Abstract
In the present review, we discuss the occurrence of ellagitannins (ETs) and ellagic acid (EA) and methods for their isolation from plant materials. We summarize analytical methods, including high-performance liquid chromatography–ultraviolet (HPLC–UV) and liquid chromatography–mass spectrometry (LC–MS), for the determination of ETs, EA and their bioactive metabolites urolithins (Uros) in samples of plant and food origin, as well as in biological samples, such as plasma, urine and feces. In addition, the current interest in the bioactivities of Uros is discussed in brief.
1. Introduction
Ellagitannins (ETs) are a group of tannins widely spread in plants, in particular in fruits, including pomegranate, cloudberries and strawberries [1,2,3]. They are complex molecules characterized by structural diversity. When foods rich in ETs are consumed, ETs are first hydrolyzed into ellagic acid (EA) in the stomach and small intestine [4]. Then, EA may be converted into urolithins (Uros) by the action of the intestinal flora [4,5]. The consumption of foods rich in ETs and EA has been associated with beneficial effects on human health. As a consequence, a plethora of studies have been performed to shed light on the bioactivities of ETs, EA and Uros [6,7,8,9,10]. Recently, an increasing interest has been focused on the bioactivities of Uros and clinical trials have led Uro supplements to enter into the market [11].
Due to the diverse biological effects of ETs, EA and Uros, there is a high interest in the isolation of such components from various natural sources, their bio-transformations in the human organism, the study of their bioactivities and analytical methods for their detection and determination. The aim of the present review is to summarize the natural sources of ETs and EA, discussing methods for the extraction and isolation of ETs and EA from various sources. Furthermore, a detailed summary of the analytical methods developed for the determination of ETs, EA and Uros in plants, foods and biological samples is presented.
2. Ellagitannins and Ellagic Acid
2.1. Natural Sources and Occurrence of ETs and EA
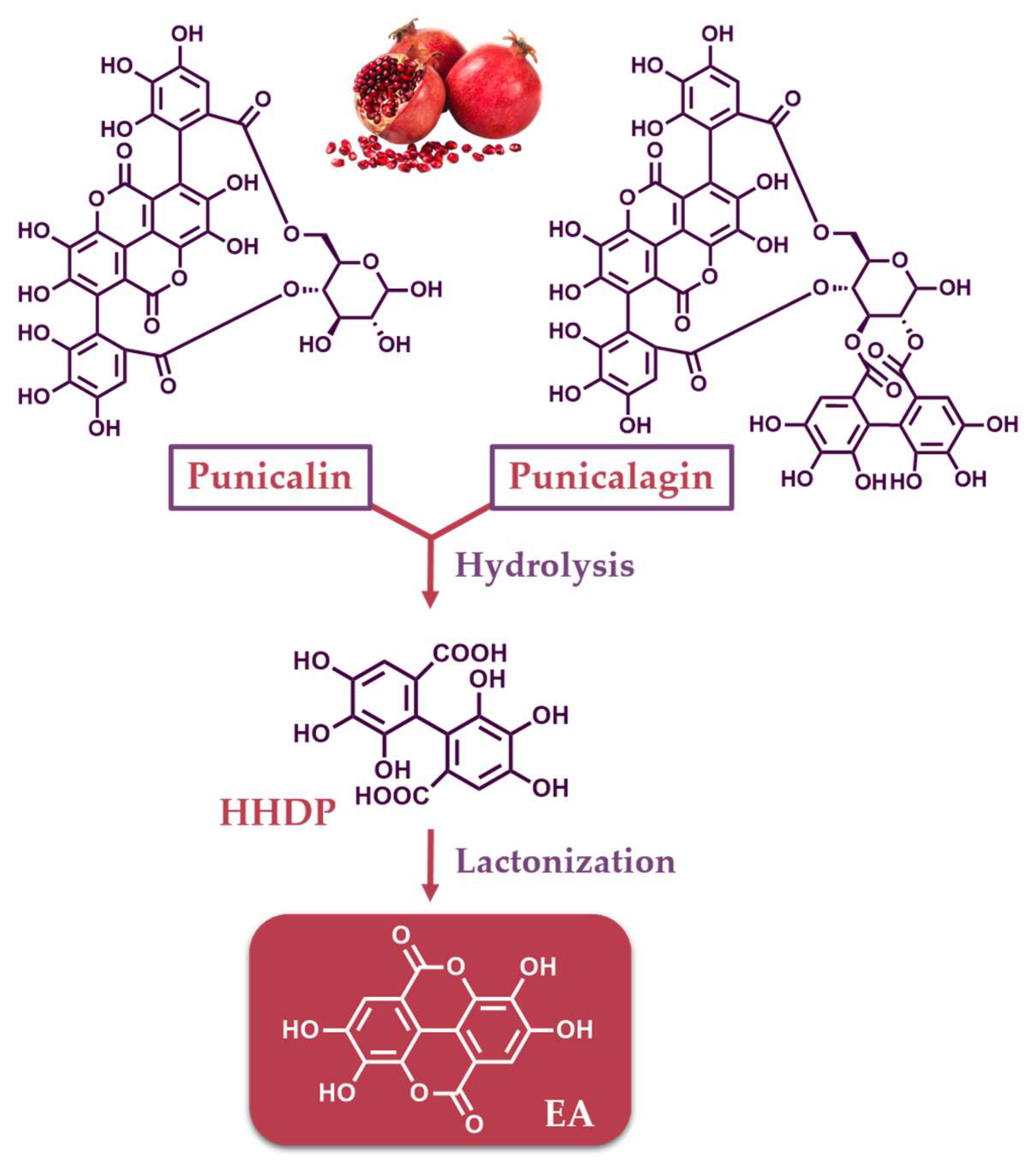
ETs are naturally occurring compounds of medicinal and biological interest found in dicotyledonous and angiosperms plants [12]. They belong to the hydrolyzable tannins and possess a complex chemical structure, which renders them susceptible to various chemical reactions, including transformation, isomerization, and oligomerization [2]. ETs are esters of gallic acid and are typically constructed by hexahydroxydiphenic acid units (HHDP), connected usually to a D-glucose moiety. However, fructose, xylose, galactose and quinic acid have also been reported to connect to HHDP [1,13,14]. The HHDP units may be linked to more than one sugar moiety; therefore, monomeric, dimeric, oligomeric and polymeric ETs are formed [15]. The exposure of ETs to an acidic or basic environment result in the formation of EA via hydrolysis/lactonization reactions. Representative ETs are punicalin and punicalagin, which are abundant in pomegranate, and their structures are depicted in Figure 1. Upon their hydrolysis, followed by dehydration/lactonization, EA is formed (Figure 1).
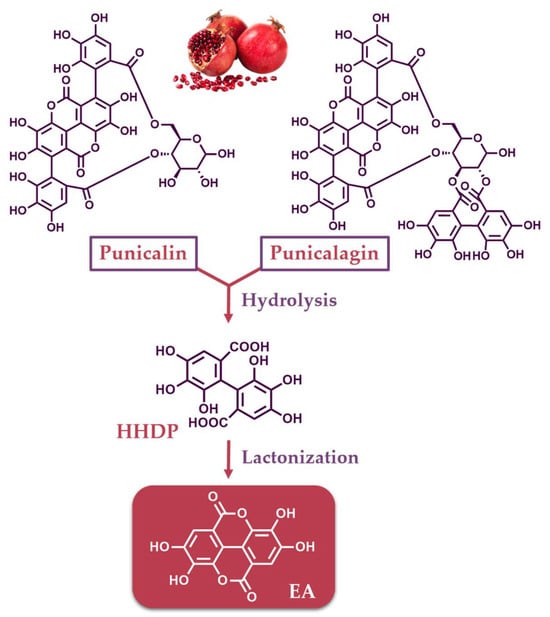
Figure 1.
Structures of ETs found in pomegranate (punicalin and punicalagin) and their conversion to EA.
EA, first described two centuries ago [16], is a chromene-dione derivative, which can be considered as a dimeric derivative of gallic acid. It is named 2,3,7,8-tetrahydroxy-chromeno [5,4,3-cde]chromene-5,10-dione according to IUPAC nomenclature, However, it is also known as 4,4′,5,5′,6,6′-hexahydroxydiphenic acid 2,6,2′,6′-dilactone (Figure 1). It consists of a pair of lactone rings as a part of a tetracyclic core, carrying four free-hydroxyl phenolic groups, therefore exhibiting an amphiphilic character. Due to the lactone functionalities and the phenolic groups, it may act as a hydrogen bond acceptor or donor, respectively.
More than 1000 ETs have been detected to occur in many plants; nevertheless, few of them are fit for human consumption. Fruits including strawberries, berries, pomegranate, and nuts and seeds are rich in edible ETs, within which raspberries and cloudberries are the most abundant sources, as are processed foods, including fruit-derived beverages, jams and cakes (Table 1) [1,3,17]. In general, fruits of the genus Rubus are the most studied for their content in ETs and EA. Among them, raspberries of different species, such as R. idaeus [18,19,20,21], R. occidentalis, and R. ursinusxidaeus [21], have been investigated showing an ETs/EA content ranging from 16 to 17.92 mg/g. Similarly, strawberries from different varieties, for example, Honeoye, Jonsok, Polka [22], and Senga saengana [23], showed a diversity in their content of ETs/EA that ranges from 0.12 to 5.64 mg/g. Impressive is the content in ETs/EA of Artic bramble, a perennial plant whose fruits can also been used for the preparation of jam, liqueur, and tea. According to Määttä-Riihinen et al., the concentration of ETs/EA reaches 24.91 mg/g [22]. In addition, other kinds of fruits native to tropical countries have been found to contain significant amounts of ETs and EA. Myrciaria jaboticaba (Myrtaceae) and Myrciaria dubia (Myrtaceae), commonly known as camu-camu, have been reported as rich sources of EA and ETs [24,25]. Another source of ETs and EA is wine. Oak wood releases tannins which are transferred to wine during its storage for maturation purposes in oak barrels [26,27].
The use of herbs containing ETs dates back to traditional medicine. Some examples are Geranium thunbergia (Geraniaceae) and Mallotus japonicus (Euphorbiaceae). Both plants produce geranin and have been widely used in Japan for treatment against diarrhea and to treat stomach ulcers, respectively [28]. Furthermore, the fruits of Terminalia chebula (Combretaceae) that contain chebulinic acid, chebulagic acid and terchebin, have been traditionally used in Indian and Iranian folk medicine to combat chronic diseases, such as dementia, diabetes, etc. [29].
