Essential Oil of Ocimum basilicum against Aedes aegypti and Culex quinquefasciatus: Larvicidal Activity of a Nanoemulsion and In Silico Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Botanical Material
2.2. Preparation and Characterization of Botanical Material
2.3. Extraction of the EO
2.4. Chemical Composition Analysis
2.5. Preparation of Nanoemulsions
2.6. Physicochemical Characterization of Nanoemulsion
2.7. Larvicidal Bioassay
2.8. Statistical Analysis
2.9. In Silico Analysis
2.9.1. Prediction of Biological Activity
2.9.2. Selection of the Proteins and Ligand Structures
2.9.3. Molecular Binding Affinity Statistics
3. Results and Discussion
3.1. Chemical Composition
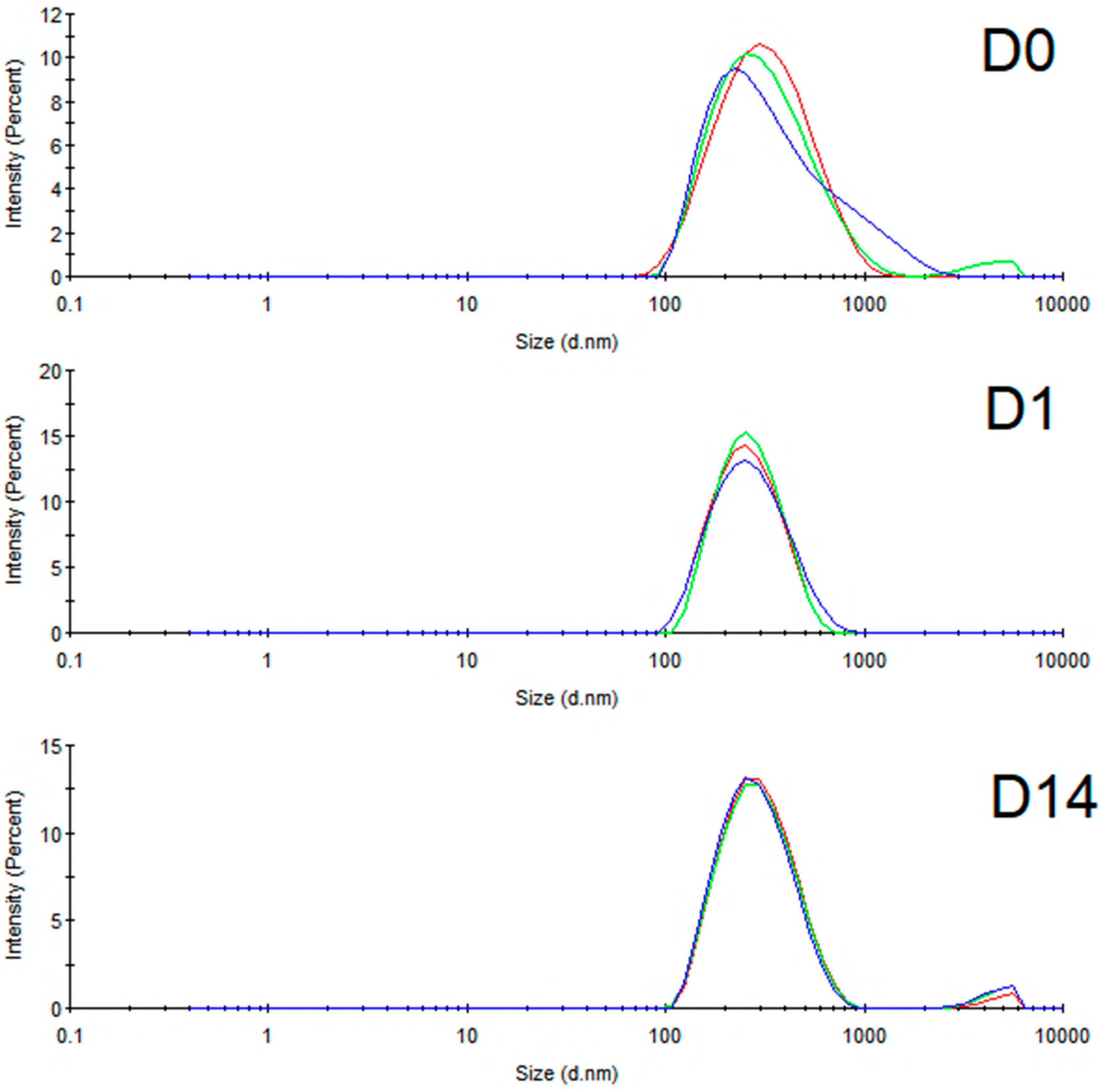
3.2. Preparation and Physical Characterization of Nanoemulsion
3.3. Larvicidal Bioassay
3.4. Predictions In Silico of Biological Activity
3.5. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muzammil, S.; Neves Cruz, J.; Mumtaz, R.; Rasul, I.; Hayat, S.; Khan, M.A.; Khan, A.M.; Ijaz, M.U.; Lima, R.R.; Zubair, M. Effects of Drying Temperature and Solvents on In Vitro Diabetic Wound Healing Potential of Moringa Oleifera Leaf Extracts. Molecules 2023, 28, 710. [Google Scholar] [CrossRef]
- Achee, N.L.; Grieco, J.P.; Vatandoost, H.; Seixas, G.; Pinto, J.; Ching-Ng, L.; Martins, A.J.; Juntarajumnong, W.; Corbel, V.; Gouagna, C.; et al. Alternative Strategies for Mosquito-Borne Arbovirus Control. PLoS Negl. Trop. Dis. 2019, 13, e0006822. [Google Scholar] [CrossRef]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula Officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef]
- Estrada-Franco, J.G.; Fernández-Santos, N.A.; Adebiyi, A.A.; López-López, M.d.J.; Aguilar-Durán, J.A.; Hernández-Triana, L.M.; Prosser, S.W.J.; Hebert, P.D.N.; Fooks, A.R.; Hamer, G.L.; et al. Vertebrate-Aedes Aegypti and Culex Quinquefasciatus (Diptera)-Arbovirus Transmission Networks: Non-Human Feeding Revealed by Meta-Barcoding and Nextgeneration Sequencing. PLoS Negl. Trop. Dis. 2020, 14, e0008867. [Google Scholar] [CrossRef]
- Zucchi, M.I.; Cordeiro, E.M.G.; Wu, X.; Lamana, L.M.; Brown, P.J.; Manjunatha, S.; Viana, J.P.G.; Omoto, C.; Pinheiro, J.B.; Clough, S.J. Population Genomics of the Neotropical Brown Stink Bug, Euschistus Heros: The Most Important Emerging Insect Pest to Soybean in Brazil. Front. Genet. 2019, 10, 1035. [Google Scholar] [CrossRef]
- Horikoshi, R.J.; Dourado, P.M.; Berger, G.U.; Fernandes, D.d.S.; Omoto, C.; Willse, A.; Martinelli, S.; Head, G.P.; Corrêa, A.S. Large-Scale Assessment of Lepidopteran Soybean Pests and Efficacy of Cry1Ac Soybean in Brazil. Sci. Rep. 2021, 11, 15956. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, K.S.; Galúcio, J.M.; Da Costa, C.H.S.; Santana, A.R.; Dos Santos Carvalho, V.; Do Nascimento, L.D.; Lima E Lima, A.H.; Neves Cruz, J.; Alves, C.N.; Lameira, J. Exploring the Potentiality of Natural Products from Essential Oils as Inhibitors of Odorant-Binding Proteins: A Structure- And Ligand-Based Virtual Screening Approach to Find Novel Mosquito Repellents. ACS Omega 2019, 4, 22475–22486. [Google Scholar] [CrossRef]
