Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Bat Feces Preparation
2.2. Preparation of Standards
2.3. Determination of Antioxidant Capacity of Fecal Samples
2.4. Vitamin Analysis
2.4.1. Water-Soluble Vitamins
- (A)
- Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
- Quaternary pump: Shimadzu LC-20AD;
- (2)
- Autosampler: Shimadzu SIL-20AC;
- (3)
- Photodiode array detector: Shimadzu SPD-M20;
- (4)
- Mass detector: Shimadzu LCMS-8040.
- (B)
- Sample preparation:
- (1)
- Place 1 g of sample powder in a 50 mL centrifuge tube, add 9 mL of 10 mM ammonium acetate aqueous solution, vortex for 1 min, and then ultrasonicate for 15 min;
- (2)
- Add another 10 mL of chloroform (chloroform) to the centrifuge tube and vortex for 1 min;
- (3)
- Centrifuge at 3500 rpm for 10 min, take out the supernatant, filter it with a 0.22 μm filter membrane, and use the filtrate as the test solution.
- (C)
- LC/MS/MS analysis method:Chromatography column: Raptor Biphenyl (2.7 μm, 100 × 2.1 mm);Column temperature: 35 °C.Mobile phase: as shown in Table 1;A: 5 mM ammonium acetate, 0.1% formic acid in water;B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
| Time (min) | A, % | B, % |
|---|---|---|
| Initial | 100 | 0 |
| 1.00 | 100 | 0 |
| 6.80 | 0 | 100 |
| 8.80 | 0 | 100 |
| 9.00 | 100 | 0 |
| 12.00 | 100 | 0 |
- Flow rate: 0.4 mL/min;
- Injection volume: 15 μL.
- Mass spectrometry conditions:
- Ion source: electrospray ionization (ESI+);
- Ion source interface voltage (probe voltage): 4.5 kV;
- Nebulizing gas flow: nitrogen, 3.0 mL/min;
- Drying gas flow: 15.00 L/min;
- Collision gas: argon, 230 kPa;
- Desolventization tube temperature (DL temp.): 250 °C;
- Heating module temperature (heat block temp.): 400 °C;
- Quantitative ion pair: as shown in Table 2.
| Vitamin | Quantitative Ion Pair | Qualitative Ion Pair |
|---|---|---|
| Precursor Ion (m/z)→Product Ion (m/z) | Precursor Ion (m/z)→Product Ion (m/z) | |
| B1 (thiamine) | 265.00→122.10 | 265.00→144.10 |
| B2 (riboflavin) | 377.20→243.10 | 377.20→198.10 |
| B3 (nicotinamide) | 123.00→80.10 | 123.00→96.10 |
| B3 (nicotinic acid) | 123.90→80.10 | 123.90→78.10 |
| B5 (pantothenic acid) | 220.20→90.10 | 220.20→202.10 |
| B6 (pyridoxine) | 170.00→152.10 | 170.00→134.10 |
| B8 (biotin) | 245.20→227.20 | 245.20→123.10 |
| B9 (folic acid) | 441.90→295.10 | 441.90→176.20 |
| B12 (cyanocobalamin) | 678.60→147.10 | 678.60→359.20 |
2.4.2. Vitamin C
- (A)
- Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
- Quaternary pump: Shimadzu LC-20AD;
- (2)
- Autosampler: Shimadzu SIL-20AC;
- (3)
- Photodiode array detector: Shimadzu SPD-M20;
- (4)
- Mass detector: Shimadzu LCMS-8040.
- (B)
- Sample preparation:
- (1)
- Place 1 g of sample powder in a 50 mL centrifuge tube, add 9 mL of 10 mM ammonium acetate aqueous solution, vortex for 1 min, and then ultrasonicate for 15 min;
- (2)
- Add another 10 mL of chloroform (chloroform) to the centrifuge tube and vortex for 1 min;
- (3)
- Centrifuge at 3500 rpm for 10 min, take out the supernatant, filter it with a 0.22 μm filter membrane, and use the filtrate as the test solution.
