuEChERS-based LC-MS-MS method for a water matrix; however, information on method bias and uncertainty was not provided [32].
Ideally, the trials for assessing accuracy should differ from those for estimating bias. Preferably, the bias assessments should rely on an external, independent source such as certified reference materials (CRMs) and proficiency testing (PT) reference values [39]. Since these were not available for the fungicides analyzed, ongoing precision results from validation experiments were used for estimating measurement uncertainty within this study.
Therefore, for a comprehensive future study, an experiment utilizing isotopically labelled standards and certified reference material for each compound could be employed to provide a more accurate estimate of uncertainty or to lower bias in the extraction and associated recoveries to achieve lower expanded uncertainty values in our method.
3.4. Method Application to Real-World Samples
Our method for analyzing samples revealed the presence of all four pesticides in surface water samples collected over a three-month period. The four fungicides investigated in this study are currently registered and approved for use in Victoria, Australia, a status that has been maintained for at least the past two years according to the Australian Pesticides and Veterinary Medicines Authority [24]. These fungicides are currently registered for a number of pests for a range of hosts and land uses. This includes a broad range of vegetables including brassica and bulb varieties as well as fruits such as different citruses and berries [24].
The mean concentrations of each pesticide found in the water samples are presented in Table 4. The highest concentration among all detected pesticides occurred in May 2023, with fluopyram reaching a mean concentration of 7.3 µg/L; however, no detectable levels were observed in July and September. Fluopicolide was only detected at a trace level of 0.15 µg/L in September, while pydiflumetofen was found at a trace level of 0.19 µg/L, exclusively in June. Penthiopyrad consistently showed trace levels across all three sampling periods. An example MRM chromatogram from a sample collected in May 2023, highlighting analyte detections, is available in Supplementary Figure S3.

Table 4.
The mean pesticide concentrations within surface water samples from the Greater Melbourne area.
Fluopyram is authorized in Victoria for application on various fruits, including apples and strawberries. The increased concentrations observed in May at our sampling site, situated near numerous strawberry fields typically cultivated during early autumn and late winter, may be linked to this result. A similar hypothesis was explored in another study where consistent occurrences of fluopyram detected within winter periods coincided with greenhouse cultivation, as well as apple and strawberry harvesting [14]. This study did not conduct sampling during the previous summer and spring periods; however, monitoring the presence of fluopyram throughout the entire year is recommended—especially with its potential to occur at higher concentrations in spring and summer periods coinciding with increases agricultural use [56].
Fluopyram has been detected in both surface and groundwater systems globally, with maximum reported concentrations reaching 0.3 µg/L [15], 1.5 µg/L [14], and 6.0 µg/L [57] within surface water systems and >0.1 µg/L in groundwater [58]. The concentration detected in this study is comparatively higher than other reported values (Table 4).
Fluopyram is moderately mobile in soil and potentially contaminates surface waters through runoff or groundwater leaching [59,60]. It is expected to be more persistent in water–sediment environments and be stable within aquatic systems under anaerobic conditions [59]. As discussed above, its heightened concentrations in May could be directly linked to its use during this time or during previous summer periods, on produce such as strawberries. Non-detectable levels in the following months may be due to its binding to sediment, fast breakdown, and low persistency associated with surface water. Fluopyram can potentially decompose into toxic fluorine-containing compounds that may contaminate the environment [59,61]. Additionally, as highlighted by Hu, et al. [62] the photolytic degradation products of fluopyram can potentially induce higher toxicity than the parent compound [63]. The study by Li, et al. [64] showed that fluopyram and three of its breakdown products were toxic to non-target aquatic organisms including fish and Daphnia. Further toxicity studies on the effects of fluopyram on non-target aquatic species is not currently available, especially at chronic and sublethal concentrations [10].
The detection of fluopyram at 7 µg/L suggests low persistence in flowing water systems. However, its relatively high concentration could still impact local aquatic organisms, especially considering that chronic exposure levels as low as 30 µg/L have previously been reported to affect fish [64]. As such, in addition to long-term monitoring studies, a detailed ecotoxicological study involving both acute and low-level concentrations including fluopyram and its degradation products is recommended to better understand risks to local waterways.