- Purushothaman, B.; Prasannasrinivasan, R.; Suganthi, P.; Ranganathan, B.; Gimbun, J.; Shanmugam, K. A Comprehensive Review on Ocimum Basilicum. J. Nat. Remedies 2018, 18, 71–85. [Google Scholar] [CrossRef]
- Aminian, A.R.; Mohebbati, R.; Boskabady, M.H. The Effect of Ocimum Basilicum L. and Its Main Ingredients on Respiratory Disorders: An Experimental, Preclinical, and Clinical Review. Front. Pharmacol. 2022, 12, 805391. [Google Scholar] [CrossRef] [PubMed]
- Dharsono, H.D.A.; Putri, S.A.; Kurnia, D.; Dudi, D.; Satari, M.H. Ocimum Species: A Review on Chemical Constituents and Antibacterial Activity. Molecules 2022, 27, 6350. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Gonçalves, M.J.; Alves, J.; Falconieri, D.; Porcedda, S.; Maxia, A.; Salgueiro, L. Ocimum Tenuiflorum L. and Ocimum Basilicum L., Two Spices of Lamiaceae Family with Bioactive Essential Oils. Ind. Crops Prod. 2018, 113, 89–97. [Google Scholar] [CrossRef]
- Kalaivani, K.; Senthil-Nathan, S.; Murugesan, A.G. Biological Activity of Selected Lamiaceae and Zingiberaceae Plant Essential Oils against the Dengue Vector Aedes Aegypti L. (Diptera: Culicidae). Parasitol. Res. 2012, 110, 1261–1268. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide Research: Current Trends and Future Priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic Emulsification of Food-Grade Nanoemulsion Formulation and Evaluation of Its Bactericidal Activity. Ultrason. Sonochem. 2013, 20, 338–344. [Google Scholar] [CrossRef]
- Bruxel, F.; Laux, M.; Wild, L.B.; Fraga, M.; Koester, L.S.; Teixeira, H.F. Nanoemulsões Como Sistemas de Liberação Parenteral de Fármacos. Quim. Nova 2012, 35, 1827–1840. [Google Scholar] [CrossRef]
- Pey, C.M.; Maestro, A.; Solé, I.; González, C.; Solans, C.; Gutiérrez, J.M. Optimization of Nano-Emulsions Prepared by Low-Energy Emulsification Methods at Constant Temperature Using a Factorial Design Study. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 288, 144–150. [Google Scholar] [CrossRef]
- Boehm, A.L.; Martinon, I.; Zerrouk, R.; Rump, E.; Fessi, H. Nanoprecipitation Technique for the Encapsulation of Agrochemical Active Ingredients. J. Microencapsul. 2003, 20, 433–441. [Google Scholar] [CrossRef]
- da Silva Júnior, O.S.; Franco, C.d.J.P.; de Moraes, A.A.B.; Cruz, J.N.; da Costa, K.S.; do Nascimento, L.D.; Andrade, E.H.d.A. In Silico Analyses of Toxicity of the Major Constituents of Essential Oils from Two Ipomoea L. Species. Toxicon 2021, 195, 111–118. [Google Scholar] [CrossRef]
- Adam, R. Identification of the Essential Oil Components by GC/MS, 4th ed.; Adams, R.P., Ed.; Allured Pub Corp: Carol Stream, IL, USA, 1995; ISBN 978-1-932()33-11-4. [Google Scholar]
