High-Efficiency Selective Adsorption of Rubidium and Cesium from Simulated Brine Using a Magnesium Ammonium Phosphate Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Adsorbents
2.3. Adsorption Experiments
2.3.1. Experiments on the Effect of pH and Coexisting Ions
2.3.2. Adsorption Kinetics Experiments
2.3.3. Adsorption Isotherms Experiments
3. Results and Discussion
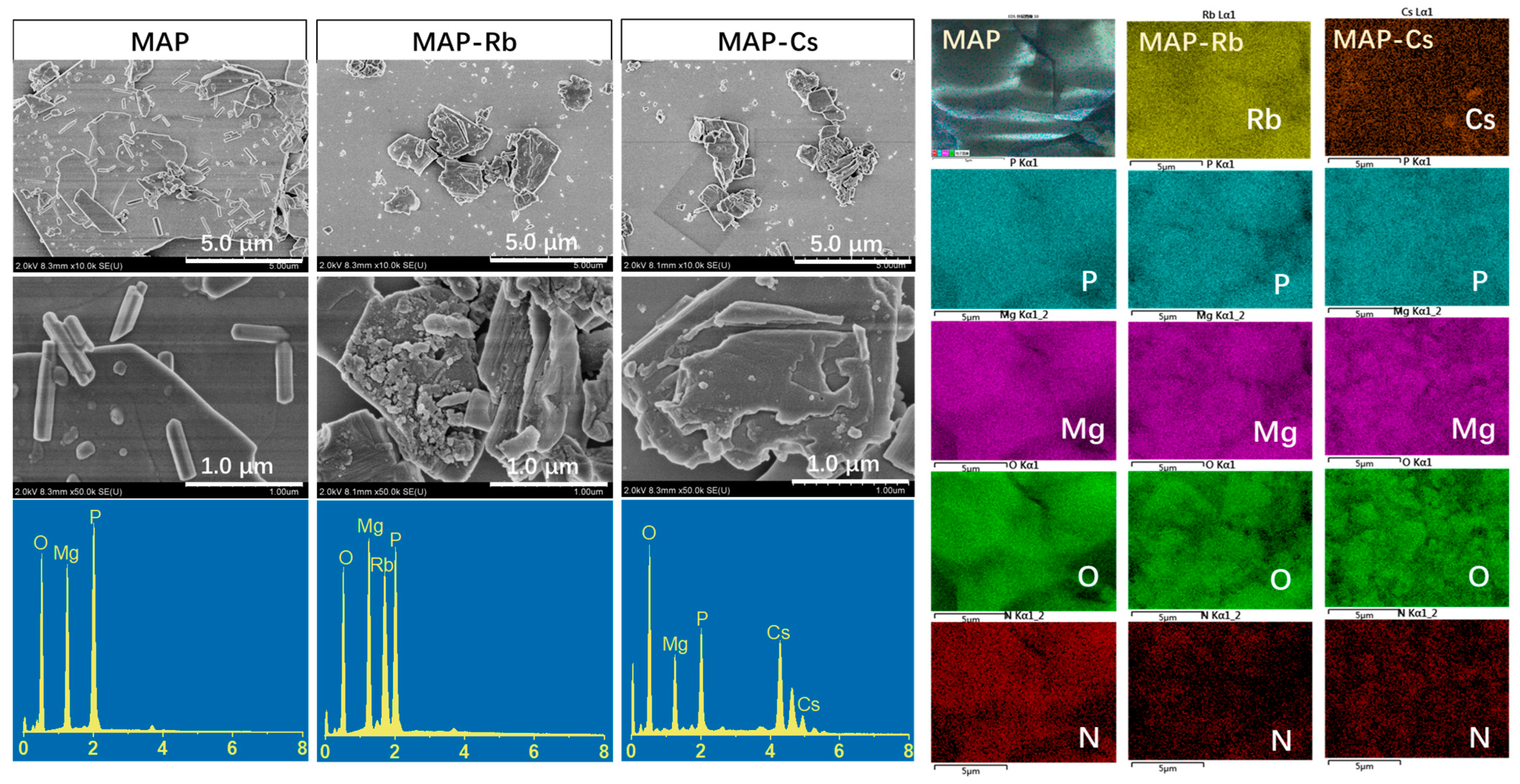
3.1. Characterization of the Adsorbents
3.2. Adsorption Performance of the Adsorbents for Rb+ and Cs+
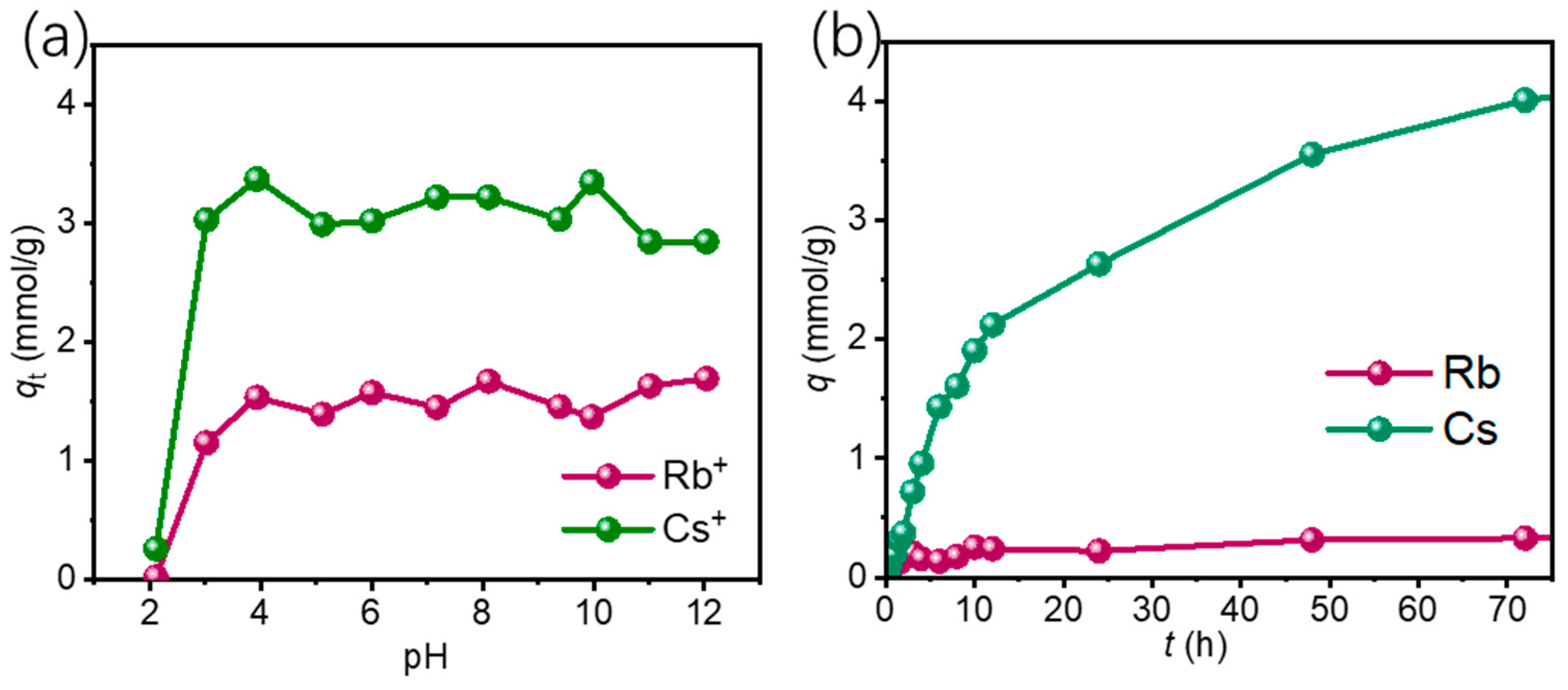
3.2.1. Effect of pH and Coexisting Ions
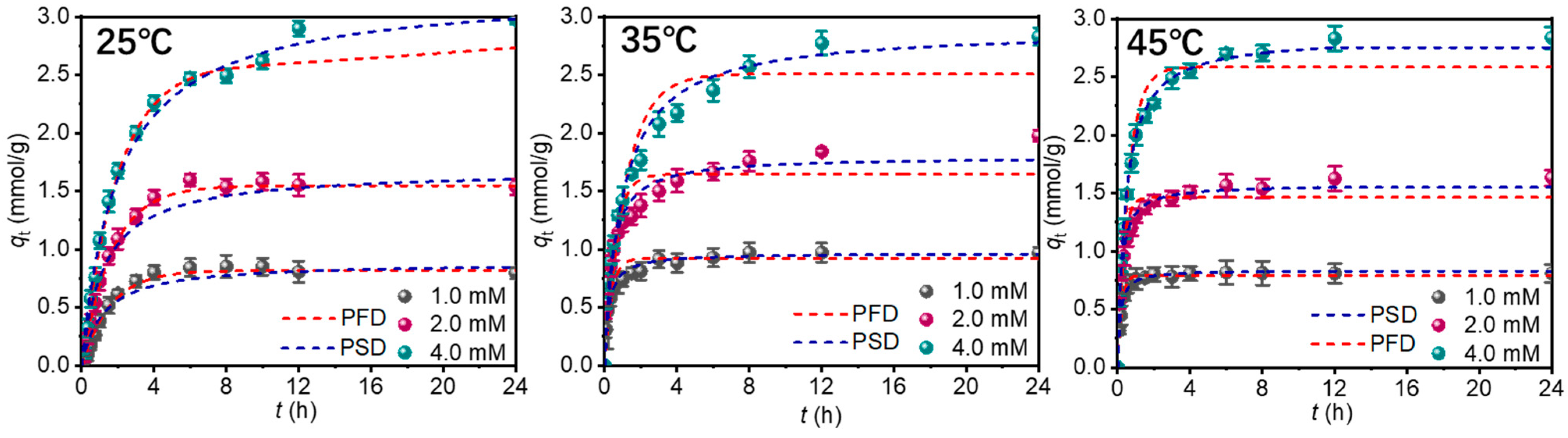
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherms
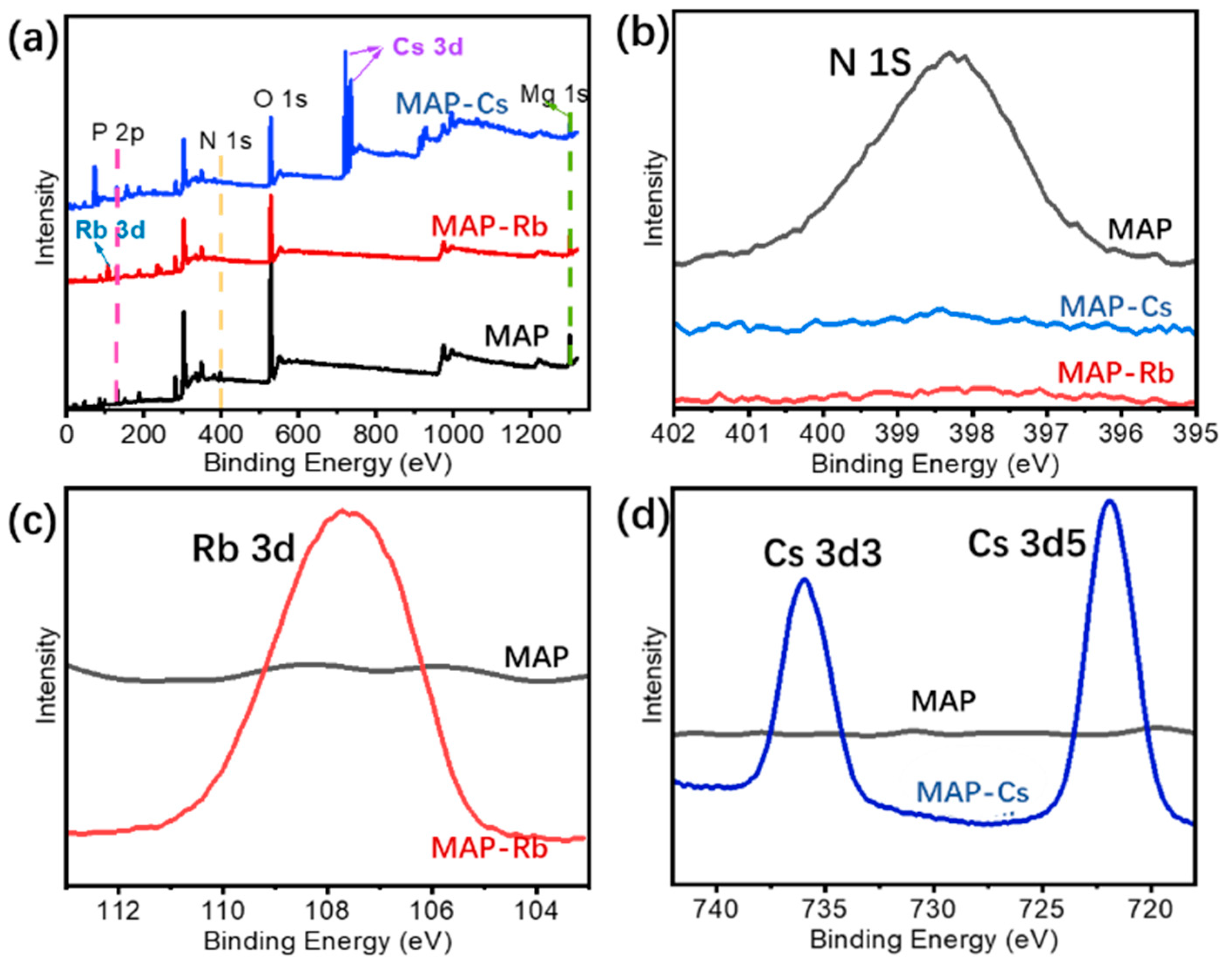
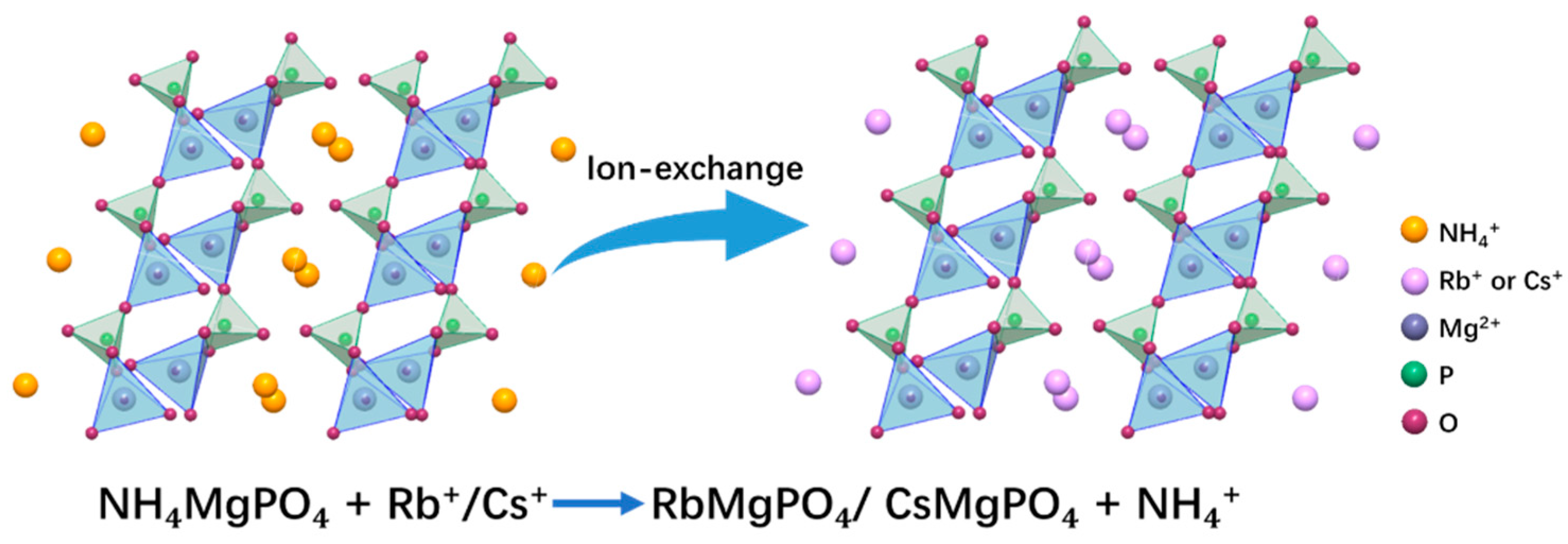
3.3. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, Y.; Ma, B.; Liu, Y.; Wang, C.; Chen, Y. A novel adsorbent potassium magnesium ferrocyanide for selective separation and extraction of rubidium and cesium from ultra-high salt solutions. J. Water Process Eng. 2023, 55, 104225. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Li, J.; Yang, Y.; Wang, Y.-N.; Zhang, Y.-D.; Liu, X.-Z. Rapid and Selective Uptake of Radioactive Cesium from Water by a Microporous Zeolitic-like Sulfide. Inorg. Chem. 2023, 62, 12843–12850. