A Robust and Reliable UPLC Method for the Simultaneous Quantification of Rosuvastatin Calcium, Glibenclamide, and Candesartan Cilexetil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.2.1. Design of Experiment (DoE)
2.2.2. Analytical Procedures and Conditions
2.2.3. Method Validation
Linearity
Accuracy and Precision
Limit of Detection (LOD) and Limit of Quantification (LOQ)
Robustness
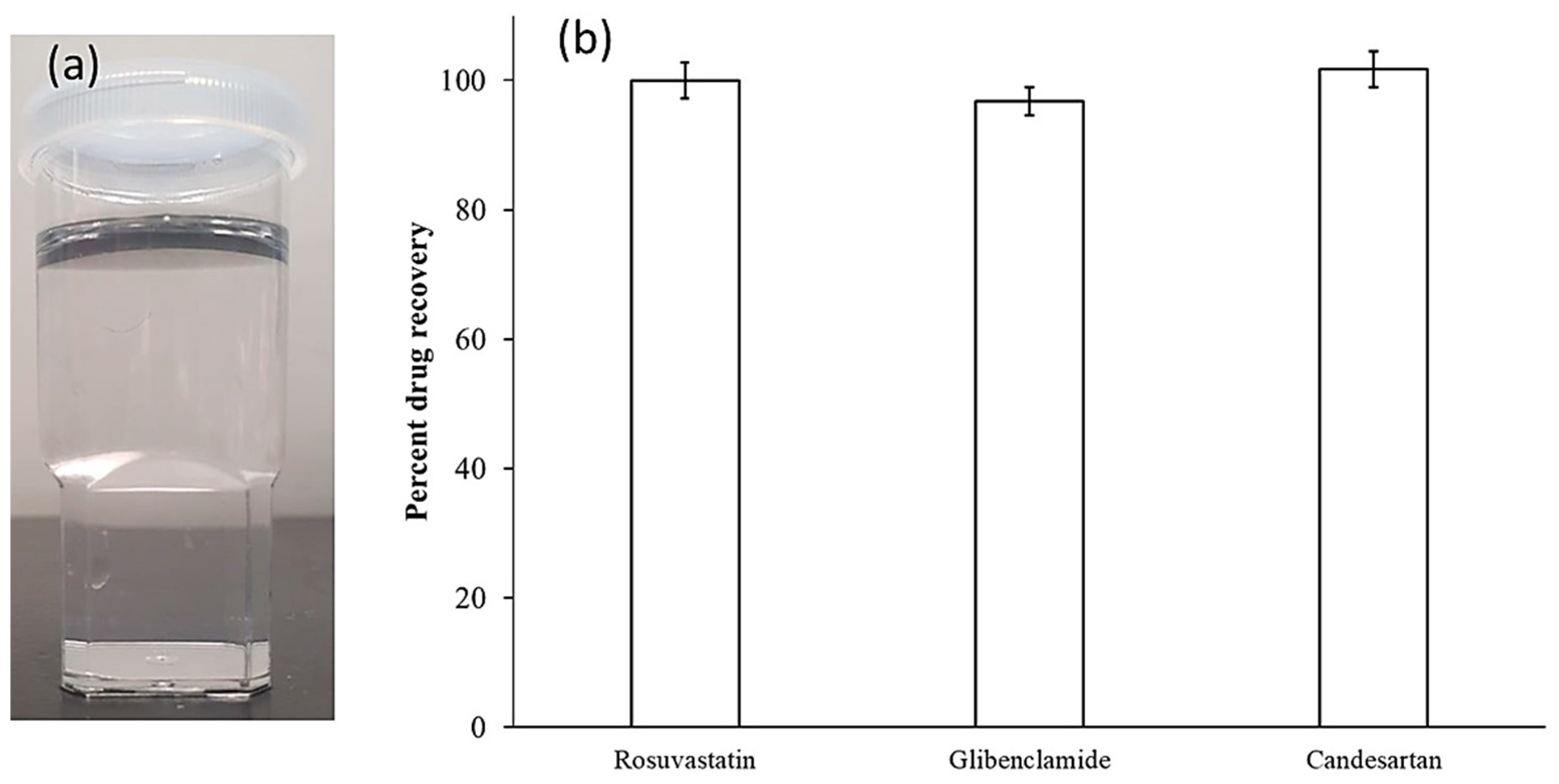
2.2.4. Preparation and Characterization of SNEDDS Formulation
Application of the Developed UPLC Method
3. Results
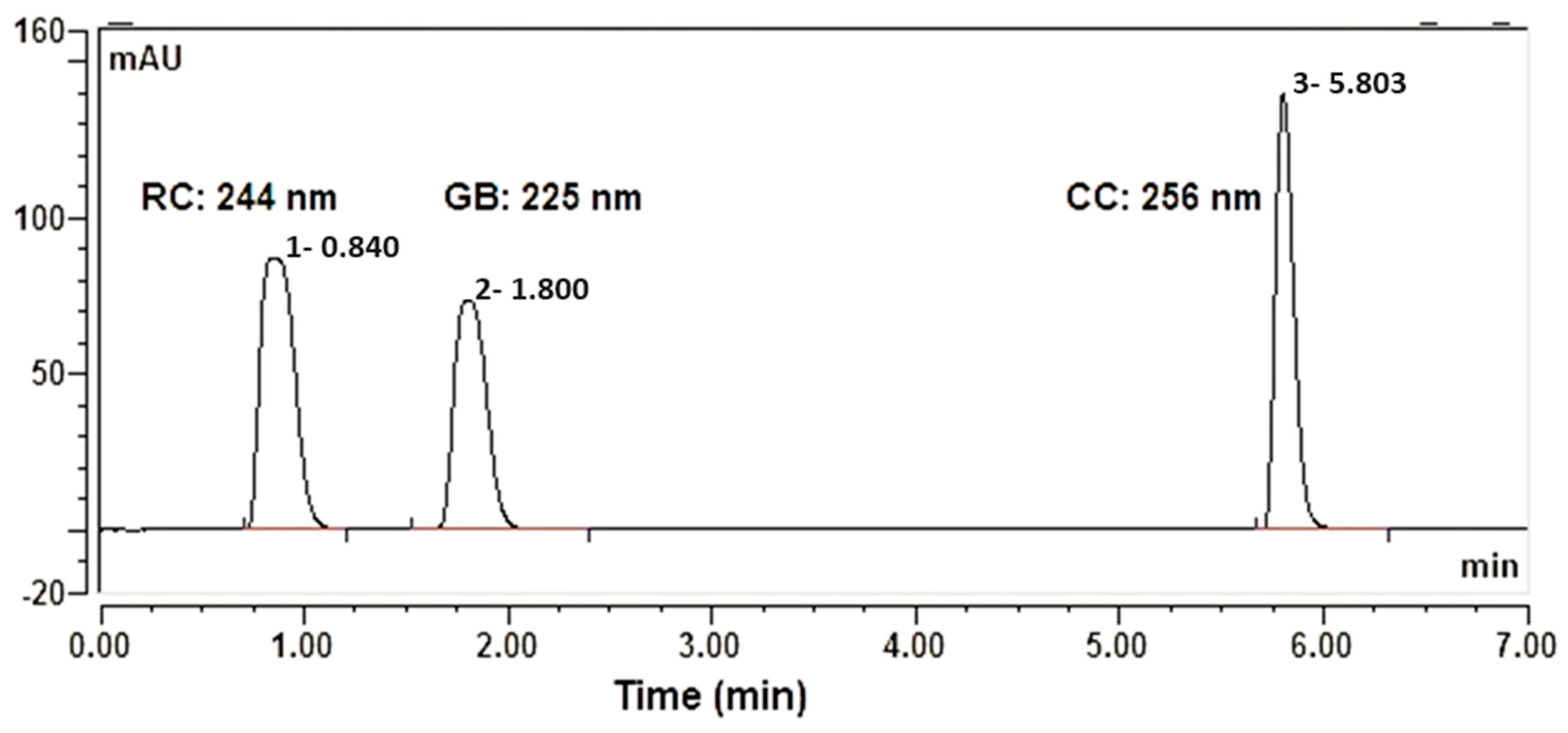
3.1. Effect of Analytical Conditions on the Simultaneous Separation of the Three APIs
3.1.1. Rosuvastatin Calcium (RC)
3.1.2. GB Separation
3.1.3. CC Separation
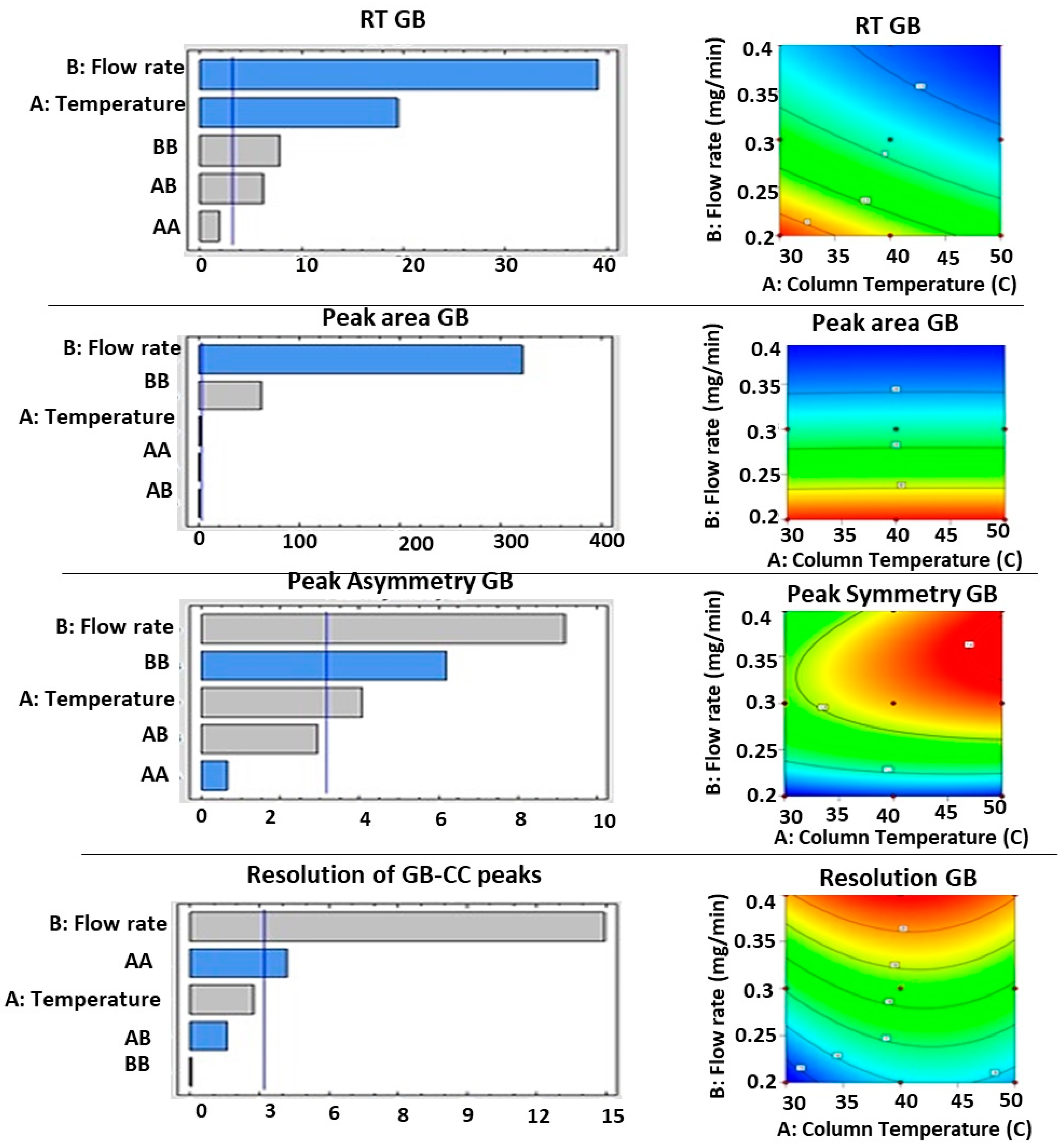
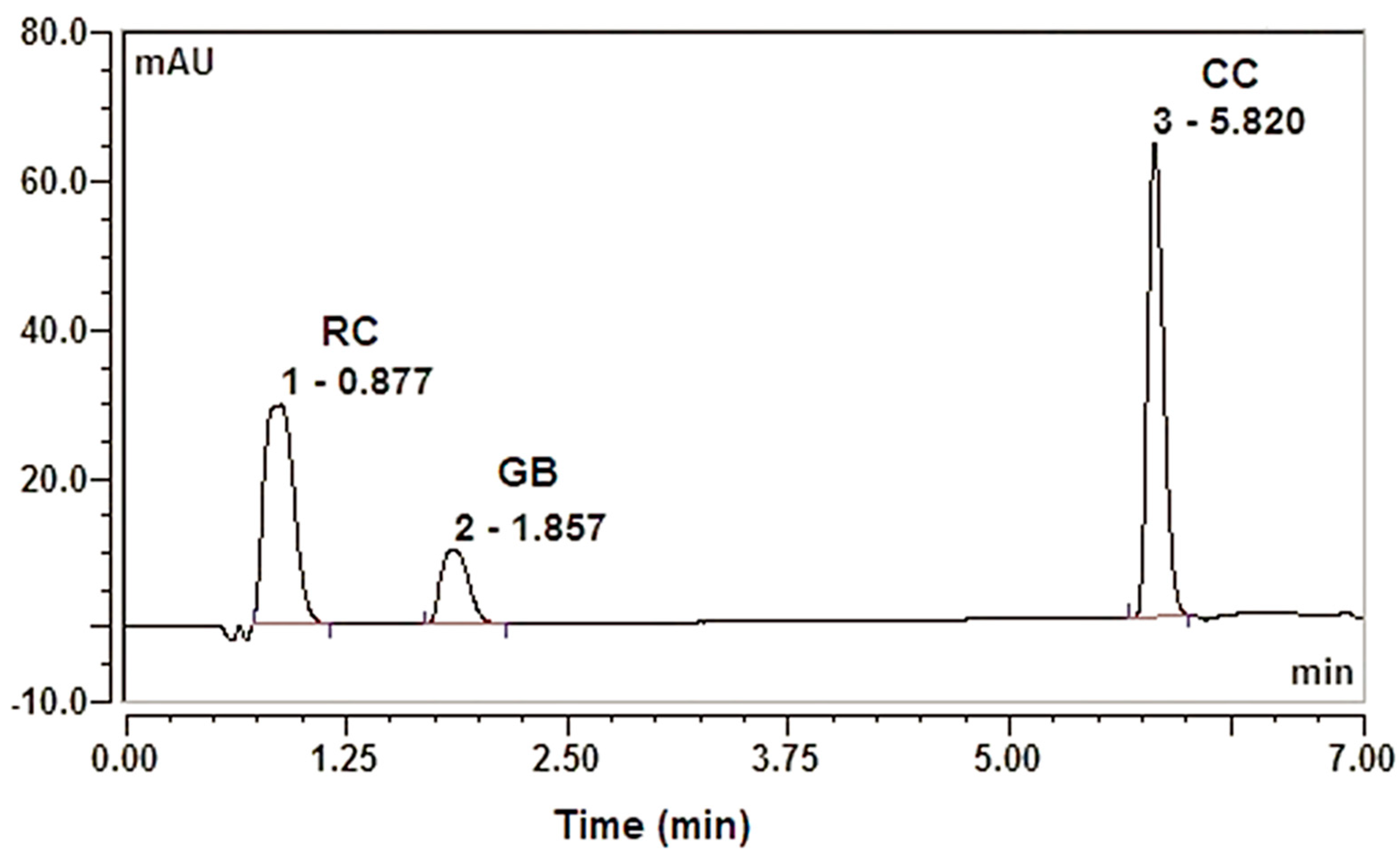
3.2. Optimization of UPLC Conditions for Simultaneous Analysis of RC, GB, and CC
3.3. Method’s Validation
3.3.1. Linearity
3.3.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.3.3. Accuracy and Precision
3.3.4. Robustness
3.4. Application of the Developed UPLC Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Ambroselli, D.; Masciulli, F.; Romano, E.; Catanzaro, G.; Besharat, Z.M.; Massari, M.C.; Ferretti, E.; Migliaccio, S.; Izzo, L.; Ritieni, A.; et al. New Advances in Metabolic Syndrome, from Prevention to Treatment: The Role of Diet and Food. Nutrients 2023, 15, 640. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, I.; Després, J.-P. Metabolic syndrome: Past, present and future. Nutrients 2020, 12, 3501. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Metabolic Syndrome Treatment. Available online: https://www.nhlbi.nih.gov/health/metabolic-syndrome/treatment (accessed on 6 January 2024).