Fluopicolide residues were detected at trace concentrations of up to 0.15 µg/L in September. Similarly, several global studies have reported fluopicolide residues, like fluopyram, in surface water systems, with levels as low as 0.02 µg/L [12,57,65]. Our study also found low-level detections (Table 4). Similar to fungicides from the SDHI class, the benzamide class, fluopicolide, has also been associated with moderate toxicities to non-target aquatic species including zebrafish and Daphnia and chronic effects to tadpoles, including developmental deformities [66,67,68].
Furthermore, there have been multiple reports on the presence of 2,6-dichlorobenzamide (BAM), a potential transformative product of fluopicolide, occurring with water systems [16,69,70]. Accordingly, consideration of the presence of fluopicolide and its degradation products, such as BAM, is warranted as part of further monitoring studies. In particular, consideration of pesticide breakdown products may reveal unknown or higher toxicological properties compared to the parent compound [71].
Penthiopyrad was detected at trace levels in the range of 0.11–0.16 µg/L during the three sampling periods. Pydiflumetofen was only detected in June at a trace concentration of 0.19 µg/L. Environmental detections of penthiopyrad and pydiflumetofen, especially within water systems, are very limited. As such, no comparison within surface water systems could be conducted. This may be attributed to their relatively recent entry into the pesticide market and limited analytical capabilities within monitoring programs [20,72]. Penthiopyrad has been associated with acute toxicity to fish. Several studies have highlighted the toxic effects induced by penthiopyrad through its mode of action, potentially exhibiting mitochondrial, metabolic disruption and developmental abnormalities within tested species such as zebrafish [73]. Although occurring at trace levels, its consistent presence could be associated with its long hydrolysis half-life (46–68 d) within aquatic systems [74]. While data on toxic effects of pydiflumetofen are limited, similar to other fungicides discussed within this study, it is also highly toxic to fish [75].
Both pydiflumetofen and penthiopyrad are chiral pesticides, existing as enantiomers. However, limited studies have investigated their enantioselective activities, including toxicities of specific isomers [76,77]. Available toxicological data on aquatic organisms recommend exploring their enantioselective effects for risk assessments [77]. Given the presence of pydiflumetofen in June and the continuous presence of penthiopyrad throughout all sampling periods in this study, further monitoring of these pesticides in surface water systems is justified. The proposed methodology may be used in conjunction with detailed future analysis, including novel enantioselective separations, as demonstrated by another recent study [72].
4. Conclusions
This study developed an optimized QuEChERS-UHPLC-MS/MS method to detect four recently registered fungicides in surface waters in Victoria, Australia. These fungicides were selected due to their limited global and Australian data and their high toxicity to aquatic organisms. The method was validated using blank surface water samples with matrix-matched calibrations, showing excellent linearity. Detection limits ranged from 0.1 to 0.2 µg/L, with recoveries between 90% and 108.8%, and precision from 2.8% to 18.7%. Expanded uncertainties for each compound were ≤35%. When applied to surface water samples from the Victorian GMA, all four fungicides were detected and accurately quantified for the first time, highlighting their persistence in aqueous environments. These findings underscore the need for ultra-trace fungicide monitoring, especially for novel compounds with limited detection and ecotoxicological data. The method will aid in assessing ecotoxicological risks and may inform regulatory monitoring for these and similar classes of fungicides in aquatic environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11100279/s1, Figure S1: Ultra-high performance liquid chromatography multiple reaction monitoring chromatogram of four fungicides -fluopyram (a), fluopicolide (b), penthiopyrad (c), pydiflumetofen (d) in spiked surface water sample (50 µg/L), showcasing their qualifier ion transition including their respective raw count intensities; Figure S2: Ultra-high performance liquid chromatography multiple reaction monitoring chromatogram of four fungicides -fluopyram (a), fluopicolide (b), penthiopyrad (c), pydiflumetofen (d) and internal standard -triphenylphosphate (e)in spiked surface water sample (5 µg/L), showcasing their quantification transition including their respective raw count intensities; Figure S3: Ultra-high performance liquid chromatography multiple reaction monitoring chromatogram of four fungicides -fluopyram (a), fluopicolide (b), penthiopyrad (c), pydiflumetofen (d) in contaminated surface water grab sample collected in May 2023, showcasing their quantification transition including their respective raw count intensities.