- Mondello, L. Mass Spectra of Flavors and Fragrances of Natural and Synthetic Compounds; Wiley Blackwell: Hoboken, NJ, USA, 2015; Volume 10, pp. 4–5. [Google Scholar]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of Activity Spectra for Biologically Active Substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software News and Updates AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- da Costa, G.V.; Ferreira, E.F.B.; Ramos, R.d.S.; da Silva, L.B.; de Sá, E.M.F.; da Silva, A.K.P.; Lobato, C.M.; Souto, R.N.P.; da Silva, C.H.T.d.P.; Federico, L.B.; et al. Hierarchical Virtual Screening of Potential Insectides Inhibitors of Acetylcholinesterase and Juvenile Hormone from Temephos. Pharmaceuticals 2019, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- da Silva Ramos, R.; da Silva Costa, J.; Campos Silva, R.; Vilhena da Costa, G.; Bruno Lobato Rodrigues, A.; de Menezes Rabelo, É.; Nonato Picanço Souto, R.; Anthony Taft, C.; Tomich de Paula da Silva, C.H.; Campos Rosa, J.M.; et al. Identification of Potential Inhibitors from Pyriproxyfen with Insecticidal Activity by Virtual Screening. Pharmaceuticals 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical Composition and Antibacterial Activity of Essential Oil Isolated from Omani Basil (Ocimum Basilicum Linn.). Asian Pacific J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Satyal, P.; Ali, N.A.A.; Pokharel, S.; Zhang, H.; Wink, M.; Kukaniev, M.A.; Setzer, W.N. The Essential Oil Compositions of Ocimum Basilicum from Three Different Regions: Nepal, Tajikistan, and Yemen. Chem. Biodivers. 2016, 13, 241–248. [Google Scholar] [CrossRef]
- Sajjadi, S.E. Analysis of the Essential Oils of Two Cultivated Basil (Ocimum Basilicum L.) from Iran. Daru 2006, 14, 128–130. [Google Scholar]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and Oil Composition of 38 Basil (Ocimum Basilicum L.) Accessions Grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical Composition, Antioxidant and Antimicrobial Activities of Basil (Ocimum Basilicum) Essential Oils Depends on Seasonal Variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Sirousmehr, A.; Arbabi, J.; Asgharipour, M.R. Effect of Drought Stress Levels and Organic Manures on Yield, Essential Oil Content and Some Morphological Characteristics of Sweet Basil (Ocimum basilicum). Adv. Environ. Biol. 2014, 8, 1322–1327. [Google Scholar]
- Ding, Y.; Huffaker, A.; Köllner, T.G.; Weckwerth, P.; Robert, C.A.M.; Spencer, J.L.; Lipka, A.E.; Schmelz, E.A. Selinene Volatiles Are Essential Precursors for Maize Defense Promoting Fungal Pathogen Resistance. Plant Physiol. 2017, 175, 1455–1468. [Google Scholar] [CrossRef]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.; Bhushan, B.; Kumar, M.; Pant, A. β-Selinene-Rich Essential Oils from the Parts of Callicarpa Macrophylla and Their Antioxidant and Pharmacological Activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Schalechet, F.; Wilkinson, J.; Matsui, K.; Tadmor, Y.; Nam, K.H.; Amar, O.; Lastochkin, E.; Larkov, O.; Ravid, U.; et al. Enhanced Levels of the Aroma and Flavor Compound S-Linalool by Metabolic Engineering of the Terpenoid Pathway in Tomato Fruits. Plant Physiol. 