- Larsen, J.R.; Dima, L.; Correll, C.U.; Manu, P. The pharmacological management of metabolic syndrome. Expert Rev. Clin. Pharmacol. 2018, 11, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Swislocki, A.L.; Siegel, D.; Jialal, I. Pharmacotherapy for the metabolic syndrome. Curr. Vasc. Pharmacol. 2012, 10, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Xu, W.; Yang, Y.; Yang, Z.; Lv, H.; Yang, S.; Lin, Z.; Li, J.; Gu, J.; Hill, C.L.; et al. Three candesartan salts with enhanced oral bioavailability. Cryst. Growth Des. 2015, 15, 3707–3714. [Google Scholar] [CrossRef]
- Khan, S.; Madni, A.; Rahim, M.A.; Shah, H.; Jabar, A.; Khan, M.M.; Khan, A.; Jan, N.; Mahmood, M.A. Enhanced in vitro release and permeability of glibenclamide by proliposomes: Development, characterization and histopathological evaluation. J. Drug Deliv. Sci. Technol. 2021, 63, 102450. [Google Scholar] [CrossRef]
- Schelz, Z.; Muddather, H.F.; Zupkó, I. Repositioning of HMG-CoA Reductase Inhibitors as Adjuvants in the Modulation of Efflux Pump-Mediated Bacterial and Tumor Resistance. Antibiotics 2023, 12, 1468. [Google Scholar] [CrossRef]
- Badila, E.; Frunza, S.A.; Tirziu, C.M.; Bartos, D.; Dorobantu, M. The effect of candesartan on blood pressure, metabolic profile and renal function in hypertensive patients. Int. J. Cardiol. 2009, 137, S134–S135. [Google Scholar] [CrossRef]
- Franco, C.C.S.; Prates, K.V.; Previate, C.; Moraes, A.M.P.; Matiusso, C.C.I.; Miranda, R.A.; de Oliveira, J.C.; Tófolo, L.P.; Martins, I.P.; Barella, L.F.; et al. Glibenclamide treatment blocks metabolic dysfunctions and improves vagal activity in monosodium glutamate-obese male rats. Endocrine 2017, 56, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Stalenhoef, A.F.; Ballantyne, C.M.; Sarti, C.; Murin, J.; Tonstad, S.; Rose, H.; Wilpshaar, W. A Comparative study with rosuvastatin in subjects with METabolic Syndrome: Results of the COMETS study†. Eur. Hearth J. 2005, 26, 2664–2672. [Google Scholar] [CrossRef]