- (C)
- LC/MS/MS analysis method:LC analysis conditions:Chromatography column: Raptor Biphenyl (2.7 μm, 100 × 2.1 mm);Column temperature: 35 °C:Mobile phase: as shown in Table 3;A: 5 mM ammonium acetate, 0.1% formic acid in water;B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
| Time (min) | A, % | B, % |
|---|---|---|
| Initial | 100 | 0 |
| 2.40 | 100 | 0 |
| 4.40 | 89 | 11 |
| 4.60 | 70 | 30 |
| 6.50 | 68 | 32 |
| 6.70 | 0 | 100 |
| 7.00 | 100 | 0 |
- Flow rate: 0.2 mL/min;
- Injection volume: 5 μL.
- Mass spectrometry conditions:
- Ion source: electrospray ionization (ESI+);
- Ion source interface voltage (probe voltage): 4.5 kV;
- Nebulizing gas flow: nitrogen, 3.0 mL/min;
- Drying gas flow: 15.00 L/min;
- Collision gas: argon, 230 kPa;
- Desolventization tube temperature (DL temp.): 250 °C;
- Heating module temperature (heat block temp.): 400 °C;
- Quantitative ion pair: as shown in Table 4.
| Vitamin | Quantitative Ion Pair | Qualitative Ion Pair |
|---|---|---|
| Precursor Ion (m/z)→Product Ion (m/z) | Precursor Ion (m/z)→Product Ion (m/z) | |
| C | 177.10→95.10 | 177.10→141.20 |
2.4.3. Fat-Soluble Vitamins
- (A)
- Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
- Quaternary pump: Shimadzu LC-20AD;
- (2)
- Autosampler: Shimadzu SIL-20AC;
- (3)
- Photodiode array detector: Shimadzu SPD-M20;
- (4)
- Mass detector: Shimadzu LCMS-8040.
- (B)
- Sample preparation:
- (1)
- Take 0.25 g of sample powder and place it in a 15 mL centrifuge tube, add 1.5 mL of pure water and 1.5 mL of methanol (methanol), shake with a vortex mixer for 1 min, and then shake with ultrasonic for 20 min;
- (2)
- Add another 10 mL of n-Hexane to the centrifuge tube and vortex for 5 min;
- (3)
- Centrifuge at 3500 rpm for 10 min, and place 1 mL of supernatant into a glass centrifuge tube;
- (4)
- Blow dry with nitrogen in a 40 °C water bath and add 1 mL of methanol (methanol) to dissolve and mix evenly;
- (5)
- Filter with a 0.22 μm filter membrane and use the filtrate as the test solution.
- (C)
- LC/MS/MS analysis method:Chromatography column: Raptor Biphenyl (2.7 μm, 100 × 2.1 mm);Column temperature: 35 °C;Mobile phase: as shown in Table 5;A: 5 mM ammonium acetate, 0.1% formic acid in water;B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
| Time (min) | A, % | B, % |
|---|---|---|
| Initial | 100 | 0 |
| 2.40 | 100 | 0 |
| 4.40 | 89 | 11 |
| 4.60 | 70 | 30 |
| 6.50 | 68 | 32 |
| 6.70 | 0 | 100 |
| 7.00 | 100 | 0 |
- Flow rate: 0.4 mL/min;
- Injection volume: 5 μL.
- Mass spectrometry conditions:
- Ion source: electrospray ionization (ESI+);
- Ion source interface voltage (probe voltage): 4.5 kV;
- Nebulizing gas flow: nitrogen, 3.0 mL/min;
- Drying gas flow: 15.00 L/min;
- Collision gas: argon, 230 kPa;
- Desolventization tube temperature (DL temp.): 250 °C;
- Heating module temperature (heat block temp.): 400 °C;
- Quantitative ion pair: as shown in Table 6.
| Vitamin | Quantitative Ion Pair | Qualitative Ion Pair |
|---|---|---|
| Precursor Ion (m/z)→Product Ion (m/z) | Precursor Ion (m/z)→Product Ion (m/z) | |
| A | 269.30→93.10 | 269.30→119.10 |
| D3 | 385.20→367.40 | 385.20→259.30 |
| E | 431.10→165.20 | 431.10→137.10 |
| K1 | 451.30→187.20 | 451.30→185.10 |
2.5. Heavy Metals Analysis
- (A)
- Inductively coupled plasma–mass spectrometry (ICP-MS) conditions:Agilent 7500a.
