Natural Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Flavonoids from Fagopyrum tataricum Bran
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Identification of Flavonoid Compounds Using UPLC-Q-TOF-MS/MS
2.3. Quantification of Flavonoid Compounds Using HPLC
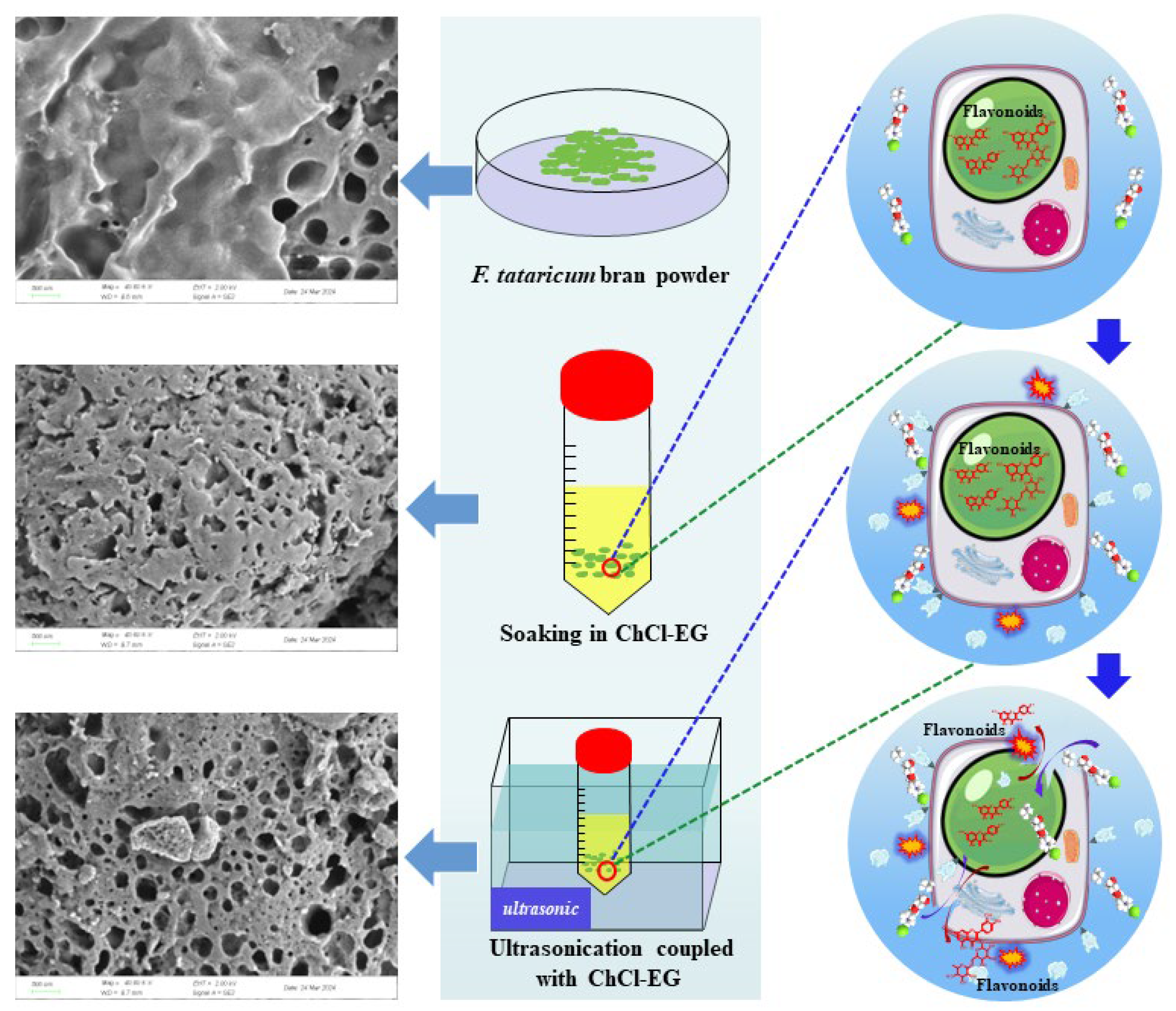
2.4. Preparation of NADES
2.5. Physico-Chemical Properties of NADES
2.6. Screening Extraction Solvent
2.7. Experimental Design of Extraction
2.7.1. Single Factor Experiment
2.7.2. Response Surface Method
2.8. Fourier-Transform Infrared Spectroscopy
2.9. COSMOtherm Simulations
2.10. Scanning Electron Microscopy (SEM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Identification of Flavonoid Compounds by UPLC-Q-TOF-MS
3.2. Physicochemical Properties of NADES
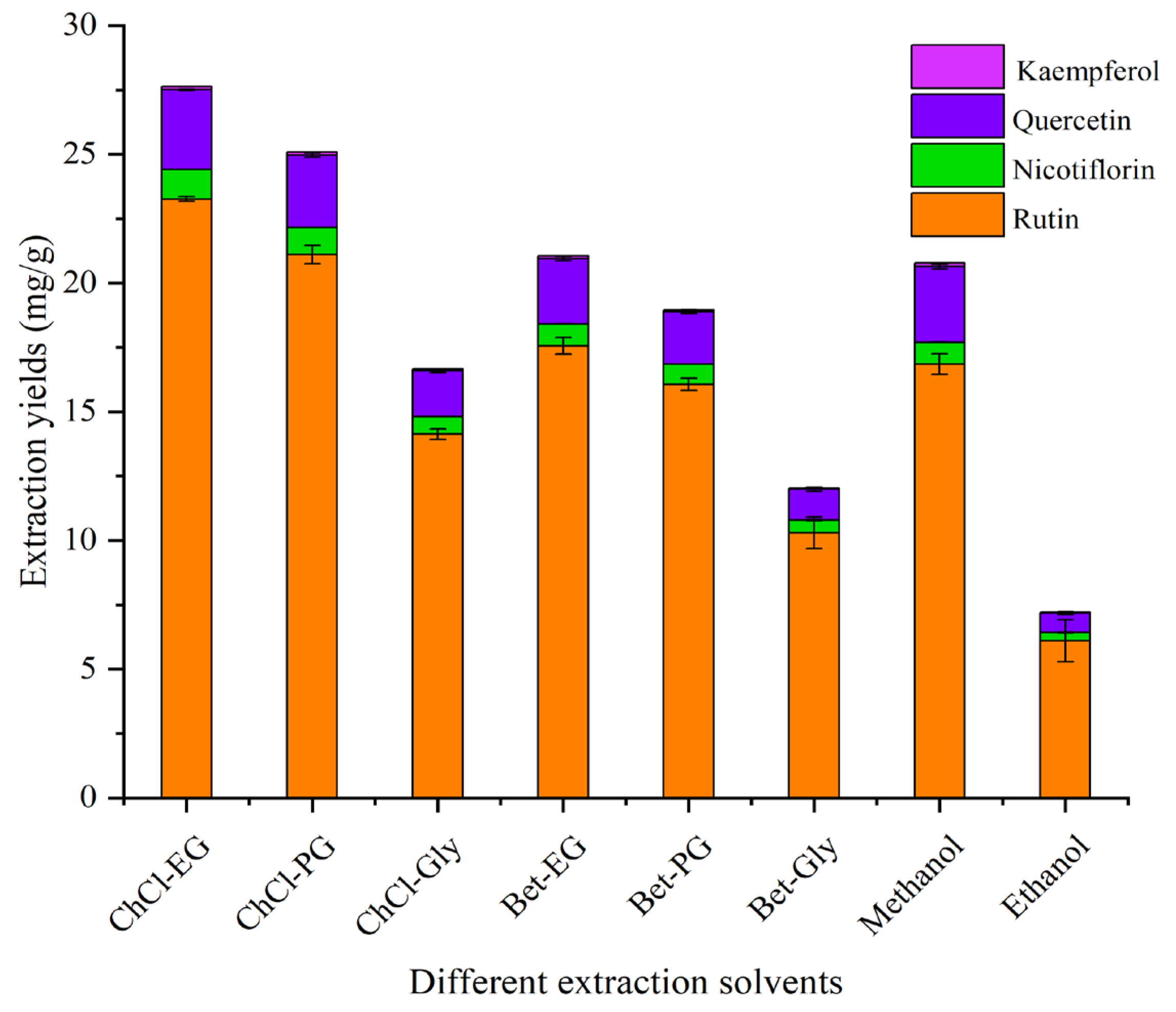
3.3. Screening the Optimal NADES
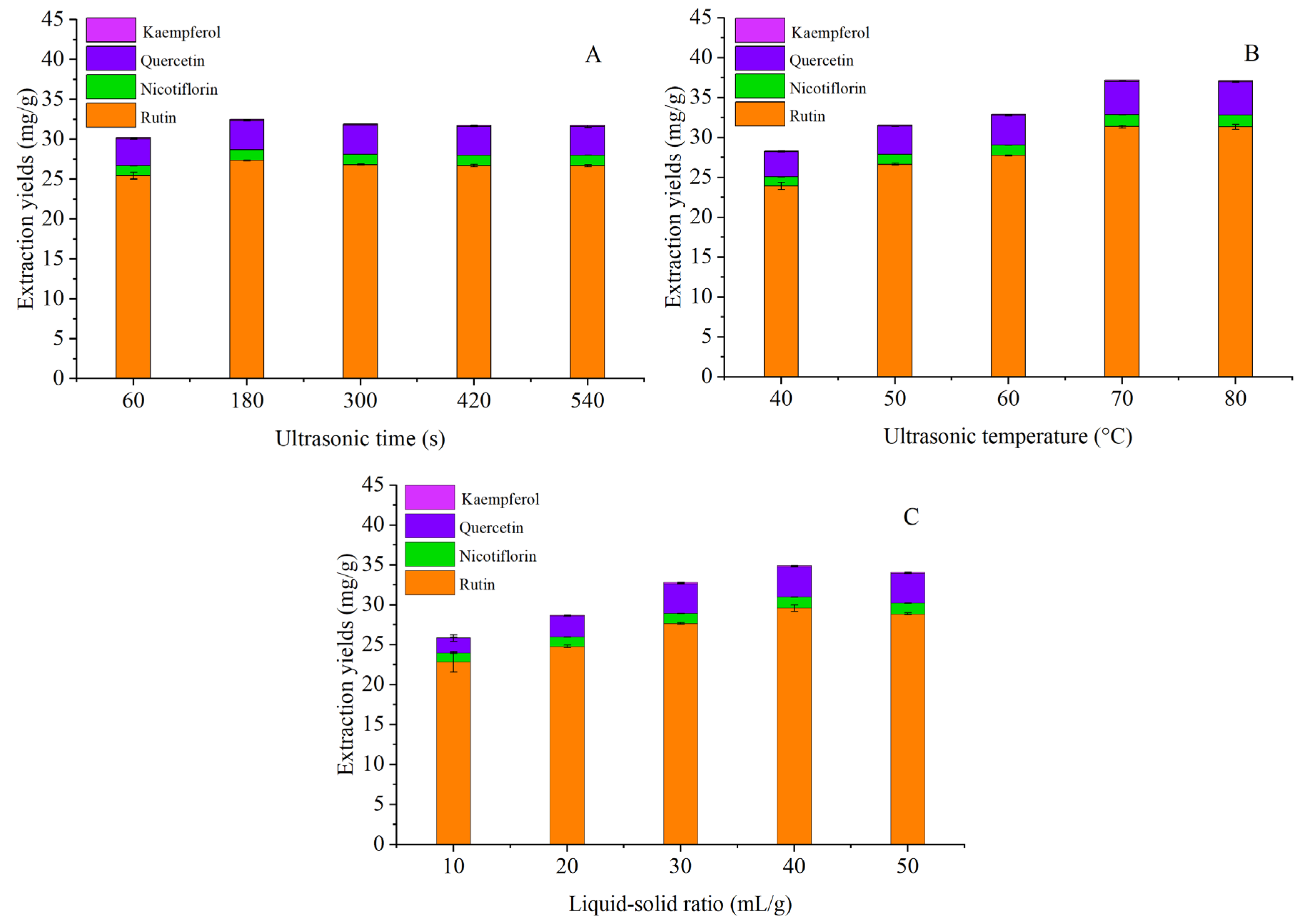
3.4. Single Factor Experiment
3.4.1. Ultrasonic Time
3.4.2. Ultrasonic Temperature
3.4.3. Liquid–Solid Ratio
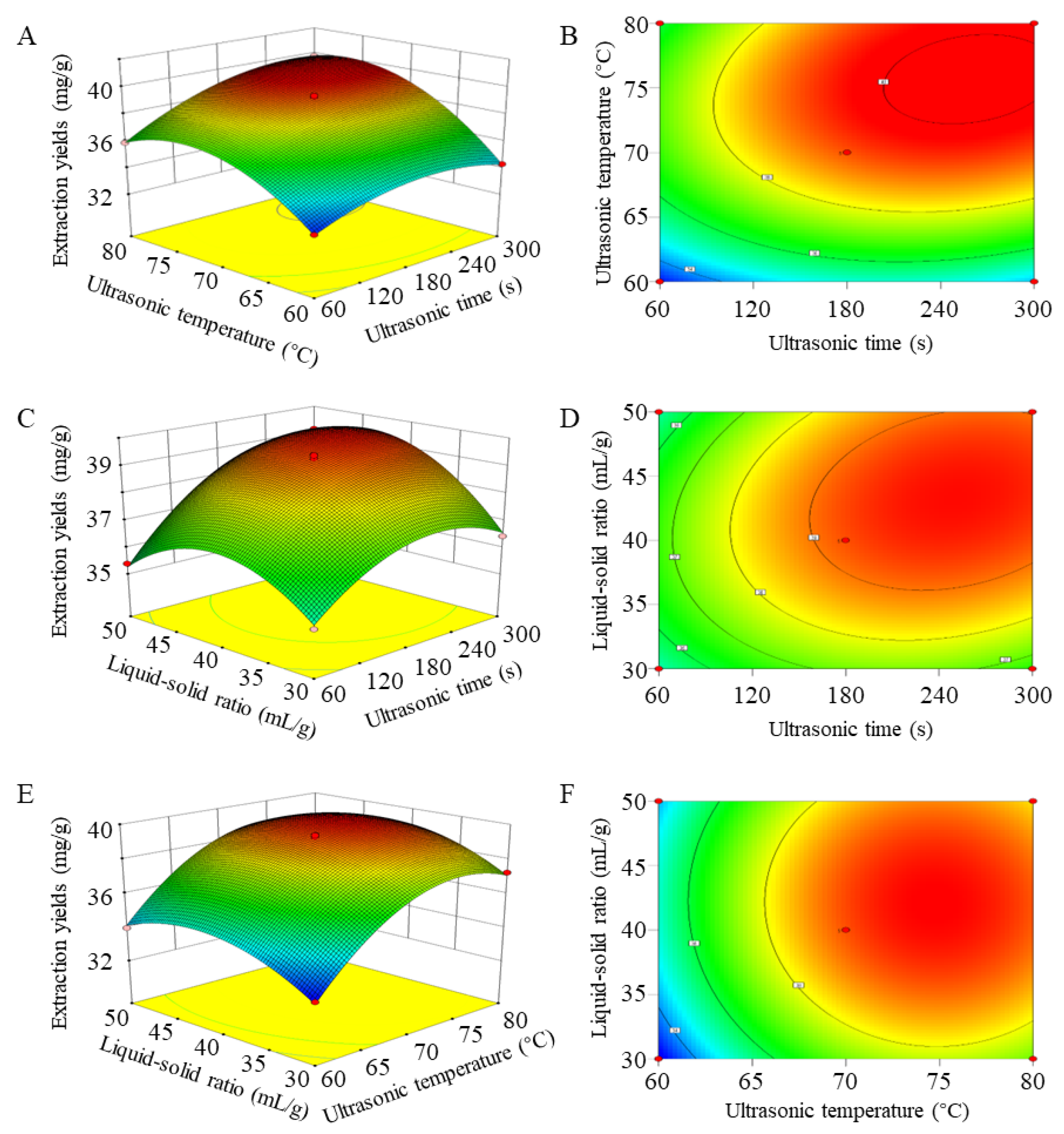
3.5. RMS-BBD Model Fitting and Response Surface Analysis
3.5.1. Predicted Model and Statistical Analysis
3.5.2. Analysis of the Response Surface
3.5.3. Verification of Predictive Model
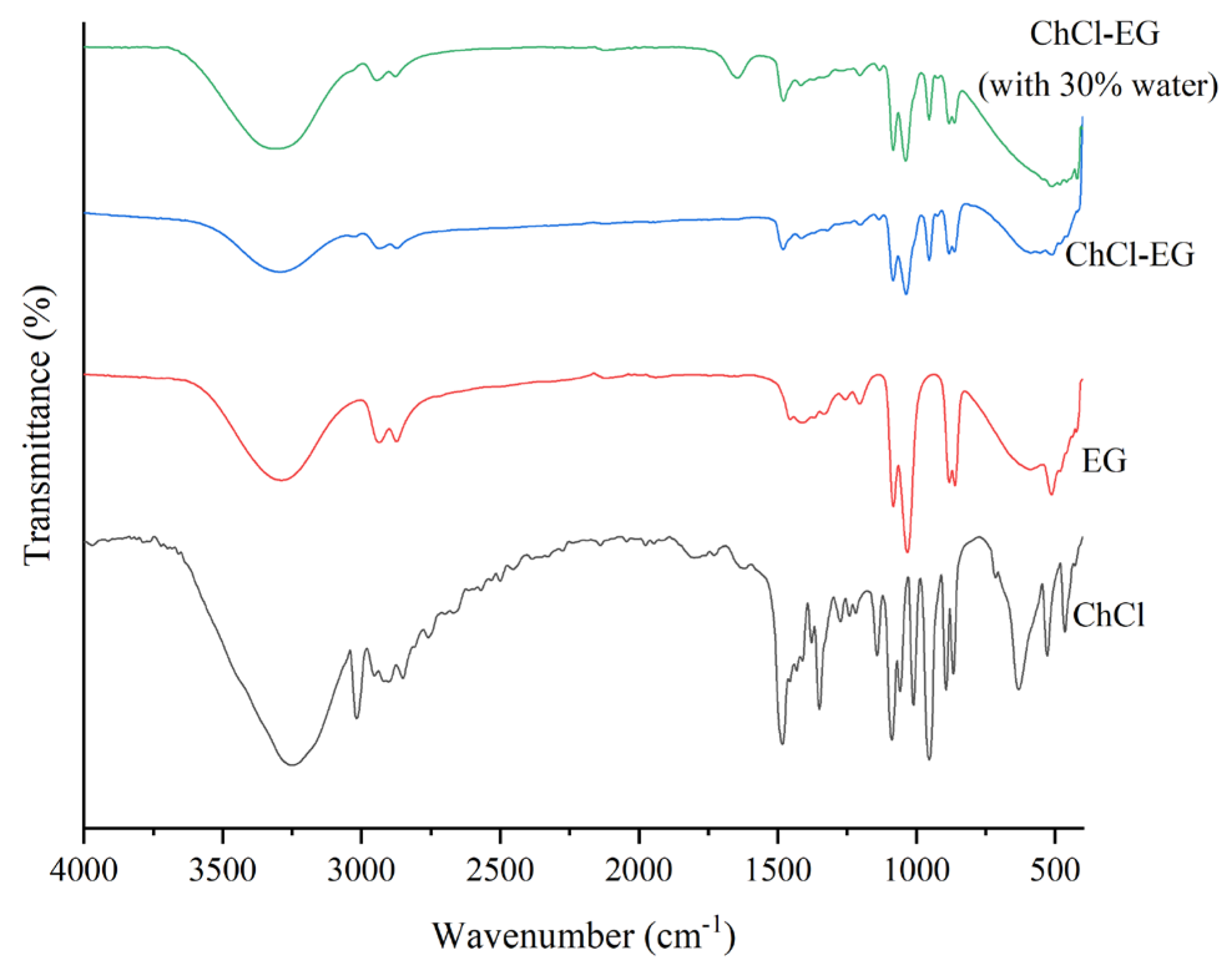
3.6. FT-IR Spectra of ChCl-EG and Single Component
3.7. Theoretical Support with COSMO Model Analysis
3.8. Microstructural Analysis of Extraction Residue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Zhao, J.; Mao, Y.; Liu, L.; Li, C.; Wu, H.; Zhao, H.; Wu, Q. Tartary buckwheat rutin: Accumulation, metabolic pathways, regulation mechanisms, and biofortification strategies. Plant Physiol. Biochem. 2024, 208, 108503. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.X.; Wei, L.J.; Yi, Q.; Chen, G.H.; Yao, Z.D.; Yan, Z.Y.; Zhao, G. In vitro potential of flavonoids from tartary buckwheat on antioxidants activity and starch digestibility. Int. J. Food Sci. Technol. 2019, 54, 2209–2218. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Chen, Z.-D.; Zhou, Y.-M.; Shi, R.-H.; Li, Z.