Variations in Essential Oil Biological Activities of Female Cones at Different Developmental Stages from Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
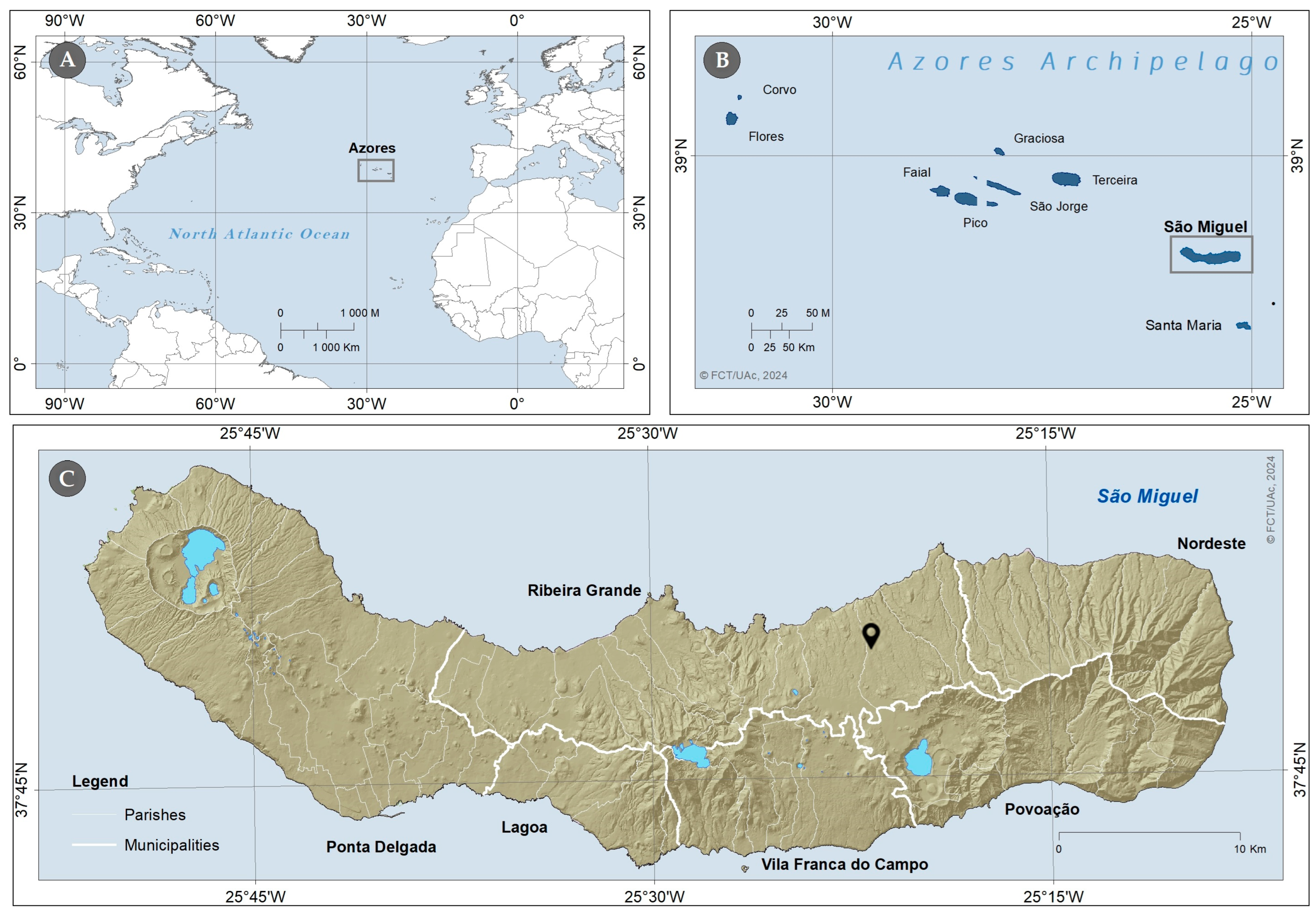
2.2. Plant Material and Collection Site
2.3. Essential Oil Extraction by Hydrodistillation Method
2.4. Essential Oil Composition Analysis
2.5. In Vitro Antioxidant Activity Evaluation
2.5.1. DPPH Free-Radical-Scavenging Activity (FRSA) Assay
2.5.2. β-Carotene-Linoleic Acid Bleaching Activity (BCBA) Assay
2.6. In Vitro Antimicrobial Activity
2.6.1. Microbial Strains and Culture Media
2.6.2. Disc Diffusion Method (DDM)
2.7. Brine Shrimp Lethality Activity (BSLA) Assay
2.8. Statistical Analysis
3. Results and Discussion
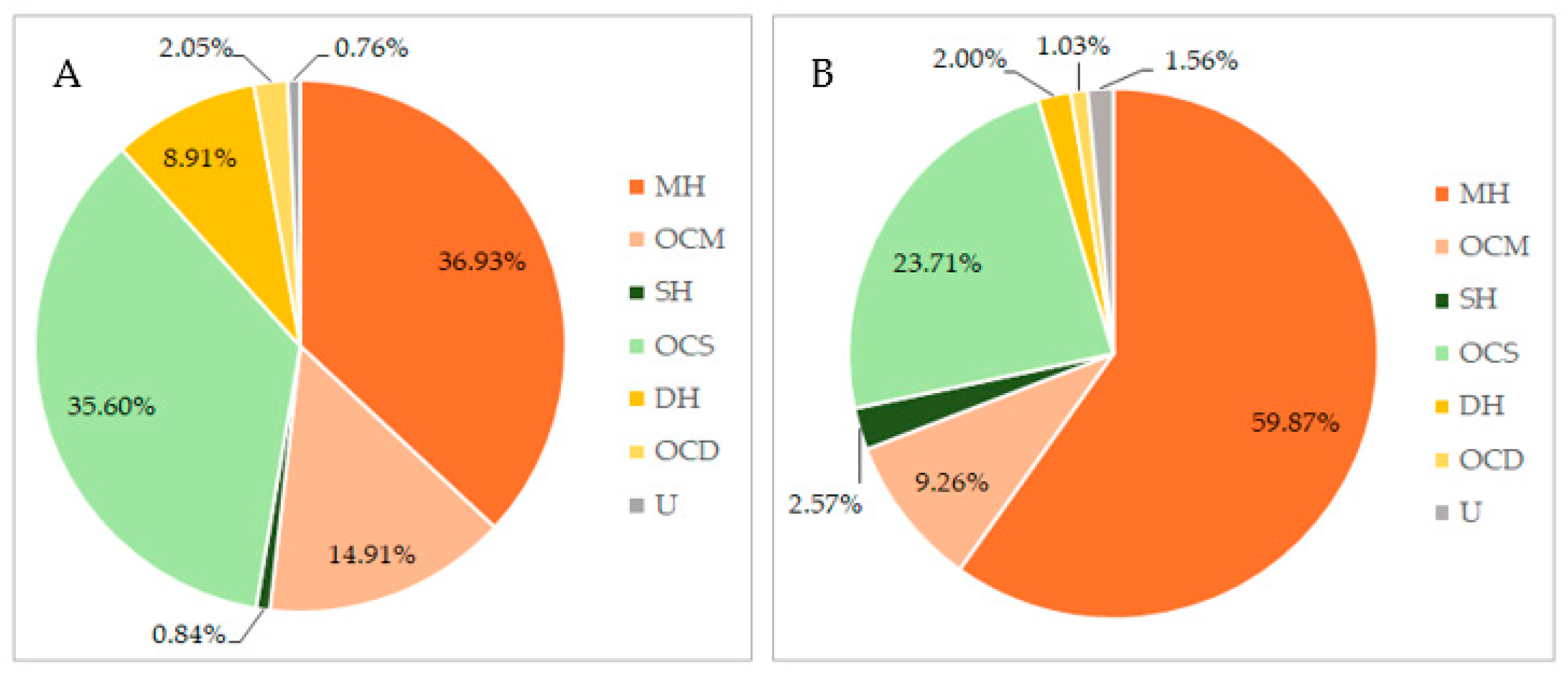
3.1. Essential Oils’ Isolation and Chemical Composition of Immature and Mature Female Cones (IFC and MFC) from Azorean C. japonica
3.2. Biological Activities of Immature and Mature Female Cones (IFC and MFC) from Azorean C. japonica
3.2.1. In Vitro Antioxidant Activities Evaluated by Radical Scavenging and Lipid Peroxidation Assays
3.2.2. In Vitro Antimicrobial Activities
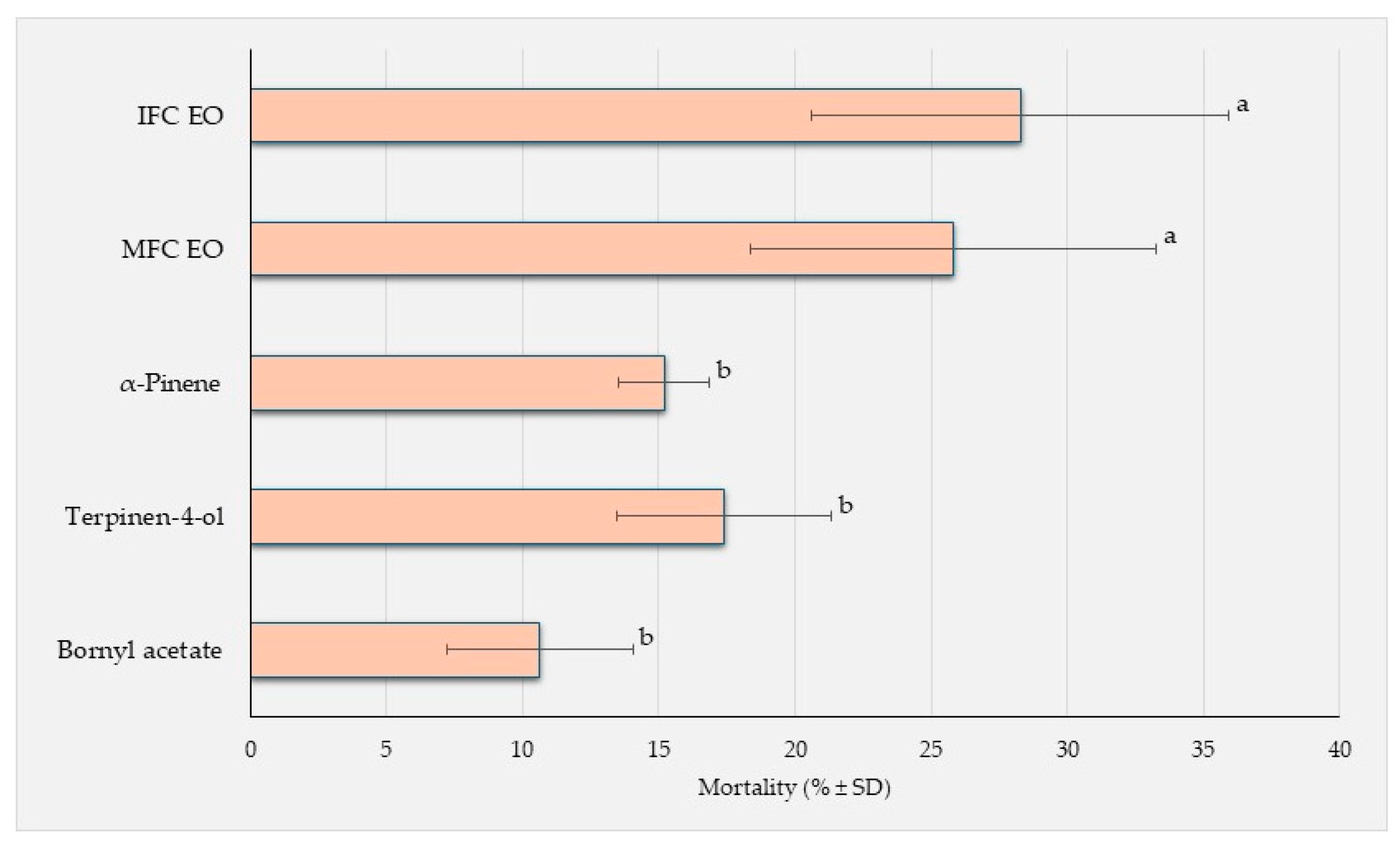