- Asrani, S.; Bacharach, J.; Holland, E.; McKee, H.; Sheng, H.; Lewis, R.A.; Kopczynski, C.C.; Heah, T. Fixed-dose combination of netarsudil and latanoprost in ocular hypertension and open-angle glaucoma: Pooled efficacy/safety analysis of phase 3 MERCURY-1 and -2. Adv. Ther. 2020, 37, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.; Oh, E. Rationale and strategies for formulation development of oral fixed dose combination drug products. J. Pharm. Investig. 2016, 46, 615–631. [Google Scholar] [CrossRef]
- Böhm, A.-K.; Schneider, U.; Aberle, J.; Stargardt, T. Regimen simplification and medication adherence: Fixed-dose versus loose-dose combination therapy for type 2 diabetes. PLoS ONE 2021, 16, e0250993. [Google Scholar] [CrossRef] [PubMed]
- Alshora, D.H.; Ibrahim, M.A.; Sherif, A.Y. Optimization and Validation of Sensitive UPLC-PDA Method for Simultaneous Determination of Thymoquinone and Glibenclamide in SNEDDs Formulations Using Response Surface Methodology. Separations 2023, 10, 577. [Google Scholar] [CrossRef]
- Ibrahim, M.; Alhabib, N.A.; Alshora, D.; Bekhit, M.M.S.; Taha, E.; Mahdi, W.A.; Harthi, A.M. Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products. Separations 2023, 10, 318. [Google Scholar] [CrossRef]
- Tarek, M.; Ghoniem, N.S.; Hegazy, M.A.; Wagdy, H.A. Design of Experiment-Based Green UPLC-DAD Method for the Simultaneous Determination of Indacaterol, Glycopyrronium and Mometasone in their Combined Dosage Form and Spiked Human Plasma. J. Chromatogr. Sci. 2023, 61, bmad072. [Google Scholar] [CrossRef] [PubMed]
