Solid–Liquid Phase Equilibria of the Aqueous Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at T = 323.2 K
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Experimental Instruments
2.3. Experimental Procedure and Analytical Method
3. Results and Discussion
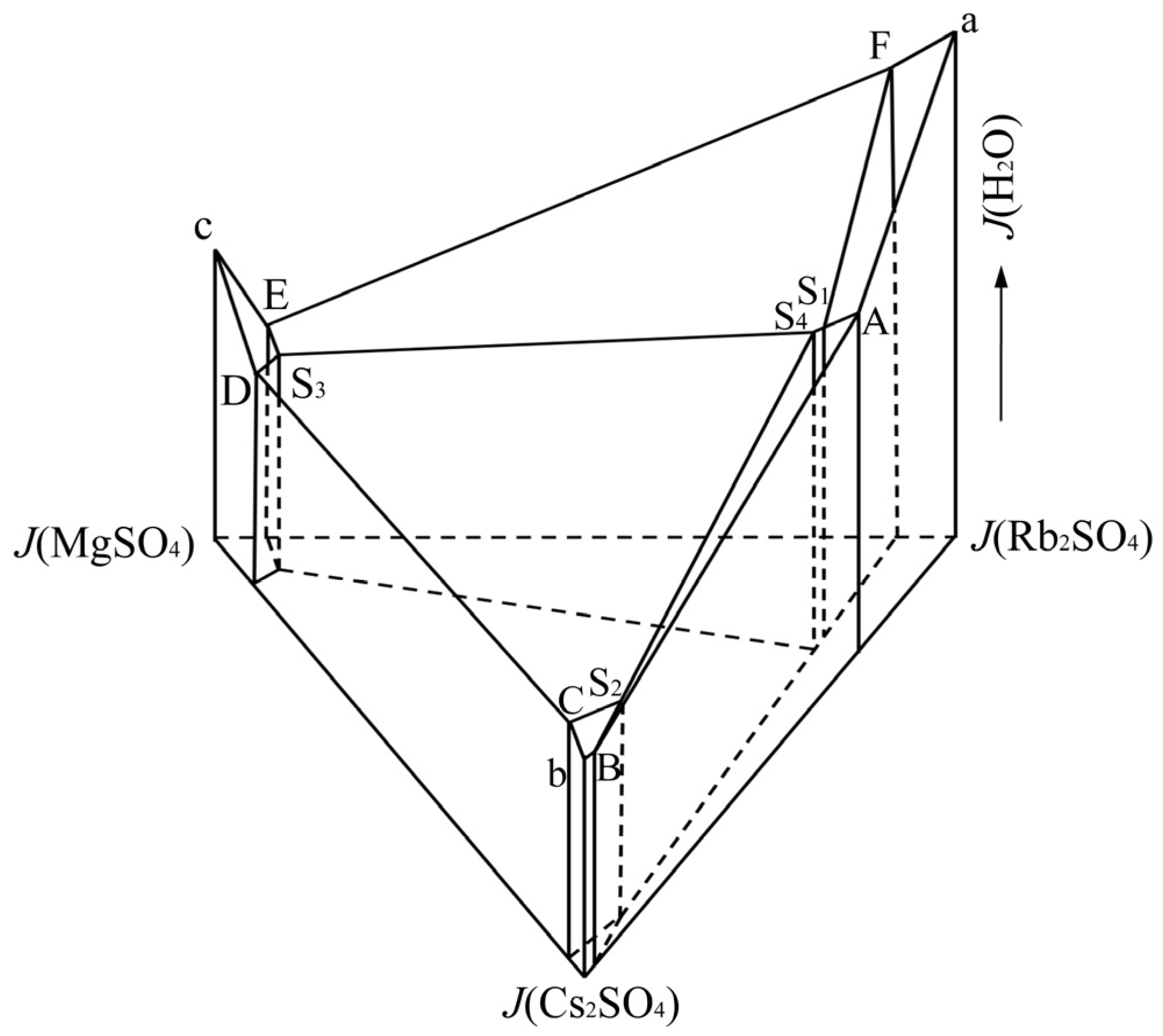
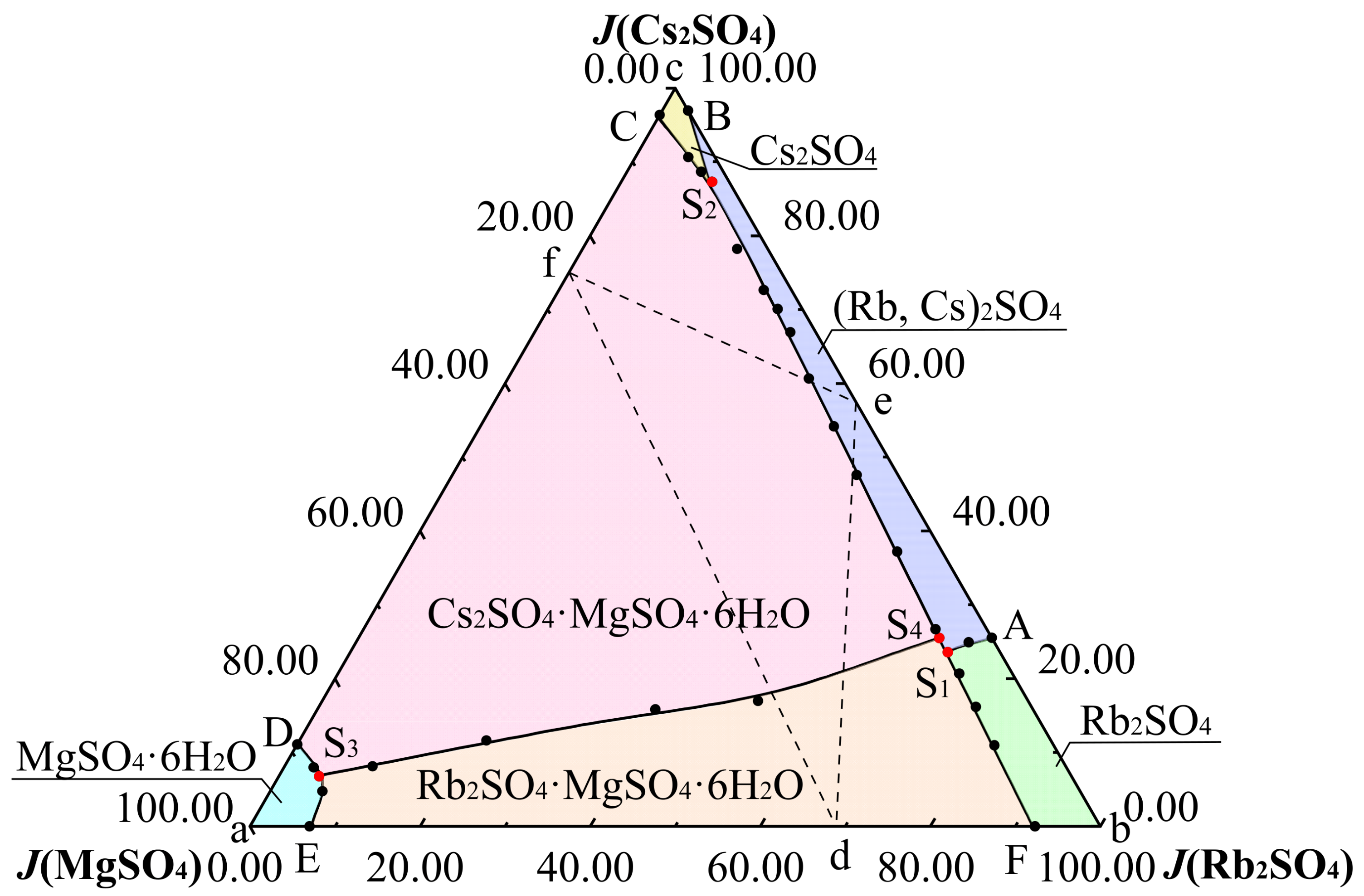
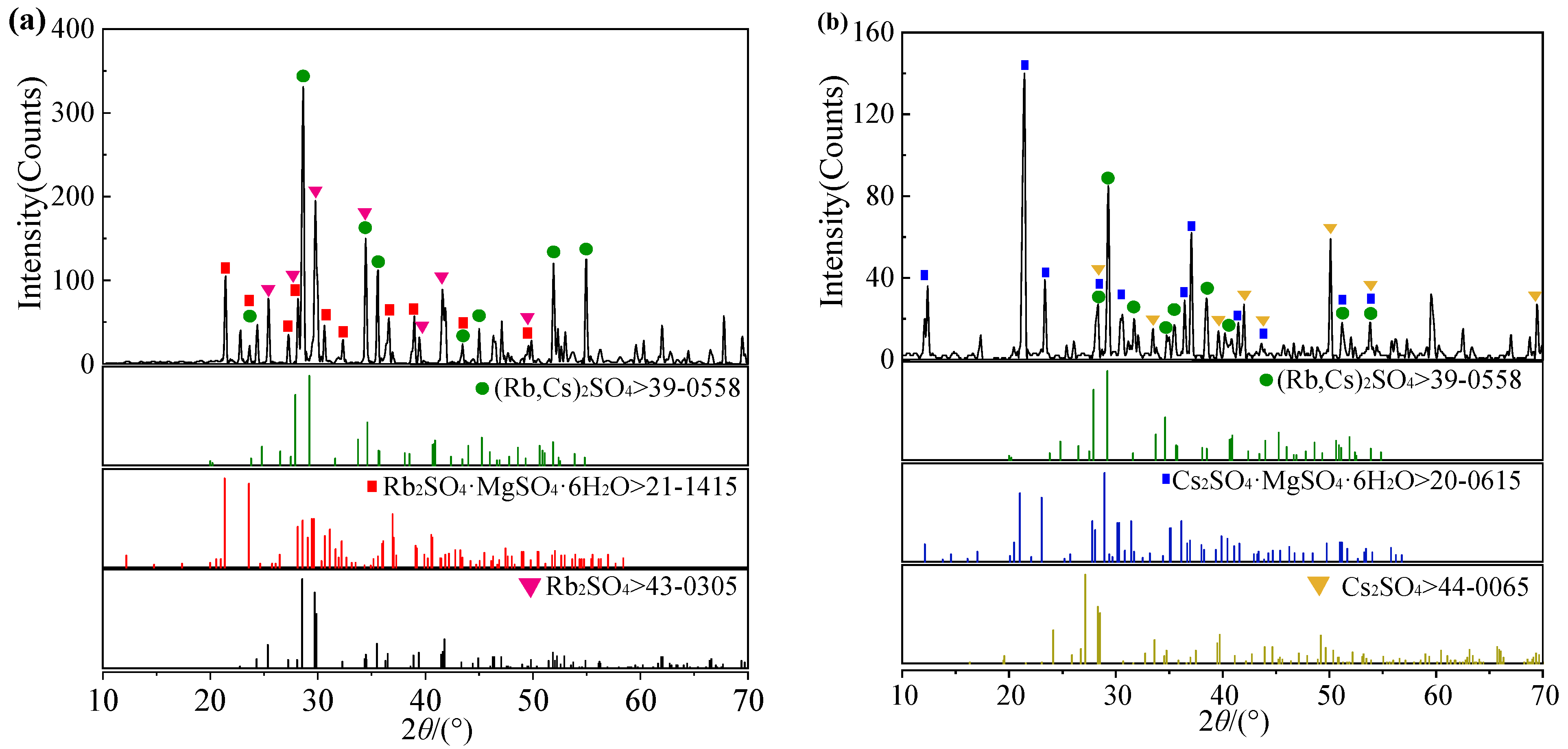
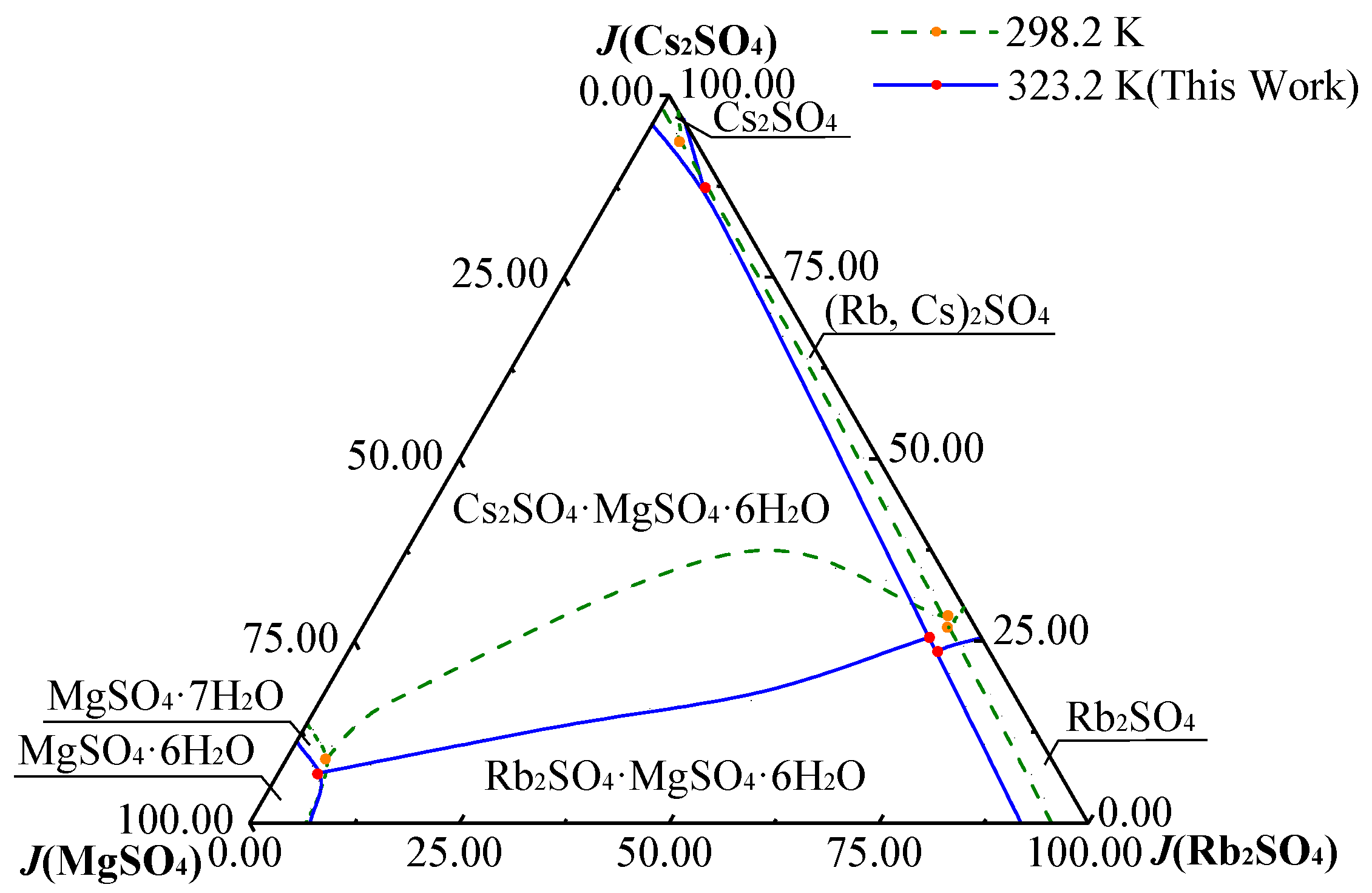
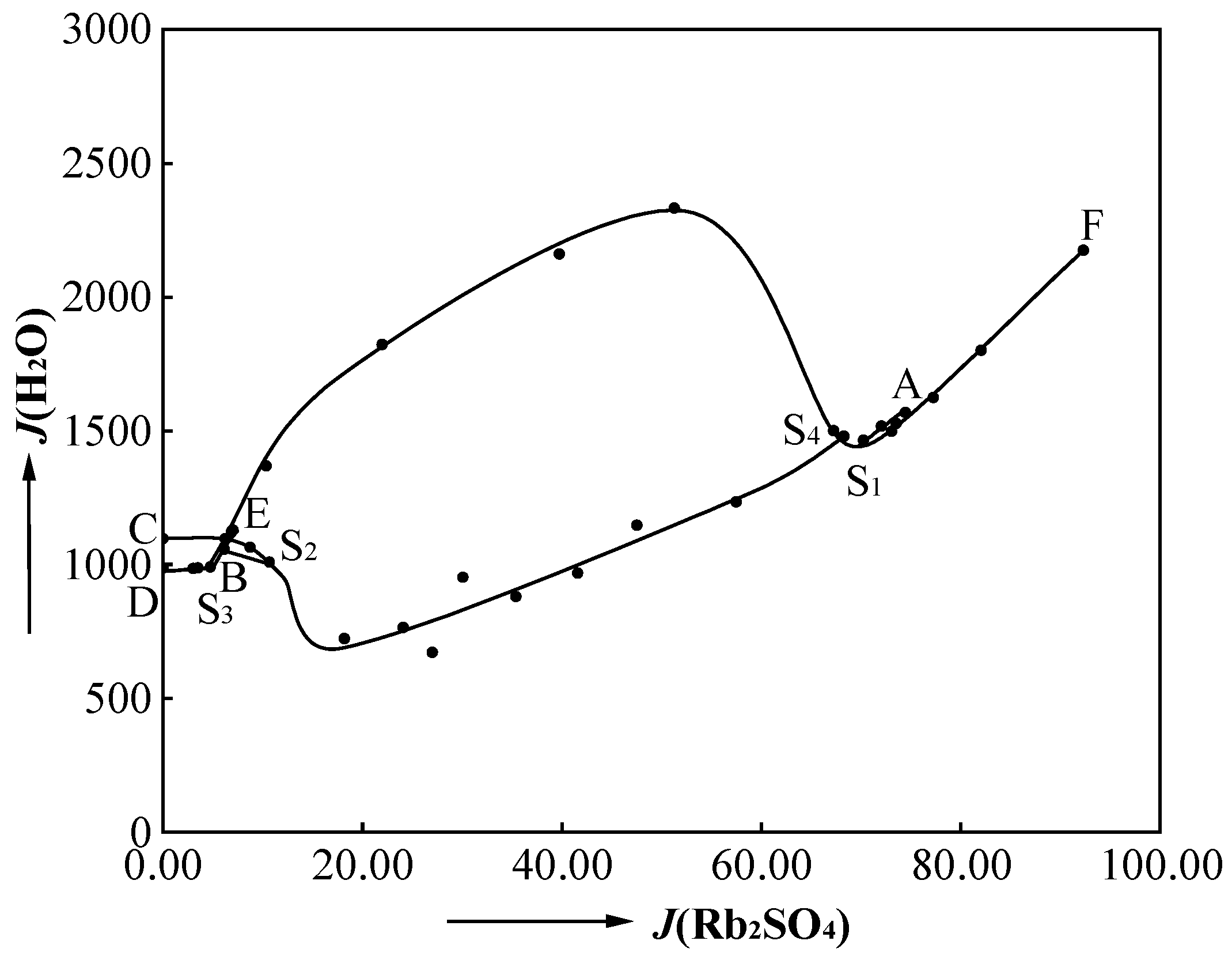
3.1. Phase Diagram of Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at 323.2 K
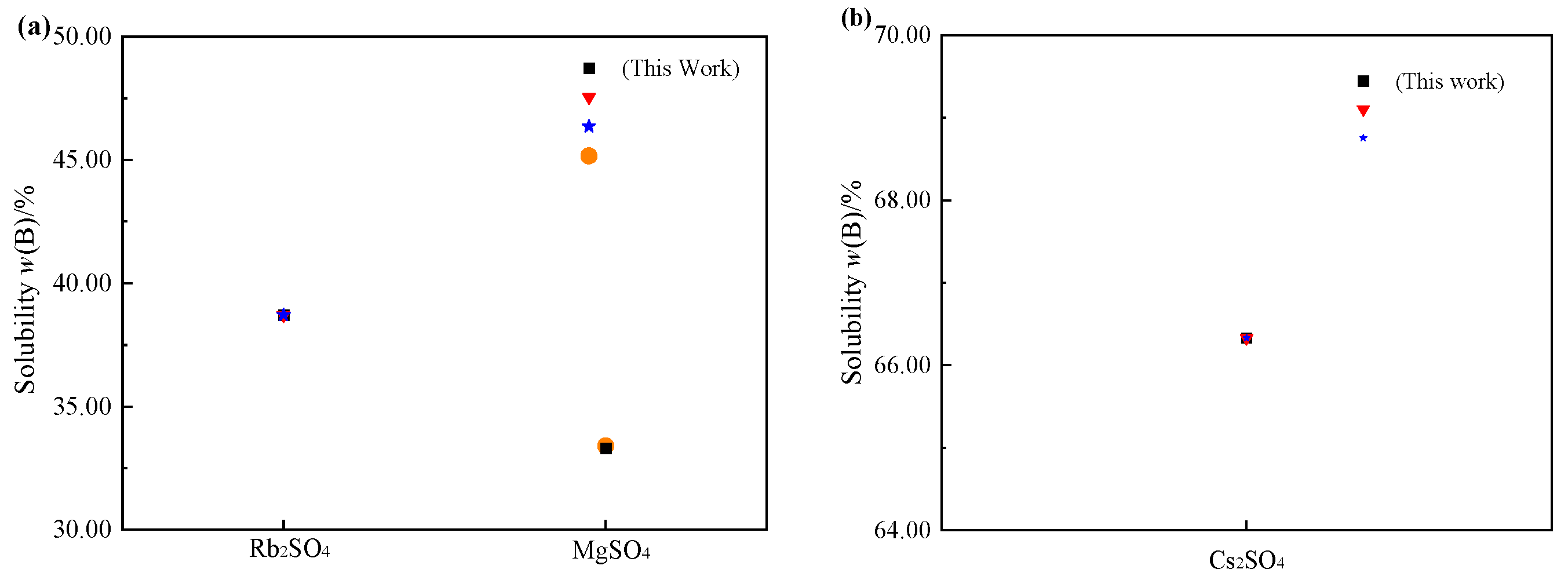
3.2. Comparison of Rb+, Cs+, Mg2+//SO42− - H2O at 298.2 K and 323.2 K
3.3. Physicochemical Properties of Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at 323.2 K
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.S.; Guo, J.; Liu, X.F.; Wu, Q.; Nie, Z.; Bu, L.Z.; Yu, J.J. Research on rubidium and cesium resources, extraction technology, development, and utilization in China. Acta Geol. Sin.