Author Contributions
P.S.: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing—original draft, Visualization. D.T.: Investigation, Writing—review and editing, Data curation. H.T.K.N.: Investigation, Validation, Resources, Project administration. D.N.: Supervision, Methodology, Writing—review and editing. V.P.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—original draft, Visualization, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a stipend funded by Melbourne Water through the Aquatic Pollution Prevention (A3P) partnership with RMIT University.
Data Availability Statement
The data presented in this study are available within the article and its Supplementary Materials.
Acknowledgments
We would like to thank Rhys Coleman (Melbourne Water) for providing this research opportunity and his comments on the draft manuscript, Saman Buddhadasa for coordinating and supporting the collaboration between NMI and RMIT University, and a special thanks to Monica Tewman for her invaluable support in gathering and managing key documents for the copyright and submission requirements. We would also like to thank the Aquatic Environmental Stress Research Group (AQUEST), RMIT for their assistance in field and laboratory work conducted during this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vryzas, Z.; Ramwell, C.; Sans, C. Pesticide prioritization approaches and limitations in environmental monitoring studies: From Europe to Latin America and the Caribbean. Environ. Int. 2020, 143, 105917. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Kadiru, S.; Patil, S.; D’Souza, R. Effect of pesticide toxicity in aquatic environments: A recent review. Int. J. Fish Aquat. Stud 2022, 10, 113–118. [Google Scholar] [CrossRef]
- Cuevas, N.; Martins, M.; Costa, P.M. Risk assessment of pesticides in estuaries: A review addressing the persistence of an old problem in complex environments. Ecotoxicology 2018, 27, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.C.; Chen, H.M.; Dong, A.Y.; Huang, G.Y.; Liu, Y.W.; Zhang, X.; Wang, W.; Hao, G.F.; Yang, G.F. Pesticide Informatics Platform (PIP): An International Platform for Pesticide Discovery, Residue, and Risk Evaluation. J Agric Food Chem 2022, 70, 6617–6623. [Google Scholar] [CrossRef]
- Das, S.K. Mode of action of pesticides and the novel trends—A critical review. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 393–401. [Google Scholar]
- Duarte Hospital, C.; Tête, A.; Debizet, K.; Imler, J.; Tomkiewicz-Raulet, C.; Blanc, E.B.; Barouki, R.; Coumoul, X.; Bortoli, S. SDHi fungicides: An example of mitotoxic pesticides targeting the succinate dehydrogenase complex. Environ. Int. 2023, 180, 108219. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- US EPA. US EPA—Pesticides—Fact Sheet for Fluopicolide; US EPA: Washington, DC, USA, 2007. [Google Scholar]
- Yanicostas, C.; Soussi-Yanicostas, N. SDHI Fungicide Toxicity and Associated Adverse Outcome Pathways: What Can Zebrafish Tell Us? Int. J. Mol. Sci. 2021, 22, 12362. [Google Scholar] [CrossRef]
- Marchand, P.A. EU Chemical Plant Protection Products in 2023: Current State and Perspectives. Agrochemicals 2023, 2, 106–117. [Google Scholar] [CrossRef]
- Gulkowska, A.; Buerge, I.; Poiger, T. Online solid phase extraction LC–MS/MS method for the analysis of succinate dehydrogenase inhibitor fungicides and its applicability to surface water samples. Anal. Bioanal. Chem. 2014, 406, 6419–6427. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T.J.; Smalling, K.L.; Orlando, J.L.; Kuivila, K.M. Occurrence of boscalid and other selected fungicides in surface water and groundwater in three targeted use areas in the United States. Chemosphere 2012, 89, 228–234. [Google Scholar] [CrossRef] [PubMed]
- la Cecilia, D.; Dax, A.; Ehmann, H.; Koster, M.; Singer, H.; Stamm, C. Continuous high-frequency pesticide monitoring in a small tile-drained agricultural stream to reveal diel concentration fluctuations in dry periods. Front. Water 2023, 4, 1062198. [Google Scholar] [CrossRef]
- Nanusha, M.Y.; Frøkjær, E.E.; Liigand, J.; Christensen, M.R.; Hansen, H.R.; Hansen, M. Unravelling the occurrence of trace contaminants in surface waters using semi-quantitative suspected non-target screening analyses. Environ. Pollut. 2022, 315, 120346. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Manamsa, K.; Talbot, J. Persistent and emerging micro-organic contaminants in Chalk groundwater of England and France. Environ. Pollut. 2015, 203, 214–225. [Google Scholar] [CrossRef]