Given the importance of ETs for their health beneficial properties, as has been documented by traditional medicine, some authors examined their presence in the non-edible parts of fruits. In a recent study, Torgbo et al. found that the peel of Nephelium lappaceum L., (Sapindaceae), a tropical fruit of Southeast Asia, contains in major quantities, with respect to other compounds, ETs such as geraniin and corilagin and their metabolites, including gallic acid and EA [30]. Similarly, de Vasconcelos et al. showed that ETs and EA are abundant in the pericarp and the integument of chestnut fruit [31]. In addition, Kaneshima et al. reported the presence of ETs, such as grandinin, vescalagin, castalagin and others, in camu-camu seeds and peels [32]. Therefore, obviously, the rational use of these by-products may lead the pharmaceutical, nutraceutical and cosmetic industries to the development of valuable products for human health. Furthermore, many scientific papers discuss various plant species for their significant amount in ETs and EA [1,2,3,17]. Plant families, such as Myrtaceae, Rosaceae, Asteraceae and Fagaceae, are reported to include several plant genera, which produce ETs and EA [33,34].
Apparently, a diversity between the concentration of these secondary metabolites is evident between the various fruits, foods, and the plant species, as presented in Table 1. The presence of many raspberries and other fruit varieties as well as the species diversity and the genetic diversity within the species contribute to these differences. However, there are also other factors to consider, including the handling of the sample for specific experimental purposes, climate variations and the developmental stage of the material under study.
2.2. Bioavailability of ETs and EA
Generally, data on the recommended daily intake of ETs and EA are not yet available. Though toxicity studies evaluate ETs and EA as safe, their pharmacokinetic behavior is still obscure. Therefore, caution should be taken by consumers, although the no-observed-effect level (NOEL) of EA in in vivo studies involving rats has been established at 3.254 mg/kg/day [35].
Although ETs are found in many natural products, their low bioavailability limits their use. ETs are generally large molecules, among which the molecular weight of Lambertianin D reaches 3740 Da [36]. The presence of the HHDP moiety(ies) in their structure, formed by C-C bond connection between adjacent galloyl residues, in addition to their polar nature, renders them components with low bioavailability. As mentioned above, most ETs are subjected to acidic or basic hydrolysis at the gastrointestinal tract to form EA through the hydrolysis of the ester bonds of ETs, enhanced by the enzyme ellagitannin acyl hydrolase [37]. However, ETs resistant to hydrolysis end intact to the large intestine [38]. Similarly to ETs, EA has also a low bioavailability both because of its poor water solubility and its capacity to bind irreversibly to cellular DNA and serum proteins, which results in complexes of large molecular weight that do not penetrate cell membranes [39,40]. However, in vivo under physiological conditions, EA is metabolized in the gut microbiota to Uros.

Table 1.
Ellagitannins and ellagic acid content.
Table 1.
Ellagitannins and ellagic acid content.
| Source | Family/Genus | Content | Solvent System | Extraction Method | Reference |
|---|---|---|---|---|---|
| Fruits | |||||
| Cloudberries | Rosaceae/Rubus | 3.15 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] |
| 18.25 mg/g a | Ethyl acetate/Methanol | Vortex | [22] | ||
| 10.90–16.30 mg/g a | 70% aqueous acetone | Solid–liquid extraction | [41] | ||
| Raspberries | Rosaceae/Rubus | 2.63–3.30 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] |
| 1.55 mg/g a | 70% aqueous acetone | Solid–liquid extraction | [42] | ||
| 0.16–3.26 mg/g a | - | - | [13] | ||
| 16.92 mg/g a | 60% acetone (acidified) | Sonication (5 min) | [19] | ||
| 1.5 mg/g b | Methanol | Soxhlet | [43] | ||
| 10.65–13.84 mg/g a | Ethyl acetate/Methanol | Vortex | [22] | ||
| 7.67–9.31 mg/g a | Methanol (acidified) | - | [44] | ||
| 1.35–5.47 mg/g a | Methanol (acidified) | Magnetic stirring (1 h) | [20] | ||
| 16.92–17.54 mg/g a | 70% aqueous acetone | Solid–liquid extraction | [41] | ||
| 47–90 mg/g b | Methanol | Solid–liquid extraction (24 h) | [21] | ||
| 1.6 mg/g b | Reflux (20 h) | ||||
| 0.71 mg/g b | Aqueous methanol (1:2) (acidified) | Reflux (2 h) | [45] | ||
| 8.58–17.92 mg/g a | Aqueous methanol(acidified) | Vortex + sonication (5 min) | [23] | ||
| 70% aqueous acetone (acidified) | [46] | ||||
| Rose Hip | Rosaceae/Rosa | 1.09 a | Aqueous methanol (acidified) | Reflux (20 h) | [18] |
| Strawberries | Rosaceae/Fragaria | 0.68–0.85 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] |
| 0.71–0.83 mg/g a | - | - | [13] | ||
| 0.63 mg/g b | Methanol | Soxhlet | [43] | ||
| 0.64 mg/g a | Methanol | Sonication (10 min) | [47] | ||
| 4.51–5.64 mg/g a | Ethyl acetate | Vigorous mixing | [22] | ||
| 0.13–0.32 mg/g a | Methanol | Agitation (30 min) | [48] | ||
| 0.12–1.29 mg/g a | 70% aqueous acetone | Solid–liquid extraction | [49] | ||
| 0.31 mg/g a | Aqueous methanol (1:2) (acidified) | Reflux (20 h) | [45] | ||
| 0.40 mg/g a | Aqueous methanol (acidified) | Reflux (2 h) | [23] | ||
| 0.81–1.84 mg/g a | 70% aqueous acetone | Solid–liquid extraction | [41] | ||
| 0.29 mg/g a | 70% aqueous acetone | Sonication (10 min) | [50] | ||
| 0.77 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] | ||
| 0.75–0.79 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] | ||
| Blackberry | Rosaceae/Rubus | 3.43 mg/g a | 70% aqueous acetone | Solid–liquid extraction | [42] |
| 1.5–2.7 mg/g a | - | - | [13] | ||
| 0.63 mg/g a | 80% aqueous methanol (acidified) | Sonication (10 min) | [51] | ||
| Arctic Bramble | Rosaceae/Rubus | 24.91 mg/g a | Ethyl acetate | Vigorous mixing | [22] |
| Caneberries | Rosaceae/Rubus | 8.7–32.2 mg/g b | Methanol | Solid–liquid extraction (24 h) | [52] |
| Rose Hip | Rosaceae/Rosa | 1.09 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] |
| Boysenberry | Rubeae/Rubus | 1.68 mg/g a | Methanol | Sonication (10 min) | [47] |
| Cranberries | Ericaceae/Vaccinium | 0.12 mg/g b | Methanol | Soxhlet | [43] |
| Pomegranate | Lythraceae/Punica | 0.58–1.77 mg/g a | - | - | [13] |
| 40.59 mg/g (mesocarp) a | 80% aqueous methanol (acidified) | Stirring | [53] | ||
| 43.98 mg/g (peel) a | |||||
| 1.25 mg/g a | Deionized water | Pressurized water extraction | [54] | ||
| 46.63 mg/g (fruit) a | Ethyl acetate | - | [55] | ||
| 81.23 mg/g (peel) a | Solid–liquid extraction | ||||
| 5.21–26.25 mmol/L a | Water | [56] | |||
| Guava | Myrtaceae/Psidium | 0.20–0.25 mg/g a | - | - | [13] |
| Sea Buckthorn | Elaeagnaceae/Hippophae | 0.01 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] |
| Kakadu Plum Fruit | Combretaceae/Terminalia | 8.80 mg/g a | Methanol | Sonication (10 min) | [47] |
| 10.69 mg/g b | Methanol | Sonication (10 min) | [57] | ||
| Grapes | Vitaceae/Vitis | 0.43 mg/g (seeds) b | Methanol: water (4:1) acidified | Sonication (1 h) | [58] |
| 0.46–0.49 mg/g (skin) a | |||||
| 0.16–0.22 mg/g a | |||||
| Longan Seed | Sapindaceae/Dimocarpus | 1.56 mg/g b | 50% aqueous ethanol | Water bath (1 h) | [59] |
| Mango Kernel | Anacardiaceae/Mangifera | 0.031–1.18 mg/g b | 50% aqueous ethanol | Water bath (1 h) | [59] |
| 0.34–0.74 mg/g b | 50% aqueous methanol | Water bath (1 h) | |||
| Pecan Kernels | Juglandaceae/Carya | 20.96–86.21 mg/g b | 80% aqueous methanol | Solid–liquid extraction | [60] |
| 0.33 mg/g b | Methanol | Soxhlet | [43] | ||
| Nuts | Fagaceae/Castanea | 1.61–24.9 mg/kg (raw) b | 70% aqueous methanol | Vortex mixer (30 min) | [61] |
| 4.30–22.1 mg/kg (boiled) b | |||||
| 4.31–21.1 mg/kg (roasted) b | |||||
| 1.49 mg/g a | |||||
| 8.23 mg/g a | 70% aqueous acetone | Ice bath | [62] | ||
| 0.36–0.59 mg/g a | - | - | [13] | ||
| 0.59 mg/g b | Methanol | Soxhlet | [43] | ||
| 0.40 mg/g a | Aqueous acetone (acidified) | Soxhlet | [63] | ||
| Pecan | Juglandaceae/Carya | 3.01 mg/g a | 70% aqueous acetone | Ice bath | [62] |
| 0.11–0.33 mg/g a | - | - | [13] | ||
| 0.70 mg/g a | Water | Reflux | [64] | ||
| 0.22 mg/g a | Aqueous acetone (acidified) | Soxhlet | [63] | ||
| Medicinal and aromatic plants | |||||
| Psidium friedrichsthalianum-Nied | Myrtaceae/Psidium | 2.43 mg/g (peel) a | Methanol: water (9:1) acidified | Sonication | [65] |
| 3.06 mg/g (flesh) a | |||||
| (Psidium guajava L.) | Myrtaceae/Psidium | 5.72–30.6 mg/100 g b | Methanol | Shaking (30 min) | [66] |
| Myrciaria jaboticaba (Vell.) Berg | Myrtaceae/Plinia | 9.1 mg/g a | 70% aqueous methanol | Εxtraction (2 h) | [67] |
| Myrciaria cauliflora | Myrtaceae/Plinia | 45.5–124.4 mg/g a | 50% aqueous acetone | Overhead stirrer | [68] |
| 7.6 mg/g (peel) a | 50% aqueous acetone | Overhead stirrer | [69] | ||
| 161.9 mg/g (seed) a | |||||
| 8.78 mg/g (pulp) a | |||||
| Myrciaria dubia | Myrtaceae/Plinia | 7.14 mg/100 g (peel) a | 50% aqueous methanol (acidified) | Vortex + sonication (15 min) | [70] |
| 6.73 mg/100 g (pulp) a | 50% aqueous methanol (acidified) | ||||