-Engl. 2024, 45, 823–829. [Google Scholar]
- Gao, X.R.; Jia, H.X.; Li, T.J.; Li, W.D.; Wang, A.J. Perspective of rubidium and cesium resource demand in China. Acta Geol. Sin.-Engl. 2023, 44, 279–285. [Google Scholar]
- Zhang, X.F.; Zhang, J.H.; Tan, X.M.; Yin, Y.J.; Zhang, Z.L.; Shi, L.J. Review on extraction of rubidium and cesium from ores. J. Salt Lake Res. 2024, 32, 89–96. [Google Scholar] [CrossRef]
- Li, Q.K.; Wang, J.P.; Fan, Q.S.; Qin, Z.J.; Wei, H.X.; Shan, F.S.; Yuan, S.; Du, Y.S. 2023 Rubidium and cesium enrichment in lacustrine sediments from Tibetan salt lakes: A potential resource. Acta Geol. Sin.-Engl. 2023, 97, 3410–3420. [Google Scholar]
- Zhou, X.; Zhao, Y.Y.; Chen, W.X. Rubidium and cesium resources current status, enrichment pattern and extraction technology in salt lakes of Qinghai-Xizang(Tibet) Plateau, China. Int. Geol. Rev. 2024, 70, 705–716. [Google Scholar]