-J. The effect of tartary buckwheat flavonoids in inhibiting the proliferation of MGC80-3 cells during seed germination. Molecules 2019, 24, 3092. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2023, 63, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.H.; Wang, H. Nutrient components and bioactive compounds in tartary buckwheat bran and flour as affected by thermal processing. Int. J. Food Prop. 2020, 23, 127–137. [Google Scholar] [CrossRef]
- Aktaş, H.; Kurek, M.A. Deep eutectic solvents for the extraction of polyphenols from food plants. Food Chem. 2024, 444, 138629. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Kumar, A.; Panesar, P.S.; Thakur, A. Potential of deep eutectic solvents in the extraction of value-added compounds from agro-industrial by-products. Appl. Food Res. 2022, 2, 100211. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Álvarez-Rivera, G.; Mendiola, J.A. Deep Eutectic Solvents for the Extraction of Bioactive Compounds from Natural Sources and Agricultural By-Products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Liu, Z.-T.; Chen, X.-Q.; Zhang, T.-T.; Zhang, Y. Deep eutectic solvent combined with ultrasound technology: A promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food Chem. 2023, 422, 136224. [Google Scholar] [CrossRef]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green extraction of bioactive compounds from apple pomace by ultrasound assisted natural deep eutectic solvent extraction: Optimisation, comparison and bioactivity. Food Chem. 2023, 398, 133871. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly efficient extraction of anthocyanins from grape skin using deep eutectic solvents as green and tunable media. Arch. Pharmacal Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-Assisted Extraction of Polyphenols from Crude Pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef] [PubMed]