| 381.98 mg/100 g (seeds) a | |||||
| Eucalyptus grandis | Myrtaceae/Eucalyptus | 47.75 mg/g (extract) b | Dichloromethane/50% aqueous methanol | Soxhlet/Stirring (24 h) | [33] |
| 2.22 mg/g (drywood) b | |||||
| Eucalyptus globulus | Myrtaceae/Eucalyptus | 4.95–5.08 mg/g (extract) b | Dichloromethane/50% aqueous methanol | Soxhlet/Stirring (24 h) | [71] |
| 0.42–0.71 mg/g (bark) b | |||||
| Myrtus communis L. | Myrtaceae/Myrtus | 1.028–2.584 mmol/L (leaves) a | Water | Solid–liquid extraction | [56] |
| Feijoa sellowiana | Myrtaceae/Feijoa | 12.04 μg/g (leaves) b | 70% aqueous acetone/ethyl acetate/n-butanol | Solid–liquid extraction | [72] |
| 7.64 μg/g (flowerbuds) b | |||||
| 4.77 μg/g (branches) b | |||||
| 4,53 μg/g (fruits) b | |||||
| Plinia peruviana | Myrtaceae/Plinia | 152.3 μg/mL b | 50% aqueous ethanol | Ultra pressure | [73] |
| Fragaria × ananassa Duch | Rosaceae/Fragaria | 33.18–151.78 mg/g (leaves) a | Acetone:water (3:1) acidified | Vortex + sonication (15 min) | [74] |
| 1.79–19.3 mg/g (roots) a | |||||
| 2.96–18.56 mg/g (fruits) a | |||||
| Prunus avium | Rosaceae/Prunus | 0.059 mg/g a | Methanol | Sonication (30 min) | [34] |
| Potentilla tormentilla | Rosaceae/Potentilla | 6.8–49.33 mg/g (rhizomes) a | 50% aqueous methanol | Sonication (15 min) | [75] |
| Agrimonia asiatica | Rosaceae/Agrimonia | 63.61 mg/g a | Water | Stirring + sonication (60 min) | [76] |
| Mangifera indica L. | Anacardiaceae/Mangifera | 0.018–0.13 mg/g b | 80% aqueous methanol | Sonication (15 min) | [77] |
| 0.14 mg/g (peel) b | Ethanol: Water (1:1) | - | [78] | ||
| 0.41 mg/g (seed) b | Acetone: Water (1:1) | - | |||
| Syzygium cumini Lam | Myrtaceae/Syzygium | 0.00–0.26 mg/g a | Methanol:Water (60:37) (acidified) | Homogenization | [79] |
| Myrciaria floribunda | Myrtaceae/Myrciaria | 2.21 mg/g b | 80% aqueous methanol | Liquid-solid extraction | [80] |
| Myrtus communis L. | Myrtaceae/Myrtus | 8.54 mg/g a | 71% aqueous ethanol | Pressurized-liquid extraction | [81] |
| Syzygium cumini L. | Myrtaceae/Syzygium | 0.13–0.36 μg/g (pulp) b | Petroleum ether/ethyl acetate/methanol/water | Soxhlet | [82] |
| 18.65–32.70 μg/g (seed) b | |||||
| 7.14–15.30 μg/g (seed coat) b | |||||
| 34.60–48.37 μg/g (kernel) b | |||||
| Juglans regia L. | Juglandaceae/Juglans | 412.9–552.9 mg/g a | Methanol | Vortex + sonication ice water (60 min) | [83] |
| Terminalia ferdinandiana | Combretaceae/Terminalia | 30.51–140.25 mg/g a | 80% aqueous methanol (acidified) | Vortex + sonication (15 min) | [84] |
| Quercus alba | Fagaceae/Quercus | 1.06 mg/g a | 80% aqueous methanol | Sonication (30 min) | [34] |
| 3.95 mg/g a | Ethanol:Water (62.5: 37.5) | Stirring | [85] | ||
| Quercus petraea | Fagaceae/Quercus | 2.51 mg/g a | 80% aqueous methanol | Sonication (30 min) | [34] |
| Quercus pyrenaica | Fagaceae/Quercus | 2.94 mg/g a | 80% aqueous methanol | Sonication (30 min) | [34] |
| Quercus robur | Fagaceae/Quercus | 4.07 mg/g a | 80% aqueous methanol | Sonication (30 min) | [34] |
| 8.36 mg/g a | Ethanol:Water (62.5: 37.5) | Stirring | [85] | ||
| Castanea sativa | Fagaceae/Castanea | 8.91 mg/g a | 80% aqueous methanol | Sonication (30 min) | [34] |
| Castanea crenata | Fagaceae/Castanea | 2.26 mg/g b | 80% aqueous methanol | Maceration (48 h) | [86] |
| Terminalia chebula Retz | Combretaceae/Terminalia | 174.43 mg/g a | Water | Boil | [87] |
| Phyllanthus amarus | Phyllanthaceae/Phyllanthus | 444.21 μg/mL a | 80% aqueous ethanol | Soak (9 days) | [88] |
| Quassia undulata | Simaroubacea/Quassia | 2.49 mg/g b | Cold water | Soak (24 h) | [89] |
| Acalypha hispida | Euphorbiaceae/Acalypha | 1.19–5.41 mg/g b | Ethanol | Soak (72 h) | [90] |
| Baccharis trinervis | Asteraceae/Baccharis | 1.35–9.74 mg/g b | Hot water | Infusion (15 min) | [91] |
| Carpobrotus edulis | Aizoaceae/Carpobrotus | 0.45 μg/g b | Water | Stirring (30 min) | [92] |
| 0.55 μg/g b | Aqueous ethanol (1:1) | ||||
| Clematis orientalis | Ranunculaceae/Clematis | 0.46 mg/g b | 80% aqueous methanol.Hexane | Shaking | [93] |
| Clematis ispahanica | Ranunculaceae/Clematis | 0.81 mg/g b | Chloroform | Shaking | [93] |
| Hippophae rhamnoides L. | Elaeagnaceae/Hippophae | 4.94–6.72 mg/g b | 80% aqueous methanol | Homogenization + sonication (20 min) | [94] |
| Euterpe edulis | Arecaceae/Euterpe | 1.40 mg/g a | 70% aqueous ethanol (acidified) | Shaking | [95] |
| Juglans nigra L. | Juglandaceae/Juglans | 9.05–98.41 μg/g b | methanol | Sonication in cool water (60 min) | [96] |
| Sterculia striata | Malvaceae/Sterculia | 0.049 mg/g (nut) b | water | Sonication (60 min) | [97] |
| 0.046 mg/g (shell) b | Sonication (45 min) | ||||
| 0.032 mg/g (pelliche) b | Sonication (45 min) | ||||
| Juices/Wines/Liquors | |||||
| Pomegranate Juice | Lythraceae/Punica | 26.5–33.2 mg/L b | - | - | [98] |
| 5.58 g/L a | - | - | [99] | ||
| 90.4–2071.0 mg/L a | - | - | [53] | ||
| 0.035–2.03 mmol/L a | Methanol | Shaking (3 min) | [100] | ||
| 1242.95 mg/L a | - | - | [101] | ||
| Jabuticaba juice | Myrtaceae/Plinia | 24.37–143 mg/L b | Water | Steam extraction (30 min) | [102] |
| Eugenia brasiliensis Lam | Myrtaceae/Eugenia | 146.1 mg/L a | Water | Homogenization | [103] |
| Muscadine juice | Vitaceae/Vitis | 9.08–107.31 mg/L a | Ethyl acetate | - | [104] |
| guava juice | Myrtaceae/Psidium | 1.41–1.48 mg/g a | - | Pasteurization | [65] |
| Raspberry juice | Rosaceae/Rubus | 2.17–3.24 mg/g a | - | - | [46] |
| Wine | Vitaceae/Vitis | 2.27–77.76 mg/L a | [104] | ||
| 20–50 mg/L a | - | - | [13] | ||
| 0.53–23.8 mg/L a | - | - | [105] | ||
| 7.88–11.61 mg/L b | Diethyl ether/ethyl acetate | - | [106] | ||
| 4.54–4.55 mg/L a | Diethyl ether/ethyl acetate | - | |||
| Pomegranate wine lees | Lythraceae/Punica | 4.36 mg/g a | 70% aqueous methanol | Vortex/sonication (10 min) | [107] |
| Eucalyptus globulus | Myrtaceae/Eucalyptus | 1165.5 mg/L b | Ethyl acetate | Liquid–liquid extraction (30 min) | [108] |
| Others | |||||
| Fruit pureé | 8.8–43 mg/100 g a | 80% ethanol | - | [109] | |
| 8.5–44.1 mg/100 g a | - | ||||
| Strawberry Pureé | 0.14–0.35 mg/g a | 70% aqueous acetone | Sonication (10 min) | [50] | |
| Kakadu Plum Fruit Pureé | 11.65–14.96 mg/g a | Acetone | Sonication (10 min) | [57] | |
| Strawberry Cake | 25.21 mg/g a | 70% aqueous acetone | Vortex/sonication (15 min) | [110] | |
| Vortex/sonication (5 min), Kept in dark (15 min) | |||||
| Strawberry Cake | 17.70–81.01 mg/g a | 70% aqueous acetone (acidified) | [46] | ||
| Strawberry Jam | 0.17–0.29 mg/g b | Methanol:water (70:30) (acidified) | Homogenization in ice bath | [111] | |
| 0.24 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] | ||
| Raspberry jam | 0.76 mg/g a | Aqueous methanol (acidified) | Reflux (20 h) | [18] | |
a Sum of ETs, EA and EA derivates; b EA.
2.3. Extraction ETs and EA from Natural Sources
Various solvents and methods have been used to extract ETs and EA from diverse sources. For example, Aaby et al. [50] extracted ETs and EA from strawberries through ultrasound-assisted extraction, using aqueous acetone as the extraction solvent. On the other hand, Abe et al. [24] observed that a treatment in an ice bath containing nuts and pecans with 80% aqueous acetone yielded higher amounts of total EA, compared to 80% aqueous ethanol. Similarly, Alagan et al. [88] successfully extracted a significant quantity of EA from the plant Phyllanthus amarus through soaking using 80% ethanol. Furthermore, Alañón et al. [34] extracted EA from three species of oak wood using methanol, while in another study by the same author, EA was extracted from mango seed kernel using an aqueous solution of methanol [77]. Both extractions were performed using a sonication process.
As shown in Table 1, the most useable extraction techniques are solid–liquid extraction, followed in some cases by sonication, or sonication alone. Solid–liquid extraction is a simple extraction technique, allowing the direct contact of the solid plant material with the solvent. Thus, it separates the soluble compounds found in a plant material and does not require further mechanical treatment of the samples. A Soxhlet apparatus is often used to achieve the isolation of the compounds; however, other procedures including maceration, stirring, and circular shaking have also been reported (Table 1). Pressurized water extraction has also been used to extract tannins. The main advantage of this method lies in its “green chemistry” characteristics. However, an important disadvantage to consider is that the high temperature used may deteriorate the presence of the analytes of interest. Indeed, Çam et al. [54] observed in their study that the optimal temperature to extract tannins is 40 °C. When the temperature was increased to 65 °C or 90 °C, the number of compounds decreased. Finally, ultrasound-assisted extraction is a common technique used to isolate plant secondary metabolites. In this case, time and sonication power are two variables of high importance, since they affect the yield of extractable compounds. Also critical is the selection of the extraction solvent, as well as the temperature of the water bath.