- Zhao, L.; Wang, M.M.; Xia, Y.G.; Gao, J. Research progress on the extraction and separation technology of rubidium and cesium ions. J. Salt Lake Res. 2024, 32, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.H.; Ding, X.J.; Zhou, Y.L.; Wang, S.L.; Wang, F.; Ma, J.H.; Liu, W.P. Research on the enrichment regularity of rubidium and cesium in Qarhan Salt Lake. Acta Petrol. Mineral. 2023, 42, 701–710. [Google Scholar]
- Cheng, H.G.; Cheng, F.Q. Phase Separation of Salt-Water System; Metallurgical Industry Press: Beijing, China, 2022. [Google Scholar]
- Yu, Z.F.; Zeng, Y.; Li, X.Q.; He, Y.H.; Lv, X.X.; Yu, X.D. Solid-liquid phase equilibria in the aqueous ternary system Rb+(Cs+), Mg2+//SO42− - H2O at 273.2 K. J. Salt Lake Res. 2024, 32, 35–41. [Google Scholar] [CrossRef]
- Merbach, A.; Gonella, J. Contribution à l’etude du systéme quaternaire KCl-RbCl-CsCl-H2O: I. Les isothermes de 25° des systéme sternaireslimités. Helv. Chim. Acta 1969, 52, 69–76. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Ma, L.C.; Liu, C.L.; Chen, L.F.; Zhao, H.T. Research on Isothermal Equilibrium of Ternary System of RbCl-CsCl-H2O at 50 °C. Inorg. Chem. Ind. 2014, 46, 21–23. [Google Scholar]
- Guo, L.J.; Li, H.X. Solubility Measurement of the Solid Solution Containing the RbCl—CsCl—H2O System from T = 298.15 to 348.15 K. J. Chem. Eng. Data 2020, 65, 1663–1668. [Google Scholar] [CrossRef]
- Donald, A.P.; Joseph, A.R.; Simon, L.C. Isopiestic determination of the osmotic and activity coefficients of Rb2SO4(aq) and Cs2SO4(aq) at T = (298.15 and 323.15) K, and representation with an extended ion-interaction (Pitzer) model. J. Chem. Thermodyn. 2002, 34, 63–102. [Google Scholar]