- Milićević, N.; Kojić, P.; Sakač, M.; Mišan, A.; Kojić, J.; Perussello, C.; Banjac, V.; Pojić, M.; Tiwari, B. Kinetic modelling of ultrasound-assisted extraction of phenolics from cereal brans. Ultrason. Sonochem. 2021, 79, 105761. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Xiang, Z.; Xia, C.; Feng, S.; Chen, T.; Zhou, L.; Liu, L.; Kong, Q.; Yang, H.; Ding, C. Assessment of free and bound phenolics in the flowers and floral organs of two Camellia species flower and their antioxidant activities. Food Biosci. 2022, 49, 101905. [Google Scholar] [CrossRef]
- Huo, J.; Ni, Y.; Li, D.; Qiao, J.; Huang, D.; Sui, X.; Zhang, Y. Comprehensive structural analysis of polyphenols and their enzymatic inhibition activities and antioxidant capacity of black mulberry (Morus nigra L.). Food Chem. 2023, 427, 136605. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, L.; Qi, Y.; Xie, C.; Zhao, W.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Effect of processing on polyphenols in butternut pumpkin (Cucurbita moschata). Food Biosci. 2022, 49, 101925. [Google Scholar] [CrossRef]
- Huang, H.; Ni, Z.-J.; Wu, Z.-F.; Ma, Y.-L.; Hu, F.; Thakur, K.; Zhang, J.-G.; Khan, M.R.; Wei, Z.-J. Comparison of polyphenols in Goji (Lycium barbarum L.) leaves at different leaf positions under different extraction methods. Ind. Crops Prod. 2024, 209, 117982. [Google Scholar] [CrossRef]
- Amaya-Cruz, D.M.; Pérez-Ramírez, I.F.; Delgado-García, J.; Mondragón-Jacobo, C.; Dector-Espinoza, A.; Reynoso-Camacho, R. An integral profile of bioactive compounds and functional properties of prickly pear (Opuntia ficus indica L.) peel with different tonalities. Food Chem. 2019, 278, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Spronsen, J.V.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Nian, B.; Li, Y.; Wu, S.; Liu, Y. Multiple Hydrogen-Bonding Interactions Enhance the Solubility of Starch in Natural Deep Eutectic Solvents: Molecule and Macroscopic Scale Insights. J. Agric. Food. Chem. 2019, 67, 12366. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Ijardar, S.P.; Singh, V.; Gardas, R.L. Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules 2022, 27, 1368. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Eng, J.J.; Khairuzi, K.; Kristianto, S.; Asyraf Wan Mahmood, W.M.; Al-Fakih, A.M.; Matmin, J.; Wahab, R.A.; Abdullah, F.; Mohamad, M.F.; et al. Effectiveness of ammonium-based deep eutectic solvents in extracting polyphenol from Chlorella vulgaris. Algal Res. 2024, 79, 103436. [Google Scholar] [CrossRef]