Most of the studies summarized in the present review use aqueous methanol as the extraction solvent in various % proportions. Both solvents’ polarity is high, and their use can be explained by the fact % yield is increased when a polar solvent is used [112,113]. For this reason, some researchers use multiple solvents during the extraction process to facilitate the extraction of ETs and EA. For example, in the study of Määttä-Riihinen et al. [22], samples were first extracted with ethyl acetate, followed by methanol, which was applied to the solid residue. The same applies for the study of Häkkinen et al. [23] who used acidified aqueous methanol and then the solid residue was diluted to methanol. Nonetheless, data gathered in Table 1 also indicate that another solvent with intermediate polarity such as acetone, in combination with water, results in a high yield of ETs and EA content [41,46].
2.4. Analytical Techniques for the Determination of ETs and EA
The analytical identification, separation and quantification of ETs poses difficulties due to their structural complexity (high molecular weight), their high polarity, and in some cases the lack of commercial standards. Acidic hydrolysis has often been applied for the analysis of ETs with their quantification as equivalents of EA [114]. Theocharis et al. indicated that a maximum yield of EA can be obtained from strawberry samples with the use of a mixture of formic acid/water (80:20, v/v) and heating at 200 °C for 30 min, followed by microwave-assisted extraction (MAE) [115].
On some occasions, adsorption on macroporous resin columns can be used as an initial fractionation step. Usually, the column is washed with water for the removal of water- soluble impurities (i.e., sugar, proteins, etc.) and then gradually eluted with mixtures of ethanol and water [116,117]. This method has been applied to different samples, such as pomegranate husk extract, and for the phenolic profiling of Duchesnea indica, also known as the Indian strawberry [116,117].
High-performance liquid chromatography (HPLC) appears as the most utilized method for the separation and isolation of ETs and EA. Commonly, reverse phase RP-C18 chromatography is employed with polar mobile phases consisting of acidified acetonitrile or methanol and water containing formic or acetic acid [114,118]. Less frequently used columns include C6 phenyl columns [100] and diol columns [119]. Alternatively, fused-core columns have also been employed for the analysis of pomegranate polyphenols, where an improvement in the separation of constituents in a short time was reported, compared to conventional stationary phases [120,121]. Furthermore, a fused-core C18 column was used for the quantification of ETs in oak-aged red wine, achieving the simultaneous analysis of free EA [122]. Solely in the case of EA, high-performance thin layer chromatography (HPTLC) has been employed for quantitative analysis in different extracts and formulations with the use of mobile phases, usually consisting of toluene, ethyl acetate and formic acid [118,123].
In terms of detection, UV detectors such as diode array (DAD) detectors have been widely utilized for the routine identification and quantification of ETs and EA [114,118]. ETs and EA display characteristic UV spectra with maximum absorbance below 270 nm [124]. They have been frequently determined in pomegranate [124,125], strawberries [126,127], Rubus berries and their leaves [128,129], chestnuts [130], as well as waste products of walnuts, chestnuts, pomegranates [131], and pomegranate peels [132]. Mass spectrometry detectors also proved to be valuable tools mainly for the identification of ETs and EA, usually in combination with HPLC/DAD systems. Generally, the negative ion mode of electrospray ionization (ESI) has been employed. Some typical losses during the fragmentation of ETs include galloyl (152 Da), HHDP (302 Da), galloylglucose (332 Da), HHDP-glucose (482 Da), and galloyl-HHDP-glucose (634 Da) [133,134], while fragment ions of EA are frequently observed at m/z 284, 257, 229 and 201 [134]. Importantly, while ET commercial standards are scarce, several scientific groups focusing on the characterization and quantification of ETs often possess isolated compounds that can be used as standards for accurate quantitative determination [53,128,135,136,137]. Collectively, the tandem use of HPLC/DAD and ESI-MS is a technique that has been widely applied to the identification and quantification of ETs and EA found in different matrices, including raspberries [138], pomegranates [53,120], walnuts [139], wine [140], Madagascar’s almonds [141], chestnut trunk samples [142], blueberries [143], Jabuticaba fruits [144], northern red oak (Quercus rubra L.) seeds [145], small burnet (Sanguisorba minor L.) [146] and Kakadu plum (Terminalia ferdinandiana) [137].
Finally, EA was previously determined through Capillary Electrophoresis (CE) in pomegranate rinds [147], industrial pulp samples and their filtrates [148], and Argentinian wines [149]. Fused-silica capillaries of 50 to 60 cm length were the most commonly used and the pH ranged between 8.4 and 9.1. All systems were equipped with a simple UV or DAD detector and the buffers used mostly consisted of boric acid or sodium tetraborate [147,148,149].
3. Urolithins
3.1. Production of Uros from ETs and EA Metabolism
Uros are bioactive metabolites containing a benzo-coumarin scaffold with differences in hydroxylation patterns. They are primarily produced by the gut microbiota in humans and some animals that receive ETs and EA through their diet [5]. Specifically, urolithin A (Uro-A) is one of the major metabolites of EA and it has exhibited a wide range of bioactivities, such as anti-inflammatory, antioxidant, anticancer, anti-diabetic and neuroprotective, among others [9,10,150,151,152].
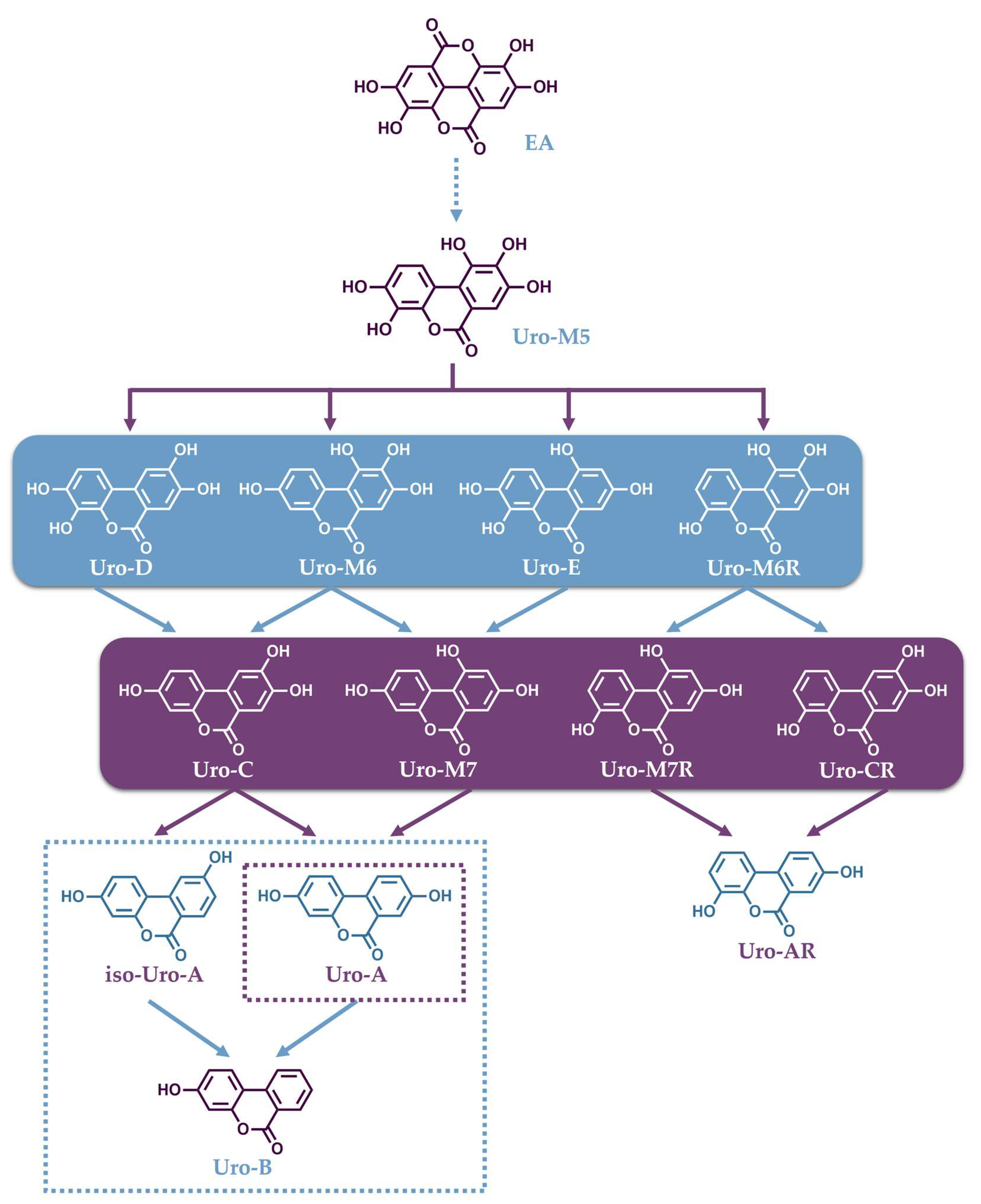
The identification of the Uros formation pathway in humans has been studied and described by Tomas-Barberan et al. in human fecal fermentation studies, as well as in a gastrointestinal simulator model (TWIN-SHIME) [153,154]. The first step in this pathway is a lactone ring cleavage and a subsequent decarboxylation of EA, which is then converted into a pentahydroxy urolithin (Uro-M5) (Figure 2). Consecutive dehydroxylations, catalyzed by different dehydroxylase enzymes, lead to the formation of tetrahydroxy Uros (Uro-E, Uro-M6, Uro-M6R and Uro-D), trihydroxy Uros (Uro-M7, Uro-M7R, Uro-C and Uro-CR) and dihydroxy Uros (Uro-A, isoUro-A and Uro-AR). Finally, the dehydroxylation of Uro-A or isoUro-A may lead to the monohydroxy Uro (Uro-B) [154].
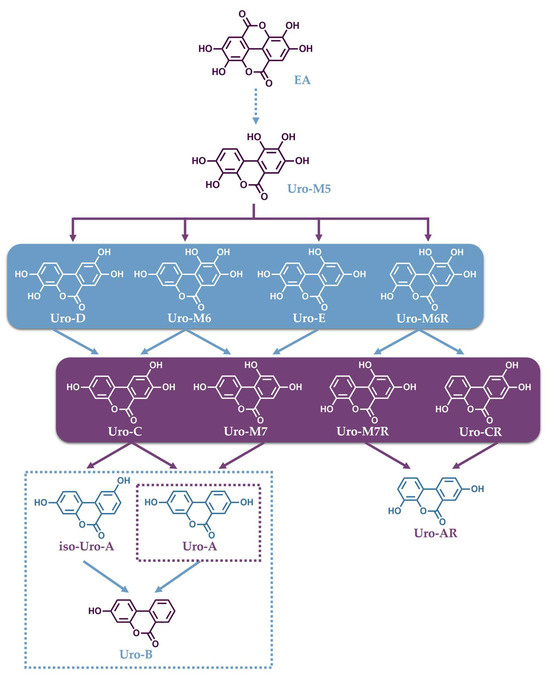
Figure 2.
Catabolic pathway for the conversion of ellagic acid into urolithins.