- Elmansi, H.; Belal, F. Development of an Eco-friendly HPLC method for the simultaneous determination of three benzodiazepines using green mobile phase. Microchem. J. 2019, 145, 330–336. [Google Scholar] [CrossRef]
- Alghazi, M.; Alanazi, F.; Mohsin, K.; Siddiqui, N.A.; Shakeel, F.; Haq, N. Simultaneous separation of antihyperlipidemic drugs by green ultrahigh-performance liquid chromatography–diode array detector method: Improving the health of liquid chromatography. J. Food Drug Anal. 2017, 25, 430–437. [Google Scholar] [CrossRef]
- Cvjetko, B.M.; Vidović, S.; Radojčić, R.I.; Jokić, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Ameta, R.K.; Soni, K.; Bhattarai, A. Recent Advances in Improving the Bioavailability of Hydrophobic/Lipophilic Drugs and Their Delivery via Self-Emulsifying Formulations. Colloids Interfaces 2023, 7, 16. [Google Scholar] [CrossRef]
- International Federation of Pharmaceutical Manufactures & Associations I. Validation of analytical procedures: Text and methodology, Methodology Q2 (R1). In Proceedings of the International Conference on Harmonization (ICH ’96), Geneva, Switzerland, 6 November 1996. [Google Scholar]
- Shamim, A.; Ansari, M.A.; Aodah, A.; Iqbal, M.; Aqil, M.; Mirza, M.A.; Iqbal, Z.; Ali, A. QbD-Engineered Development and Validation of a RP-HPLC Method for Simultaneous Estimation of Rutin and Ciprofloxacin HCl in Bilosoma Nanoformulation. ACS Omega 2023, 8, 21618–21627. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.M. RP-UPLC method development and validation for simultaneous estimation of vildagliptin with metformin hydrochloride and ciprofloxacin hydrochloride with dexamethasone sodium phosphate. World J. Pharm. Sci. 2015, 3, 1755–1762. [Google Scholar]