- Mero, A.; Koutsoumpos, S.; Giannios, P.; Stavrakas, I.; Moutzouris, K.; Mezzetta, A.; Guazzelli, L. Comparison of physicochemical and thermal properties of choline chloride and betaine-based deep eutectic solvents: The influence of hydrogen bond acceptor and hydrogen bond donor nature and their molar ratios. J. Mol. Liq. 2023, 377, 121563. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Zhan, A.; Niu, D.; Li, K.; Li, J. Characterization of some sucrose-based deep eutectic solvents and their effect on the solubility of piroxicam. J. Mol. Liq. 2023, 377, 121556. [Google Scholar] [CrossRef]
- Rodrigues, L.A.; Cardeira, M.; Leonardo, I.C.; Gaspar, F.B.; Radojčić Redovniković, I.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Deep eutectic systems from betaine and polyols—Physicochemical and toxicological properties. J. Mol. Liq. 2021, 335, 116201. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Ke, J.; Ran, B.; Sun, P.; Cheng, Y.; Chen, Q.; Li, H. An Evaluation of the Absolute Content of Flavonoids and the Identification of Their Relationship with the Flavonoid Biosynthesis Genes in Tartary Buckwheat Seeds. Agronomy 2023, 13, 3006. [Google Scholar] [CrossRef]
- Liu, Y.; Zhe, W.; Zhang, R.; Peng, Z.; Wang, Y.; Gao, H.; Guo, Z.; Xiao, J. Ultrasonic-assisted extraction of polyphenolic compounds from Paederia scandens (Lour.) Merr. Using deep eutectic solvent: Optimization, identification, and comparison with traditional methods. Ultrason. Sonochem. 2022, 86, 106005. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.; Zhao, J.; Wang, Y.; Yao, Q.; Li, S. Development and optimization of green extraction of polyphenols in Michelia alba using natural deep eutectic solvents (NADES) and evaluation of bioactivity. Sustain. Chem. Pharm. 2024, 37, 101425. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. TrAC Trend. Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Zhen, S.; Chen, S.; Geng, S.; Zhang, H.; Chen, Y.; Liu, B. Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction and Bioactivities of Flavonoids in Ampelopsis grossedentata Leaves. Foods 2022, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Dey, S.; Chakraborty, R. Effect of choline chloride-oxalic acid based deep eutectic solvent on the ultrasonic assisted extraction of polyphenols from Aegle marmelos. J. Mol. Liq. 2019, 287, 110956. [Google Scholar] [CrossRef]
- Wang, W.; An, M.; Zhao, G.; Wang, Y.; Yang, D.; Zhang, D.; Zhao, L.; Han, J.; Wu, G.; Bo, Y. Ultrasonic-assisted customized natural deep eutectic solvents extraction of polyphenols from Chaenomeles speciosa. Microchem. J. 2023, 193, 108952. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Hao, C.; Chen, L.; Dong, H.; Xing, W.; Xue, F.; Cheng, Y. Extraction of Flavonoids from Scutellariae Radix using Ultrasound-Assisted Deep Eutectic Solvents and Evaluation of Their Anti-Inflammatory Activities. ACS Omega 2020, 5, 23140–23147. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Hong, S.M.; Kamaruddin, A.H.; Nadzir, M.M. Sustainable ultrasound-assisted extraction of polyphenols from Cinnamomum cassia bark using hydrophilic natural deep eutectic solvents. Process Biochem. 2023, 132, 323–336. [Google Scholar] [CrossRef]
- Hayyan, M.; Abo-Hamad, A.; AlSaadi, M.A.; Hashim, M.A. Functionalization of graphene using deep eutectic solvents. Nanoscale Res. Lett. 2015, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Y.; Jin, T.; Yan, K.; Liu, X.; He, S.; Li, L.; Gong, Y.; Ma, J.; Yu, H.; et al. Extraction of triterpene acids from loquat leaves via a novel hydrophobic deep eutectic solvent screened by COSMO-SAC model. J. Clean. Prod. 2023, 427, 139274. [Google Scholar] [CrossRef]
