Kinetic Study and Process Optimization of Plutonium Barrier Units for Enhanced Plutonium Stripping in the PUREX Process
Abstract
:1. Introduction
2. Experimental Setup
2.1. Reagents and Instruments
2.2. Extraction and Stripping Experiments
2.3. Methods of Analysis
2.4. Analyses of Simulation Software
3. Results
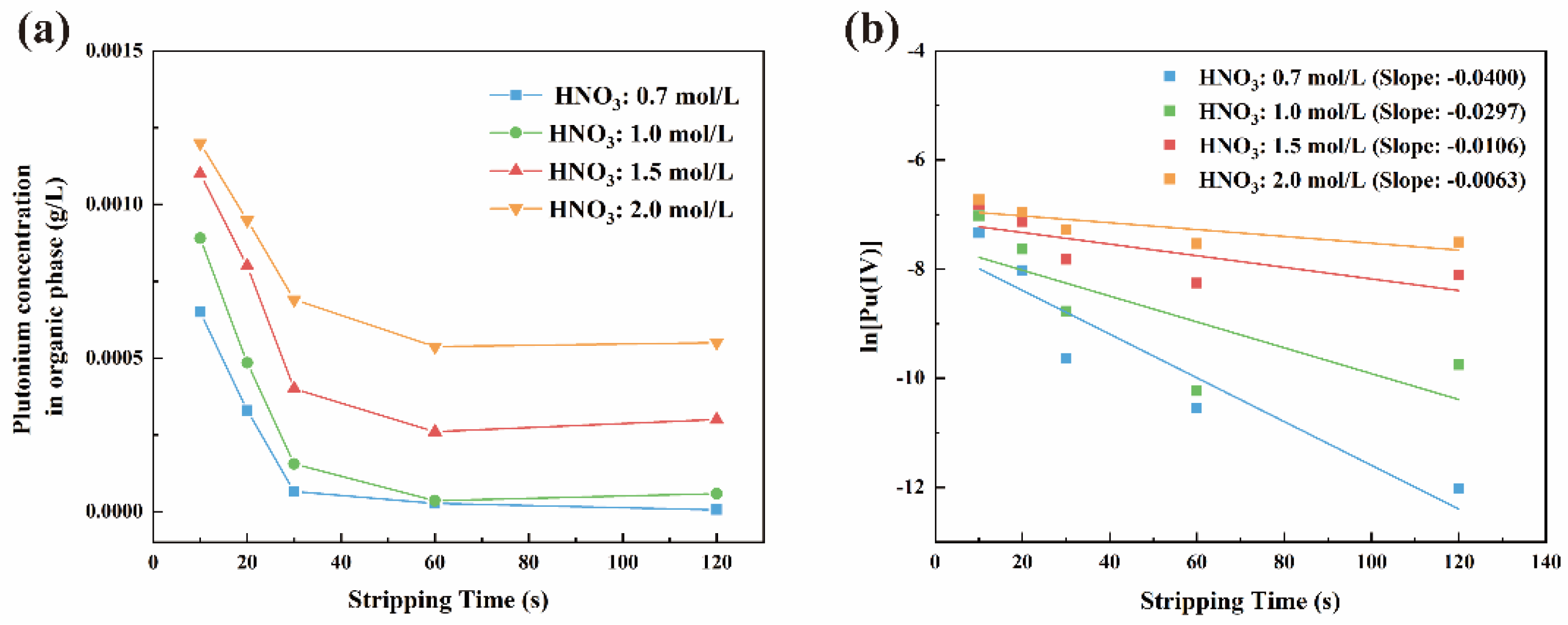
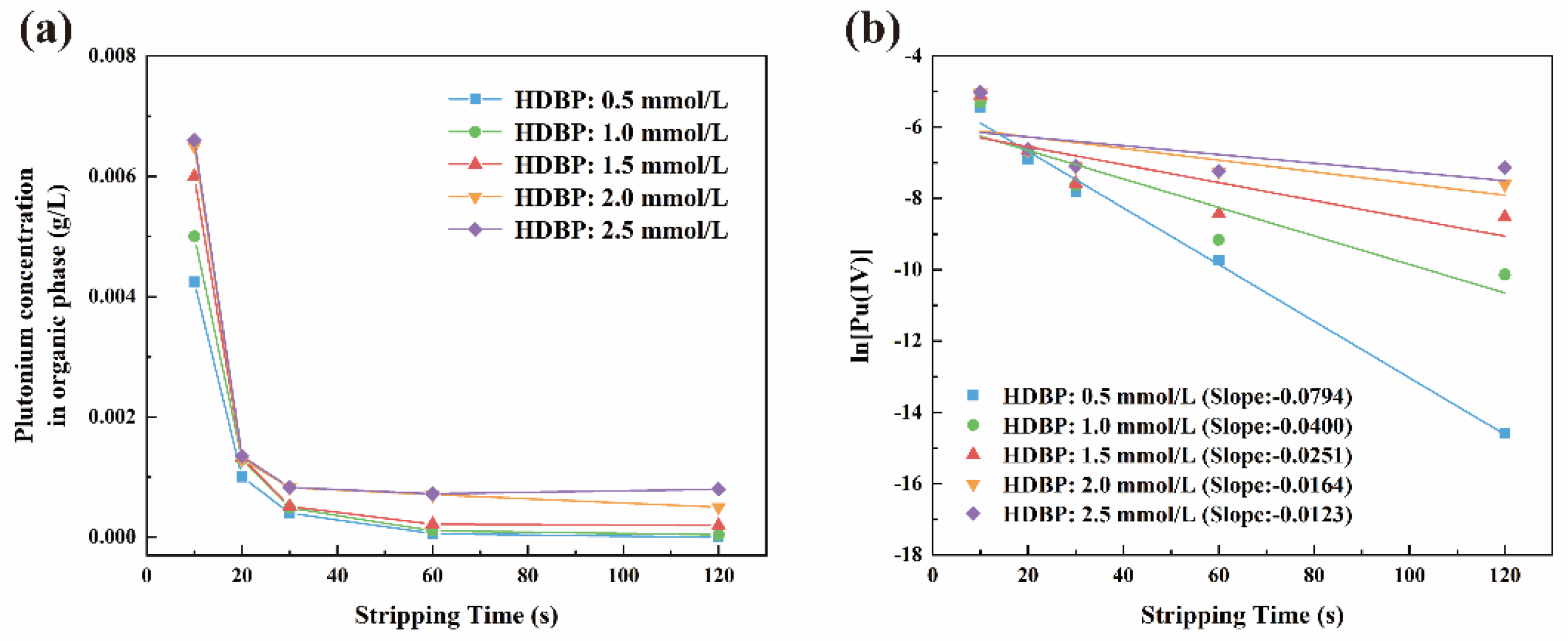
3.1. Kinetic Study of U(IV) Reduction and Stripping of Pu(IV)
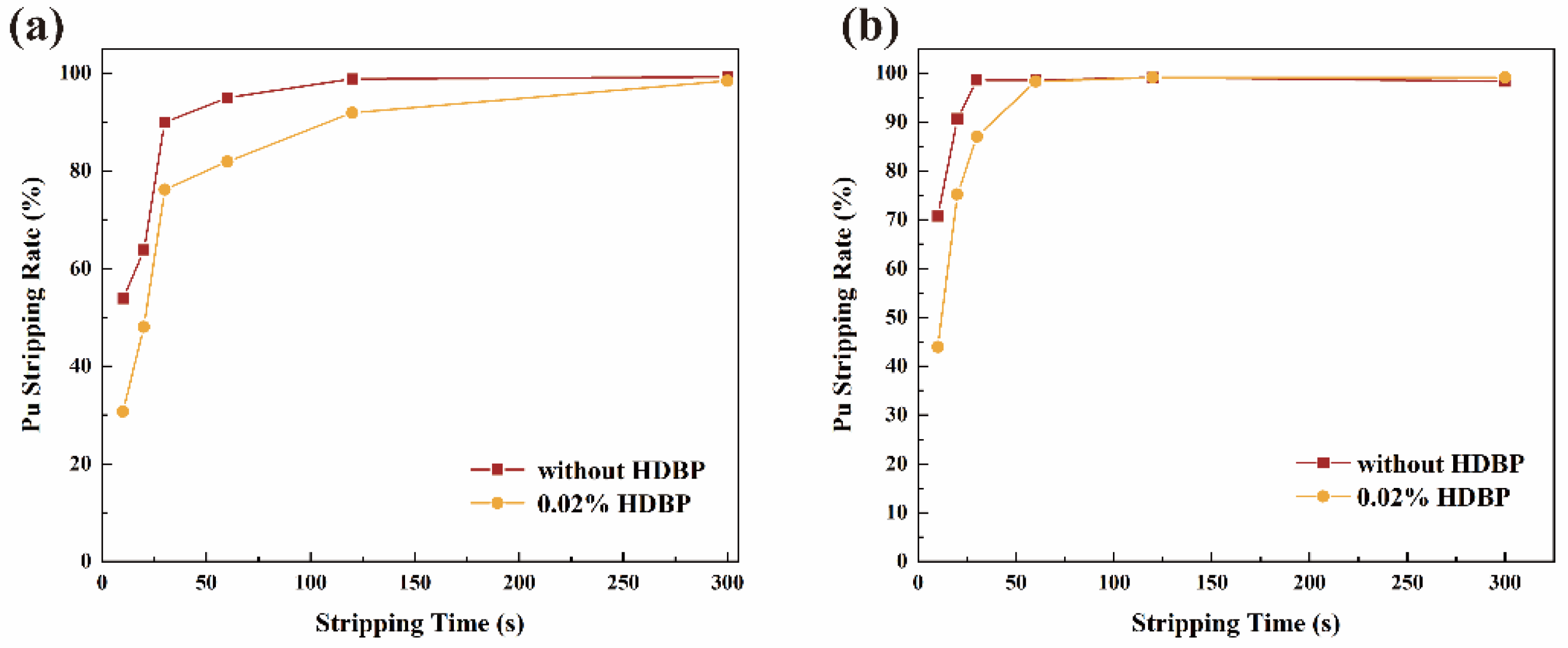
3.1.1. Reaction Equilibrium Time
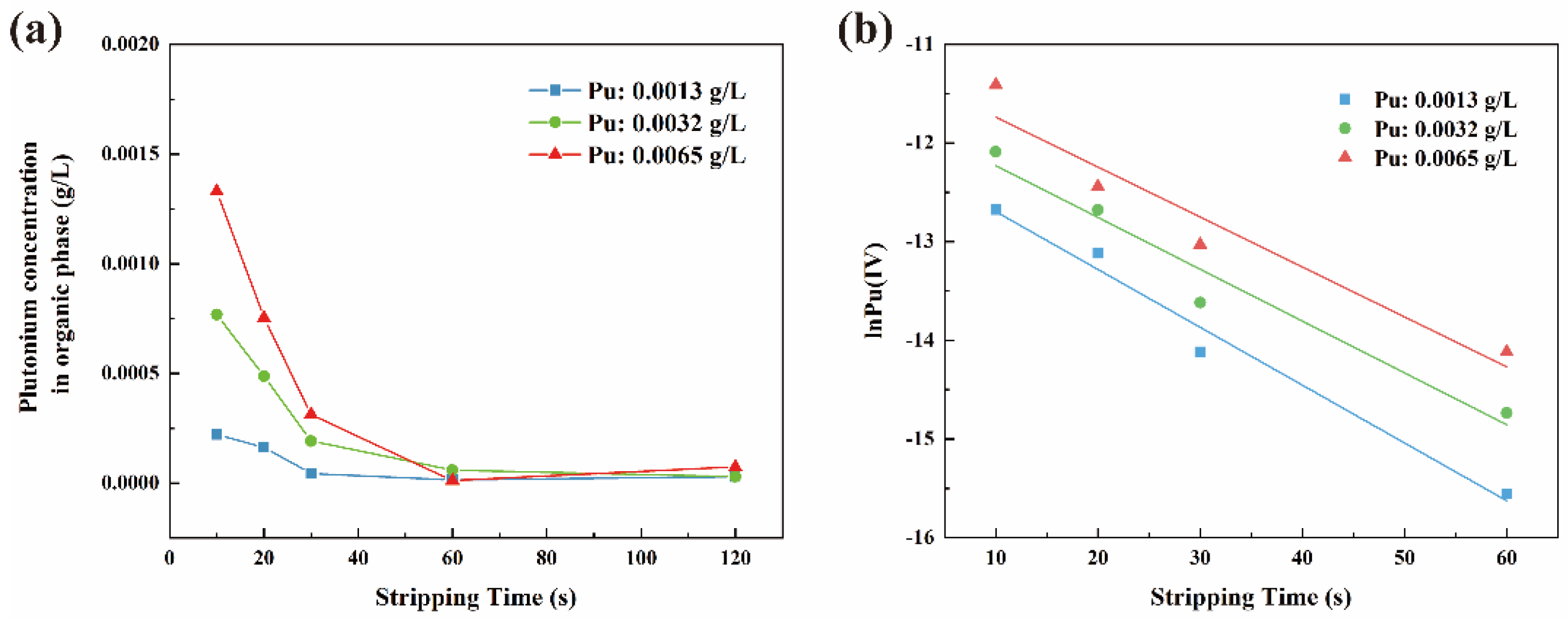
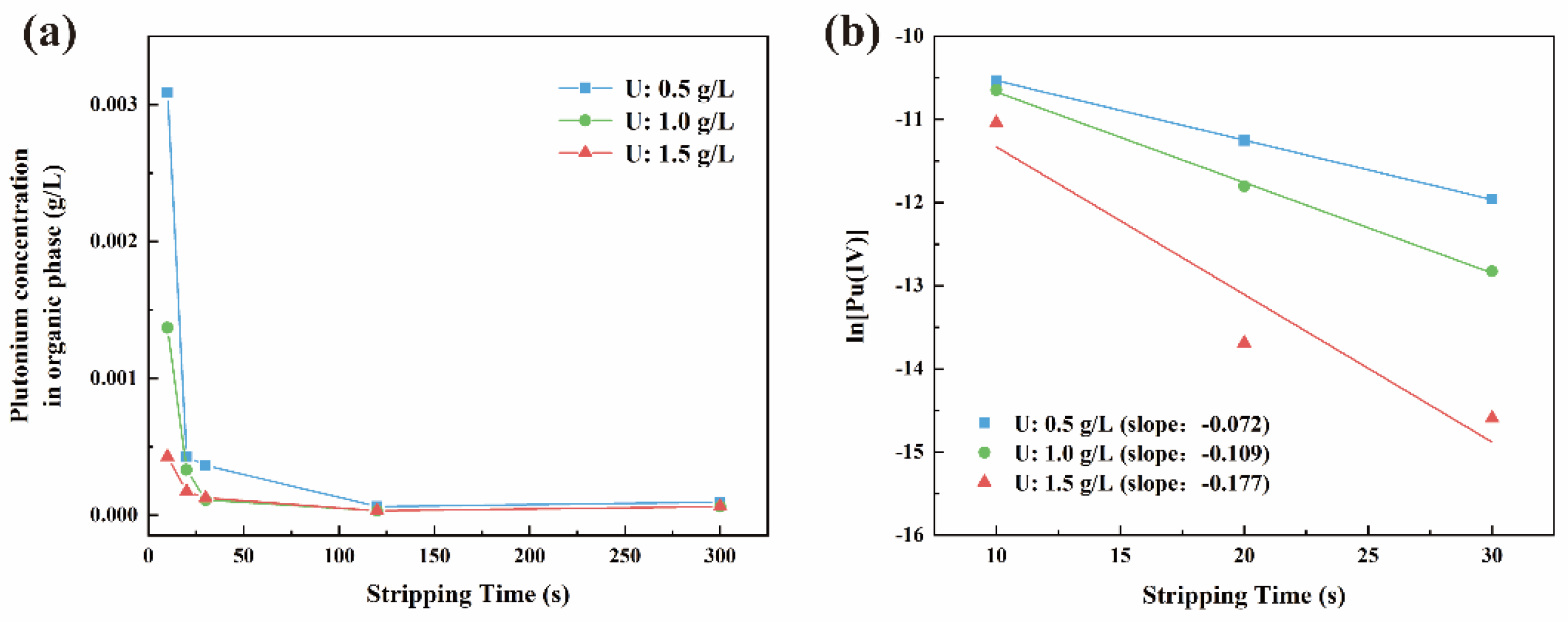
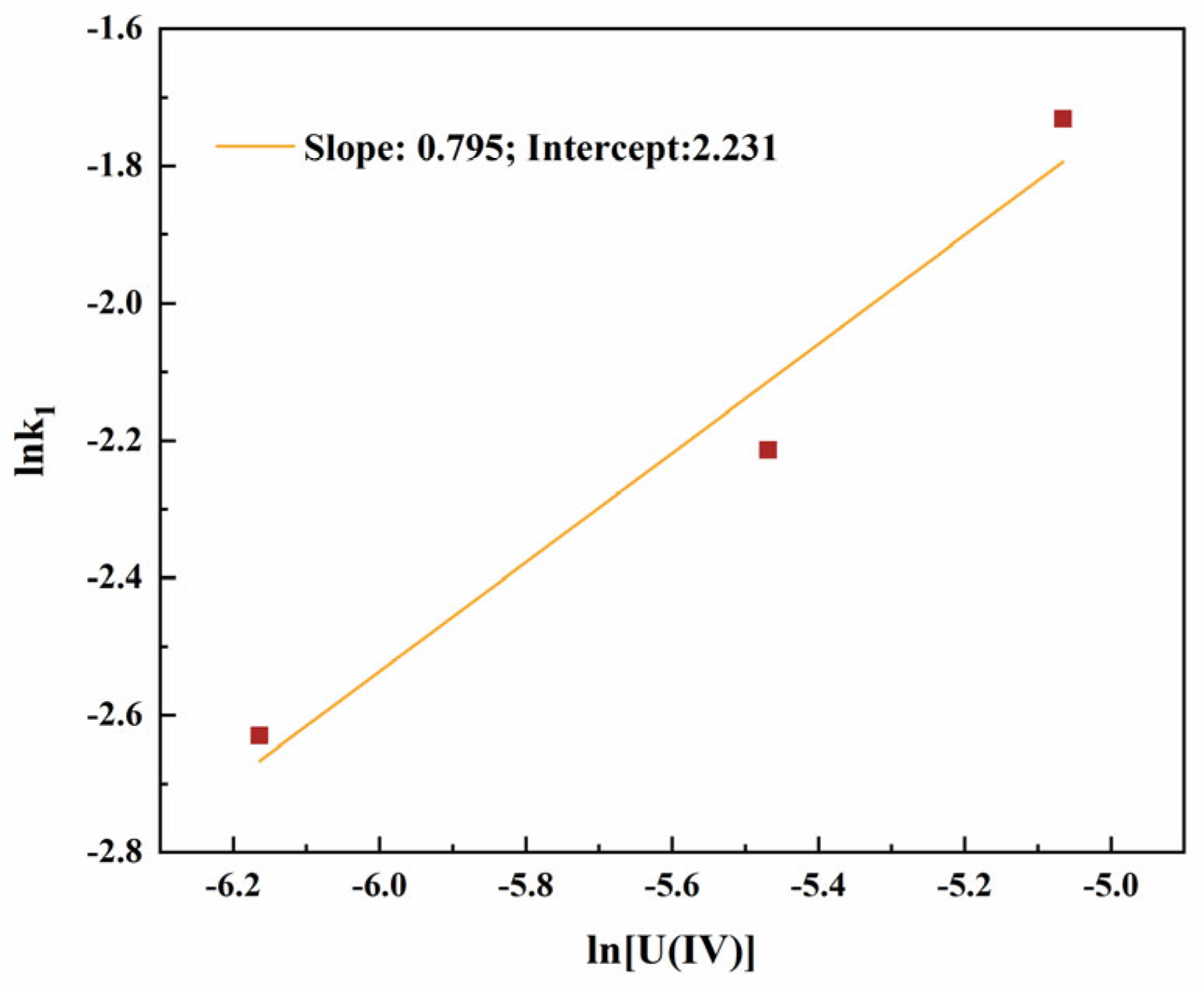
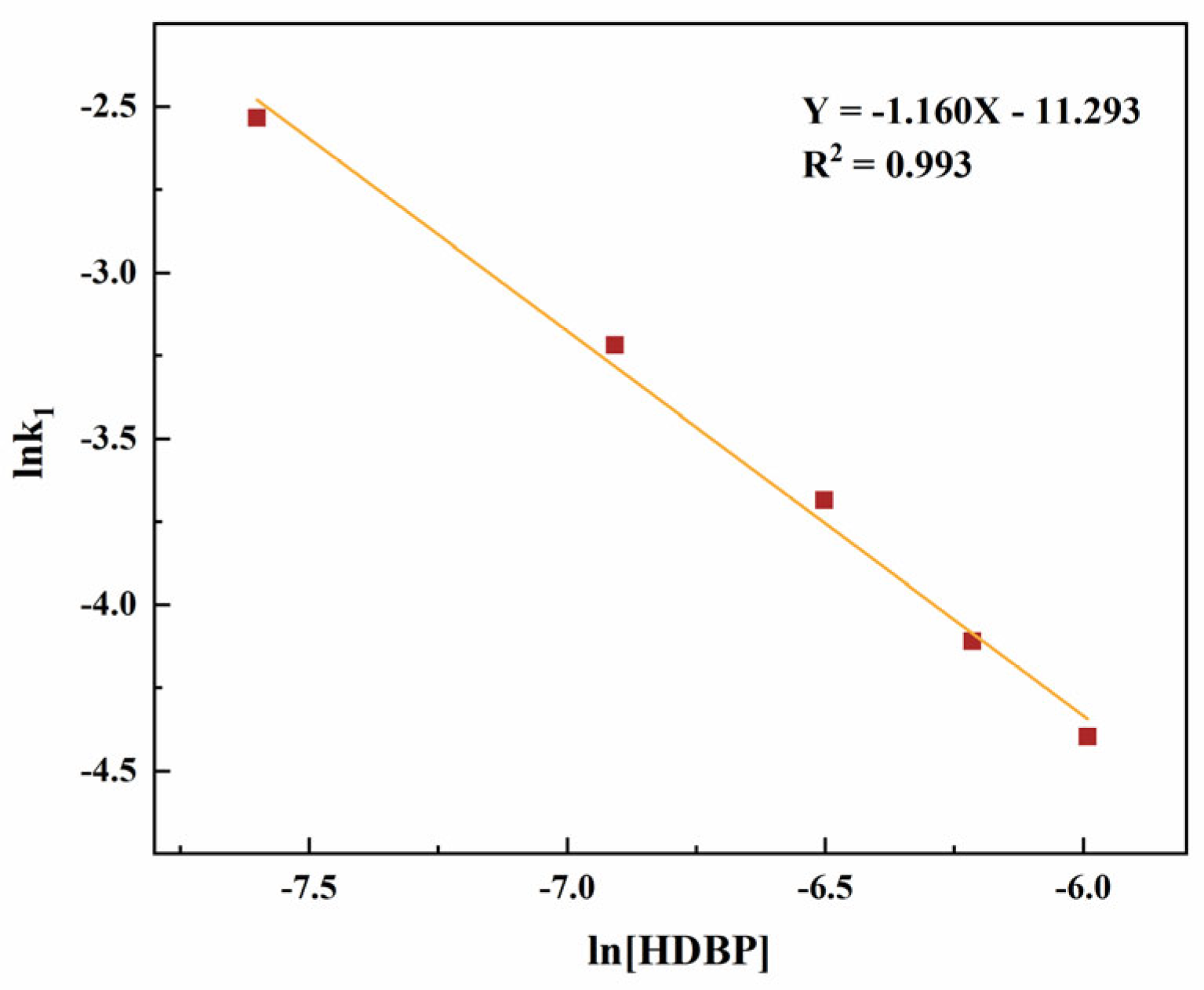
3.1.2. Reaction Kinetics Equation
3.1.3. The Effect of Phase Ratio on the Reaction
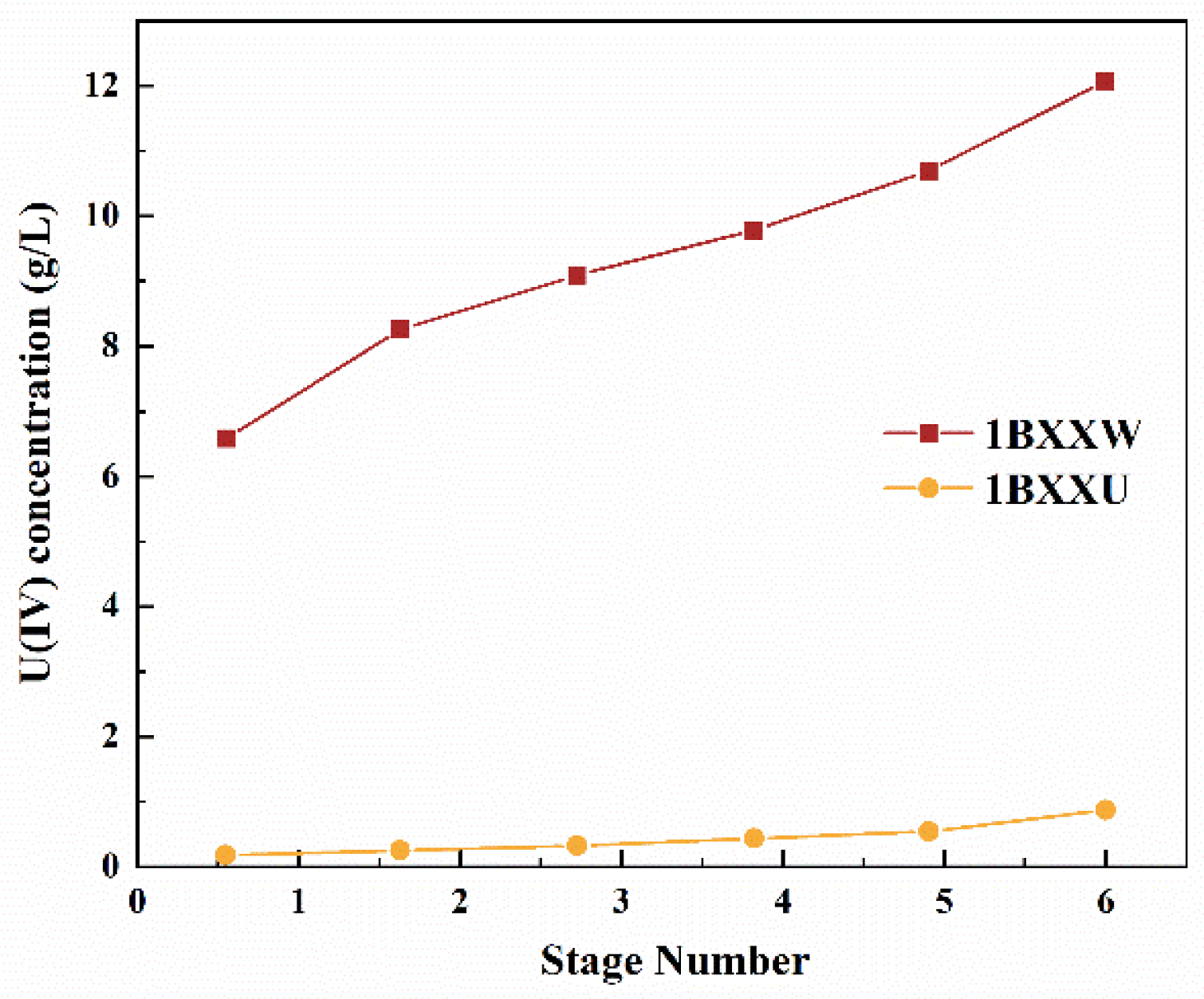
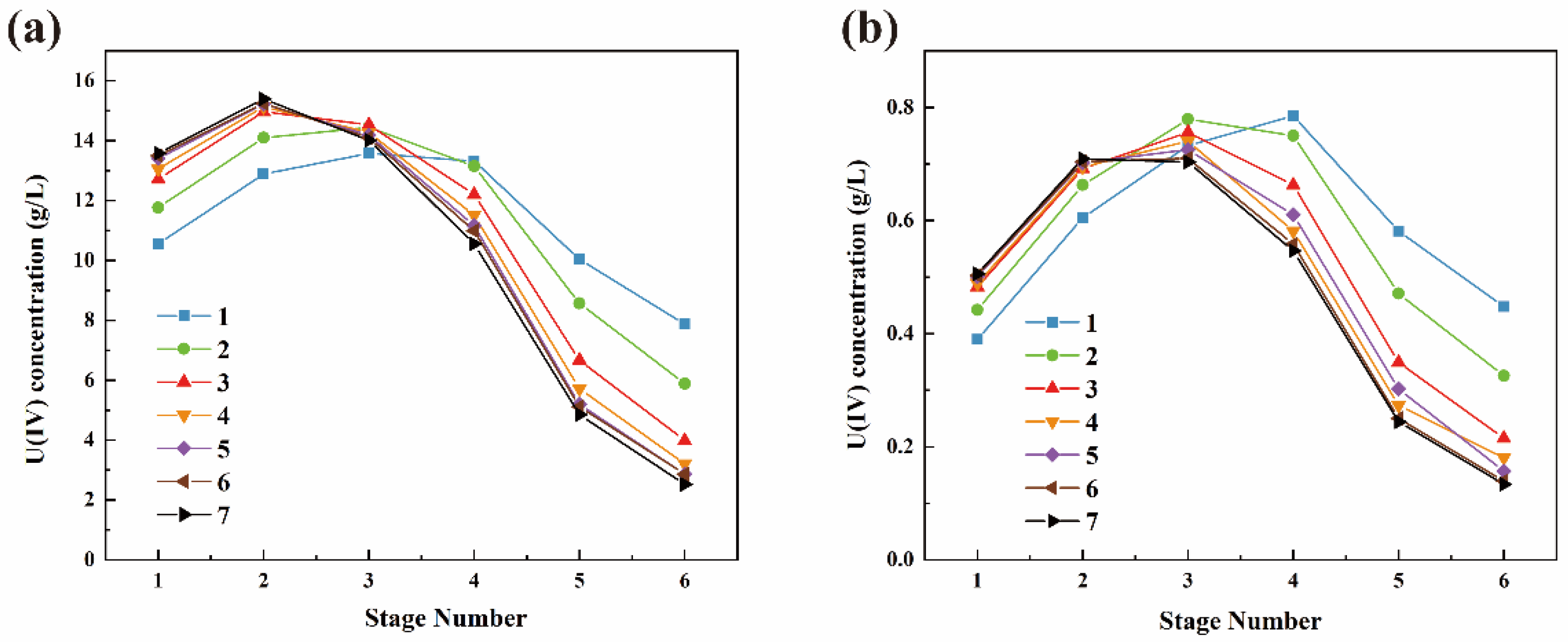
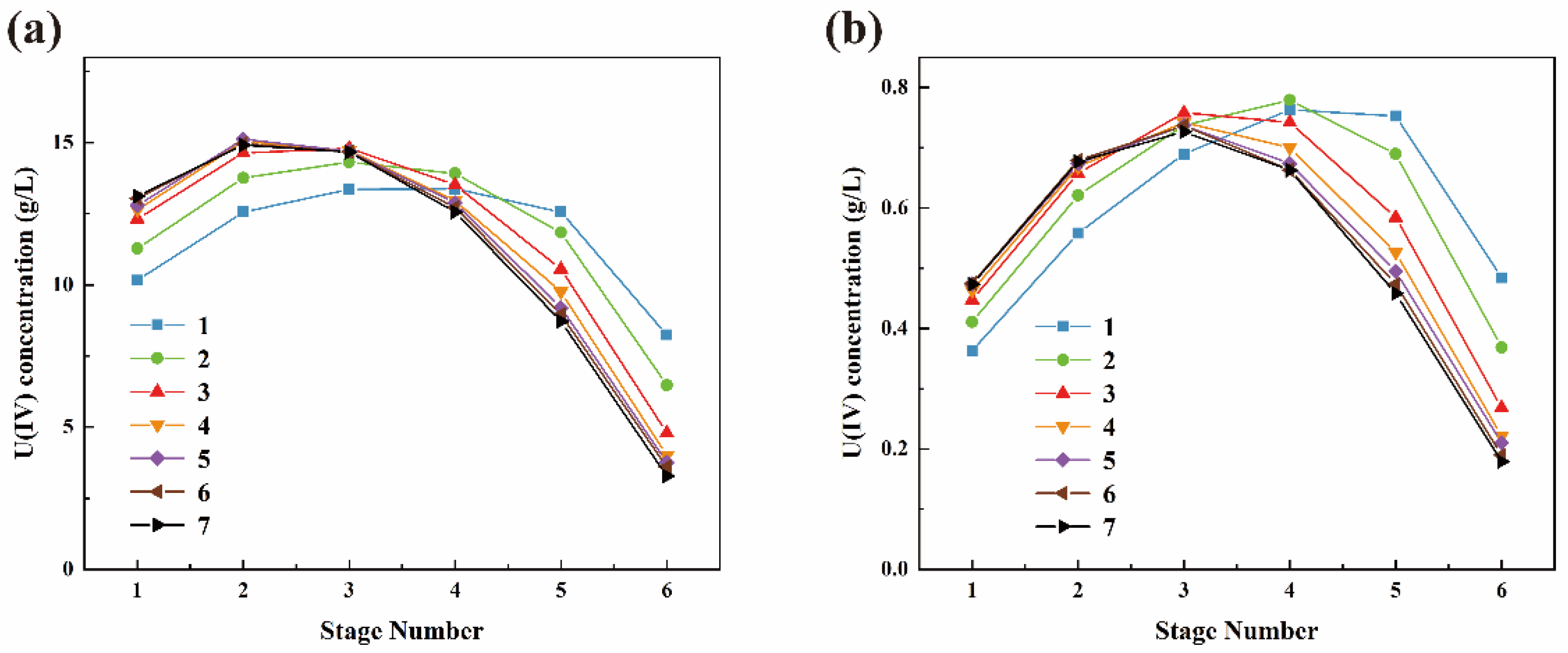
3.2. U(IV) Extraction Losses Study
3.3. Study on Oxidative Loss of U(IV)
3.3.1. Stability Studies of U(IV) in Aqueous Phases
3.3.2. Stability Studies of U(IV) in Organic Phases
3.3.3. Oxidative Loss of U(IV) in Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carboni, M.; Abney, C.W.; Liu, S.; Lin, W. Highly porous and stable metal--organic frameworks for uranium extraction. Chem. Sci. 2013, 4, 2396–2402. [Google Scholar] [CrossRef]