The production of Uros, following ellagitannin-rich food intake, exhibits large variability throughout the population, because it is a process that depends on the gut microbiota composition of each individual. To date, three urolithin-producing metabotypes have been assigned to humans regarding their ability to produce Uros [155]. “Metabotype A” is assigned to individuals who only produce Uro-A conjugates, “metabotype B” to those who produce isoUro-A and/or Uro-B as well as Uro-A, and in individuals categorized as “metabotype 0”, none of these urolithins are detected. As mentioned, the different metabotypes are associated with differences in the gut microbiome composition in healthy humans [156], which reportedly depends on aging [157]. In their study, Cortés-Martín et al. reported that individuals assigned with metabotype A represent approximately 70% and metabotype B 20% of the population at the ages between 5 and 30 years. In individuals older than 30 years, these two metabotypes tend to equate with a decline in metabotype A and an increase in metabotype B, while the percentage of metabotype 0 remained close to 10% [157].
The known gut bacteria that characterize different metabotypes by transforming EA to Uros mainly belong to the genera Gordonibacter and Ellagibacter, which belong to the Eggerthellaceae family. In a recent study, it was confirmed that the genus Gordonibacter metabolizes EA into Uro-M5, Uro-M6, and Uro-C, while Ellagibacter can convert EA into Uro-M5, Uro-M6, Uro-C and isoUro-A. Both genera could also convert Uro-D and Uro-M6 into Uro-C [158]. Importantly, a novel bacterium Enterocloster bolteae from the family Lachnospiraceae was isolated from human feces that could convert Uro-C and isoUro-A into Uro-A and Uro-B [158]. Then, co-cultures of these bacteria, which have complementary activities, were capable of cooperatively reproducing the Uro formation profiles associated with metabotype A and B individuals upon in vitro fermentation. The patented bacterial combinations could pave the way for future probiotic supplements for metabotype 0 individuals [158]. Finally, a new Uro, namely Uro-G, was discovered by the same team, and its occurrence was validated in human fecal samples following the intake of pomegranate ETs. Furthermore, it was indicated that Uro-G is produced from Uro-D in vivo by human Enterocloster species and it is the first urolithin bearing a catechol group in the A ring as well as solely one hydroxyl in the B ring, a feature that has not been found in human and animal samples until now [159]. In summary, the genera Gordonibacter and Ellagibacter can transform EA into different Uros through lactone-ring cleavage, decarboxylation, and further catechol dehydroxylations at 4- and 10-positions, as well as 8- only in the case of Ellagibacter, which can also produce Uro-A from Uro-G. In contrast, the Enterocloster genera is able to catalyze the dehydroxylation of hydroxyl groups at 9- and 10-positions in both aromatic rings, while Uro-G can only be obtained after the dehydroxylation of Uro-D catalyzed by the Enterocloster species that possesses 9-dehydroxylase activity [158].
3.2. Bioavailability of Uros and Their Metabolites
Uros are easily absorbed in the colon and undergo extensive phase II metabolism, resulting in glucuronide and sulfate conjugates that can reach plasma and systemic tissues, eventually being excreted in urine. In animals, the presence of a Uro conjugating in urine and plasma samples has been widely studied. Animals included in such studies were mainly rodents (rats and mice) [160,161,162,163], ruminants [164,165], pigs [166], and other mammals [164]. In humans, the levels of Uro-A and Uro-B conjugates have been determined in plasma and urine samples after the intake of ellagitannin-rich foods, such as raspberries [167,168,169,170,171,172], strawberries [167,173,174], walnuts [167,175,176,177,178,179], oak-aged wine [167], black tea [180], pomegranate products [175,178,181,182,183,184,185,186,187] and blackberry juice [188]. In studies regarding human plasma, the main Uro conjugates are detected at micromolar concentrations (Uro-A glucuronide up to 35 μM, Uro-B glucuronide 7.3 μM, and isoUro-A 0.745 μM, respectively) [153]. Moreover, in human urine samples, these metabolites reached concentrations up to 100 μM. Importantly, other conjugates of intermediate metabolites, such as Uro-C and Uro-D glucuronides, have been detected, but are generally less studied due to lack of commercial standards [188]. In some studies, the enzymatic hydrolysis of Uro glucuronides and sulfates was employed, with the samples being treated with a β-glucuronidase/sulfatase solution and the Uros determined in total (conjugated and non-conjugated) [188,189,190,191,192,193]. The most common Uros, Uro-A, Uro-B and isoUro-A, have been identified and quantified in their non-conjugated form or as aglycones in the fecal samples or cecal digesta of rats and mice [189,190,191,194,195], ruminants [165], and other mammals, such as squirrels, beavers [164], and pigs [166]. In humans, the same non-conjugated metabolites have been determined in feces after the consumption of pomegranate extracts or walnuts [5,196,197]. In other human tissues, relatively high levels of Uro-A, -B, -C, -D and isoUro-A, and their glucuronide and sulfate conjugates (42 to 1671 ng/g tissue), were identified in normal and malignant colon tissues following pomegranate extract intake [183]. Furthermore, in human prostate samples, Uro-A glucuronide and Uro-B glucuronide were identified in trace amounts, the following consumption of walnuts or pomegranate juice [175].
Due to the inter-individual differences in Uro pharmacokinetics, bioavailable concentrations vary greatly between individuals. In recent years, physiologically based pharmacokinetic (PBPK) modeling has become an interesting alternative to animal testing, making use of existing physicochemical data and in vitro metabolism data in order to determine the tissue concentrations of chosen compounds. As an example, in a recent study of a PBPK model regarding the post-biotic supplementation of Uro-A, it was suggested that peak concentrations in most tissues were low (nanomolar range), supporting the safety of Uro-A to be used as a post-biotic supplement [198].
3.3. Extraction of Uros from Biological Samples
The importance of Uros and their health benefits as a result of ET and EA metabolism led to the development of appropriate analytical methods regarding their determination, mainly in biological samples. To serve this purpose, the first step was the selection of suitable extraction protocols considering the occurrence of conjugated and non-conjugated forms of Uros in combination with the distinct properties of different matrices. As a result, urine samples were often directly injected after dilution and/or filtration [164,170,172,175,176,178,179,180,182,183,184,185,187,190,197], while plasma, breast milk and tissue samples were extracted with solvents, such as MeOH and ACN (acidified with formic or hydrochloric acid in some cases), to facilitate protein precipitation [162,165,168,169,170,172,175,176,177,178,179,182,183,186,187,189,191,192,199,200,201,202]. Fecal samples were extracted with mixtures of MeOH/H2O/HCl or formic acid [5,162,164,165,176,178,179,196], and in vitro fecal fermentation cultures were extracted with organic solvents, such as acetone, ethyl acetate or diethyl ether acidified with formic acid in some cases [203,204,205,206,207]. Solid-phase extraction (SPE) was also used as a cleanup technique in more complex matrices. Different cartridges (C18 Sep-pack, Supelclean LC-C18, Bond Elut C18, Oasis HLB, MCX) were chosen for the different samples, and in all cases, water was used as the washing solvent and MeOH as the elution solvent. In some cases, ninety-six well micro-elution SPE was employed, as it is useful for low volumes of samples and it can facilitate high-throughput sample processing [103,165,166,167,168,180,181,184,185,188,208,209,210].
3.4. Analytical Techniques for the Determination of Uros
The methodologies that have been employed throughout the years for the identification and quantification of Uros in biological fluids are summarized in Table 2 and Table 3. Methods reported for the determination of Uros in samples originated from animals or in vitro cultures are shown in Table 2, while Table 3 summarizes studies on human biological samples. HPLC and UHPLC were the most common methods employed for the separation of Uros, using reverse phase columns with octadecyl-bonded stationary phases and isocratic or gradient mobile phases consisting of water mixed with acetonitrile or methanol with the addition of formic or acetic acid. In rare cases, a pentafluorophenylpropyl (F5) phase column and an ether-linked phenyl phase column were also successfully utilized for the separation of Uro conjugates [170,208]. The detection and quantification of analytes was usually achieved with the use of UV-diode array detectors (DAD) or photodiode array detectors (PDA) coupled in series with mass spectrometers, such as ion traps (IT) [5,162,165,166,167,173,175,180,204,205,208,211], triple quadrupoles (QqQ) [168,171,172,174,189,190,192,193,196,199,200,209,212,213], and quadrupole-time of flight (QTOF) systems with electrospray ionization (ESI) sources [169,177,178,179,182,183,191,203,207]. In detail, the quantification of common Uros was primarily carried out in UV at specific wavelengths (305, 280, 330, 360 nm), and MS was employed for the identification of similar compounds based on differences in their fragmentation patterns. Intermediate Uros (Uro-D, Uro-M6 and Uro-M7), which have not been widely studied, were successfully characterized by Tomás-Barberán et al. with the use of three systems consisting of an LC coupled to a DAD and a QqQ or a QTOF detector. Following the validation of their methodology, it was applied in different biological samples, namely, urine, feces and plasma, after the consumption of ellagitannin-rich sources [178]. These metabolites have since been included in other studies; for example, Uro-M6, Uro-M7, Uro-C, and Uro-D were determined as increasing products of the catabolism of ETs from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro colonic fermentation [203]. In a recent study investigating the supplementation of Gordonibacter urolithinfaciens in combination with EA in C57BL/6J mice, the predominant metabolites found in cecal content included Uro-C, Uro-M6/Uro-D, and Uro-A [190]. In fact, the co-administration of Gordonibacter with EA dramatically promoted EA catabolism and enhanced the production and systemic circulation of Uro-C and Uro-A in particular, especially when compared to the sole administration of EA, overcoming its known low bioavailability and poor absorption [190].
Finally, the presence of different isomers of Uro gluconorides has been previously described [178]. Their determination has not been achieved due to the limitations of the existing methods, mainly their resolution by reversed-phase HPLC. Recently, a novel method for the separation of such isomers using supercritical fluid chromatography (SFC) coupled to a UV detector was reported by Ares et al. utilizing a (S,S) Whelk-O 1 (150 × 4.6 mm, 3.5 μm) column with a mobile phase consisting of a mixture of carbon dioxide and 0.1% (v/v) trifluoroacetic acid in isopropanol (70:30; v/v) [214]. They were able to separate Uro-A 3- and 8-glucuronide, isoUro-A 3- and 9- glucuronide, and Uro-B 3-glucuronide in less than 15 min. This method was successfully applied in the analysis of these metabolites in urine samples from volunteers with different metabotypes and for the first time it was indicated that in metabotype B volunteers, the most common isomer of isoUro-A glucoronide was 3-, while both Uro-A glucuronide isomers were found to exist in similar quantities.

Table 2.
Summary of reported analytical methods for the determination of urolithins in biological samples derived from animals or in vitro cultures.
Table 2.