3.2.3. Brine Shrimp Lethality Activity (BSLA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Singh, G.; Agrawal, H.; Bednarek, P. Specialized metabolites as versatile tools in shaping plant-microbe associations. Mol. Plant 2023, 16, 122–144. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zha, W.; Li, W.; Wang, J.; You, A. Advances in the biosynthesis of terpenoids and their ecological functions in plant resistance. Int. J. Mol. Sci. 2023, 24, 11561. [Google Scholar] [CrossRef] [PubMed]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, I.; Guillamón, E.; Ruiz, A.; Esteban, L.S. Essential oils from residual foliage of forest tree and shrub species: Yield and antioxidant capacity. Molecules 2021, 26, 3257. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Silva, A.S.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.A.; Dhanjal, D.S.; Nicoletti, M.; et al. Conifers phytochemicals: A valuable forest with therapeutic potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, K.; Islam, M.T.; Jayasena, V.; Sharma, B.; Sharma, S.; Sharma, P.; Kuča, K.; Bhardwaj, P. Review on essential oils, chemical composition, extraction, and utilization of some conifers in Northwestern Himalayas. Phyther. Res. 2020, 34, 2889–2910. [Google Scholar] [CrossRef]
- Vázquez-González, C.; Zas, R.; Erbilgin, N.; Ferrenberg, S.; Rozas, V.; Sampedro, L. Resin ducts as resistance traits in conifers: Linking dendrochronology and resin-based defences. Tree Physiol. 2020, 40, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.; Araújo, C.; Mendes, J.; Elias, R.; Mendes, C.; Melo, C. Espécies Florestais das Ilhas—Açores. In Árvores e Florestas de Portugal; Silva, J.S., Ed.; Público, Comunicação Social, SA/Fundação Luso-Americana/Liga para a Protecção da Natureza: Lisboa, Portugal, 2007; Volume 6, pp. 199–254. [Google Scholar]
- Arruda, F.; Rosa, J.S.; Rodrigues, A.; Oliveira, L.; Lima, A.; Barroso, J.G.; Lima, E. Essential oil variability of Azorean Cryptomeria japonica leaves under different distillation methods, Part 1: Color, yield and chemical composition analysis. Appl. Sci. 2022, 12, 452. [Google Scholar] [CrossRef]
- Mizushina, Y.; Kuriyama, I. Cedar (Cryptomeria japonica) Oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Preedy, V.R., Ed.; Academic Press: London, UK, 2016; pp. 317–324. [Google Scholar]
- Farjon, A. Plate 371. Cryptomeria japonica. Curtis’s Bot. Mag. 1999, 16, 212–228. [Google Scholar] [CrossRef]
- Hosoo, Y. Development of pollen and female gametophytes in Cryptomeria japonica. Int. J. Plant Dev. Biol. 2007, 1, 116–121. [Google Scholar]