- Chen, Q.; Li, B.; Wang, J.; Zhu, H.; Chen, X.; Hu, Y.; Zhou, J.; Li, X.; Zheng, W.; Yan, T. Extraction of Uranium in Nitric Media with Novel Asymmetric Tetra-Alkylcarbamide. Molecules 2022, 27, 5527. [Google Scholar] [CrossRef] [PubMed]
- Kumari, I.; Kumar, B.; Khanna, A. A review on UREX processes for nuclear spent fuel reprocessing. Nucl. Eng. Des. 2020, 358, 110410. [Google Scholar] [CrossRef]
- Veliscek-Carolan, J. Separation of actinides from spent nuclear fuel: A review. J. Hazard Mater. 2016, 318, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, F.; Ren, P.; Yuan, L.; Liu, K.; Geng, J.; Tang, H.; Chai, Z.; Shi, W. Selective separation between UO22+ and Pu4+ by novel tetradentate chelate phenanthroline diamide ligand in 1-octanol. Sep. Purif. Technol. 2021, 277, 119521. [Google Scholar] [CrossRef]
- Ebert, K. Reprocessing of spent nuclear fuel: Status and prospects. Nucl. Energy 1988, 27, 361–365. [Google Scholar]
- Yan, T.; Zhen, W.; Ye, G.; Zhang, Y.; Xian, L.; Di, Y.; Bian, X. Synthesis of dihydroxyurea and its application to the U/Pu split in the PUREX process. J. Radioanal. Nucl. Chem. 2009, 279, 293–299. [Google Scholar] [CrossRef]
- He, H.; Ye, G.; Tang, H.; Zheng, W.; Li, G.; Lin, R. An advanced purex process based on salt-free reductants. Radiochim. Acta 2014, 102, 127–133. [Google Scholar] [CrossRef]