Summary of reported analytical methods for the determination of urolithins in biological samples derived from animals or in vitro cultures.
| Analyte | Sample Type— Origin | Analytical Technique | Instrumental Analysis | Column/Mobile Phase | Sample Preparation— Solvent Extraction | Ref. |
|---|---|---|---|---|---|---|
| Uro-A, Uro-B | Liver, kidney, heart, brain tissue and biofluids (blood and urine) of adult male rats. | UHPLC–MS/MS | Waters Acquity UPLC (Milford, MA, USA) equipped with a binary pump, autosampler, column compartment and an Acquity PDA eλ detector, coupled to a Waters Xevo TQ (Milford, MA, USA) triple quadrupole MS with an electrospray interface. | Waters Acquity UPLC column HSS T3 (100 mm × 2.1 mm, 1.8 mm, Milford, MA, USA). The mobile phase consisted of (A) water/formic acid (99.9:0.1, v/v) and (B) ACN/formic acid (99.9:0.1, v/v; flow rate 0.4 mL/min. | Extraction with 95% MeOH. | [212] |
| Uro-A Uro-A, Uro-B Uro-A-glucuronide | Cecal digesta (Wistar rats): intake of strawberry. Urine, plasma, cecal digesta (Wistar rats): intake of strawberry. | HPLC-PDA | HPLC Knauer Smartline system with photoDAD, (Knauer, Berlin, Germany). | Gemini C18 column (250 × 4.60 mm, 5 μm, Phenomenex, Torrance, CA USA). The mobile phase consisted of (A) 0.05% phosphoric acid in H2O and (B) 0.05% phosphoric acid in 80% ACN; flow rate 1.25 mL/min. | Extraction with acetone. | [160,161,194] |
| Uro-A | Cecal digesta (Wistar rats): intake of blackberry. | HPLC-ESI-MS | Dionex UltiMate 3000 UHPLC coupled to a Thermo Scientific Q Exactive quadrupole ion trap MS (Thermo Fisher Scientific, Waltham, MA, USA). | Kinetex 110A C18 column (150 × 2.1 mm, 2.6 μm, Phenomenex, Torrance, CA USA). The mobile phase consisted of (A) 0.1% formic acid in H2O and (B) 0.1% formic acid in ACN; flow rate 0.5 mL/min. | Extraction with acetone. | [195] |
| Uros (A, B, C, D, M5, M6 and M7) | Colonic fermentation samples. | HPLC-DAD-QTOFMS/MS | HPLC (CBM-20A Prominence, Shimadzu, Kyoto, Japan) equipped with a degasser (DGU20A5 prominence, Shimadzu, Japan) and column oven (CTO-20A Prominence, Shimadzu, Japan), coupled to DAD (SPDM-20A Prominence, Shimadzu, Japan) and connected to a QTOF MS analyzer and ESI (micrOTOF-QIII, Bruker Daltonics, Bremen, Germany). | C-18 Hypersil Gold column (150 mm × 4.6 mm; 5 μm, Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase consisted of (A) 5% (v/v) methanol in acidified water (0.1% (v/v) of formic acid) and (B) 0.1% (v/v) of formic acid in ACN; flow rate 1.0 mL/min. | Extraction using an acidified acetone solution (0.35% formic acid, v/v). | [203] |
| Uro-A, -B, -C, -D | Plasma, liver, prostate, colon tissue and luminal content (C57BL/6 mice): intake of raspberries. | UPLC-MS/MS | UPLC system (ACQUITY, Waters, Milford, MA, USA) coupled to a triple quadrupole MS (Quattro Ultima, Waters, Milford, MA, USA). | BEH C18 Reverse Phase column (2.1 × 50 mm, 1.7 μm, ACQUITY UPLC, Waters). The mobile phase consisted of (A) 1% aqueous formic acid (v/v) and (B) 1% formic acid in ACN; flow rate 0.3 mL/min. | Samples were treated with β-glucuronidase/sulfatase (S9296, Sigma-Aldrich, St. Louis, MO, USA). Extraction with diethyl ether. | [189] |
| Uro-A and uro-B | Plasma (CD1 Harlan-Nossan male mice): intake of E. angustifolium extract. | UHPLC-MS/MS | Shimadzu Nexera UHPLC system with two LC 30 CE pumps, a SIL 30AC autosampler, a CTO 20AC column oven, and a CBM 20A controller. Coupled to a triple quadrupole LCMS 8050 (Shimadzu, Kyoto, Japan) with an ESI source. | ACQUITY UPLC® BEH C18 column (50 mm × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 0.1% aqueous acetic acid and (B) ACN plus 0.1% acetic acid; flow rate 0.5 mL/min. | Extraction with ice-cold acetonitrile acidified with 98% HCN and 2% HCOOH. | [199] |
| Uro-B, Uro-C | In vitro gastrointestinal digestion of raspberry extract. | HPLC-MS | Exactive™ Plus Orbitrap MS with an ESI Interface (Thermo Fisher Scientific Inc, USA). | Thermo Hypersil GOLD C18 column (100 mm × 2.1 mm, 3 μm, Thermo Fisher Scientific Inc., USA). The mobile phase consisted of (A) 1% formic acid in water and (B) 1% formic acid in ACN; flow rate 0.35 mL/min. | Filtration by 0.22 μm membrane and direct injection. | [211] |
| Uro-A | Samples of in vitro unfermented/fermented pomegranate juice. | HPLC LC-MS/MS | HPLC Waters 1525 Q-Exactive LC-MS/MS (Thermo Fisher Scientific, Shanghai, China). | Hypersil GOLD C18 column (2.1 mm × 100 mm, 1.9 μm, Thermo Fisher Scientific, Shanghai, China). The mobile phase consisted of (A) 0.1% formic acid in water, and (B) ACN; flow rate 0.3 mL/min. | Extraction with diethyl ether and ethyl acetate. | [206] |
| Uro-A, -B, -C, -M5, -M6, isoUro-A | Samples of in vitro unfermented/fermented pomegranate peels. | HPLC-MS | LC-MS system (G2-XS QTof, Waters Corporation, Milford, MA, United States). | ACQUITY UPLC® BEH C18 column (100 mm × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 0.1% aqueous formic acid and (B) ACN plus 0.1% formic acid; flow rate 0.35 mL/min. | Extraction with ethyl acetate acidified with 1.5% formic acid. | [207] |
| Uro-M7, Uro-M6, Uro-D, -C, -A | Plasma, liver, cecal content, urine, brain, adipose tissue (C57BL/6J mice): supplementation of Gordonibacter urolithinfaciens. | UPLC-MS | Agilent 1290 Infinity II UHPLC system coupled to an Agilent 6460 Triple Quadrupole MS with an ESI source (Agilent Technologies Inc., Santa Clara, CA, USA). | ACQUITY UPLC® BEH C18 column (50 mm × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 0.05% aqueous formic acid and (B) ACN plus 0.05%; flow rate 0.45 mL/min. | Plasma, liver, cecal content, urine samples were treated with β-glucuronidase/sulfatase (S9296, Sigma-Aldrich, St. Louis, MO, USA). Extraction with ethyl acetate. Brain and adipose tissue were treated with β-glucuronidase/sulfatase. Extraction using an EMR-lipid 96-well plate. | [190] |
| Uro-A and conjugates | Plasma, liver, and feces (C57BL/6 mice). | HPLC-UV | HPLC system (SPD-M20A DAD, Shimadzu, Kyoto, Japan). UPLCESIMS (Thermo Scientific Orbitrap Elite Mass Spectrometer). | C18 HQ column (4.6 mm × 250 mm, 5 μm, Interchim, Montluçon, France). The mobile phase consisted of 50% MeOH and 50% ddH2O (0.05% phosphoric acid); flow rate 1.0 mL/min. | Plasma: extraction with MeOH. Liver and feces: ultrasonication and extraction with MeOH:12N HCl:water (79.9: 0.1: 20, v/v/v). | [162] |
| Uros and their conjugates | Plasma, urine, feces, ruminal content from animals and beaver castoreum. | HPLC-DAD-MS/MS and HPLC-TOF-MS/MS | HPLC system with a binary pump (G1312A), an autosampler (G1313 A), a degasser (G1322A) and an Agilent 1100 series diode array and a mass detector in series (Agilent Technologies, Waldbronn, Germany). HPLC-TOF-MS Agilent 6220 system with an HPLC system Agilent 1200 series DAD (Agilent Technologies, Waldbronn, Germany). | LiChroCART (C18) column (25 cm × 0.4 cm, 5 μm, Merck, Darmstadt, Germany). The mobile phase consisted of (A) 5% aqueous formic acid and (B) ACN; flow rate 1.0 mL/min. | Feces: extraction with MeOH/HCl/water (79.9:0.1:20, v/v/v). | [164] |
| Uro-A | Plasma, hippocampus and cortex (C57BL/6J mice). | HPLC-ESI-MS/MS | LC-MS/MS-18, TQ6500+ Triple quad (AB Sciex Pte. Ltd., USA) with an ESI interface (Waters, Milford, MA, USA). | Waters CORTECS T3 column (2.1 mm × 100 mm, 2.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 0.1% formic acid in Milli-Q water and (B) 0.1% formic acid in MeOH; flow rate 0.50 mL/min. | Extraction with MeOH. | [201] |
| Uro-C | Rat plasma. | LC-ESI–MS/MS | Agilent 1100 LC system (Agilent Technologies, Les Ulis, France) coupled to an API 3000 tandem triple quadrupole MS (ABSciex, Courtaboeuf, France). | C18 Kinetex EVO column (2.1 × 150 mm, 2.6 μm, Phenomenex, Le Pecq, France). The mobile phase consisted of (A) 1% formic acid in water and (B) ACN; flow rate 0.20 mL/min. | Extraction with ethyl acetate. | [213] |
| Uro-A, -B, -C, -D and their conjugates | Plasma, urine, bile, intestinal lumen, feces, organs and tissues (iberian pigs). | HPLC-DAD-MS/MS | HPLC system (Agilent Technologies, Waldbronn, Germany) equipped with a DAD and an ion-trap mass detector in series with a binary pump and autosampler and an ESI system (Agilent Technologies, Waldbronn, Germany). | LiChroCART (C18) column (25 cm × 0.4 cm, 5 μm, Merck, Darmstadt, Germany) The mobile phase consisted of (A) 1% aqueous formic acid and (B) ACN; flow rate 1.0 mL/min. | Filtration through a reverse phase C18 Sep-Pak cartridge (Millipore Corp., Burlington, MA, USA). Wash with distilled water (10 mL), elution with MeOH. | [166] |
| Uro-A and conjugates | Plasma and tissues (C57BL/6 wild-type mice). intake of pomegranate juice and extract. | HPLC-ESI/MS | LCQ Classic Finnigan system (ThermoFinnigan, San Jose, CA, USA), equipped with an Agilent HP 1100 series HPLC (Santa Clara, CA, USA) system consisting of an autosampler/injector, quaternary pump, column heater, and DAD. | Symmetry C18 column (100 mm × 2.1 mm, 3.5 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 2% formic acid in water and (B) 2% formic acid in MeOH; flow rate 0.15 mL/min. | Homogenization in MeOH with 0.1% acetic acid. | [163] |
| Uro-A, -B, -C, -M6, isoUro-A and their conjugates | Rumen, feces, plasma and urine (brown swiss bulls). | HPLC-DAD-MS-MS | A HPLC system equipped with a photo-DAD (1100 series, Agilent Technologies, Waldbronn, Germany) in series with an ion-trap MS detector (Bruker Daltonics, Bremen, Germany). | LiChroCART (C18) column (25 cm × 0.4 cm, 5 μm, Merck, Darmstadt, Germany). The mobile phase consisted of (A) 1% aqueous formic acid and (B) ACN; flow rate 1.0 mL/min. | Rumen: Sep-Pak C18 cartridge (Waters, Milford, MA, USA). Wash with distilled water, elution with MeOH. Feces: homogenization with MeOH:HCl:water (79.9/0.1/20, v/v/v). Plasma: extraction with ACN:formic acid (99:1, v/v). | [165] |
| Uro-A | Plasma and brain tissue (albino Wistar rats with PD) | UPLC-ESI-QTOF-MS | Agilent 1290 Infinity (Agilent, Les Ulis, France) equipped with an ESI-QTOF-MS (Agilent 6530 Accurate Mass, Agilent, Les Ulis, France). | Eclipse Plus C18 column (2.1 × 100 mm, 1.8 μm, Agilent, Les Ulis, France). The mobile phase consisted of (A) water with 0.1% formic acid and (Β) methanol with 0.1% formic acid; flow rate 0.3 mL/min. | Plasma: extraction with ACN: formic acid (98:2, v/v) Brain: extraction with methanol:HCl (99.9:0.1 v/v). Samples were treated with β-glucuronidase/sulfatase (S9296, Sigma-Aldrich, Poznań, Poland). | [191] |
| Uro-M5, -M6, -A, -C, isoUro-A | In vitro cultures of G. urolithinfaciens and E. isourolithinifaciens. | HPLC-DAD-ESI-IT | Agilent 1100 HPLC system coupled to DAD (Agilent Technologies, Waldbronn, Germany) and an ion trap MS (Esquire 1100 with an ESI source, Brüker Daltoniks). | Poroshell 120 EC-C18 column (3 × 100 mm, 2.7 μm, Agilent Technologies, Waldbronn, Germany). The mobile phase consisted of (A) 1% aqueous formic acid (v/v) and (B) ACN; flow rate 0.5 mL/min. | Extraction with ethyl acetate. | [205] |

Table 3.