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ran, W.; Li, J.; Khan, A.; Zhong, H.; He, Z. Efficient Leaching of Rubidium from Biotite by Ion Exchange without Destroying the Lamellar Crystal Structure. ACS Sustain. Chem. Eng. 2023, 11, 8822–8835. [Google Scholar] [CrossRef]
- Xu, Z.; Rong, M.; Ni, S.; Meng, Q.; Wu, X.; Liu, H.; Yang, L. Self-Polycondensing Hypercross-Linked Polymers from Hydroxybenzyl Alcohols for Efficient Cesium Adsorption. ACS Appl. Polym. Mater. 2023, 5, 8315–8325. [Google Scholar] [CrossRef]
- Xing, P.; Wang, C.; Ma, B.; Wang, L.; Zhang, W.; Chen, Y. Rubidium and Potassium Extraction from Granitic Rubidium Ore: Process Optimization and Mechanism Study. ACS Sustain. Chem. Eng. 2018, 6, 4922–4930. [Google Scholar] [CrossRef]
- Chu, W.-F.; Yuan, Z.; Lin, P.; Jin, G.-P. Recovery of Rubidium Using Hydrogel Beads Encapsulating Potassium Copper Hexacyanoferrate from Saline Lake Brines. Ind. Eng. Chem. Res. 2024, 63, 1988–1999. [Google Scholar] [CrossRef]
- Li, Z.; Pranolo, Y.; Zhu, Z.; Cheng, C.Y. Solvent extraction of cesium and rubidium from brine solutions using 4-tert-butyl-2-(α-methylbenzyl)-phenol. Hydrometallurgy 2017, 171, 1–7. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, H.; Qian, W.; Tang, A.; Cao, H.; Feng, W.; Zheng, G. Synthesis and assessment of spherogranular composite tin pyrophosphate antimonate adsorbent for selective extraction of rubidium and cesium from salt lakes. Desalination 2024, 581, 117540. [Google Scholar] [CrossRef]