- Zhang, A.; Hu, J.; Zhang, X.; Wang, F. Hydroxylamine derivative in PUREX process. VI. Study on the partition of uranium/neptunium and uranium/plutonium with N, N-diethylhydroxylamine in the purification cycle of uranium contactor. Solvent Extr. Ion Exch. 2001, 19, 965–979. [Google Scholar] [CrossRef]
- Ren, F.; Zhou, Z. Foreign Nuclear Fuel Reprocessing; Atomic Energy Press: Beijing, China, 2006. [Google Scholar]
- Jiang, S.; Ren, F.; Ma, R.; et al. Nuclear Fuel Reprocessing Engineering; Atomic Energy Press: Beijing, China, 1995. [Google Scholar]
- Zhang, Z.; Wang, J.; Zhang, T. Nuclear Fuel Reprocessing Engineering for Power Reactors; Atomic Energy Press: Beijing, China, 2013. [Google Scholar]
- Jenkins, E. The effect of dissolved air on the reduction of tracer level plutonium-IV by uranium-IV. J. Inorg. Nucl. Chem. 1960, 13, 323. [Google Scholar] [CrossRef]
- Taylor, R.; Koltunov, V.; Marchenko, V.; Baranov, S. The kinetics and mechanism of the reduction of neptunium (VI) ions by uranium (IV) ions in nitric acid. In Proceedings of the International Conference on the Scientific Research on the Back-End of the Fuel Cycle for the 21st Century, Avignon, France, 24–26 October 2000; pp. 149–156. [Google Scholar]