- Lima, A.; Arruda, F.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Variations in essential oil chemical composition and biological activities of Cryptomeria japonica (Thunb. ex L.f.) D. Don from different geographical origins: A critical review. Appl. Sci. 2021, 11, 11097. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Moiteiro, C.; Rodrigues, M.C.S.M.; Almeida, A.J.R.M. Essential oil composition from Cryptomeria japonica D. Don grown in Azores: Biomass valorization from forest management. Nat. Prod. Commun. 2021, 16, 1934578X211038431. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Janeiro, A.; Rodrigues, T.; Baptista, J.; Figueiredo, A.C.; Lima, E. Essential oils from different parts of Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae): Comparison of the yields, chemical compositions, and biological properties. Appl. Sci. 2023, 13, 8375. [Google Scholar] [CrossRef]
- Janeiro, A.; Lima, A.; Arruda, F.; Wortham, T.; Rodrigues, T.; Baptista, J.; Lima, E. Essential oils from Azorean Cryptomeria japonica female cones at different developmental stages: Variations in the yields and chemical compositions. Separations 2024, 11, 62. [Google Scholar] [CrossRef]
- ISO 9235:2013; Aromatic Natural Raw Materials—Vocabulary. Available online: https://www.iso.org/obp/ui/#iso:std:iso:9235:ed-2:v1:en. (accessed on 19 March 2024).