- (B)
- Sample preparation:
- (1)
- Take 0.4 g of the sample powder and place it in a microwave digestion bottle, add 8 mL of nitric acid, let it stand for about 10 min, and then digest it in a microwave digester. The operating conditions of microwave digestion are as shown in the table below;
Stage # Max Power (W) Ramp (min) Temperature (°C) Hold (min) 1 1200 15 175 05:00 2 1200 5 200 15:00 - (2)
- After the digestion is completed, cool to room temperature and transfer to a 100 mL quantitative flask. Wash the microwave digestion flask with pure water. Put the washing liquid into the quantitative flask, dilute it with pure water to a constant volume, mix evenly, and filter through a 0.45 μm filter membrane. The filtrate was the finished product sample solution, and this solution was used as the test solution.
- (C)
- ICP-MS analysis method.
- (1)
- Method settings:Acquisition mode: spectrum;Peak pattern: full quant;Every mass integration time: 0.33 s;Repetition: three times.
- (2)
- Peristaltic pump program:Uptake speed: 0.35 rps;Uptake time: 30 s;Stabilization time: 30 s.
- (3)
- Analysis conditions.Plasma Parameters:
- Plasma radio frequency power: 500~1600 W, normal setting 1200 W;
- Sampling depth: 3.0–23.0 mm, normal setting 10 mm;
- Carrier gas flow rate: 0.00–2.00 L/min, normal setting is 1 L/min;
- Auxiliary gas flow rate: 0.00–2.00 L/min, normal setting is 0.22 L/min;
- Nebulizer pump: 0.00–0.50 rps, normal setting is 0.1 rps;
- Premix chamber temperature (S/C temp): 2 °C.
Ion Lenses:- Extract 1: −200–10 V, normal setting −120 V;
- Extract 2: −200–0 V, normal setting −39 V;
- Einzel 1,3: −200–100 V, normal setting −80 V;
- Einzel 2: −200–100 V, normal setting 8 V;