- Yu, E.; Liu, L.; Fei, H. Study on the use of U(IV) as Pu(IV) reducing agent in the process of Purex. J. Nucl. Radiochem. 1992, 14, 207–214. [Google Scholar]
- Newton, T. The kinetics of the reaction between Pu(IV) and U(IV). J. Phys. Chem. 1959, 63, 1493–1497. [Google Scholar] [CrossRef]
- Koltunov, V.; Zhuravleva, G.; Marchenko, V.; Dvoeglazov, K.; Savilova, O.; Koltunov, G.; Taylor, R.; Bankhead, M. Kinetics and mechanism of the plutonium catalysed nitric acid oxidation of U(IV) ions in 30% tributyl phosphate solution. Radiochim. Acta 2007, 95, 559–567. [Google Scholar] [CrossRef]
- Biddle, P.; Miles, J.; Waterman, M. Catalysis in the reduction of plutonium (IV) by uranium (IV). J. Inorg. Nucl. Chem. 1966, 28, 1736–1739. [Google Scholar] [CrossRef]
- Herbst, R.; Baron, P.; Nilsson, M. Standard and advanced separation: PUREX processes for nuclear fuel reprocessing. In Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Elsevier: Amsterdam, The Netherlands, 2011; pp. 141–175. [Google Scholar]
- Kolarik, Z. Oxidation of U(IV) nitrate in liquid-liquid dispersions and separate phases containing tributyl phosphate and nitric acid. J. Inorg. Nucl. Chem. 1981, 43, 1633–1645. [Google Scholar] [CrossRef]
- Mincher, B.; Elias, G.; Martin, L.; Mezyk, S. Radiation chemistry and the nuclear fuel cycle. J. Radioanal. Nucl. Chem. 2009, 282, 645–649. [Google Scholar] [CrossRef]