- Lei, Z.; Li, X.; Huang, P.; Hu, H.; Li, Z.; Zhang, Q. Mechanochemical activation of antigorite to provide active magnesium for precipitating cesium from the existences of potassium and sodium. Appl. Clay Sci. 2019, 168, 223–229. [Google Scholar] [CrossRef]
- Yuan, T.; Chen, Q.; Shen, X. Adsorption of cesium using mesoporous silica gel evenly doped by Prussian blue nanoparticles. Chin. Chem. Lett. 2020, 31, 2835–2838. [Google Scholar] [CrossRef]
- Ge, K.; Ji, Y.; Tang, S. Crystallization Kinetics and Mechanism of Magnesium Ammonium Phosphate Hexahydrate: Experimental Investigation and Chemical Potential Gradient Model Analysis and Prediction. Ind. Eng. Chem. Res. 2020, 59, 13799–13809. [Google Scholar] [CrossRef]
- Li, Z.; Yang, C.; Cho, K. Dittmarite-type magnesium phosphates for highly efficient capture of Cs+. J. Hazard. Mater. 2023, 453, 131385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Li, K.; Wang, C.; Fang, D.; Han, W.; Lu, M.; Ye, X.; Zhang, H.; Liu, H.; et al. Efficient Selective Adsorption of Rubidium and Cesium from Practical Brine Using a Metal–Organic Framework-Based Magnetic Adsorbent. Langmuir 2024, 40, 9688–9701. [Google Scholar] [CrossRef]