- Omega bias: −200–100 V, normal setting −41 V;
- Omega (+): −200–100 V, normal setting 9 V;
- Omega (−): −200–100 V, normal setting 9 V;
- QF focus: −200–100 V, normal setting 9 V;
- Plate bias: −50–50 V, normal setting −10 V.
2.6. Statistical Analysis
3. Results
3.1. Antioxidant Capacity of Bat Feces Treatment
3.2. Quantification of Vitamins in Bat Feces Using LC/MS/MS Analysis
3.3. Quantification of Heavy Metals in Bat Feces Using ICP/MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, R.; Zhang, B.; Xue, C.M.; Liu, S.M.; Zhao, Q.; Li, K. [Classification of 365 Chinese medicines in Shennong’s Materia Medica Classic based on a semi-supervised incremental clustering method]. Zhong Xi Yi Jie He Xue Bao 2011, 9, 665–674. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z. Compendium of Materia Medica: Bencao Gangmu; Luo, X.W., Ed.; Beijing Foreign Languages Press: Beijing, China, 2003; ISBN 7119032607. [Google Scholar]
- Wu, Y. Rihuazi Materia Medica; Anhui Science and Technology Press: Hefei, China, 2005; ISBN 13:9787533732455. [Google Scholar]
- Dierenfeld, E.S.; Seyjagat, J. Plasma fat-soluble vitamin and mineral concentrations in relation to diet in captive pteropodid bats. J. Zoo Wildl. Med. 2000, 31, 315–321. [Google Scholar] [PubMed]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Conservation. Economic importance of bats in agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Bosso, L.; Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Environ. 2018, 266, 31–38. [Google Scholar] [CrossRef]
- Emerson, J.K.; Roark, A.M. Composition of guano produced by frugivorous, sanguivorous, and insectivorous bats. Acta Chiropterol. 2007, 9, 261–267. [Google Scholar] [CrossRef]