- Serasinghe, P.; Nguyen, H.T.K.; Hepburn, C.; Nugegoda, D.; Pettigrove, V. Use of passive sampling and high-resolution mass spectrometry for screening emerging pesticides of concern within surface waters. J. Hazard. Mater. Adv. 2024, 13, 100408. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2018, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, X.; Zhang, H.; Li, W. Chronic toxic effects of isoflucypram on reproduction and intestinal energy metabolism in zebrafish (Danio rerio). Environ. Pollut. 2022, 315, 120479. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Cao, F.; Zhang, J.; Li, C.; Song, M.; Wang, C. Effects of penthiopyrad on the development and behaviour of zebrafish in early-life stages. Chemosphere 2019, 214, 184–194. [Google Scholar] [CrossRef]
- Teng, M.; Zhu, W.; Wang, D.; Yan, J.; Qi, S.; Song, M.; Wang, C. Acute exposure of zebrafish embryo (Danio rerio) to flutolanil reveals its developmental mechanism of toxicity via disrupting the thyroid system and metabolism. Environ. Pollut. 2018, 242, 1157–1165. [Google Scholar] [CrossRef]
- Qian, L.; Qi, S.; Wang, Z.; Magnuson, J.T.; Volz, D.C.; Schlenk, D.; Jiang, J.; Wang, C. Environmentally relevant concentrations of boscalid exposure affects the neurobehavioral response of zebrafish by disrupting visual and nervous systems. J. Hazard. Mater. 2021, 404, 124083. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lv, L.; Di, S.; Li, X.; Weng, H.; Wang, X.; Wang, Y. Combined toxic impacts of thiamethoxam and four pesticides on the rare minnow (Gobiocypris rarus). Environ. Sci. Pollut. Res. 2021, 28, 5407–5416. [Google Scholar] [CrossRef] [PubMed]
- APVMA. Public Chemical Registration Information System—PUBCRIS. Available online: https://portal.apvma.gov.au/pubcris (accessed on 18 June 2020).
- Beretov, J.; Wasinger, V.C.; Graham, P.H.; Millar, E.K.; Kearsley, J.H.; Li, Y. Proteomics for breast cancer urine biomarkers. Adv. Clin. Chem. 2014, 63, 123–167. [Google Scholar] [CrossRef]
- Hajeb, P.; Zhu, L.; Bossi, R.; Vorkamp, K. Sample preparation techniques for suspect and non-target screening of emerging contaminants. Chemosphere 2022, 287, 132306. [Google Scholar] [CrossRef] [PubMed]
- Suseela, M.N.L.; Viswanadh, M.K.; Mehata, A.K.; Priya, V.; Vikas; Setia, A.; Malik, A.K.; Gokul, P.; Selvin, J.; Muthu, M.S. Advances in solid-phase extraction techniques: Role of nanosorbents for the enrichment of antibiotics for analytical quantification. J. Chromatogr. A 2023, 1695, 463937. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, J.H.; Abd El-Aty, A.; Chung, H.S.; Lee, H.S.; Kim, S.W.; Rahman, M.M.; Park, B.J.; Kim, J.E.; Shin, H.C. Development of a single-run analytical method for the detection of ten multiclass emerging contaminants in agricultural soil using an acetate-buffered QuEChERS method coupled with LC–MS/MS. J. Sep. Sci. 2017, 40, 415–423. [Google Scholar] [CrossRef]
- Li, L.; Yin, Y.; Zheng, G.; Liu, S.; Zhao, C.; Xie, W.; Ma, L.; Shan, Q.; Dai, X.; Wei, L. Determination of multiclass herbicides in sediments and aquatic products using QuECHERS combined with ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) and its application to risk assessment of rice-fish co-culture system in China. Microchem. J. 2021, 170, 106628. [Google Scholar] [CrossRef]
- Song, N.-E.; Jung, Y.S.; Choi, J.Y.; Koo, M.; Choi, H.-K.; Seo, D.-H.; Lim, T.-G.; Nam, T.G. Development and application of a multi-residue method to determine pesticides in agricultural water using QuEChERS extraction and LC-MS/MS analysis. Separations 2020, 7, 52. [Google Scholar] [CrossRef]
- Bian, C.; Gao, M.; Liu, L.; Zhou, W.; Li, Y.; Wan, C.; Li, B. Determination of Pydiflumetofen Residues in Rice and its Environment by an Optimized QuEChERS Method Coupled with HPLC-MS. Bull. Environ. Contam. Toxicol. 2021, 107, 239–247. [Google Scholar] [CrossRef]
- Englert, B. Method 1699: Pesticides in Water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS; US Environmental Protection Agency (EPA): Washington, DC, USA, 2007; pp. 1–96. [Google Scholar]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organisation for Standardisation, Croatian Standard Institute: Zagreb, Croatia, 2007.