- Chen, P.J.; Pan, J.J.; Song, X.; Chen, L.; Chen, Y.; Wu, L.X.; Zeng, Y.; Yu, X.D. Stable phase equilibrium in ternary system Rb2SO4 + Cs2SO4 + H2O at 323 K. Ind. Miner. Process. 2018, 47, 12–15+19. [Google Scholar]
- Fan, Y.F.; Zhuang, Z.Y.; Gao, D.D.; Zeng, D.W.; Li, D.D. Thermodynamic modeling of the solid-liquid phase equilibrium in the Na+, K+, Rb+, Cs+//SO42− - H2O system. J. Salt Lake Res. 2022, 30, 83–101. [Google Scholar]
- Zeng, Y.; Wang, K.; Tong, H.L.; Lv, X.X.; He, Y.H.; Yu, X.D. Stable-phase diagram of the quaternary water-salt system K+, Rb+, Cs+//SO42− - H2O at T = 323.2 K. J. Chem. Eng. Data 2022, 67, 491–499. [Google Scholar] [CrossRef]
- Chen, P.J.; Zeng, Y.; Wu, L.X.; Huang, P.; Chen, Y.; Sun, J.; Yu, X.D. Measurements and Calculations of Stable Phase Equilibria in Ternary Systems MgSO4(Rb2SO4) + Cs2SO4 + H2O at T = 298.2 K. J. Chem. Eng. Data 2018, 63, 3418–3426. [Google Scholar] [CrossRef]
- Wu, L.X.; Zeng, Y.; Chen, Y.; Yu, X.D.; Chen, P.J.; Huang, P.; Sun, J. The stable phase equilibria of the ternary systems Na2SO4 + Rb2SO4(Cs2SO4) + H2O at 298.2 K. J. Chem. Eng. Data 2019, 64, 529–535. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, P.J.; Yu, X.D. Phase equilibria for quaternary system Rb+, Cs+, Mg2+//SO42− - H2O at 298.2 K. CIESC J. 2020, 71, 3460–3468. [Google Scholar]
- Institute of Qinghai Salt Lakes of Chinese Academy of Science. Analytical Methods of Brines and Salts; Beijing Science Press: Beijing, China, 1988. [Google Scholar]
- Standardization Administration of the People’s Republic of China. GB/T 13747.9-2022; Methods for Chemical Analysis of Zirconium and Zirconium Alloys-Part 9: Determination of Magnesium Content—Flame Atomic Absorption Spectrometry and Inductively Coupled Plasma Atomic Emission Spectrometry. Standards Press of China: Beijing, China, 2022.