2001, 127, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, G.M.; Annies, V.; de Oliveira, C.F.; Lara, R.A.; Gabriel, M.M.; Betim, F.C.M.; Nadal, J.M.; Farago, P.V.; Dias, J.F.G.; Miguel, O.G.; et al. Evaluation of Larvicidal Activity and Ecotoxicity of Linalool, Methyl Cinnamate and Methyl Cinnamate/Linalool in Combination against Aedes Aegypti. Ecotoxicol. Environ. Saf. 2017, 139, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ahn, Y.J. Contact and Fumigant Activities of Constituents of Foeniculum Vulgare Fruit against Three Coleopteran Stored-Product Insects. Pest Manag. Sci. 2001, 57, 301–306. [Google Scholar] [CrossRef]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella Anisum Essential Oil Nanoemulsions against Tribolium Castaneum—Insecticidal Activity and Mode of Action. Environ. Sci. Pollut. Res. 2018, 25, 18802–18812. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; He, Y.; Bhandari, B. Effectiveness of Encapsulating Biopolymers to Produce Sub-Micron Emulsions by High Energy Emulsification Techniques. Food Res. Int. 2007, 40, 862–873. [Google Scholar] [CrossRef]
- Teo, A.; Goh, K.K.T.; Wen, J.; Oey, I.; Ko, S.; Kwak, H.S.; Lee, S.J. Physicochemical Properties of Whey Protein, Lactoferrin and Tween 20 Stabilised Nanoemulsions: Effect of Temperature, PH and Salt. Food Chem. 2016, 197, 297–306. [Google Scholar] [CrossRef]
- Silva, M.B. Percepção Da População Assistida Sobre a Inserção de Estudantes de Medicina Na Unidade Básica de Saúde. In Trabalho de Conclusão de Curso; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2016; Volume 1, pp. 1–10. ISBN 9788578110796. [Google Scholar]
- Sundararajan, B.; Moola, A.K.; Vivek, K.; Kumari, B.D.R. Formulation of Nanoemulsion from Leaves Essential Oil of Ocimum Basilicum L. and Its Antibacterial, Antioxidant and Larvicidal Activities (Culex Quinquefasciatus). Microb. Pathog. 2018, 125, 475–485. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Formation of Flavor Oil Microemulsions, Nanoemulsions and Emulsions: Influence of Composition and Preparation Method. J. Agric. Food Chem. 2011, 59, 5026–5035. [Google Scholar] [CrossRef]
- Anjali, C.; Sharma, Y.; Mukherjee, A.; Chandrasekaran, N. Neem Oil (Azadirachta Indica) Nanoemulsion-a Potent Larvicidal Agent against Culex Quinquefasciatus. Pest Manag. Sci. 2012, 68, 158–163. [Google Scholar] [CrossRef]
- Paul, S.; Panda, A.K. Physico-Chemical Studies on Microemulsion: Effect of Cosurfactant Chain Length on the Phase Behavior, Formation Dynamics, Structural Parameters and Viscosity of Water/(Polysorbate-20 + n-Alkanol)/n-Heptane Water-in-Oil Microemulsion. J. Surfactants Deterg. 2011, 14, 473–486. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-Energy Formation of Edible Nanoemulsions: Factors Influencing Droplet Size Produced by Emulsion Phase Inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef]
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of Selected Plant Essential Oils against the Yellow Fever Mosquito Aedes Aegypti Larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef]
- Silva, W.J.; Dória, G.A.A.; Maia, R.T.; Nunes, R.S.; Carvalho, G.A.; Blank, A.F.; Alves, P.B.; Marçal, R.M.; Cavalcanti, S.C.H. Effects of Essential Oils on Aedes Aegypti Larvae: Alternatives to Environmentally Safe Insecticides. Bioresour. Technol. 2008, 99, 3251–3255. [Google Scholar] [CrossRef]