- Mincher, B.; Mezyk, S. Radiation chemical effects on radiochemistry: A review of examples important to nuclear power. Radiochim. Acta 2009, 97, 519–534. [Google Scholar] [CrossRef]
- Berthon, L.; Charbonnel, M. Radiolysis of solvents used in nuclear fuel reprocessing. Solvent Extr. Ion Exch. 2010, 19, 429–513. [Google Scholar] [CrossRef]
- Mishra, S.; Rama, S.K.; Rajesh, P.; Desigan, N.; Venkatesan, K. Effect of di-butyl phosphate on the distribution behavior of uranyl ions in PUREX solvent. Sep. Sci. Technol. 2024, 59, 112–121. [Google Scholar] [CrossRef]
- Yu, T.; Ye, G.; He, H.; Chen, Y.; Liu, J.; Zhang, L. Present Situation of Research of Computer Simulation of Stripping of Plutonium by Chemical Reduction in PUREX Process. J. Nucl. Radiochem. 2017, 39, 257–267. (In Chinese) [Google Scholar] [CrossRef]
- Yu, T.; He, H.; Hong, Z.; Liu, Z.; Li, F.; Ye, G. Computer Simulation and Software Development of Uranium/Plutonium Separation Unit (1B) in Purex Process. At Energy Sci. Technol. 2019, 53, 193–199. (In Chinese) [Google Scholar] [CrossRef]
- Yu, T.; He, H.; Hong, Z.; Chen, Y.; Ye, G. Computer Simulation of Single-Stage Reductive Stripping of U(IV) With Pu(IV). J. Nucl. Radiochem. 2017, 39, 413–421. (In Chinese) [Google Scholar] [CrossRef]