Summary of reported analytical methods for the determination of urolithins in human biological samples.
Table 3.
Summary of reported analytical methods for the determination of urolithins in human biological samples.
| Analyte | Sample Type— Origin | Analytical Technique | Instrumental Analysis | Column/Mobile Phase | Sample Preparation— Solvent Extraction | Ref. |
|---|---|---|---|---|---|---|
| Uro-B-glucuronide and aglycone Uro-A | Urine (healthy volunteers): intake of strawberries, raspberries, walnuts, and oak-aged wine. Feces (healthy volunteers): intake of walnuts. Fecal suspensions. | LC-MS/MS | HPLC binary pump, autosampler, and degasser (Agilent Technologies, Waldbronn, Germany) coupled to an ion-trap MS equipped with an ESI system (Agilent Technologies, Waldbronn, Germany). | LiChroCART (C18) column (25 cm × 0.4 cm, 5 μm, Merck, Darmstadt, Germany). The mobile phase consisted of (A) 5% aqueous formic acid and (B) MeOH; flow rate 1.0 mL/min. | Urine: Sep-Pak C-18 solid phase extraction cartridge (Waters Millipore, United States). Wash with water elution with MeOH. Feces: extraction with MeOH:H2O:HCOOH (80:19.9:0.1, v/v). Fecal suspensions: extraction with diethyl ether. | [5,167] |
| Uro-A-glucuronide, Uro-B-glucuronide | Prostate, urine and plasma samples (prostate cancer patients): intake of pomegranate or walnuts. | HPLC-DAD-MS/MS | HPLC-DAD system (1200 series, Agilent) coupled to an HTC Ultra ion-trap mass detector (Bruker Daltonics, Bremen, Germany). | SB C18 Zorbax column (150 mm × 0.5 mm, 5 mm, Agilent Technologies, Waldbronn, Germany). The mobile phase consisted of (A) water/formic acid (99:1, v/v) and (B) ACN; flow rate 10.0 mL/min. | Prostate samples: extraction with cold MeOH:HCl:H2O (79.9:0.1:20, v/v/v). Plasma: extraction with ACN. | [175] |
| Uro-A-glucuronide, Uro-B-glucuronide | Urine (human subjects): black tea intake | HPLC-PDA-FTMSn | Accela HPLC tower connected to an LTQ/Orbitrap hybrid MS (Thermo Fisher Scientific). | Luna C18 column (2.0 × 150 mm, 3 mm, Phenomenex, Torrance, CA, USA). The mobile phase consisted of (A) water/formic acid (99.9:0.1, v/v) and (B) ACN/formic acid (99.9:0.1, v/v); flow rate 0.19 mL/min. | HLB SPE cartridge (OASIS, Waters, Milford, MA, USA). Wash with water, elution with MeOH. | [180] |
| Uro-A, B, C, D and their glucuronides | Urine, plasma, fecal samples (healthy volunteers): intake of walnuts. | HPLC-ESI-MS | Agilent 1100 HPLC, coupled to a HP1101 single-quadrupole, mass-selective detector (Agilent Technologies, Waldbronn, Germany). | RP-18 (250 mm × 4.5 mm, 5 μm, Latek, Eppelheim, Germany). The mobile phase consisted of (A) 2% acetic acid in water and (B) ACN. | Plasma: extraction with 0.2 M hydrochloric acid and EtOH. Feces: extraction with MeOH. | [176] |
| Uro-A, B, C, D, isoUro-A | Feces (healthy volunteers): intake of pomegranate juice. | UPLC–MS/MS | Waters Acquity Ultra-PerformanceTM LC system (Waters, Milford, MA, USA), equipped with a binary pump system, coupled to a triple quadrupole detector (TQD) MS (Waters, Milford, MA, USA) with a Z-spray electrospray interface. | Acquity BEH C18 (100 mm × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) Milli-Q water:acetic acid (99.8:0.2, v/v) and (B) ACN; flow rate 0.3 mL/min. | Extraction with MeOH/HCl/H2O (79.9:0.1:20, v/v/v). | [196] |
| Uro-A | Stool and urine (healthy volunteers): intake of pomegranate juice. | HPLC-DAD | Surveyor HPLC system equipped with DAD, and an autosampler (Thermo Finnigan, San Jose, USA). | Agilent Zorbax SB C-18 column (250 × 4.6 mm, 5 μm, Agilent Technologies, Waldbronn, Germany). The mobile phase consisted of (A) 0.1% phosphoric acid in H2O and (B) ACN; flow rate 0.75 mL/min. | Stool: extraction with DMSO. Samples were treated with β-glucuronidase/sulfatase. | [197] |
| Uro-A, -B, -C, -D | Urine and plasma (healthy men) Urine and plasma (prostate cancer patients) | UPLC-ESI-MS/MS | UPLC system (Acquity UPLC, Waters Corp., Milford, MA, USA) coupled to a triple quadrupole MS (Quattro Ultima, Waters Corp., Beverley, MA, USA). | BEH C18 (50 × 2.1 mm, 1.7 μm). The mobile phase consisted of (A) 1% formic acid in H2O and (B) 1% formic acid in ACN; flow rate 0.75 mL/min. | Samples were treated with β-glucuronidase/sulfatase (S9626, Sigma Chem. Co., St Louis, MO, USA). Urine: extraction with diethyl ether. Plasma: extraction with 2:1 ACN:water. | [192] |
| Uro-A, -B, -C, -D and their conjugates | Urine and plasma (healthy subjects): intake of grumixama. | HPLC-MS | Prominence LC (Shimadzu, Japan) coupled to microTOF-Q II (Bruker Daltonics, Billerica, MA, USA). | Prodigy ODS3 column (250 × 4.60 mm, 5 μm, Phenomenex Ltd., Cheshire, UK). The mobile phase consisted of (A) 0.5% formic acid in H2O and (B) 0.5% formic acid in ACN; flow rate 1.0 mL/min. | SPE in a C18 column (0.3 g, Supelclean LC-C18alkyl, Supelco, Bellefonte, PA, USA) and a CC6 polyamide column (Macherey-Nagel GmbH and Co., Duren, Germany). Wash with oxalic acid, elution with MeOH (5% TFA). | [103] |
| Uro-A, -B, isoUro-A glucuronides | Plasma (healthy older volunteers): intake of strawberry. | HPLC-MS | Agilent 1290 Infinity UHPLC system coupled to an Agilent 6460 Triple Quadrupole MS (Agilent Technologies, Santa Clara, CA, USA). | Poroshell 120 stablebond C18 column (2.1 mm × 150 mm, 2.7 μm). The mobile phase consisted of (A) 1% formic acid in H2O and (B) ACN; flow rate 0.3 mL/min. | C18 SPE cartridges (Agilent Technologies, Santa Clara, CA, USA). Wash with water (1% formic acid). Elution with methanol (1% formic acid) and acetone (1% formic acid). | [174] |
| Uro-A, -B, isoUro-A and their conjugates | Plasma and urine (adults with prediabetes and insulin resistance): intake of fructo-oligosaccharide supplemepnts. Intake of red raspberries | UHPLC-QQQ | UHPLC system coupled with a triple quadrupole tandem MS model 6460 (UHPLC-QQQ, Agilent Technologies, Santa Clara, CA, USA) | Poroshell 120 SB-C18 Stable Bond column (2.1 × 150 mm, 2.7 μm). The mobile phase consisted of (A) 1% aqueous formic acid (v/v) and (B) ACN; flow rate 0.3 mL/min. | SPE C18 cartridges (Agilent Technologies, Waldbronn, Germany). | [168,209] |
| UA and conjugates | Fecal samples and plasma (healthy subjects): intake of pomegranate juice. | HPLC MS/MS | Agilent 1200 HPLC system (Agilent Technologies, Waldbronn, Germany) coupled to a TSQ Vantage triple-stage quadropole MS/MS (ThermoFisher Scientific, San Jose, CA, USA) | C18 reverse phase column (YMC Co., Ltd., Kyoto, Japan). | Plasma: SPE with a Bond-Elut focus plate (Agilent Technologies, Waldbronn, Germany). Wash with water, elution with MeOH. | [181] |
| Urolithin A, B, C, D, M6, M7, isoUroA and conjugates | Human breast milk: walnut Intake. | UPLC-ESI-QTOF | Agilent 1290 Infinity UPLC system coupled to a 6550 Accurate-Mass QTOF (Agilent Technologies, Waldbronn, Germany) | Poroshell 120 EC-C18 column (3 × 100 mm, 2.7 μm). The mobile phase consisted of (A) 0.1% aqueous formic acid (v/v) and (B) ACN plus 0.1% formic acid; flow rate 0.5 mL/min. | Extraction with ACN/formic acid (99:1, v/v). | [177] |
| Uro-A, -B, isoUro-A and conjugates | Plasma (subjects with T2DM): intake of red raspberry. Urine (subjects with metabolic syndrome): intake of pomegranate extract. | UPLC-ESI-QTOF-MS/MS | Agilent 1290 Infinity UPLC system coupled to a 6550 Accurate-Mass QTOF (Agilent Technologies, Waldbronn, Germany) | Poroshell 120 EC-C18 column (3 × 100 mm, 2.7 μm). The mobile phase consisted of (A) 0.1% aqueous formic acid (v/v) and (B) ACN plus 0.1% formic acid; flow rate 0.4 mL/min. | Extraction with ACN/formic acid (98:2, v/v). | [169,182] |
| Uro-A, -B | Plasma (healthy subjects): intake of pomegranate extract. | UHPLC-MS/MS | Agilent 1290 Infinity II LC (Agilent Technologies, Santa Clara, CA, USA), equipped with a binary solvent manager, sample manager, and heated column compartment coupled to a 6470 triple quadrupole MS detector. | Agilent ZORBAX Eclipse Plus C18 column (50 mm × 2.1 mm, 1.8 μm, Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of (A) 0.1% aqueous formic acid and (B) ACN plus 0.1% formic acid; flow rate 0.4 mL/min. | Extraction with ACN (2% formic acid). | [200] |