- Ramos, R.; Rodrigues, A.; Souto, R.; Almeida, S. Chemical Study, Antioxidant Analysis and Evaluation of the Larvicidal Potential against Aedes Aegypti Larvae of Essential Oil of Ocimum Basilicum Linn. European J. Med. Plants 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Oliveira, A.E.M.F.M.; Duarte, J.L.; Amado, J.R.R.; Cruz, R.A.S.; Rocha, C.F.; Souto, R.N.P.; Ferreira, R.M.A.; Santos, K.; Da Conceição, E.C.; De Oliveira, L.A.R.; et al. Development of α Larvicidal Nanoemulsion with Pterodon Emarginatus Vogel Oil. PLoS ONE 2016, 11, e0145835. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz, M.H.; Zubair, M.; Aslam, B.; Ashraf, A.; Siddique, M.H.; Hayat, S.; Cruz, J.N.; Muzammil, S.; Khurshid, M.; Sarfraz, M.F.; et al. Comparative Analysis of Phyto-Fabricated Chitosan, Copper Oxide, and Chitosan-Based CuO Nanoparticles: Antibacterial Potential against Acinetobacter Baumannii Isolates and Anticancer Activity against HepG2 Cell Lines. Front. Microbiol. 2023, 14, 1188743. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Formulation and Characterization of Plant Essential Oil Based Nanoemulsion: Evaluation of Its Larvicidal Activity against Aedes Aegypti. Asian J. Chem. 2013, 25, 18–20. [Google Scholar]
- Lim, C.J.; Basri, M.; Omar, D.; Abdul Rahman, M.B.; Salleh, A.B.; Raja Abdul Rahman, R.N.Z. Green Nanoemulsion-Laden Glyphosate Isopropylamine Formulation in Suppressing Creeping Foxglove (A. gangetica), Slender Button Weed (D. ocimifolia) and Buffalo Grass (P. conjugatum). Pest Manag. Sci. 2013, 69, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Borrin, T.R. Nanoemulsões Produzidas Pelo Método Do Ponto de Inversão Da Emulsão (EIP) Para Encapsulação de Curcumina: Parâmetros de Produção, Estabilidade Físico-Química e Incorporação Em Sorvete. Doctoral Dissertation, Universidade de São Paulo, Pirassununga, Brazil, 2015. [Google Scholar]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A.T. Anti-Acetylcholinesterase Activity of Essential Oils and Their Major Constituents from Four Ocimum Species. Zeitschrift fur Naturforsch.—Sect. C J. Biosci. 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Teixeira, M.G.; Alvarenga, E.S.; Lopes, D.T.; Oliveira, D.F. Herbicidal Activity of Isobenzofuranones and in Silico Identification of Their Enzyme Target. Pest Manag. Sci. 2019, 75, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.R.L.; Melo, M.A.; Cardoso, A.V.; Santos, R.L.C.; de Sousa, D.P.; Cavalcanti, S.C.H. Structure-Activity Relationships of Larvicidal Monoterpenes and Derivatives against Aedes Aegypti Linn. Chemosphere 2011, 84, 150–153. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.B.M.; Barbosa, D.B.; do Bomfim, M.R.; Amparo, J.A.O.; Andrade, B.S.; Costa, S.L.; Campos, J.M.; Cruz, J.N.; Santos, C.B.R.; Leite, F.H.A.; et al. Identification of a Novel Dual Inhibitor of Acetylcholinesterase and Butyrylcholinesterase: In Vitro and In Silico Studies. Pharmaceuticals 2023, 16, 95. [Google Scholar] [CrossRef]
- Silva, L.B.; Ferreira, E.F.B.; Maryam; Espejo-Román, J.M.; Costa, G.V.; Cruz, J.V.; Kimani, N.M.; Costa, J.S.; Bittencourt, J.A.H.M.; Cruz, J.N.; et al. Galantamine Based Novel Acetylcholinesterase Enzyme Inhibitors: A Molecular Modeling Design Approach. Molecules 2023, 28, 1035. [Google Scholar] [CrossRef]