- Yu, T.; Ye, G.; Hong, Z.; He, H.; Chen, Y.; Lan, T.; Liu, J. Computer Simulation of Single-stage Reductive Stripping of Plutonium with Hydroxylamine Nitrate. Energy Sci. Technol. 2017, 51, 1928–1935. (In Chinese) [Google Scholar] [CrossRef]
- Yu, T.; He, H.; Liu, Z.; Zhang, L.; Li, Z.; Zhao, H.; Ye, G. Transient Behavior and Mathematical Model of Solvent Extraction Process in Mixer-Settler. J. Nucl. Radiochem. 2020, 42, 214–225. (In Chinese) [Google Scholar] [CrossRef]
- Baron, P.; Boullis, B.; Germain, M.; Gue, J.; Miquel, P.; Poncelet, F.; Dormant, J.; Dutertre, F. Extraction cycles design for La Hague plants (No. CEA-CONF-11650). In Proceedings of the GLOBAL’93, Seattle, WA, USA, 12–17 September 1993; pp. 12–17. [Google Scholar]
- Natarajan, R. Reprocessing of FBTR mixed carbide fuel-some process chemistry aspects. In Proceedings of the INSAC-2005:16, Annual Conference of Indian Nuclear Society, Mumbai, India, 15–18 November 2005; pp. 1–9. [Google Scholar]
- Kawaguchi, Y.; Morimoto, K.; Kitao, T.; Ohyama, K.; Omori, E. Study of solvent degradation in reprocessing MOX spent fuel solvent degradation and its effect on Pu purification cycle. Trans. At. Energy Soc. Jpn. 2009, 8, 221–229. [Google Scholar] [CrossRef]


| U(IV) mol/L | HDBP mol/L | HNO3 mol/L | k1 s−1 | k (mol/L)2.4s−1 |
|---|---|---|---|---|
| 0.0021 | 0.0010 | 1.0 | 0.0720 | 0.0029 |