- Oliveira, G.; Wojeicchowski, J.P.; Farias, F.O.; Igarashi-Mafra, L.; de Pelegrini Soares, R.; Mafra, M.R. Enhancement of biomolecules solubility in aqueous media using designer solvents as additives: An experimental and COSMO-based models’ approach. J. Mol. Liq. 2020, 318, 114266. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Law, C.L.; Zhang, L. Natural deep eutectic solvents for the extraction of lentinan from shiitake mushroom: COSMO-RS screening and ANN-GA optimizing conditions. Food Chem. 2024, 430, 136990. [Google Scholar] [CrossRef]
- Cui, Z.; Djocki, A.V.E.; Yao, J.; Wu, Q.; Zhang, D.; Nan, S.; Gao, J.; Li, C. COSMO-SAC-supported evaluation of natural deep eutectic solvents for the extraction of tea polyphenols and process optimization. J. Mol. Liq. 2021, 328, 115406. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Lazović, M.; Cvijetić, I.; Jankov, M.; Milojković-Opsenica, D.; Trifković, J.; Ristivojević, P. COSMO-RS in prescreening of Natural Eutectic Solvents for phenolic extraction from Teucrium chamaedrys. J. Mol. Liq. 2 2023, 387, 122649. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, C.; Zhang, J.; Chen, L.; Zhang, B.; Qi, Z. Screening deep eutectic solvents for extractive desulfurization of fuel based on COSMO-RS model. Chem. Eng. Process.—Process Intensif. Chem. Eng. Process. 2018, 125, 246–252. [Google Scholar] [CrossRef]
- Cao, Y.; Song, Z.; Dong, C.; Ni, W.; Xin, K.; Yu, Q.; Han, L. Green ultrasound-assisted natural deep eutectic solvent extraction of phenolic compounds from waste broccoli leaves: Optimization, identification, biological activity, and structural characterization. LWT 2023, 190, 115407. [Google Scholar] [CrossRef]

| Run | X1 | X2 | X3 | Extraction Yields (mg/g) | ||||
|---|---|---|---|---|---|---|---|---|
| Ultrasonic Time (s) | Ultrasonic Temperature (°C) | Liquid–Solid Ratio (mL/g) | Rutin | Nicotiflorin | Quercetin | Kaemferol | Total Flavonoids | |
| 1 | 180 | 70 | 40 | 33.28 | 1.55 | 4.37 | 0.18 | 39.37 |
| 2 | 180 | 70 | 40 | 33.13 | 1.54 | 4.40 | 0.18 | 39.25 |
| 3 | 300 | 70 | 50 | 33.03 | 1.53 | 4.37 | 0.18 | 39.11 |
| 4 | 60 | 80 | 40 | 30.46 | 1.40 | 3.89 | 0.16 | 35.91 |
| 5 | 180 | 60 | 30 | 27.73 | 1.28 | 3.73 | 0.15 | 32.88 |