- Banskar, S.; Mourya, D.T.; Shouche, Y.S. Bacterial diversity indicates dietary overlap among bats of different feeding habits. Microb. Res. 2016, 182, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Kuang, T.T.; Qiu, S.; Xu, T.; Gang Huan, C.L.; Fan, G.; Zhang, Y. Fecal medicines used in traditional medical system of China: A systematic review of their names, original species, traditional uses, and modern investigations. Chin. Med. 2019, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Fan, B.; Li, Y.L.; Zuo, Z.Y.; Li, G.Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Gong, J.; Zheng, H.; Jia, X.; Wang, M.; Wu, Y.; Zhang, X.; Chen, G.; Ni, S. Research on the traditional Chinese medicine bat feces. Jilin J. Tradit. Chin. Med. 2012, 32, 1047–1049. [Google Scholar]
- Steinert, R.E.; Lee, Y.K.; Sybesma, W. Vitamins for the Gut Microbiome. Trends Mol. Med. 2020, 26, 137–140. [Google Scholar] [CrossRef]
- Xia, Y.; Ji, C.; Li, M.; Zhang, W.; Cheng, X.; Qiu, Y.; Ge, W. Simultaneous quantification of seven B vitamins in human feces by stable isotope label-based high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2024, 237, 115784. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Ghisi, D.; Westermann, B.; Bettoni, A.; Fanelli, A.; La Colla, L.; Danelli, G.; Cesana, B.M. The use of vitamin B1 as a perineural adjuvant to middle interscalene block for postoperative analgesia after shoulder surgery. Acta Biomed. 2016, 87, 22–27. [Google Scholar] [PubMed]
- Nebbioso, M.; Rusciano, D.; Pucci, B.; Zicari, A.M.; Grenga, R.; Pescocolido, N. Treat-ment of glaucomatous patients by means of food supplement to reduce the ocular dis-comfort: A double blind randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1117–1122. [Google Scholar] [PubMed]