- Cerofolini, G.F.; Meda, L. A theory of multilayer adsorption on rough surfaces in terms of clustering and melting BET piles. Surf. Sci. 1998, 416, 403–422. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, G.; Yang, X.; Chen, L.; Chen, G.Z. Controllable synthesis of (NH4)Fe2(PO4)2(OH)·2H2O using two-step route: Ultrasonic-intensified impinging stream pre-treatment followed by hydrothermal treatment. Ultrason. Sonochem. 2018, 42, 452–463. [Google Scholar] [CrossRef]
- Gezović, A.; Milović, M.; Bajuk-Bogdanović, D.; Grudić, V.; Dominko, R.; Mentus, S.; Vujković, M.J. An effective approach to reaching the theoretical capacity of a low-cost and environmentally friendly Na4Fe3(PO4)2(P2O7) cathode for Na-ion batteries. Electrochim. Acta 2024, 476, 143718. [Google Scholar] [CrossRef]
- Sugiyama, S.; Yokoyama, M.; Ishizuka, H.; Sotowa, K.-I.; Tomida, T.; Shigemoto, N. Removal of aqueous ammonium with magnesium phosphates obtained from the ammonium-elimination of magnesium ammonium phosphate. J. Colloid Interface Sci. 2005, 292, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Zhang, G.H.; Guo, J.B.; Qi, D.W.; Liu, R.Q. An insight into the thermal properties of struvite-k by Rietveld refinement method. J. Mater. Res. Technol. 2023, 24, 3683–3690. [Google Scholar] [CrossRef]