- Council of Europe. European Directorate for the Quality of Medicines. In European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2010. [Google Scholar]
- Chen, X.; Shang, S.; Yan, F.; Jiang, H.; Zhao, G.; Tian, S.; Chen, R.; Chen, D.; Dang, Y. Antioxidant activities of essential oils and their major components in scavenging free radicals, inhibiting lipid oxidation and reducing cellular oxidative stress. Molecules 2023, 28, 4559. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.E. A simplified method for the evaluation of antioxidants. J. Am. Oil Chem. Soc. 1971, 48, 91–97. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food- and Airborne Fungi, 6th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; 389p. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; CBS–KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; 390p. [Google Scholar]
- André, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical characteristics of patients with Micrococcus luteus bloodstream infection in a chinese tertiary-care hospital. Pol. J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Khater, D.F.; Lela, R.A.; El-Diasty, M.; Moustafa, S.A.; Wareth, G. Detection of harmful foodborne pathogens in food samples at the points of sale by MALDT-TOF MS in Egypt. BMC Res. Notes 2021, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [PubMed]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and antioxidant activities of bornyl acetate and nezukol fractionated from Cryptomeria japonica essential oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Chaijaroenkul, W.; Na-Bangchang, K. Therapeutic potential and pharmacological activities of β-eudesmol. Chem. Biol. Drug Des. 2021, 97, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil: A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Ancuceanu, R.; Anghel, A.I.; Hovanet, M.V.; Ciobanu, A.-M.; Lascu, B.E.; Dinu, M. Antioxidant activity of essential oils from Pinaceae species. Antioxidants 2024, 13, 286. [Google Scholar] [CrossRef]
- Emami, S.A.; Javadi, B.; Hassanzadeh, M.K. Antioxidant activity of the essential oils of different parts of Juniperus communis. subsp. hemisphaerica and Juniperus oblonga. Pharm. Biol. 2007, 45, 769–776. [Google Scholar] [CrossRef]
- Ruas, A.; Graça, A.; Marto, J.; Gonçalves, L.; Oliveira, A.; Nogueira da Silva, A.; Pimentel, M.; Moura, A.M.; Serra, A.T.; Figueiredo, A.C.; et al. Chemical characterization and bioactivity of commercial essential oils and hydrolates obtained from Portuguese forest logging and thinning. Molecules 2022, 27, 3572. [Google Scholar] [CrossRef] [PubMed]
- Bruna, F.; Fernández, K.; Urrejola, F.; Touma, J.; Navarro, M.; Sepúlveda, B.; Larrazabal-Fuentes, M.; Paredes, A.; Neira, I.; Ferrando, M.; et al. The essential oil from Drimys winteri possess activity: Antioxidant, theoretical chemistry reactivity, antimicrobial, antiproliferative and chemical composition. Front. Nat. Prod. 2022, 1, 958425. [Google Scholar] [CrossRef]
- Hofmann, T.; Visi-Rajczi, E.; Albert, L. Antioxidant properties assessment of the cones of conifers through the combined evaluation of multiple antioxidant assays. Ind. Crops Prod. 2020, 145, 111935. [Google Scholar] [CrossRef]
- Lima, A.; Arruda, F.; Janeiro, A.; Medeiros, J.; Baptista, J.; Madruga, J.; Lima, E. Biological activities of organic extracts and specialized metabolites from different parts of Cryptomeria japonica (Cupressaceae): A critical review. Phytochemistry 2023, 206, 113520. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.; Yiap, B.C.; Ping, H.C.; Lim, S.H. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils: Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Živković, M.; Miljković, M.S.; Ruas-Madiedo, P.; Markelić, M.B.; Veljović, K.; Tolinački, M.; Soković, S.; Korać, A.; Golić, N. EPS-SJ Exopolisaccharide produced by the strain Lactobacillus paracasei subsp. paracasei BGSJ2-8 is involved in adhesion to epithelial intestinal cells and decrease on E. coli association to Caco-2 cells. Front. Microbiol. 2016, 7, 286. [Google Scholar] [CrossRef]