- Koçyiğit, H.; Sinanoğlu, F. Method validation for the analysis of pesticide residue in aqueous environment. Environ. Monit. Assess 2020, 192, 567. [Google Scholar] [CrossRef] [PubMed]
- Zaidon, S.Z.; Ho, Y.B.; Hamsan, H.; Hashim, Z.; Saari, N.; Praveena, S.M. Improved QuEChERS and solid phase extraction for multi-residue analysis of pesticides in paddy soil and water using ultra-high performance liquid chromatography tandem mass spectrometry. Microchem. J. 2019, 145, 614–621. [Google Scholar] [CrossRef]
- Magnusson, B. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics (2014); Euracheml: Lisboa, Portugal, 2014; p. 2. [Google Scholar]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; SANTE/12682/2019; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Spatial Economics. Land Use Projections Greater Metropolitan Melbourne 2016 to 2051. 2016. Available online: https://www.portphillip.vic.gov.au/media/iu5h5tdo/11-2-att-1-draft-yarra-catchment-integrated-water-management-plan.pdf (accessed on 20 September 2024).
- Wang, S.; Wang, X.; He, Q.; Lin, H.; Chang, H.; Sun, H.; Liu, Y. Simultaneous Determination of Seven Pesticides and Metabolite Residues in Litchi and Longan through High-Performance Liquid Chromatography-Tandem Mass Spectrometry with Modified QuEChERS. Molecules 2022, 27, 5737. [Google Scholar] [CrossRef] [PubMed]
- Rizzetti, T.M.; Kemmerich, M.; Martins, M.L.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Optimization of a QuEChERS based method by means of central composite design for pesticide multiresidue determination in orange juice by UHPLC–MS/MS. Food Chem. 2016, 196, 25–33. [Google Scholar] [CrossRef]
- Su, Y.; Lu, J.; Liu, J.; Li, F.; Wang, N.; Lei, H.; Shen, X. Optimization of a QuEChERS-LC-MS/MS method for 51 pesticide residues followed by determination of the residue levels and dietary intake risk assessment in foodstuffs. Food Chem 2024, 434, 137467. [Google Scholar] [CrossRef]
- Guillarme, D.; Dong, M. UHPLC, Part II: Benefits. LCGC N. Am. 2017, 35, 486–495. [Google Scholar]
- Behnoush, B.; Sheikhazadi, A.; Bazmi, E.; Fattahi, A.; Sheikhazadi, E.; Saberi Anary, S.H. Comparison of UHPLC and HPLC in benzodiazepines analysis of postmortem samples: A case-control study. Medicine 2015, 94, e640. [Google Scholar] [CrossRef]
- Frenich, A.G.; Romero-González, R.; del Mar Aguilera-Luiz, M. Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. TrAC Trends Anal. Chem. 2014, 63, 158–169. [Google Scholar] [CrossRef]
- Swetha, S.R.; Bhavya, S.K.; Mounika, C. A Review on Comparative study of HPLC and UPLC. Res. J. Pharm. Technol. 2020, 13, 1570–1574. [Google Scholar] [CrossRef]
- Chawla, G.; Ranjan, C. Principle, instrumentation, and applications of UPLC: A novel technique of liquid chromatography. Open Chem. J. 2016, 3. [Google Scholar] [CrossRef]
- Ren, D.-B.; Yang, Z.-H.; Liang, Y.-Z.; Fan, W.; Ding, Q. Effects of injection volume on chromatographic features and resolution in the process of counter-current chromatography. J. Chromatogr. A 2013, 1277, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hogenboom, A.C.; Hofman, M.P.; Kok, S.J.; Niessen, W.M.; Brinkman, U.A. Determination of pesticides in vegetables using large-volume injection column liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2000, 892, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Dacal, A.; Rial-Berriel, C.; Díaz-Díaz, R.; Bernal-Suárez, M.d.M.; Luzardo, O.P. Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci. Total Environ. 2021, 753, 142015. [Google Scholar] [CrossRef] [PubMed]