- Ahmad, M.; Yang, K.; Li, L.; Fan, Y.; Shah, T.; Zhang, Q.; Zhang, B. Modified Tubular Carbon Nanofibers for Adsorption of Uranium (VI) from Water. ACS Appl. Nano Mater. 2020, 3, 6394–6405. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Qiu, F.; Li, Z.; Yan, Y. Dual-Functional Mesoporous Films Templated by Cellulose Nanocrystals for the Selective Adsorption of Lithium and Rubidium. J. Chem. Eng. Data 2019, 64, 926–933. [Google Scholar] [CrossRef]
- Nxumalo, N.L.; Mahlambi, P.N. Molecularly Imprinted Polymer-Based Adsorbents for the Selective Removal of Pharmaceuticals from Wastewater: Adsorption Kinetics, Isotherms, and Thermodynamics Studies. Ind. Eng. Chem. Res. 2023, 62, 16525–16544. [Google Scholar] [CrossRef]
- Ishfaq, M.M.; Karim, H.M.A.; Khan, M.A. A radiochemical study on the thermodynamics of cesium adsorption on potassium copper nickel hexacyanoferrate (II) from aqueous solutions. J. Radioanal. Nucl. Chem. 1997, 222, 177–181. [Google Scholar] [CrossRef]
- Dan, H.; Xian, Q.; Chen, L.; Xiong, T.H.; Jiang, Z.D.; Yi, F.C.; Ding, Y. One-step direct synthesis of mesoporous AMP/SBA-15 using PMA as acid media and its use in cesium ion removal. J. Nucl. Mater. 2019, 527, 151809. [Google Scholar] [CrossRef]
- Aguila, B.; Banerjee, D.; Nie, Z.; Shin, Y.; Ma, S.Q.; Thallapally, P. Selective removal of cesium and strontium using porous frameworks from high level nuclear waste. Chem. Commun. 2016, 52, 5940–5943. [Google Scholar] [CrossRef]