- Lv, P.; Zhong, Y.; Meng, R.Y.; Sun, B.; Song, P.S. Weighing titration analysis of the sulfate content. Int. J. Salt Lake Res. 2015, 23, 5–13. [Google Scholar]
- Haynes, W.M.; Lide David, R.; Bruno Thomas, J. CRC Handbook of Chemistry and Physics 97th; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Niu, Z.D.; Cheng, F.Q. Phase Diagram of Water-Salt System and Its Application; Tianjin University Press: Tianjin, China, 2002. [Google Scholar]



| Chemical Agents | CAS No. | Purity (w/w) a | Source | Analysis Method |
|---|---|---|---|---|
| Rb2SO4 | 7488-54-2 | 0.995 | Jiangxi Dongpeng | alizarin red-S volumetric method [20] |
| Cs2SO4 | 10294-54-9 | 0.999 | Jiangxi Dongpeng | |
| MgSO4·7H2O | 10034-99-8 | 0.999 | Shanghai Bohr |
| Experimental Instruments | Precision | Producer | Type Number | Usage |
|---|---|---|---|---|
| Analytical balance | 0.0001 g | Sartorius | BSA124S | Weighing |
| Thermostat | ±0.2 K | Chongqing Inborn Experiment Instrument | SHH250 | Constant temperature |
| X-ray powder diffraction | 1° | Dandong Fangyuan Instrument Co., Ltd. | DX-2700 | Solid phase identification |
| Atomic absorption spectroscopy | 0.0001 | iCE 3300, ThermoFisher | iCE 3300 | Liquid phase identification |
| No. | Density /ρ(g·cm−3) | Refractive Index | Equilibrium Solutions Composition, w(B) × 102 | Jänecke Index of Dry Salt, J(B) (mol/100 mol S) | Equilibrium Solid Phase b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| J(Rb2SO4) + J(Cs2SO4) + J(MgSO4) = 100 | |||||||||||
| w(Rb2SO4) | w(Cs2SO4) | w(MgSO4) | w(H2O) | J(Rb2SO4) | J(Cs2SO4) | J(MgSO4) | J(H2O) | ||||
| 1,A | 1.5309 | 1.3761 | 34.65 | 16.11 | 0.00 | 49.24 | 74.46 | 25.54 | 0.00 | 1568.20 | Rb2SO4 + SS |
| 2 | 1.5406 | 1.3800 | 34.38 | 16.12 | 0.65 | 48.85 | 72.05 | 24.93 | 3.02 | 1517.33 | Rb2SO4 + SS |
| 3,S1 | 1.5502 | 1.3804 | 34.46 | 15.70 | 1.36 | 48.48 | 70.24 | 23.61 | 6.15 | 1464.55 | Rb2SO4 + SS + RM |
| 4,B | 2.0499 | 1.4109 | 1.51 | 65.40 | 0.00 | 33.09 | 3.03 | 96.97 | 0.00 | 985.51 | Cs2SO4 + SS |
| 5,S2 | 2.0794 | 1.4107 | 5.39 | 59.75 | 0.46 | 34.40 | 10.67 | 87.31 | 2.02 | 1009.68 | Cs2SO4 + SS + CM |
| 6,C | 2.0338 | 1.4104 | 0.00 | 63.32 | 0.79 | 35.89 | 0.00 | 96.38 | 3.62 | 1097.40 | Cs2SO4 + CM |
| 7 | 2.0359 | 1.4110 | 3.04 | 60.08 | 0.69 | 36.19 | 6.22 | 90.65 | 3.13 | 1096.89 | Cs2SO4 + CM |
| 8 | 2.0594 | 1.4105 | 4.32 | 59.52 | 0.58 | 35.58 | 8.72 | 88.68 | 2.60 | 1064.84 | Cs2SO4 + CM |
| 9,S2 | 2.0794 | 1.4107 | 5.39 | 59.75 | 0.46 | 34.40 | 10.67 | 87.31 | 2.02 | 1009.68 | Cs2SO4 + SS + CM |
| 10,D | 1.5375 | 1.4075 | 0.00 | 12.37 | 32.92 | 54.71 | 0.00 | 11.11 | 88.89 | 987.05 | M6 + CM |
| 11 | 1.5313 | 1.4073 | 2.89 | 8.97 | 33.02 | 55.12 | 3.49 | 8.00 | 88.51 | 987.20 | M6 + CM |
| 12,S3 | 1.5067 | 1.4074 | 3.92 | 7.61 | 33.06 | 55.41 | 4.73 | 6.78 | 88.49 | 991.01 | M6 + RM + CM |
| 13 | 1.4212 | 1.3913 | 6.87 | 7.32 | 24.43 | 61.38 | 10.34 | 8.13 | 81.54 | 1368.79 | RM + CM |
| 14 | 1.3397 | 1.3782 | 11.52 | 8.28 | 15.69 | 64.51 | 21.97 | 11.65 | 66.38 | 1823.49 | RM + CM |
| 15 | 1.3575 | 1.3690 | 17.50 | 9.46 | 8.82 | 64.22 | 39.73 | 15.85 | 44.42 | 2161.01 | RM + CM |
| 16 | 1.3779 | 1.3673 | 20.84 | 9.36 | 5.82 | 63.98 | 51.26 | 16.99 | 31.75 | 2332.36 | RM + CM |