| 6 | 300 | 80 | 40 | 33.64 | 1.55 | 4.38 | 0.18 | 39.76 |
| 7 | 300 | 70 | 30 | 30.93 | 1.42 | 3.93 | 0.16 | 36.43 |
| 8 | 180 | 60 | 50 | 28.86 | 1.29 | 3.68 | 0.16 | 33.98 |
| 9 | 180 | 70 | 40 | 33.10 | 1.51 | 4.45 | 0.18 | 39.25 |
| 10 | 180 | 70 | 40 | 33.40 | 1.52 | 4.30 | 0.18 | 39.40 |
| 11 | 180 | 80 | 50 | 32.12 | 1.49 | 4.26 | 0.17 | 38.04 |
| 12 | 180 | 80 | 30 | 31.55 | 1.46 | 4.07 | 0.17 | 37.25 |
| 13 | 300 | 60 | 40 | 29.01 | 1.32 | 3.82 | 0.15 | 34.30 |
| 14 | 60 | 70 | 50 | 29.99 | 1.36 | 3.88 | 0.16 | 35.39 |
| 15 | 60 | 60 | 40 | 27.98 | 1.26 | 3.74 | 0.15 | 33.13 |
| 16 | 180 | 70 | 40 | 32.87 | 1.51 | 4.41 | 0.18 | 38.98 |
| 17 | 60 | 70 | 30 | 29.57 | 1.36 | 3.98 | 0.16 | 35.07 |
| No. | Rt (min) | M.W. | Major Ion [M − H]− (m/z) | Molecular Formula | Fragment Ions | Identified Compounds |
|---|---|---|---|---|---|---|
| MS/MS (m/z) | ||||||
| 1 | 4.86 | 290.27 | 289.0689 | C15H14O6 | 245, 203, 179, 121, 109 | Cianidanol |
| 2 | 7.28 | 290.27 | 289.0689 | C15H14O6 | 245, 203, 179, 121, 109 | Epicatechin |
| 3 | 9.43 | 610.5 | 609.1259 | C27H30O16 | 301, 271, 255, 243 | Rutin |
| 4 | 9.56 | 464.38 | 463.0775 | C21H20O12 | 301, 300, 271, 255, 243, 227, 199, 151 | Hyperoside |
| 5 | 9.71 | 464.38 | 463.0731 | C21H20O12 | 301, 300, 271, 255, 243, 227, 199, 151 | Isoquercitrin |
| 6 | 10.20 | 594.52 | 593.1296 | C27H30O15 | 285, 255, 227 | Nicotiflorin |
| 7 | 10.52 | 448.38 | 447.0805 | C21H20O11 | 301, 271, 255, 243, 227, 179, 163, 151 | Quercitrin |
| 8 | 12.48 | 302.23 | 301.0315 | C15H10O7 | 179, 151, 121, 107, 93, 83 | Quercetin |
| 9 | 13.67 | 270.24 | 269.0428 | C15H10O5 | 225, 151, 149, 117, 107 | Apigenin |
| 10 | 13.88 | 286.24 | 285.0373 | C15H10O6 | 285 | Kaempferol |
| 11 | 14.21 | 316.26 | 315.0478 | C16H12O7 | 300, 271, 227, 163, 151, 148 | Isorhamnetin |
| Label | HBA | HBD | Viscosity (mm2/s) | Density (mg/mL) | pH |
|---|---|---|---|---|---|
| ChCl-EG | Choline chloride | ethylene glycol | 9.32 ± 0.11 f | 1105.43 ± 4.83 d | 4.75 ± 0.01 e |
| ChCl-PG | Choline chloride | 1,2-propylene glycol | 15.11 ± 0.06 d | 1082.94 ± 1.09 f | 4.87 ± 0.01 d |