- Ai, J.; Chen, F.Y.; Gao, C.Y.; Tian, H.R.; Pan, Q.J.; Sun, Z.M. Porous Anionic Uranyl-Organic Networks for Highly Efficient Cs+ Adsorption and Investigation of the Mechanism. Inorg. Chem. 2018, 57, 4419–4426. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, G.; Dai, W.; Ma, L.; Ma, N. Enhanced adsorption of rubidium ion by a phenol@MIL-101(Cr) composite material. Microporous Mesoporous Mater. 2017, 251, 51–57. [Google Scholar] [CrossRef]





| Concentration (mg/L) | Adsorption Efficiency (%) | ||
|---|---|---|---|
| Ions | Before Adsorption | After Adsorption | |
| Li+ | 355.3 | 349.7 | 1.58 |
| Na+ | 11,893.2 | 11,819.6 | 0.62 |
| K+ | 4082.3 | 4011.6 | 1.73 |
| Mg2+ | 8061.6 | 7936.2 | 1.56 |
| Rb+ | 440.4 | 405.1 | 8.02 |
| Cs+ | 744.5 | 147.2 | 80.3 |
| NH4+ | 84.9 | ||
| PFO | PSO | |||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | C (mmol/L) | qe,exp (mmol/g) | qe,cal (mmol/g) | k1 | R2 | qe,cal (mmol/g) | k2 | R2 |
| 25 | 1.00 | 0.917 | 0.889 | 0.085 | 0.996 | 1.046 | 0.090 | 0.997 |
| 2.00 | 1.374 | 1.332 | 0.113 | 0.990 | 1.515 | 0.091 | 0.998 | |
| 4.00 | 1.835 | 1.800 | 0.127 | 0.997 | 2.038 | 0.074 | 0.996 | |
| 35 | 1.00 | 0.483 | 0.481 | 0.059 | 0.982 | 0.643 | 0.080 | 0.987 |
| 2.00 | 0.934 | 0.870 | 0.068 | 0.951 | 1.099 | 0.062 | 0.967 | |
| 4.00 | 1.443 | 1.390 | 0.097 | 0.994 | 1.731 | 0.055 | 0.997 | |
| 45 | 1.00 | 0.242 | 0.243 | 0.058 | 0.981 | 0.329 | 0.150 | 0.986 |