- Ibrahim, M.A.; Alshora, D.A.; Alowayid, M.A.; Alanazi, N.A.; Almutair, R.A. Development and Validation of a Green UPLC Analytical Procedure for Glibenclamide Determination in Pharmaceutical Product Using Response Surface Methodology. Orient. J. Chem. 2022, 38, 865–874. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Shahba, A.A.-W. Development of a Multifunctional Oral Dosage Form via Integration of Solid Dispersion Technology with a Black Seed Oil-Based Self-Nanoemulsifying Drug Delivery System. Biomedicines 2023, 11, 2733. [Google Scholar] [CrossRef] [PubMed]
- Kazi, T.M.; Dandagi, P.M. A New Stability-Indicating RP-HPLC Method for Simultaneous Quantification of Diacerein and Aceclofenac in Novel Nanoemulgel Formulation and Commercial Tablet Dosage Form in the Presence of their Major Degradation Products Using DoE Approach. J. Chromatogr. Sci. 2023, 61, 918–929. [Google Scholar] [CrossRef]
- Ettre, L.S. Nomenclature for chromatography (IUPAC Recommendations 1993). Pure Appl. Chem. 1993, 65, 819–872. [Google Scholar] [CrossRef]
- Yang, Y.; Lamm, O.J.; He, P.; Kondo, T. Temperature Effect on PeakWidth and Column Efficiency in Subcritical Water Chromatography. J Chromatog Sci. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Abul Hossain, M.; Kumita, M.; Michigami, Y.; Islam, T.S.A.; Mori, S. Rapid Speciation Analysis of Cr(VI) and Cr(III) by Reversed-Phase High-Performance Liquid Chromatography with UV Detection. J. Chromatogr. Sci. 2005, 43, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Crawford Scientific. Peak Tailing in HPLC. Available online: https://www.crawfordscientific.com/chromatography-blog/post/peak-tailing-in-hplc (accessed on 27 March 2024).
- Chromatography Today. What Is Peak Tailing? Available online: https://www.chromatographytoday.com/news/autosamplers/36/breaking-news/what-is-peak-tailing/31253 (accessed on 27 March 2024).