- Carmo Bastos, M.L.; Silva-Silva, J.V.; Neves Cruz, J.; Palheta da Silva, A.R.; Bentaberry-Rosa, A.A.; da Costa Ramos, G.; de Sousa Siqueira, J.E.; Coelho-Ferreira, M.R.; Percário, S.; Santana Barbosa Marinho, P.; et al. Alkaloid from Geissospermum Sericeum Benth. & Hook.f. Ex Miers (Apocynaceae) Induce Apoptosis by Caspase Pathway in Human Gastric Cancer Cells. Pharmaceuticals 2023, 16, 765. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.F.; de Souza, J.L.; da Costa, D.M.; Costa, D.B.; Moreira, P.O.L.; da Fonseca, A.L.; Varotti, F.d.P.; Cruz, J.N.; dos Santos, C.B.R.; Alves, C.Q.; et al. Antiplasmodial Activity of Coumarins Isolated from Polygala Boliviensis: In Vitro and in Silico Studies. J. Biomol. Struct. Dyn. 2023, 41, 13383–13403. [Google Scholar] [CrossRef]
- Harel, M.; Kryger, G.; Rosenberry, T.L.; Mallender, W.D.; Lewis, T.; Fletcher, R.J.; Guss, J.M.; Silman, I.; Sussman, J.L. Three-dimensional Structures of Drosophila Melanogaster Acetylcholinesterase and of Its Complexes with Two Potent Inhibitors. Protein Sci. 2000, 9, 1063–1072. [Google Scholar] [CrossRef]
- Kua, J.; Zhang, Y.; McCammon, J.A. Studying Enzyme Binding Specificity in Acetylcholinesterase Using a Combined Molecular Dynamics and Multiple Docking Approach. J. Am. Chem. Soc. 2002, 124, 8260–8267. [Google Scholar] [CrossRef]
- Atanasova, M.; Stavrakov, G.; Philipova, I.; Zheleva, D.; Yordanov, N.; Doytchinova, I. Galantamine Derivatives with Indole Moiety: Docking, Design, Synthesis and Acetylcholinesterase Inhibitory Activity. Bioorganic Med. Chem. 2015, 23, 5382–5389. [Google Scholar] [CrossRef]
- Wan, Y.; Guan, S.; Qian, M.; Huang, H.; Han, F.; Wang, S.; Zhang, H. Structural Basis of Fullerene Derivatives as Novel Potent Inhibitors of Protein Acetylcholinesterase without Catalytic Active Site Interaction: Insight into the Inhibitory Mechanism through Molecular Modeling Studies. J. Biomol. Struct. Dyn. 2020, 38, 410–425. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Repellency effect of forty-one essential oils against Aedes, Anopheles, and Culex mosquitoes. Parasitol. Res. 2006, 99, 478–490. [Google Scholar] [CrossRef]
- Sinthusiri, J.; Soonwera, M. Oviposition deterrent and ovicidal activities of seven herbal essential oils against female adults of housefly, Musca domestica L. Parasitol. Res. 2014, 113, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Chokechaijaroenporn, O.; Bunyapraphatsara, N.; Kongchuensin, S. Mosquito repellent activities of ocimum volatile oils. Phytomedicine 1994, 1, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Murugan, P.; Noortheen, A. Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector, Aedes aegypti (Insecta:Diptera:Culicidae). Bioresour. Technol. 2007, 98, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Anees, A.M. Larvicidal activity of Ocimum sanctum Linn. (Labiatae) against Aedes aegypti (L.) and Culex quinquefasciatus (Say). Parasitol. Res. 2008, 103, 1451–1453. [Google Scholar] [CrossRef]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef]