| 2.00 | 0.378 | 0.374 | 0.064 | 0.988 | 0.512 | 0.103 | 0.992 | |
| 4.00 | 0.779 | 0.766 | 0.094 | 0.995 | 0.985 | 0.088 | 0.996 | |
| PFO | PSO | |||||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | C (mmol/L) | qe,exp (mmol/g) | qe,cal (mmol/g) | k1 | R2 | qe,cal (mmol/g) | k2 | R2 |
| 25 | 1.00 | 0.851 | 0.883 | 0.544 | 0.992 | 0.887 | 0.957 | 0.994 |
| 2.00 | 1.596 | 1.547 | 0.602 | 0.995 | 1.683 | 0.483 | 0.995 | |
| 4.00 | 3.017 | 2.99 | 0.306 | 0.959 | 3.232 | 0.153 | 0.992 | |
| 35 | 1.00 | 0.969 | 0.886 | 3.178 | 0.923 | 0.951 | 5.003 | 0.985 |
| 2.00 | 1.976 | 1.646 | 1.791 | 0.883 | 1.799 | 1.405 | 0.959 | |
| 4.00 | 2.830 | 2.508 | 0.842 | 0.921 | 2.803 | 0.399 | 0.971 | |
| 45 | 1.00 | 0.808 | 0.79 | 4.882 | 0.989 | 0.837 | 9.389 | 0.994 |
| 2.00 | 1.625 | 1.464 | 3.492 | 0.940 | 1.573 | 3.391 | 0.990 | |
| 4.00 | 2.831 | 2.584 | 1.661 | 0.964 | 2.852 | 0.803 | 0.994 | |
| Langmuir model | |||
|---|---|---|---|
| T | 25 °C | 35 °C | 45 °C |
| qm,exp (mmol/g) | 2.828 | 2.478 | 1.473 |
| qm,cal (mmol/g) | 3.037 | 2.776 | 2.186 |
| KL | 0.967 | 0.586 | 0.177 |
| R2 | 0.982 | 0.966 | 0.992 |
| Freundlich model | |||
| KF | 1.440 | 1.030 | 0.429 |
| n | 3.125 | 2.538 | 1.932 |
| R2 | 0.976 | 0.948 | 0.994 |
| Langmuir model | |||
|---|---|---|---|
| T | 25 °C | 35 °C | 45 °C |
| qm,exp (mmol/g) | 4.372 | 4.253 | 4.048 |