- Maryutina, T.A.; Savonina, E.Y.; Fedotov, P.S.; Smith, R.M.; Siren, H.; Hibbert, D.R. Terminology of separation methods (IUPAC Recommendations 2017). Pure Appl. Chem. 2018, 90, 181–231. [Google Scholar] [CrossRef]
- Kevin, E.; Van Cott, R.D. Whitley and N.-H. Linda Wang. Effects of temperature and flow rate on frontal and elution chro-matography of aggregating systems. Separ Technol. 1991, 1, 142–152. [Google Scholar]
- Abdelgawad, M.A.; Elmowafy, M.; Musa, A.; Al-sanea, M.M.; Nayl, A.A.; Ghoneim, M.M.; Ahmed, Y.M.; Hassan, H.M.; AboulMagd, A.M.; Salem, H.F.; et al. Development and Greenness Assessment of HPLC Method for and Papaya Extract. Molecules 2022, 27, 375. [Google Scholar] [CrossRef] [PubMed]
- Thurmann, S.; Lotter, C.; Heiland, J.J.; Chankvetadze, B.; Belder, D. Chip-based high-performance liquid chromatography for high-speed enantioseparations. Anal. Chem. 2015, 87, 5568–5576. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jayaseelan, K. Environmentally sustainable analytical quality by design aided RP-HPLC method for the estimation of brilliant blue in commercial food samples employing a green-ultrasound-assisted extraction technique. Green Process. Synth. 2023, 12, 20230178. [Google Scholar] [CrossRef]


| Independent Factors | Level | Dependent Factors (Response) | |||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| A: Temperature (°C) B: Flow rate (mL/min) | 30 0.2 | 40 0.3 | 50 0.4 | RC | Y1: Retention time (min) |
| Y2: Peak area (mAU/min) | |||||
| Y3: Peak Asymmetry | |||||
| GB | Y4: Retention time (min) | ||||
| Y5: Peak area (mAU/min) | |||||
| Y6: Peak Asymmetry | |||||
| Y7: Resolution of RC-GB Peaks | |||||
| CC | Y8: Retention time (min) | ||||
| Y9: Peak area (mAU/min) | |||||
| Y10: Peak Asymmetry | |||||
| Y11: Resolution of GB-CC Peaks | |||||
| Response | RT | Peak Area | Peak Assym. | Resolution of Next Peak | ||||
|---|---|---|---|---|---|---|---|---|
| RC | ||||||||
| Source | p Values | F Value | p Values | F Value | p Values | F Value | p Values * | F Value |
| A—Temperature | 0.0002 | 528.19 | 0.1965 | 2.74 | 0.0396 | 5.08 | 0.0730 | 311.48 |
| B—Flow rate | <0.0001 | 11,826.65 | <0.0001 | 1.461 × 105 | 0.0017 | 21.81 | 0.0004 | 10.06 |
| AB | 0.0095 | 35.49 | 0.5175 | 0.5350 | 0.4890 | 1.73 | 0.2095 | 3.67 |
| A² | 0.6568 | 0.2415 | 0.2890 | 1.65 | 0.6294 | 0.6246 | 0.0249 | 1.01 |
| B² | 0.0001 | 624.66 | <0.0001 | 5314.20 | 0.6294 | 10.90 | 0.9611 | 0.3797 |
| GB | ||||||||
| Source | p Values | F Value | p Values | F Value | p Values | F Value | p Values ** | F Value |
| A—Temperature | 0.0003 | 376.67 | 0.1061 | 5.24 | 0.0266 | 16.65 | 0.0730 | 7.36 |
| B—Flow rate | <0.0001 | 1531.53 | <0.0001 | 1.03 × 105 | 0.0027 | 85.23 | 0.0004 | 322.28 |
| AB | 0.0085 | 38.41 | 0.5070 | 0.5646 | 0.0607 | 8.62 | 0.2095 | 2.54 |
| A² | 0.1531 | 3.62 | 0.3027 | 1.54 | 0.5632 | 0.4197 | 0.0249 | 17.49 |
| B² | 0.0043 | 62.01 | <0.0001 | 3802.92 | 0.0084 | 38.61 | 0.9611 | 0.0028 |
| CC | ||||||||
| Source | p Values | F Value | p Values | F Value | p Values | F Value | ||
| A—Temperature | <0.0001 | 2385.74 | 0.058 | 9.02 | 0.0396 | 25.90 | ||
| B—Flow rate | 0.0002 | 558.60 | 6.507 | 486.21 | 0.0017 | 12.23 | ||
| AB | <0.0001 | 10,937.10 | 0.667 | 0.227 | 0.4890 | 116.10 | ||
| A² | 0.216 | 33.42 | 0.077 | 7.04 | 0.6294 | 0.6187 | ||