- Ren, X.; Chou, Y.; Wang, Y.; Jing, D.; Chen, Y.; Li, X. The Utility of Oral Vitamin B1 and Mecobalamin to Improve Corneal Nerves in Dry Eye Disease: An In Vivo Confocal Microscopy Study. Nutrients 2022, 14, 3750. [Google Scholar] [CrossRef]
- Pereira, A.; Adekunle, R.D.; Zaman, M.; Wan, M.J. Association Between Vitamin Deficiencies and Ophthalmological Conditions. Clin. Ophthalmol. 2023, 17, 2045–2062. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Barthes, S.; Féart, C.; Cougnard-Grégoire, A.; Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Delcourt, C. B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2022, 14, 2821. [Google Scholar] [CrossRef]
- Das, T.; Sikdar, S.; Chowdhury, M.H.U.; Nyma, K.J.; Adnan, M. SARS-CoV-2 prevalence in domestic and wildlife animals: A genomic and docking based structural comprehensive review. Heliyon 2023, 9, e19345. [Google Scholar] [CrossRef] [PubMed]
- Gerbáčová, K.; Maliničová, L.; Kisková, J.; Maslišová, V.; Uhrin, M.; Pristaš, P. The Faecal Microbiome of Building-Dwelling Insectivorous Bats (Myotis myotis and Rhinolophus hipposideros) also Contains Antibiotic-Resistant Bacterial Representatives. Curr. Microbiol. 2020, 77, 2333–2344. [Google Scholar] [CrossRef]
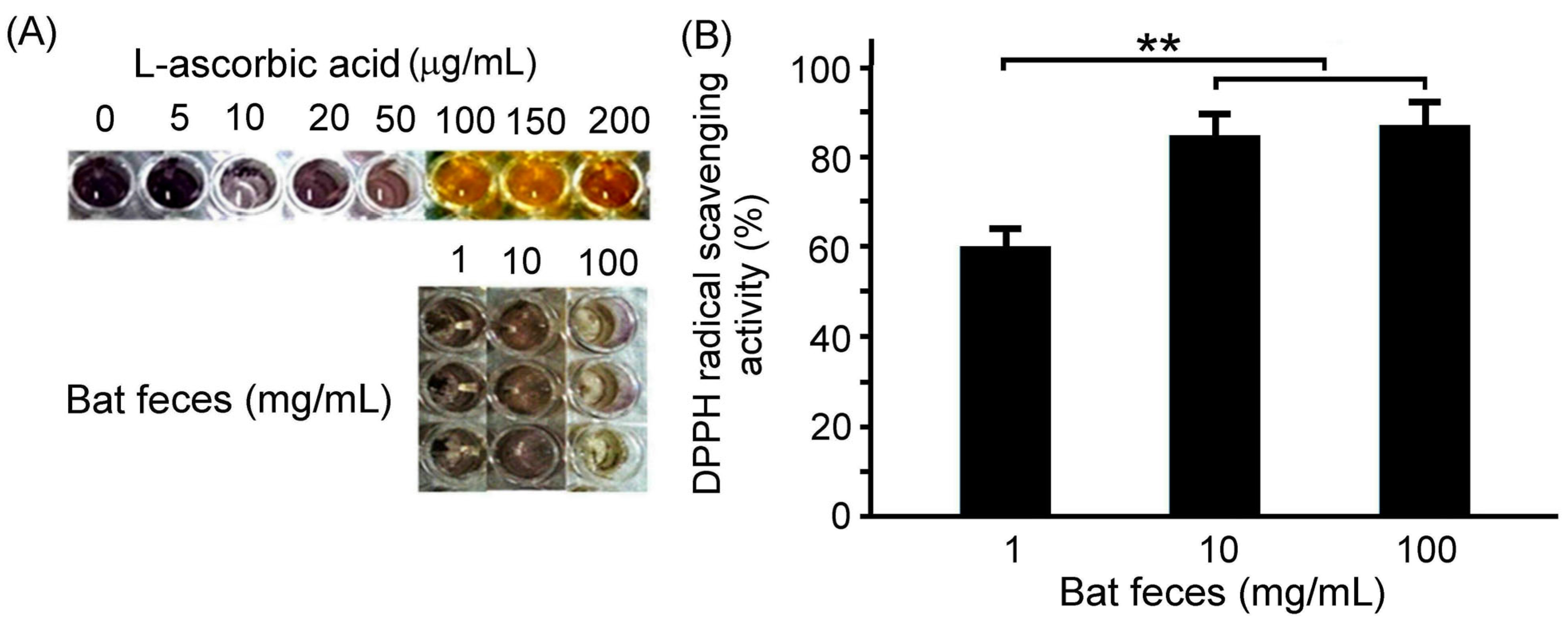
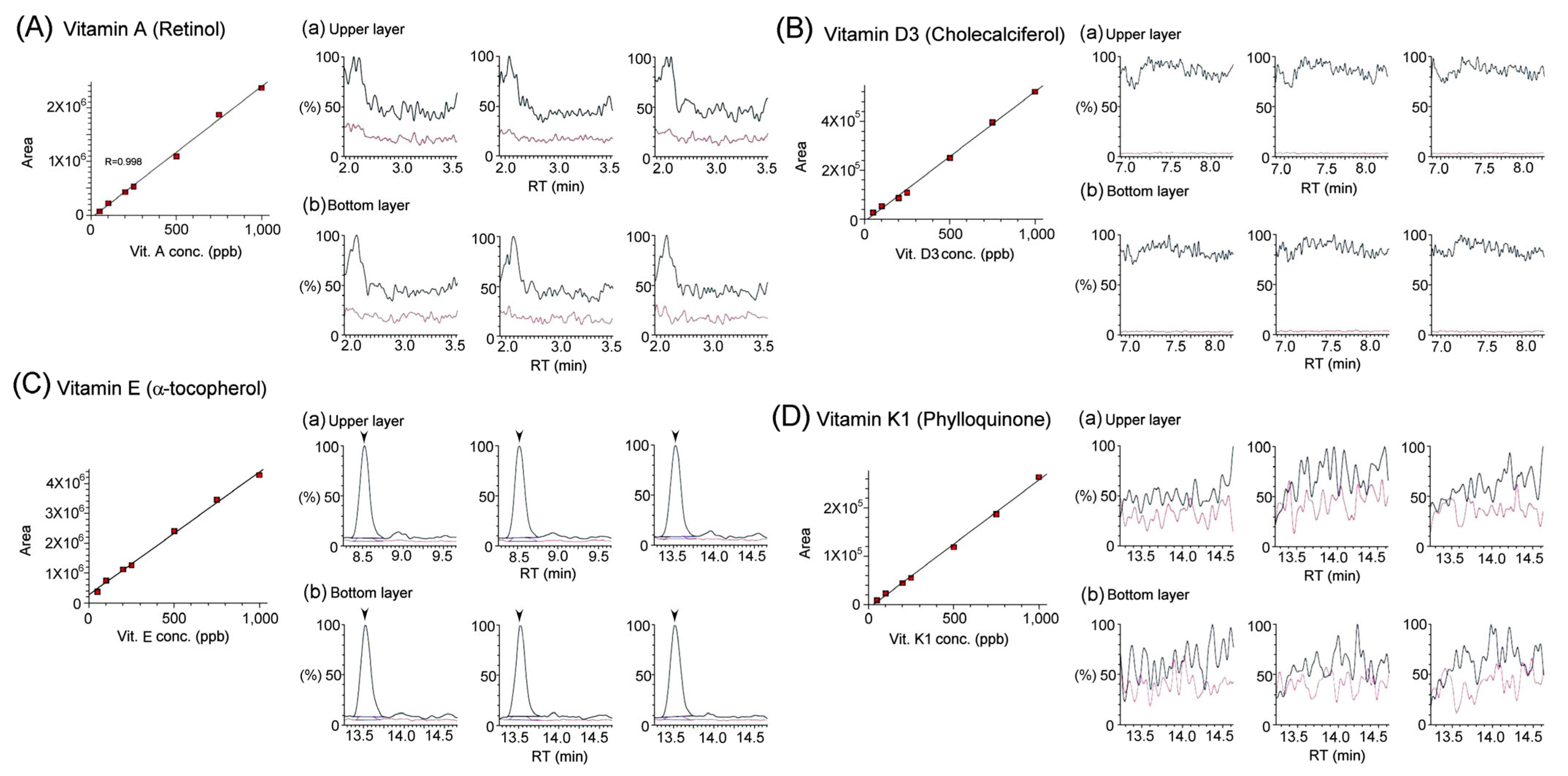

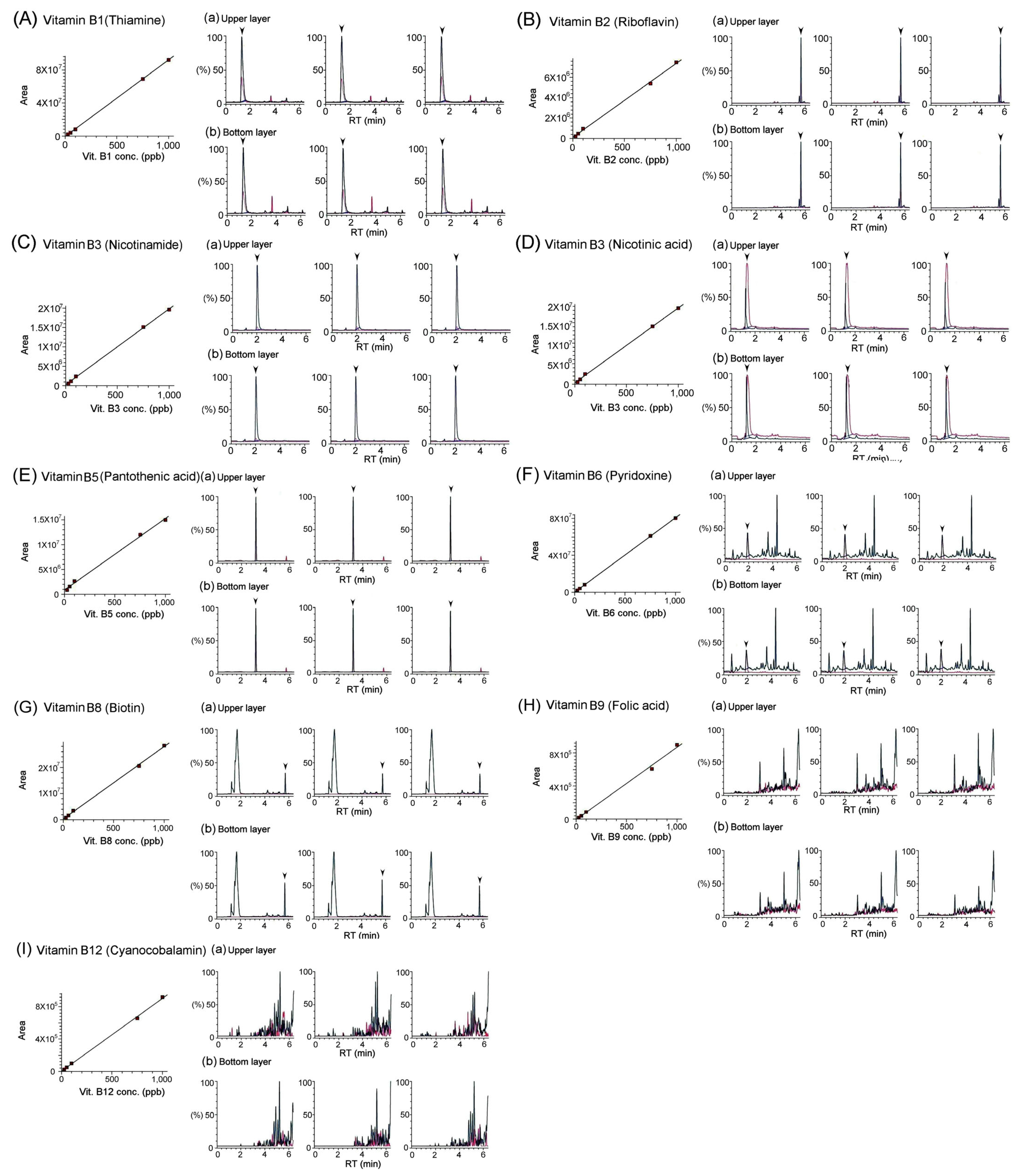
| Vitamins | Bat Feces | ||
|---|---|---|---|
| Upper Layer | Bottom Layer | ||
| Water-Soluble Vitamins | B1(thiamine) | 3.44 ± 0.05 | 2.22 ± 0.02 |
| B2 (riboflavin) | 6.75 ± 0.34 | 2.37 ± 0.21 | |
| B3 (nicotinamide) | 52.53 ± 1.50 | 70.41 ± 1.46 | |
| B3 (nicotinic acid) | 19.67 ± 0.36 | 16.13 ± 0.49 | |