- da Silveira Carvalho, J.M.; de Morais Batista, A.H.; Nogueira, N.A.P.; Holanda, A.K.M.; de Sousa, J.R.; Zampieri, D.; Bezerra, M.J.B.; Stefânio Barreto, F.; de Moraes, M.O.; Batista, A.A.; et al. A biphosphinic ruthenium complex with potent anti-bacterial and anti-cancer activity. New J. Chem. 2017, 41, 13085–13095. [Google Scholar] [CrossRef]
- Niksic, H.; Becic, F.; Koric, E.; Gusic, I.; Omeragic, E.; Muratovic, S.; Miladinovic, B.; Duric, K. Cytotoxicity screening of Thymus vulgaris L. essential oil in brine shrimp nauplii and cancer cell lines. Sci. Rep. 2021, 11, 13178. [Google Scholar] [CrossRef] [PubMed]

| No. | Class | Component | RT (min) | RI | Relative Content (%) | |
|---|---|---|---|---|---|---|
| IFC EO | MFC EO | |||||
| 1 | MH | α-Pinene | 12.63 | 929 | 19.53 ± 0.45 | 41.32 ± 1.31 |
| 2 | MH | Sabinene | 14.78 | 967 | 3.86 ± 0.04 | 5.38 ± 0.51 |
| 3 | MH | Limonene | 18.22 | 1024 | 1.54 ± 0.17 | 1.11 ± 0.03 |
| 4 | MH | γ-Terpinene | 20.11 | 1052 | 2.69 ± 0.25 | 1.02 ± 0.08 |
| 5 | OCM | Terpinen-4-ol | 28.49 | 1175 | 11.21 ± 1.08 | 5.97 ± 0.51 |
| 6 | OCM | Bornyl acetate | 35.55 | 1278 | 1.55 ± 0.09 | 0.43 ± 0.02 |
| 7 | OCS | Elemol | 52.32 | 1540 | 11.01 ± 0.05 | 7.00 ± 0.02 |
| 8 | OCS | γ-Eudesmol | 57.19 | 1623 | 11.41 ± 0.21 | 2.82 ± 0.03 |
| 9 | OCS | α+β-Eudesmol | 58.52 | 1646 | 10.32 ± 1.00 | 11.81 ± 0.29 |
| 10 | DH | Phyllocladene | 77.34 | 2009 | 6.54 ± 0.09 | 1.32 ± 0.01 |
| 11 | OCD | Nezukol | 82.41 | 2119 | 1.77 ± 0.03 | 0.45 ± 0.02 |
| Total identified components | - | - | 99.24 ± 0.89 | 98.44 ± 1.29 | ||
| EO and Compound | IC50, mg/mL | |
|---|---|---|
| DPPH–FRSA | BCBA | |
| IFC EO | 0.67 ± 0.24 a | 0.097 ± 0.09 a |
| MFC EO | 2.04 ± 0.54 b | 0.204 ± 0.04 b |
| α-Pinene | NA | NA |
| Terpinen-4-ol | NA | NA |
| Bornyl acetate | NA | NA |
| Ascorbic acid | 0.004 ± 0.0002 | - |
| Gallic acid | - | 0.02 ± 0.003 |
| Microorganism | Growth Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| EO Sample | EO Compound | Reference Compound | |||||
| IFC | MFC | α-Pinene | Terpinen-4-ol | Bornyl acetate | Kanamycin | Clotrimazole | |
| Bacillus subtilis | 12 ± 1 b | 10 ± 1 c | 8 ± 1 c | 13 ± 1 b | 21 ± 1 a | 39 ± 2 | - |
| Bacillus licheniformis | 9 ± 1 b | 8 ± 1 b | 9 ± 1 b | 16 ± 1 a | 17 ± 3 a | 34 ± 2 | - |
| Micrococcus luteus | 10 ± 1 c | 12 ± 2 b | 18 ± 1 a | 7 ± 1 d | NA | 30 ± 0 | - |
| Staphylococcus aureus | 20 ± 2 a | 9 ± 2 bc | 17 ± 1 a | 10 ± 1 b | 8 ± 0 c | 29 ± 2 | - |
| Staphylococcus epidermidis | 13 ± 3 a | 10 ± 1 ab | 10 ± 2 ab | 8 ± 2 b | NA | 36 ± 3 | - |
| Serratia marcescens | NA | NA | 8 ± 1 b | 21 ± 2 a | NA | 24 ± 0 | - |
| Enterobacter cloacae | NA | NA | 7 ± 1 b | 31 ± 3 a | NA | 21 ± 1 | - |
| Escherichia coli | NA | NA | 8 ± 1 b | 18 ± 1 a | NA | 24 ± 1 | - |
| Penicillium italicum | 9 ± 1 b | NA | 7 ± 0 b | 13 ± 0 a | NA | - | 20 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janeiro, A.; Lima, A.; Arruda, F.; Wortham, T.; Rodrigues, T.; Baptista, J.; Lima, E. Variations in Essential Oil Biological Activities of Female Cones at Different Developmental Stages from Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae). Separations 2024, 11, 102. https://doi.org/10.3390/separations11040102
Janeiro A, Lima A, Arruda F, Wortham T, Rodrigues T, Baptista J, Lima E. Variations in Essential Oil Biological Activities of Female Cones at Different Developmental Stages from Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae). Separations. 2024; 11(4):102. https://doi.org/10.3390/separations11040102
Chicago/Turabian StyleJaneiro, Alexandre, Ana Lima, Filipe Arruda, Tanner Wortham, Tânia Rodrigues, José Baptista, and Elisabete Lima. 2024. "Variations in Essential Oil Biological Activities of Female Cones at Different Developmental Stages from Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae)" Separations 11, no. 4: 102. https://doi.org/10.3390/separations11040102
APA StyleJaneiro, A., Lima, A., Arruda, F., Wortham, T., Rodrigues, T., Baptista, J., & Lima, E. (2024). Variations in Essential Oil Biological Activities of Female Cones at Different Developmental Stages from Azorean Cryptomeria japonica (Thunb. ex L.f.) D. Don (Cupressaceae). Separations, 11(4), 102. https://doi.org/10.3390/separations11040102