| 17,S4 | 1.5169 | 1.3801 | 33.23 | 16.82 | 1.36 | 48.59 | 68.29 | 25.51 | 6.20 | 1480.05 | SS + RM + CM |
| 18,E | 1.4616 | 1.4027 | 5.60 | 0.00 | 33.51 | 60.89 | 7.01 | 0.00 | 92.99 | 1129.03 | M6 + RM |
| 19 | 1.4866 | 1.4065 | 4.95 | 5.21 | 32.37 | 57.47 | 6.14 | 4.77 | 89.09 | 1056.82 | M6 + RM |
| 20,S3 | 1.5067 | 1.4074 | 3.92 | 7.61 | 33.06 | 55.41 | 4.73 | 6.78 | 88.49 | 991.01 | M6 + RM + CM |
| 21,F | 1.4030 | 1.3704 | 38.06 | 0.00 | 1.43 | 60.51 | 92.31 | 0.00 | 7.69 | 2175.04 | Rb2SO4 + RM |
| 22 | 1.4687 | 1.3730 | 37.03 | 6.71 | 1.42 | 54.84 | 82.05 | 10.97 | 6.98 | 1800.94 | Rb2SO4 + RM |
| 23 | 1.5043 | 1.3825 | 36.48 | 10.37 | 1.39 | 51.76 | 77.26 | 16.21 | 6.53 | 1624.77 | Rb2SO4 + RM |
| 24 | 1.5228 | 1.3810 | 35.64 | 13.69 | 1.37 | 49.30 | 73.06 | 20.71 | 6.23 | 1497.90 | Rb2SO4 + RM |
| 25,S1 | 1.5502 | 1.3804 | 34.46 | 15.70 | 1.36 | 48.48 | 70.24 | 23.61 | 6.15 | 1464.55 | Rb2SO4 + SS + RM |
| 26,S4 | 1.5169 | 1.3801 | 33.23 | 16.82 | 1.36 | 48.59 | 68.29 | 25.51 | 6.20 | 1480.05 | SS + RM + CM |
| 27 | 1.5273 | 1.3812 | 32.43 | 17.45 | 1.31 | 48.81 | 67.27 | 26.71 | 6.03 | 1500.51 | SS + CM |
| 28 | 1.5931 | 1.3862 | 29.69 | 26.06 | 1.23 | 43.02 | 57.49 | 37.23 | 5.28 | 1234.55 | SS + CM |
| 29 | 1.7277 | 1.3910 | 24.80 | 33.67 | 1.14 | 40.39 | 47.54 | 47.62 | 4.85 | 1147.41 | SS + CM |
| 30 | 1.7820 | 1.3961 | 22.81 | 40.29 | 1.05 | 35.85 | 41.57 | 54.18 | 4.25 | 968.41 | SS + CM |
| 31 | 1.8435 | 1.4008 | 19.79 | 45.99 | 0.99 | 33.23 | 35.39 | 60.68 | 3.93 | 880.74 | SS + CM |
| 32 | 1.9604 | 1.4087 | 16.13 | 48.68 | 0.72 | 34.47 | 30.07 | 66.95 | 2.98 | 952.34 | SS + CM |
| 33 | 1.9833 | 1.4095 | 16.01 | 56.33 | 0.78 | 26.88 | 27.00 | 70.08 | 2.92 | 671.79 | SS + CM |
| 34 | 2.0278 | 1.4110 | 13.71 | 56.06 | 0.84 | 29.39 | 24.08 | 72.65 | 3.27 | 765.05 | SS + CM |
| 35 | 2.0586 | 1.4116 | 10.41 | 60.71 | 0.93 | 27.95 | 18.18 | 78.22 | 3.60 | 723.37 | SS + CM |
| 36,S2 | 2.0794 | 1.4107 | 5.39 | 59.75 | 0.46 | 34.40 | 10.67 | 87.31 | 2.02 | 1009.68 | Cs2SO4 + SS + CM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Zeng, Y.; Li, X.; Sun, H.; Li, L.; He, W.; Chen, P.; Yu, X. Solid–Liquid Phase Equilibria of the Aqueous Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at T = 323.2 K. Separations 2024, 11, 309. https://doi.org/10.3390/separations11110309
Yu Z, Zeng Y, Li X, Sun H, Li L, He W, Chen P, Yu X. Solid–Liquid Phase Equilibria of the Aqueous Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at T = 323.2 K. Separations. 2024; 11(11):309. https://doi.org/10.3390/separations11110309
Chicago/Turabian StyleYu, Zhangfa, Ying Zeng, Xuequn Li, Hongbo Sun, Longgang Li, Wanghai He, Peijun Chen, and Xudong Yu. 2024. "Solid–Liquid Phase Equilibria of the Aqueous Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at T = 323.2 K" Separations 11, no. 11: 309. https://doi.org/10.3390/separations11110309
APA StyleYu, Z., Zeng, Y., Li, X., Sun, H., Li, L., He, W., Chen, P., & Yu, X. (2024). Solid–Liquid Phase Equilibria of the Aqueous Quaternary System Rb+, Cs+, Mg2+//SO42− - H2O at T = 323.2 K. Separations, 11(11), 309. https://doi.org/10.3390/separations11110309