- Golge, O.; Koluman, A.; Kabak, B. Validation of a Modified QuEChERS Method for the Determination of 167 Pesticides in Milk and Milk Products by LC-MS/MS. Food Anal. Methods 2018, 11, 1122–1148. [Google Scholar] [CrossRef]
- Berlioz-Barbier, A.; Vauchez, A.; Wiest, L.; Baudot, R.; Vulliet, E.; Cren-Olivé, C. Multi-residue analysis of emerging pollutants in sediment using QuEChERS-based extraction followed by LC-MS/MS analysis. Anal. Bioanal. Chem. 2014, 406, 1259–1266. [Google Scholar] [CrossRef]
- Jing, W.; Nakano, K.; Shen, Z.; Okuda, T. Optimization of the QuEChERS extraction method to determine Polycyclic Aromatic Hydrocarbons (PAHs) in powder aerosol particles collected by cyclone. Environ. Technol. Innov. 2023, 31, 103141. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS - Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Simultaneous determination of veterinary antibiotics and hormone in broiler manure, soil and manure compost by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 160–168. [Google Scholar] [CrossRef]
- Wightwick, A.M.; Bui, A.D.; Zhang, P.; Rose, G.; Allinson, M.; Myers, J.H.; Reichman, S.M.; Menzies, N.W.; Pettigrove, V.; Allinson, G. Environmental fate of fungicides in surface waters of a horticultural-production catchment in southeastern Australia. Arch. Environ. Contam. Toxicol. 2012, 62, 380–390. [Google Scholar] [CrossRef]
- Spycher, S.; Mangold, S.; Doppler, T.; Junghans, M.; Wittmer, I.; Stamm, C.; Singer, H. Pesticide Risks in Small Streams—How to Get as Close as Possible to the Stress Imposed on Aquatic Organisms. Environ. Sci. Technol. 2018, 52, 4526–4535. [Google Scholar] [CrossRef]
- Sjerps, R.M.A.; Kooij, P.J.F.; van Loon, A.; Van Wezel, A.P. Occurrence of pesticides in Dutch drinking water sources. Chemosphere 2019, 235, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Rathod, P.; Shah, P.; Parmar, K.; Kalasariya, R. The Fate of Fluopyram in the Soil–Water–Plant Ecosystem: A Review. Rev. Environ. Contam. Toxicol. 2022, 260, 1. [Google Scholar] [CrossRef]
- APVMA. Submission to the Productivity Commission inquiry into Regulation of Australian Agriculture; APVMA: Armidale, Australia, 2016. [Google Scholar]
- Dong, B.; Hu, J. Photodegradation of the novel fungicide fluopyram in aqueous solution: Kinetics, transformation products, and toxicity evolvement. Environ. Sci. Pollut. Res. 2016, 23, 19096–19106. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pang, K.; Dong, B.Z. Mechanism and identify photolysis products of fluopyram under TiO2: Experiments, DFT and ab initio Molecular dynamics study. SDRP J. Earth Sci. Environ. Stud. 2019, 4, 681–690. [Google Scholar] [CrossRef]
- Zhan, J.; Liang, Y.; Liu, D.; Liu, C.; Liu, H.; Wang, P.; Zhou, Z. Organochlorine pesticide acetofenate and its hydrolytic metabolite in rabbits: Enantioselective metabolism and cytotoxicity. Pestic. Biochem. Physiol. 2018, 145, 76–83. [Google Scholar] [CrossRef]
- Li, C.; Yuan, S.; Jiang, F.; Xie, Y.; Guo, Y.; Yu, H.; Cheng, Y.; Qian, H.; Yao, W. Degradation of fluopyram in water under ozone enhanced microbubbles: Kinetics, degradation products, reaction mechanism, and toxicity evaluation. Chemosphere 2020, 258, 127216. [Google Scholar] [CrossRef]