| B5 (pantothenic acid) | 62.63 ± 2.34 | 41.38 ± 0.33 | |
| B6 (pyridoxine) | 0.05 ± 0.02 | 0.04 ± 0.02 | |
| B8 (biotin) | N/A | N/A | |
| B9 (folic acid) | N/A | N/A | |
| B12 (cyanocobalamin) | N/A | N/A | |
| C (ascorbic acid) | N/A | N/A | |
| Fat-Soluble Vitamins | A (retinol) | N/A | N/A |
| D3 (cholecalciferol) | N/A | N/A | |
| E (α-tocopherol) | N/A | N/A | |
| K1 (phylloquinone) | N/A | N/A | |
| Types of Heavy Metals | Heavy Metals in Bat Feces (ppm) | Limitation Standards of Heavy Metals in TCM (ppm) |
|---|---|---|
| Chromium (Cr) | 2.87 ± 0.38 | -- |
| Manganese (Mn) | 55.53 ± 4.48 | -- |
| Copper (Cu) | 46.25 ± 3.51 | -- |
| Arsenic (As) | 5.57 ± 0.68 | 2.0 |
| Cadmium (Cd) | 0.39 ± 0.07 | 1.0 |
| Mercury (Hg) | 0.33 ± 0.07 | 0.2 |
| Lead (Pb) | 2.29 ± 0.37 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-T.; Lin, C.-L.; Chuang, W.-C.; Lee, M.-C.; Chen, L.-W.; Wu, C.-H. Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine. Separations 2024, 11, 76. https://doi.org/10.3390/separations11030076
Chung K-T, Lin C-L, Chuang W-C, Lee M-C, Chen L-W, Wu C-H. Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine. Separations. 2024; 11(3):76. https://doi.org/10.3390/separations11030076
Chicago/Turabian StyleChung, Kou-Toung, Ching-Lung Lin, Wu-Chang Chuang, Ming-Chung Lee, Li-Wen Chen, and Chung-Hsin Wu. 2024. "Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine" Separations 11, no. 3: 76. https://doi.org/10.3390/separations11030076
APA StyleChung, K.-T., Lin, C.-L., Chuang, W.-C., Lee, M.-C., Chen, L.-W., & Wu, C.-H. (2024). Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine. Separations, 11(3), 76. https://doi.org/10.3390/separations11030076