| qm,cal (mmol/g) | 4.379 | 4.332 | 4.227 |
| KL | 2.729 | 3.929 | 4.531 |
| R2 | 0.968 | 0.996 | 0.983 |
| Freundlich model | |||
| KF | 2.863 | 3.061 | 3.128 |
| n | 5.041 | 6.013 | 7.095 |
| R2 | 0.886 | 0.827 | 0.680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, Y.; Zhang, Q.; Han, W.; Zhang, H.; Ye, X. High-Efficiency Selective Adsorption of Rubidium and Cesium from Simulated Brine Using a Magnesium Ammonium Phosphate Adsorbent. Separations 2024, 11, 277. https://doi.org/10.3390/separations11090277
Liu H, Wang Y, Zhang Q, Han W, Zhang H, Ye X. High-Efficiency Selective Adsorption of Rubidium and Cesium from Simulated Brine Using a Magnesium Ammonium Phosphate Adsorbent. Separations. 2024; 11(9):277. https://doi.org/10.3390/separations11090277
Chicago/Turabian StyleLiu, Haining, Yanping Wang, Qiongyuan Zhang, Wenjie Han, Huifang Zhang, and Xiushen Ye. 2024. "High-Efficiency Selective Adsorption of Rubidium and Cesium from Simulated Brine Using a Magnesium Ammonium Phosphate Adsorbent" Separations 11, no. 9: 277. https://doi.org/10.3390/separations11090277
APA StyleLiu, H., Wang, Y., Zhang, Q., Han, W., Zhang, H., & Ye, X. (2024). High-Efficiency Selective Adsorption of Rubidium and Cesium from Simulated Brine Using a Magnesium Ammonium Phosphate Adsorbent. Separations, 11(9), 277. https://doi.org/10.3390/separations11090277