- Pazikowska-Sapota, G.; Galer-Tatarowicz, K.; Dembska, G.; Wojtkiewicz, M.; Duljas, E.; Pietrzak, S.; Dzierzbicka-Glowacka, L.A. The impact of pesticides used at the agricultural land of the Puck commune on the environment of the Puck Bay. PeerJ 2020, 8, e8789. [Google Scholar] [CrossRef]
- Wen, S.; Wang, Y.; Wang, X.; Liu, C.; Xue, Y.; Liu, C.; Wang, J.; Xia, X. Fluopicolide-Induced Oxidative Stress and DNA Damage in the Earthworm Eisenia foetida. Toxics 2023, 11, 808. [Google Scholar] [CrossRef]
- Jin, L.; Yun, G.; Wei, M.; Kaiyun, W.; Hui, X.; Jie, L. Acute toxicity of fluopicolide to 9 kinds of environmental organisms and its bioaccumulation in zebrafish. Asian J. Ecotoxicol. 2016, 6, 296–305. [Google Scholar]
- Lin, J.; Wang, H.; Wang, K.; Fan, W.; Xu, H.; Liu, J. Toxic effects of fluopicolide on zebrafish. China Environ. Sci. 2014, 34, 3230–3236. [Google Scholar]
- Lopez, B.; Ollivier, P.; Togola, A.; Baran, N.; Ghestem, J.-P. Screening of French groundwater for regulated and emerging contaminants. Sci. Total Environ. 2015, 518–519, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015, 503–504, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Adasakaveg, E.J.; Michailides, T.; Eskalen, A. Fungicides, Bactericides, Biocontrols, and Natural Products for Deciduous Tree Fruit and Nut, Citrus, Strawberry, and Vine Crops in California; University of California Agriculture and Natural Resources: Davis, CA, USA, 2022. [Google Scholar]
- Wang, Z.; Liu, S.; Zhao, X.; Tian, B.; Sun, X.; Zhang, J.; Gao, Y.; Shi, H.; Wang, M. Enantioseparation and stereoselective dissipation of the novel chiral fungicide pydiflumetofen by ultra-high-performance liquid chromatography tandem mass spectrometry. Ecotoxicol. Envioron. Saf. 2021, 207, 111221. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Liang, H.; Li, L.; Li, Y.; Liang, H.; Zhao, T.; Chen, H.; Zhao, Y. Enantioselective toxic effects of the novel chiral antifungal agrochemical penthiopyrad in the early life stage of zebrafish (Danio rerio). Chem.-Biol. Interact. 2023, 369, 110252. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Lan, T.; Dou, L.; Zhang, K. Potential enantioselectivity of the hydrolysation and photolysation of the chiral agrochemical penthiopyrad in aquatic environments. Environ. Sci. Water Res. Technol. 2021, 7, 1220–1229. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Y.; Li, Y.; Duan, J.; Wu, Q.; Li, R.; Shi, H.; Wang, M. Comprehensive study of pydiflumetofen in Danio rerio: Enantioselective insight into the toxic mechanism and fate. Environ. Int. 2022, 167, 107406. [Google Scholar] [CrossRef]
- Di, S.; Cang, T.; Liu, Z.; Xie, Y.; Zhao, H.; Qi, P.; Wang, Z.; Xu, H.; Wang, X. Comprehensive evaluation of chiral pydiflumetofen from the perspective of reducing environmental risks. Sci. Total Environ. 2022, 826, 154033. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, T.; Li, Y.; Liang, H.; Zhao, Y.; Chen, H.; Li, L.; Liang, H. Enantioselective bioaccumulation and toxicity of the novel chiral antifungal agrochemical penthiopyrad in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 228, 113010. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).