| ChCl-Gly | Choline chloride | glycerol | 17.57 ± 0.12 c | 1158.26 ± 0.90 b | 4.38 ± 0.01 f |
| Bet-EG | Betaine | ethylene glycol | 14.88 ± 0.06 e | 1121.55 ± 2.31 c | 8.24 ± 0.01 b |
| Bet-PG | Betaine | 1,2-propylene glycol | 24.59 ± 0.19 b | 1095.25 ± 2.29 e | 8.42 ± 0.01 a |
| Bet-Gly | Betaine | glycerol | 33.65 ± 0.08 a | 1181.16 ± 2.26 a | 7.55 ± 0.02 c |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 94.43 | 9 | 10.49 | 273.25 | <0.0001 ** |
| X1 | 12.75 | 1 | 12.75 | 332.09 | <0.0001 ** |
| X2 | 34.74 | 1 | 34.74 | 904.67 | <0.0001 ** |
| X3 | 2.99 | 1 | 2.99 | 77.85 | <0.0001 ** |
| X1X2 | 1.80 | 1 | 1.80 | 46.76 | 0.0002 ** |
| X1X3 | 1.39 | 1 | 1.39 | 36.26 | 0.0005 ** |
| X2X3 | 0.02 | 1 | 0.02 | 0.63 | 0.4549 ns |
| X12 | 6.64 | 1 | 6.64 | 173.06 | <0.0001 ** |
| X22 | 20.73 | 1 | 20.73 | 539.84 | <0.0001 ** |
| X32 | 9.39 | 1 | 9.39 | 244.68 | <0.0001 ** |
| Residual | 0.27 | 7 | 0.04 | ||
| Lack of fit | 0.16 | 3 | 0.05 | 1.93 | 0.2664 ns |
| Pure error | 0.11 | 4 | 0.03 | ||
| Cor total | 94.70 | 16 | |||
| R2 = 0.9972 | Adj R2 = 0.9935 | CV = 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Da, X.; Qu, J.; Xiao, S. Natural Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Flavonoids from Fagopyrum tataricum Bran. Separations 2024, 11, 145. https://doi.org/10.3390/separations11050145
Xu Z, Da X, Qu J, Xiao S. Natural Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Flavonoids from Fagopyrum tataricum Bran. Separations. 2024; 11(5):145. https://doi.org/10.3390/separations11050145
Chicago/Turabian StyleXu, Zhou, Xiaomei Da, Jipeng Qu, and Shiming Xiao. 2024. "Natural Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Flavonoids from Fagopyrum tataricum Bran" Separations 11, no. 5: 145. https://doi.org/10.3390/separations11050145
APA StyleXu, Z., Da, X., Qu, J., & Xiao, S. (2024). Natural Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Flavonoids from Fagopyrum tataricum Bran. Separations, 11(5), 145. https://doi.org/10.3390/separations11050145