| B² | 0.0003 | 2.44 | <0.0001 | 18,308.96 | 0.6294 | 0.2868 | ||
| Column Temperature | Flow Rate | RC | GB | CC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT RC | Peak Area RC | Symmetry RC | Resolution RC-GB Peaks | RT GB | Peak Area GB | Symmetry GB | Resolution GB-CC Peaks | RT CC | Peak Area CC | Symmetry CC | ||
| 30 | 0.2 | 1.299 ± 0.06 | 21.22 ± 1.23 | 1.43 ± 0.004 | 14.557 ± 0.07 | 3.3 ± 0.008 | 34.51 ± 0.54 | 1.12 ± 0.002 | 4.59 ± 0.02 | 7.8 ± 0.21 | 19.55 ± 0.98 | 1.44 ± 0.008 |
| 40 | 0.4 | 0.62 ± 0.03 | 10.7093 ± 0.56 | 1.533 ± 0.002 | 20.94 ± 0.02 | 1.45 ± 0.007 | 17.22 ± 1.21 | 1.31 ± 0.001 | 4.03 ± 0.07 | 4.71 ± 0.09 | 9.67 ± 0.21 | 1.25 ± 0.009 |
| 30 | 0.3 | 0.861 ± 0.04 | 14.230 ± 1.12 | 1.637 ± 0.002 | 16.713 ± 0.05 | 2.22 ± 0.006 | 22.99 ± 0.98 | 1.25 ± 0.007 | 4.46 ± 0.07 | 5.86 ± 0.21 | 12.94 ± 0.09 | 1.39 ± 0.003 |
| 50 | 0.4 | 0.573 ± 0.008 | 10.7494 ± o.98 | 1.543 ± 0.003 | 19.987 ± 0.05 | 1.24 ± 0.0001 | 17.31 ± 1.51 | 1.39 ± 0.001 | 3.39 ± 0.01 | 4.43 ± 0.09 | 9.70 ± 0.21 | 1.25 ± 0.007 |
| 50 | 0.3 | 0.743 ± 0.012 | 14.2546 ± 0.87 | 1.543 ± 0.005 | 17.967 ± 0.03 | 1.61 ± 0.003 | 23.10 ± 0.94 | 1.38 ± 0.004 | 3.25 ± 0.03 | 5.30 ± 0.21 | 13.00 ± 0.41 | 1.31 ± 0.001 |
| 40 | 0.2 | 1.2167 ± 0.016 | 21.32 ± 0.98 | 1.06 ± 0.001 | 15.783 ± 0.02 | 2.73 ± 0.002 | 34.76 ± 1.52 | 1.11 ± 0.008 | 3.67 ± 0.09 | 7.42 ± 0.12 | 19.53 ± 0.87 | 1.45 ± 0.005 |
| 30 | 0.4 | 0.663 ± 0.006 | 10.718 ± 0.21 | 1.643 ± 0.004 | 20.023 ± 0.009 | 1.67 ± 0.004 | 17.22 ± 0.87 | 1.24 ± 0.01 | 4.57 ± 0.04 | 4.89 ± 0.09 | 9.67 ± 0.13 | 1.27 ± 0.001 |
| 40 | 0.3 | 0.79 ± 0.003 | 14.294 ± 0.87 | 1.67 ± 0.007 | 18.73 ± 0.02 | 1.84 ± 0.005 | 23.10 ± 1.14 | 1.36 ± 0.009 | 3.81 ± 0.09 | 5.61 ± 0.14 | 12.95 | 1.35 ± 0.007 |
| 50 | 0.2 | 1.129 ± 0.07 | 21.30 ± 0.48 | 1.067 ± 0.001 | 15.607 ± 0.08 | 2.35 ± 0.007 | 34.70 ± 0.79 | 1.11 ± 0.02 | 3.06 ± 0.05 | 6.98 ± 0.23 | 19.61 | 1.39 ± 0.001 |
| Optimized Independent Parameters | Response | ||||
|---|---|---|---|---|---|
| Type | Desirability | Predicted | Observed | ||
| Temperature (A): 50 °C (B): Flow rate 0.26 mL/min | RC | Y1: Retention time (min) | Minimum | 0.866 | 0.837 ± 0.013 × 10−14 |
| Y2: Peak area (mAU/min) | Maximum | 16.67 | 17.003 ± 0.033 | ||
| Y3: Peak Asymmetry | In range | 1.4 | 1.59 ± 0.006 | ||
| Y4: RC-GB Peak Resolution | In range | 3.13 | 3.08 ± 0.01 | ||
| GB | Y5: Retention time (min) | Minimum | 1.83 | 1.8 ± 0.004 | |
| Y6: Peak area (mAU/min) | Maximum | 27.07 | 27.18 ± 0.0.08 | ||
| Y7: Peak Asymmetry | In range | 1.3 | 1.36 ± 0.012 | ||
| Y8: GB-CC Peak Resolution | In range | 16.95 | 17.07 ± 0.044 | ||
| CC | Y9: Retention time (min) | Minimum | 5.85 | 5.80 ± 0.005 | |
| Y10: Peak area (mAU/min) | Maximum | 15.25 | 15.35 ± 0.08 | ||
| Y11: Peak Asymmetry | In range | 1.35 | 1.32 ± 0.01 | ||
| Parameters | CC | GB | RC |
|---|---|---|---|
| Linearity range | 2–50 ppm | 2–50 ppm | 2–50 ppm |
| Line equation | Y = 0.342x − 0.1644 | Y = 0.581x − 0.257 | Y = 0.459x − 0.203 |
| Regression coefficient (r2) | 0.999 ± 0 | 0.999 ± 0 | 0.999 ± 0 |
| Slope | 0.342 ± 0.0007 | 0.581 ± 0.00121 | 0.459 ± 0.00129 |
| Standard error of the slope | 0.00026 | 0.00046 | 0.00049 |
| Intercept | 0.1644 ± 0.0027 | 0.257 ± 0.00442 | 0.257 ± 0.0057 |
| Standard error of the slope | 0.00130 | 0.00167 | 0.00218 |
| Theoretical Concentration (ppm) | RC | GB | CC | |||