- Tawatsin, A.; Thavara, U. Dengue haemorrhagic fever in Thailand: Current incidence and vector management. In Vector Biology, Ecology and Control; Springer: Berlin/Heidelberg, Germany, 2010; pp. 113–125. [Google Scholar] [CrossRef]


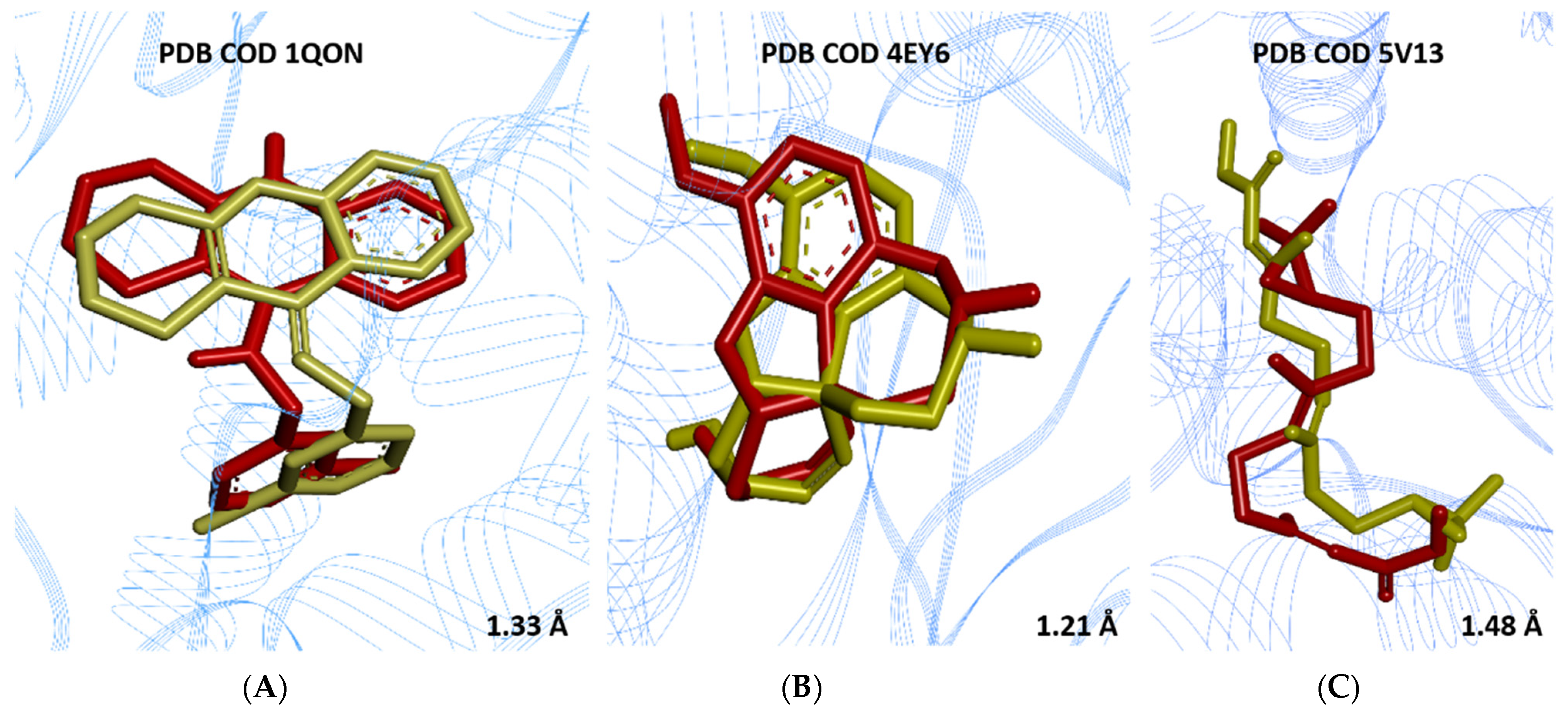
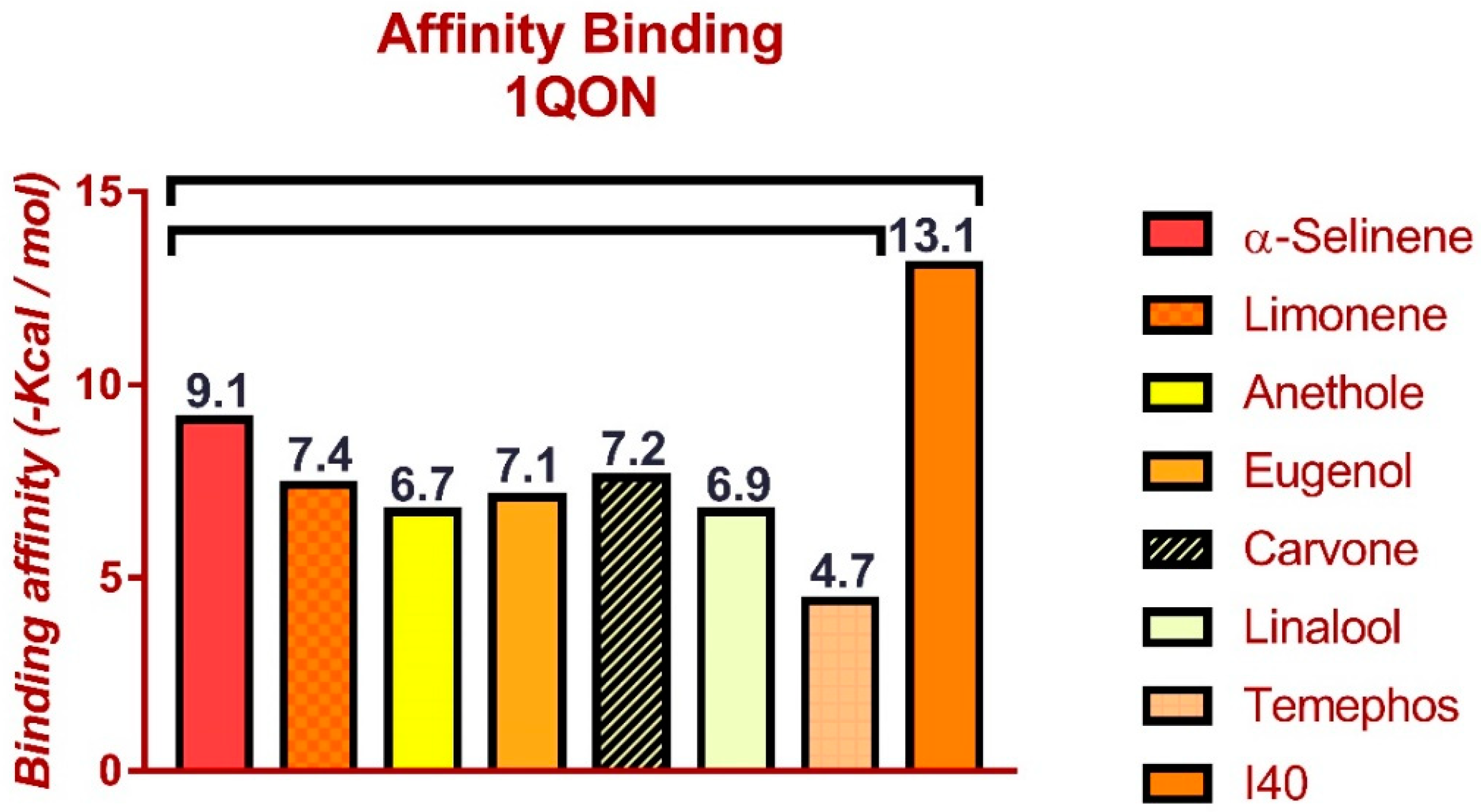
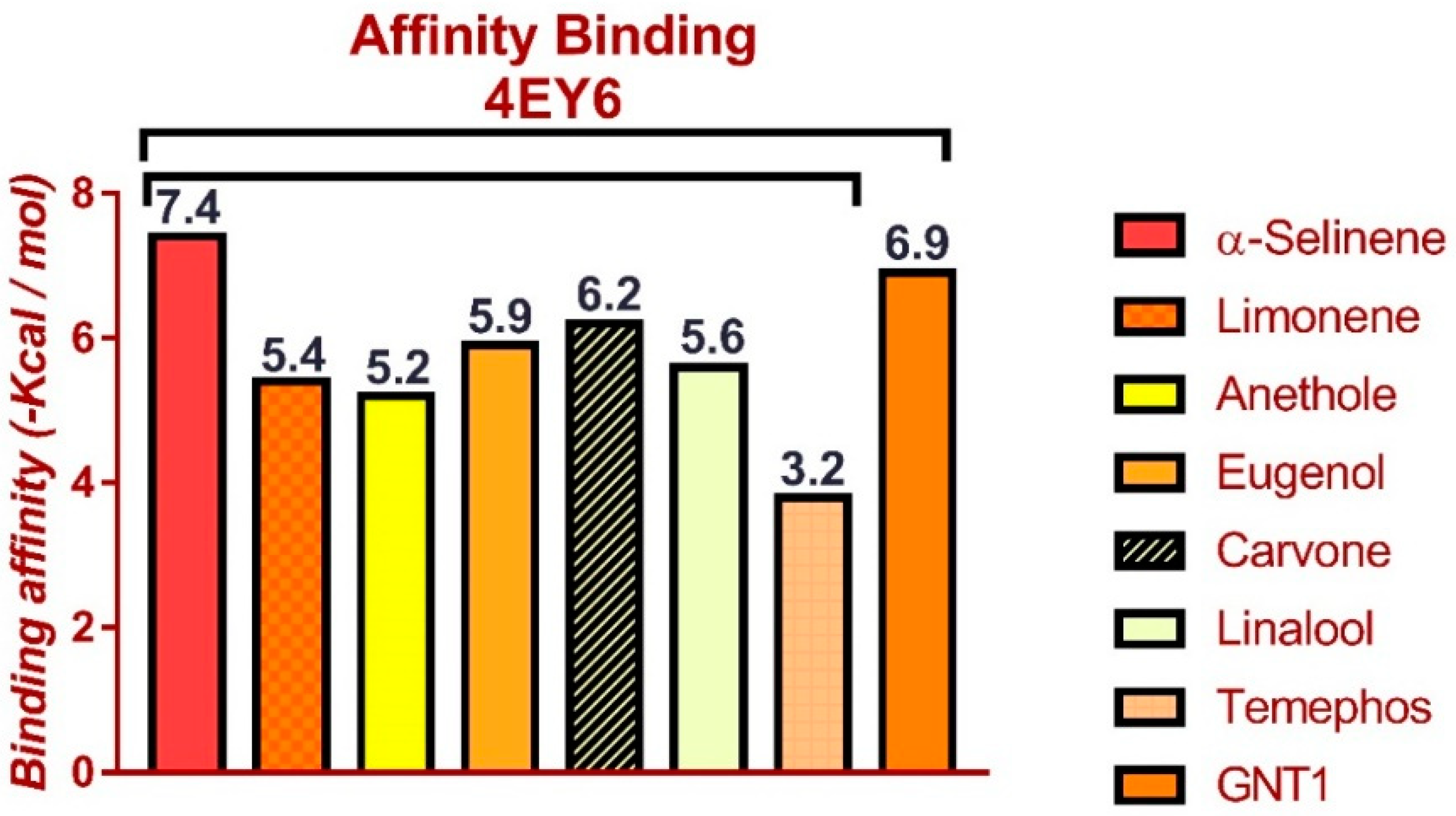
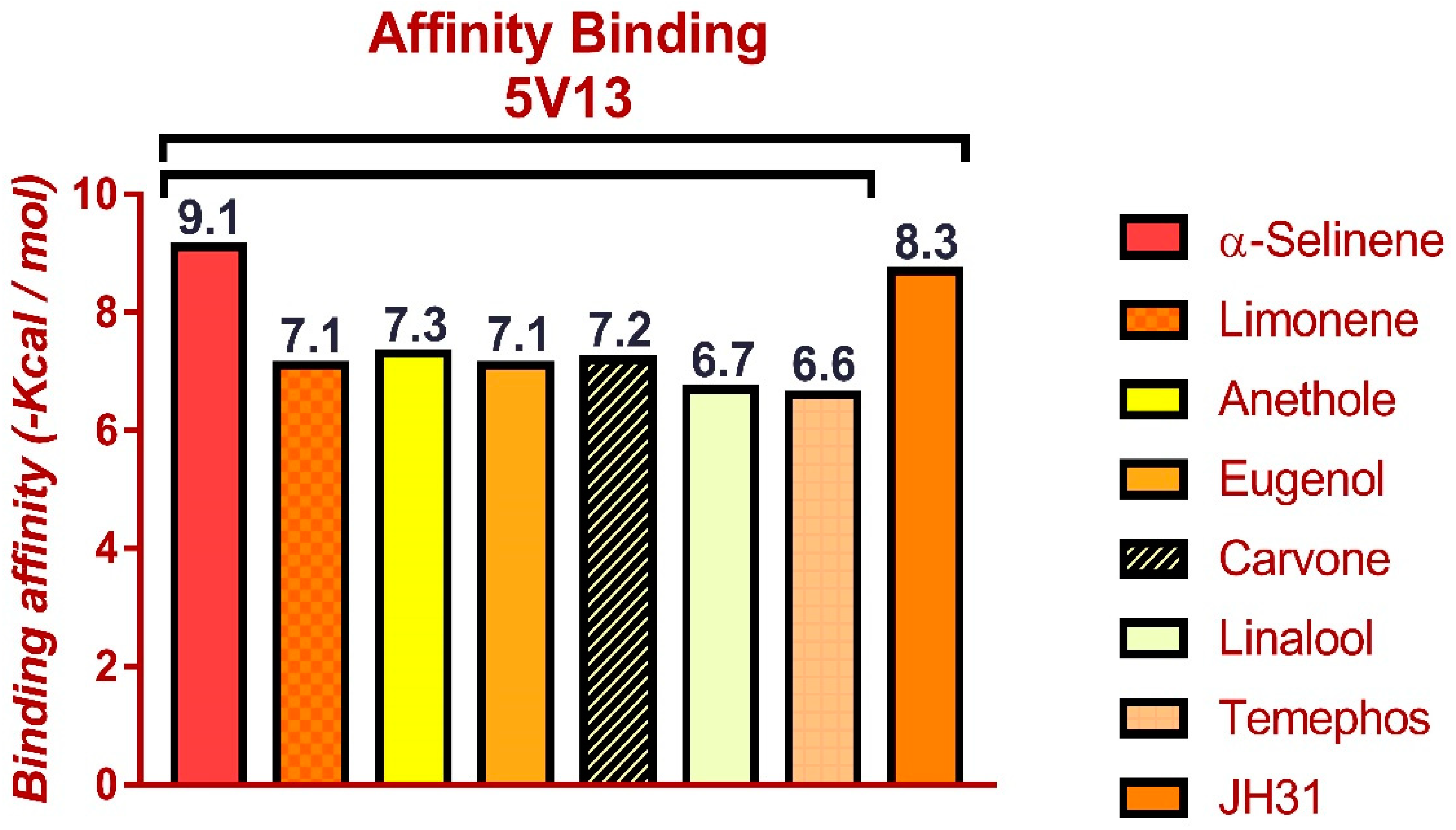
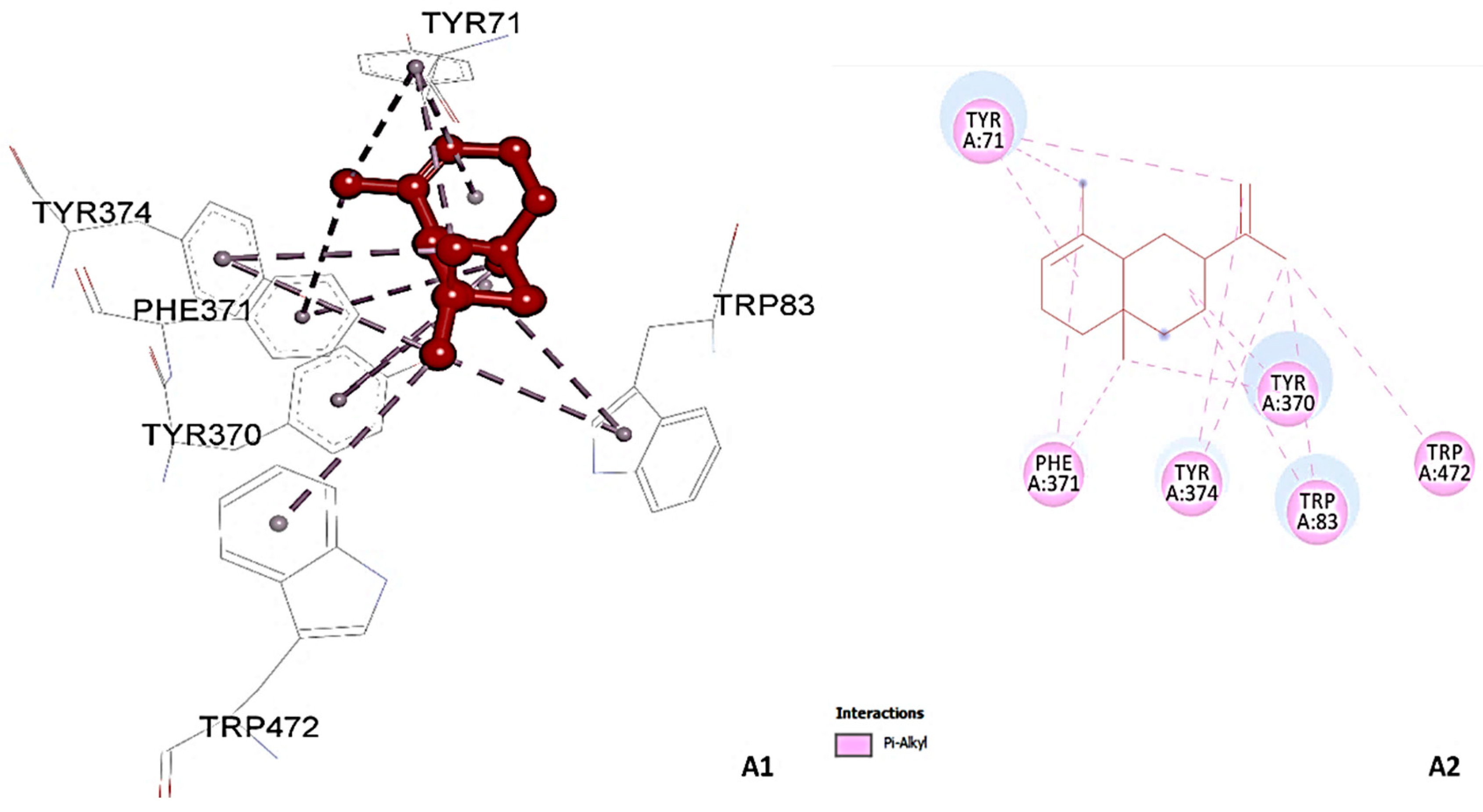

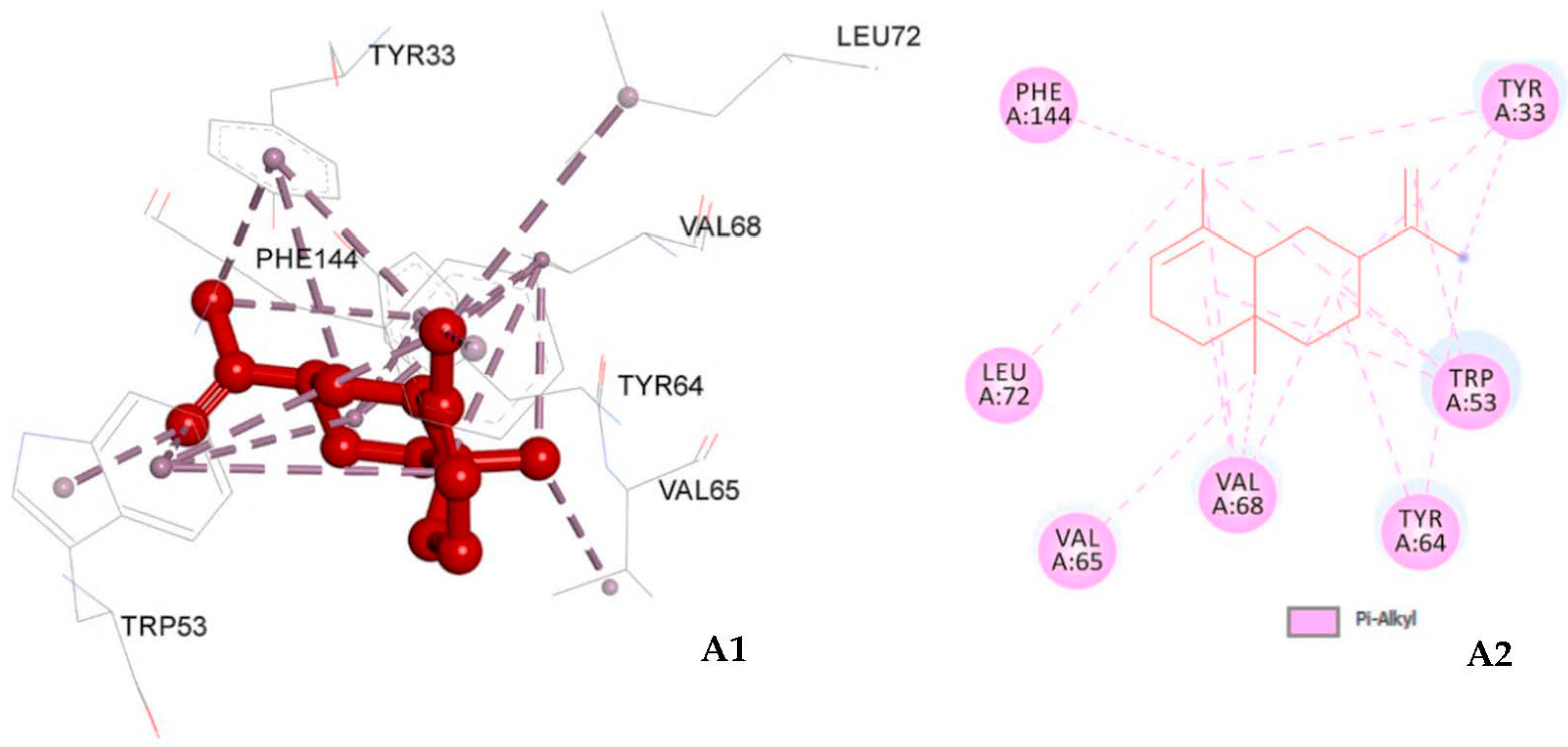
| Receptor | Ligand | Ligand Coordinates of the Grid Center | Grid Size (Points) |
|---|---|---|---|
| AChE (PDB ID 1QON) | 9-(3-Iodobenzylamino)-1,2,3,4- tetrahydroacridine | X = 33.0302 Y = 67.5629 Z = 09.9828 | 25 x 23 y 12 z |
| AChE (PDB ID 4EY6) | (–)-Galantamine | X = 09.0708 Y = 60.8558 Z = 23.9190 | 29 x 18 y 12 z |
| Juvenile hormone (PDB ID 5V13) | Methyl(2E,6E)-9-[(2R)-3,3-dimethyloxiran-2-yl]- 3,7-dimethylnona-2,6-dienoate | X = 251.9827 Y = 09.5063 Z = 353.313 | 34 x 25 y 19 z |
| RIa | Constituents | Concentration (%) |
|---|---|---|
| 831 | 3-hexanone | 0.09 |
| 833 | 2-hexanone | 0.12 |
| 835 | 3-hexanol | 0.05 |
| 837 | 2-hexanol | 0.07 |
| 867 | Trans-2-hexenal | 0.21 |
| 868 | Cis-4-hexenol | 0.21 |
| 925 | α-thujene | 0.19 |
| 932 | α-pinene | 0.61 |
| 947 | Camphene | 0.04 |
| 972 | Sabinene | 0.26 |
| 976 | β-pinene | 0.77 |
| 988 | Myrcene | 0.54 |
| 1004 | 4-carene | 0.05 |
| 1015 | α-terpinene | 0.20 |
| 1023 | p-cymene | 0.24 |
| 1027 | Limonene | 6.65 |
| 1030 | Eucalyptol | 8.64 |
| 1034 | Trans-β-ocimene | 0.10 |
| 1044 | β-ocimene | 0.66 |
| 1056 | γ-terpinene | 0.48 |
| 1065 | Sabinene hydrate | 0.07 |
| 1087 | Terpinolene | 0.20 |
| 1099 | Linalool | 32.66 |
| 1144 | Camphor | 0.61 |
| 1166 | δ-terpinolene | 0.20 |
| 1177 | L-4-terpineol | 3.38 |
| 1190 | α-terpineol | 1.02 |
| 1199 | Anethole | 32.48 |
| 1210 | Acetic acid | 0.06 |
| 1219 | Trans-carveol | 0.05 |
| 1230 | Cis-carveol | 0.05 |
| 1243 | Carvotanacetone | 3.41 |
| 1286 | Bornyl acetate | 0.03 |
| 1337 | Carveol acetate | 0.04 |
| 1358 | Eugenol | 0.13 |
| 1385 | β-bourbonene | 0.05 |
| 1391 | β-elemene | 0.40 |
| 1420 | Trans-caryophyllene | 0.64 |
| 1435 | Cis-α-bergamotene | 0.06 |
| 1439 | α-guaiene | 0.08 |
| 1455 | Trans-β-bergamotene | 0.89 |
| 1482 | β-cubebene | 0.23 |
| 1487 | β-selinene | 0.78 |
| 1496 | α-selinene | 0.82 |
| 1506 | δ-guaiene | 0.28 |
| 1515 | γ-cadinene | 0.16 |
| 1562 | Trans-nerolidol | 0.07 |
| 1615 | Epicubeol | 0.10 |
| 1641 | δ-cadinol | 0.73 |
| 1651 | β-eudesmol | 0.10 |
| Monoterpene hydrocarbons | 11.26 | |
| Oxygenated monoterpenes | 49.89 | |
| Sesquiterpene hydrocarbons | 4.39 | |
| Oxygenated sesquiterpenes | 1.00 | |
| Phenylpropanoids | 32.61 | |
| Others | 0.81 | |
| Total | 99.96 | |
| Days | Size (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|
| D0 | 278.3 ± 1.102 | 0.231 ± 0.021 | −18.6 ± 0.265 |
| D1 | 244.6 ± 3.102 | 0.152 ± 0.022 | −17.1 ± 0.0577 |
| D14 | 280.4 ± 2.957 | 0.209 ± 0.015 | −15.7 ± 0.289 |
| Concentration (mg/L) | Aedes aegypti | Culex quinquefasciatus | ||
|---|---|---|---|---|
| Mortality (%) | ||||
| 24 h | 48 h | 24 h | 48 h | |
| 10 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 4 ± 0.548 a |