| 0.0042 | 0.0010 | 1.0 | 0.1090 | 0.0025 |
| 0.0063 | 0.0010 | 1.0 | 0.1775 | 0.0029 |
| 0.0021 | 0.0005 | 1.0 | 0.1325 | 0.0023 |
| 0.0021 | 0.0010 | 1.0 | 0.0704 | 0.0028 |
| 0.0021 | 0.0020 | 1.0 | 0.0243 | 0.0022 |
| 0.0021 | 0.0025 | 1.0 | 0.0205 | 0.0024 |
| 0.0021 | 0.0010 | 0.7 | 0.1099 | 0.0019 |
| 0.0021 | 0.0010 | 2.0 | 0.0189 | 0.0026 |
| Average of rate constants | 0.0025 ± 0.0006 | |||
| Phase Ratio | Plutonium Retention in the Organic Phase, g/L | Stripping Rate, % |
|---|---|---|
| 1:1 | 0.0001 | 99.24 |
| 2:1 | 0.0002 | 99.31 |
| 5:1 | 0.0006 | 98.67 |
| 10:1 | 0.0015 | 98.22 |
| Stage Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| U(IV) concentration in organic phase of 6th stage, g/L | 0.48 | 0.36 | 0.26 | 0.22 | 0.20 | 0.19 | 0.18 |
| Wastage loss of U(IV), % | 31.89 | 24.33 | 17.55 | 14.75 | 13.32 | 12.38 | 11.83 |
| Number | 1st | 2nd | 3rd | 4th | 5th | 6th | Wastage Loss of U(IV), % |
|---|---|---|---|---|---|---|---|
| 1 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 24.33 |
| 2 | 0.3 | 0.25 | 0.2 | 0.15 | 0.1 | 0.1 | 19.22 |
| 3 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 | 0.1 | 29.19 |
| 4 | 0.2 | 0.2 | 0.1 | 0.2 | 0.3 | 0.1 | 26.08 |
| 5 | 0.3 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 22.16 |
| 6 | 0.3 | 0.2 | 0.1 | 0.1 | 0.3 | 0.1 | 20.83 |