|---|---|---|---|---|---|---|
| %Recovery | %RSD | %Recovery | %RSD | %Recovery | %RSD | |
| 2 | 104.761 | 0.268 | 105.347 | 0.548 | 107.507 | 0.141 |
| 10 | 97.609 | 0.305 | 97.419 | 0.313 | 99.094 | 0.240 |
| 50 | 100.850 | 0.301 | 100.678 | 0.231 | 102.735 | 0.222 |
| Analytes | Theoretical Concentration (ppm) | Intra-Day (Measured Concentration, RSD%) | Inter-Day (Measured Concentration, RSD%) | ||
|---|---|---|---|---|---|
| Day-1 | Day-2 | Day-3 | |||
| RC | 2 | 2.095; 0.268 | 2.095; 0.268 | 2.09; 0.094 | 2.121; 1.457 |
| 10 | 9.760; 0.305 | 9.760; 0.305 | 9.80; 0.071 | 9.849; 0.483 | |
| 50 | 50.425; 0.301 | 50.425; 0.301 | 50.78; 0.185 | 51.061; 0.372 | |
| GB | 2 | 2.106; 0.548 | 2.106; 0.548 | 2.104; 0.374 | 2.117; 0.274 |
| 10 | 9.741; 0.313 | 9.741; 0.313 | 9.78; 0.141 | 9.802; 0.157 | |
| 50 | 5.033; 0.231 | 5.033; 0.231 | 50.62; 0.123 | 50.854; 0.284 | |
| CC | 2 | 2.15; 0.141 | 2.15; 0.141 | 2.104; 0.175 | 2.115; 0.375 |
| 10 | 9.909; 0.240 | 9.909; 0.240 | 9.755; 0.167 | 9.779; 0.175 | |
| 50 | 51.36; 0.222 | 51.36; 0.222 | 50.643; 0.162 | 50.979; 0.248 | |
| Parameters | Rosuvastatin Calcium | |||
|---|---|---|---|---|
| UV Wavelength (nm) | Peak Area | Retention Time | Peak Symmetry | Resolution |
| 242 | 0.523 | 0 | 0.911 | 0.745 |
| 244 | 0.303 | 0.263 | 1.024 | 0 |
| 246 | 0.048 | 0.601 | 1.683 | 0.211 |
| Column temperature | ||||
| 48 | 0.661 | 0 | 1.274 | 0 |
| 50 | 0.303 | 0.263 | 1.024 | 0 |
| 52 | 0.409 | 0 | 1.266 | 0.439 |
| Glibenclamide | ||||
| UV Wavelength (nm) | Peak Area | Retention Time | Peak Symmetry | Resolution |
| 223 | 0.495 | 0.123 | 1.202 | 0.428 |
| 225 | 0.232 | 0.278 | 1.711 | 0.156 |
| 227 | 0.246 | 0.217 | 1.078 | 0.321 |
| Column temperature | ||||
| 48 | 0.853 | 0.398 | 2.192 | 0.763 |
| 50 | 0.232 | 0.28 | 1.711 | 0.156 |
| 52 | 0.451 | 0.420 | 1.418 | 1.244 |
| Candesartan | ||||
| UV Wavelength (nm) | Peak Area | Retention Time | Peak Symmetry | Resolution |
| 256 | 0.516 | 0 | 1.142 | |
| 258 | 0.223 | 0.086 | 1.727 | |
| 260 | 0.594 | 1.9 × 10−14 | 1.503 | |
| Column temperature | ||||
| 48 | 0.872 | 0 | 1.545 | |
| 50 | 0.223 | 0.086 | 1.727 | |
| 52 | 0.461 | 0 | 0.441 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.; Sherif, A.Y.; Alshora, D.; Alsaadi, B. A Robust and Reliable UPLC Method for the Simultaneous Quantification of Rosuvastatin Calcium, Glibenclamide, and Candesartan Cilexetil. Separations 2024, 11, 113. https://doi.org/10.3390/separations11040113
Ibrahim MA, Sherif AY, Alshora D, Alsaadi B. A Robust and Reliable UPLC Method for the Simultaneous Quantification of Rosuvastatin Calcium, Glibenclamide, and Candesartan Cilexetil. Separations. 2024; 11(4):113. https://doi.org/10.3390/separations11040113
Chicago/Turabian StyleIbrahim, Mohamed Abbas, Abdelrahman Y. Sherif, Doaa Alshora, and Badr Alsaadi. 2024. "A Robust and Reliable UPLC Method for the Simultaneous Quantification of Rosuvastatin Calcium, Glibenclamide, and Candesartan Cilexetil" Separations 11, no. 4: 113. https://doi.org/10.3390/separations11040113
APA StyleIbrahim, M. A., Sherif, A. Y., Alshora, D., & Alsaadi, B. (2024). A Robust and Reliable UPLC Method for the Simultaneous Quantification of Rosuvastatin Calcium, Glibenclamide, and Candesartan Cilexetil. Separations, 11(4), 113. https://doi.org/10.3390/separations11040113