| 20 | 2 ± 0.447 a | 2 ± 0.447 a | 0 ± 0 a | 4 ± 0.58 a |
| 30 | 4 ± 0.548 a | 4 ± 0.548 a | 26 ± 0.548 b | 30 ± 10.00 b |
| 40 | 20 ± 10.00 b | 30 ± 12.25 b | 44 ± 15.17 c | 44 ± 15.17 b |
| 50 | 98 ± 4.47 c | 100 ± 0 c | 86 ± 11.40 d | 90 ± 10.00 c |
| C1 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| C2 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Positive Control * | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a |
| Positive control ** | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a | 100 ± 0.00 a |
| Species | Time (Hours) | LC50 (mg/L) | LC90 (mg/L) | χ2 | p Value |
|---|---|---|---|---|---|
| Aedes aegypti | 24 | 42.15 | 50.35 | 34.80 | <0.0001 |
| (37.79–46.87) * | (45.93–62.81) * | ||||
| 48 | 40.94 | 48.87 | 37.45 | <0.0001 | |
| (36.64–45.42) * | (44.63–60.28) * | ||||
| Culex quinquefasciatus | 24 | 39.64 | 52.58 | 27.48 | <0.0001 |
| (34.52–46.00) * | (46.16–69.15) * | ||||
| 48 | 38.08 | 54.26 | 23.54 | <0.0001 | |
| (32.36–45.55) * | (46.47–74.23) * |
| Compounds | Acetylcholine Neuromuscular Blocking Agent | Acetylesterase Inhibitor | Insecticide | Juvenile-Hormone Esterase Inhibitor | ||||
|---|---|---|---|---|---|---|---|---|
| Pa [a] | Pi [b] | Pa [a] | Pi [b] | Pa [a] | Pi [b] | Pa [a] | Pi [b] | |
| Temephos | 0.260 | 0.255 | 0.509 | 0.031 | 0.780 | 0.002 | 0.038 | 0.005 |
| Linalool | 0.281 | 0.232 | 0.290 | 0.103 | 0.309 | 0.015 | 0.041 | 0.005 |
| Anethole | 0.594 | 0.025 | 0.446 | 0.041 | 0.416 | 0.005 | 0.043 | 0.005 |
| Carvone | 0.729 | 0.004 | 0.393 | 0.054 | 0.471 | 0.004 | - | - |
| α-Selinene | 0.741 | 0.004 | 0.274 | 0.117 | 0.475 | 0.004 | - | - |
| Eugenol | 0.630 | 0.014 | 0.229 | 0.167 | 0.444 | 0.005 | - | - |
| Limonene | 0.743 | 0.004 | 0.497 | 0.033 | 0.696 | 0.002 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa dos Santos, E.L.V.; Cruz, J.N.; da Costa, G.V.; de Sá, E.M.F.; da Silva, A.K.P.; Fernandes, C.P.; de Faria Mota Oliveira, A.E.M.; Duarte, J.L.; Bezerra, R.M.; Tavares, J.F.; et al. Essential Oil of Ocimum basilicum against Aedes aegypti and Culex quinquefasciatus: Larvicidal Activity of a Nanoemulsion and In Silico Study. Separations 2024, 11, 97. https://doi.org/10.3390/separations11040097
de Sousa dos Santos ELV, Cruz JN, da Costa GV, de Sá EMF, da Silva AKP, Fernandes CP, de Faria Mota Oliveira AEM, Duarte JL, Bezerra RM, Tavares JF, et al. Essential Oil of Ocimum basilicum against Aedes aegypti and Culex quinquefasciatus: Larvicidal Activity of a Nanoemulsion and In Silico Study. Separations. 2024; 11(4):97. https://doi.org/10.3390/separations11040097
Chicago/Turabian Stylede Sousa dos Santos, Edla Lídia Vasques, Jorddy Neves Cruz, Glauber Vilhena da Costa, Ester Martins Félix de Sá, Alicia Karine Pereira da Silva, Caio Pinho Fernandes, Anna Eliza Maciel de Faria Mota Oliveira, Jonatas Lobato Duarte, Roberto Messias Bezerra, Josean Fechine Tavares, and et al. 2024. "Essential Oil of Ocimum basilicum against Aedes aegypti and Culex quinquefasciatus: Larvicidal Activity of a Nanoemulsion and In Silico Study" Separations 11, no. 4: 97. https://doi.org/10.3390/separations11040097