| 7 | 0.3 | 0.1 | 0.1 | 0.2 | 0.3 | 0.1 | 24.97 |
| 8 | 0.3 | 0.3 | 0.2 | 0.1 | 0.1 | 0.1 | 15.94 |
| 9 | 0.2 | 0.1 | 0.1 | 0.3 | 0.3 | 0.1 | 27.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Chen, Q.; Zuo, C.; Li, T.; Yuan, J.; Zhao, Z.; Yan, T.; Zheng, W. Kinetic Study and Process Optimization of Plutonium Barrier Units for Enhanced Plutonium Stripping in the PUREX Process. Separations 2024, 11, 278. https://doi.org/10.3390/separations11090278
Zhu H, Chen Q, Zuo C, Li T, Yuan J, Zhao Z, Yan T, Zheng W. Kinetic Study and Process Optimization of Plutonium Barrier Units for Enhanced Plutonium Stripping in the PUREX Process. Separations. 2024; 11(9):278. https://doi.org/10.3390/separations11090278
Chicago/Turabian StyleZhu, Haowei, Qi Chen, Chen Zuo, Tianchi Li, Jieqiong Yuan, Ziqian Zhao, Taihong Yan, and Weifang Zheng. 2024. "Kinetic Study and Process Optimization of Plutonium Barrier Units for Enhanced Plutonium Stripping in the PUREX Process" Separations 11, no. 9: 278. https://doi.org/10.3390/separations11090278
APA StyleZhu, H., Chen, Q., Zuo, C., Li, T., Yuan, J., Zhao, Z., Yan, T., & Zheng, W. (2024). Kinetic Study and Process Optimization of Plutonium Barrier Units for Enhanced Plutonium Stripping in the PUREX Process. Separations, 11(9), 278. https://doi.org/10.3390/separations11090278