| Uro-A and Uro-B aglycone, glucuronide and sulfate conjugates | Urine (adolescents with metabolic syndrome) | HPLC -LTQ-Orbitrap-HRMS | Accela chromatograph (Thermo Scientific, Hemel Hempstead, UK) equipped with a quaternary pump and a thermostated autosampler. | Kinetex F5 100 Å (50 × 4.6 mm, 2.6 μm, Phenomenex, Torrance, CA, USA). The mobile phase consisted of (A) 0.05% aqueous formic acid and (B) ACN plus 0.05% formic acid; flow rate 0.5 mL/min. | Oasis 96-well reversed-phase phase extraction plates (Waters, MA, USA). Wash with 1.5M formic acid and 0.5% MeOH, elution with MeOH. | [208] |
| Uro-A, -B, isoUro-A and their conjugates | Plasma, urine and colon tissue (colorectal cancer patients): intake of pomegranate extract. | UPLC-ESI-QTOF-MS/MS | Agilent 1290 Infinity UPLC system coupled to the 6550 Accurate-Mass quadrupole TOF MS (Agilent Technologies, Waldbronn, Germany). | Poroshell 120 EC-C18 column (3 × 100 mm, 2.7 μm). The mobile phase consisted of (A) 0.1% aqueous formic acid (v/v) and (B) ACN plus 0.1% formic acid; flow rate 0.4 mL/min. | Colon tissue: extraction with MeOH:HCl (99.9:0.1 v/v). Plasma samples: extraction with ACN:formic acid (98:2, v/v). Urine samples: dilution with water containing 0.1% formic acid. | [183] |
| Uro-A, -B, -C, -D and their conjugates | Urine (metabolic syndrome subjects): intake of nuts. | LC-PDA-QqQ-MS/MS | API 3000 triple-quadrupole MS (ABSciex, Concord, ON, Canada) equipped with a Turbo Ionspray source coupled to an Acquity UPLC with a Waters binary pump system (Waters, Milford, MA, USA). | Luna C18 analytical column (50 × 2.0 mm, 5 μm; Phenomenex, Torrance, CA, USA). The mobile phase consisted of (A) water/ACN/formic acid, 94.9:5:0.1 (v/v/v) and (B) ACN/formic acid, 99.9:0.1 (v/v); flow rate 0.4 mL/min. | Acidification with acetic acid, incubation with β-glucuronidase/sulfatase and solid-phase extraction (Oasis MCX 96-well plates, Waters, Mildford, MA, USA) | [193] |
| Uro-A glucuronide | Human plasma and urine: intake of strawberries. | HPLC-MS/MS | HPLC system equipped with a diode array absorbance detector and an autosampler (Thermo Finnigan, San Jose, CA, USA) coupled to an LCQ Advantage ion trap MS (Thermo Finnigan). | Agilent ZORBAX SB C18 column (150 mm × 2.1 mm, 5 μm, Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of (A) 1% aqueous acetic acid and (B) ACN; flow rate 0.190 mL/min. | SPE cartridge (Sep-Pak C18 Plus, Waters) | [173] |
| Uro-A, Uro-B glucuronides | Plasma and urine (healthy volunteers): intake of pomegranate juice. Breast milk, plasma and urine (mothers and infants): intake of pomegranate juice. | LC-MS/MS | LCQ Classic Finnigan system (ThermoFinnigan, San Jose, CA, USA), equipped with an Agilent HP 1100 series HPLC (Santa Clara, CA, USA) system consisting of an autosampler/injector, quaternary pump, column heater, and DAD. | Symmetry C18 column (100 mm × 2.1 mm, 3.5 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 2% formic acid in water and (B) 2% formic acid in MeOH; flow rate 0.15 mL/min. | Extraction with ACN and SPE on C18 cartridges (Waters WAT 036945). Wash with water and elution with MeOH. | [184,185] |
| Uro-A | Breast milk (healthy volunteers) | HPLC and HPLC-MS/MS | HPLC (1260 Series, Agilent Technologies, Waldbronn, Germany). HPLC-MS/MS (Thermo Fisher, Waltham, MA, USA). | ZORBAX SB-C18 column (250 × 4.6 mm, 5.0 μm, Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of (A) 1% MeOH and (B) ACN; flow rate 1.0 mL/min. | Extraction with ACN:H2O:HCOOH (80:19.9:0.1). | [202] |
| Uro-A, -B, -C, -D, -M7, isoUro-A and their conjugates | Urine, feces and plasma (healthy volunteers): intake of walnuts and pomegranate extract. | UPLC-ESI-QTOF-MS | Agilent 1290 Infinity UPLC system coupled to a 6550 Accurate-Mass QTOF (Agilent Technologies, Waldbronn, Germany). | Poroshell 120 EC-C18 column (3 × 100 mm, 2.7 μm, Agilent Technologies, Waldbronn, Germany). The mobile phase consisted of (A) 0.5% aqueous formic acid (v/v) and (B) ACN; flow rate 0.5 mL/min. | Urine: dilution with water containing 0.1% formic acid. Feces: homogenization with MeOH/H2O (80:20) and 0.1% HCl. Plasma: extraction with ACN:formic acid (98:2, v/v). | [178,179] |
| Uro-A, -B and conjugates | Plasma (healthy volunteers): intake of pomegranate extract. | HPLC-MS | HPLC system (Agilent Technologies, Waldbronn, Germany) equipped with a DAD and mass detector in series with a binary pump and autosampler (Agilent Technologies, Waldbronn, Germany). | LiChroCART (C18) column (25 cm × 0.4 cm, 5 μm, Merck, Darmstadt, Germany) The mobile phase consisted of (A) 5% aqueous formic acid and (B) MeOH; flow rate 1.0 mL/min. | Homogenization with MeOH:0.2 M HCl (1:1, v/v). | [186] |
| Uro-A and conjugates | Plasma and urine (healthy human subjects): intake of pomegranate juice and extract. | HPLC-ESI/MS | LCQ Classic Finnigan system (ThermoFinnigan, San Jose, CA, USA), equipped with an Agilent HP 1100 series HPLC (Santa Clara, CA, USA) system consisting of an autosampler/injector, quaternary pump, column heater, and DAD. | Symmetry C18 column (100 mm × 2.1 mm, 3.5 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) 2% formic acid in water and (B) 2% formic acid in MeOH; flow rate 0.15 mL/min. | Human plasma: extraction with ACN. Human urine: dilution with H2O (2% formic acid)/methanol (9:1 v/v). | [187] |
| Uro-A, -B and conjugates | Plasma and urine (healthy volunteers and subjects with an ileostomy): intake of raspberries. | HPLC-PDA-MS2 | Surveyor HPLC system with an HPLC pump, PDA detector and an autosampler (Thermo Finnigan, San Jose, CA, USA). | Synergi RP-Polar (250 × 4.6 mm, 4 μm, Phenomenex, Macclesfield, UK). The mobile phase consisted of (A) 1% formic acid in water and (B) 1% formic acid in MeOH; flow rate 1.0 mL/min. | Homogenization in MeOH/water/formic acid (95:4:1, v/v/v). | [170] |
| Uro-A, -B, -M5, -M6, -M7, -C, isoUro-A and Uro-E and their conjugates | Feces and urine (healthy volunteers) and in vitro fermentation samples. | LC-UV/Vis and LC-MS/MS | A HPLC system equipped with a photo-DAD (1100 series, Agilent Technologies, Waldbronn, Germany) in series with an ion-trap MS detector (Bruker Daltonics, Bremen, Germany). | LiChroCART (C18) column (25 cm × 0.4 cm, 5 μm, Merck, Darmstadt, Germany). The mobile phase consisted of (A) 1% aqueous formic acid and (B) ACN; flow rate 1.0 mL/min. | Feces: homogenization with MeOH/DMSO/H2O (40:40:20) with 0.1% HCl. Human faecal suspensions: Extraction with ethyl acetate acidified with 1.5% formic acid. | [204] |
| Uro-A, -B, -C and their conjugates | Plasma (healthy volunteers): intake of French oak wood extract (Robuvit). | HPLC-ESI-MS/MS | Perkin-Elmer series 200 HPLC system coupled to an Applied Biosystems (Foster City, CA, USA) API 3200 instrument with a Turbo ion-spray source. | Restek Ultra C18 column (100 × 2.1 mm, 3 μm). The mobile phase consisted of (A) 1% aqueous formic acid and (B) ACN with 1% formic acid; flow rate 0.3 mL/min. | HLB solid-phase extraction cartridge (OASIS, Waters, Milford, MA, USA) Wash with water, elution with MeOH. | [210] |
| Uro-A, Uro-B, Uro-C, Uro-D, Uro-M5 and conjugates | Urine (healthy volunteers): intake of blackberry juice. | UPLC-DAD/ESI-Q-TOF/MS | Waters Acquity UPLC-PDA coupled to a Quadrupole Time-Of-Flight Mass Spectrometer (ESI-Q-TOF/MS) (Waters Synapt G1, Waters Corp., Milford, MA, USA). | ACQUITY UPLC C18 CSH (100 × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA). The mobile phase consisted of (A) water/formic acid (99.9:0.1, v/v) and (B) ACN/formic acid (99.9/0.1 v/v); flow rate 0.4 mL/min. | SupelcleanTM LC-18 extraction cartridges (Supelco Analytical, USA). Wash with MilliQ water, elution with MeOH. | [188] |
| Uro-A and conjugates | Plasma and urine (human subjects): intake of raspberry drink. | UHPLC-QQQ | UHPLC system coupled with a 6460 Series Triple Quadrupole (QQQ) (Agilent Technologies, Santa Clara, CA, USA). | Poroshell C18 Stable Bond column (2.1 × 150 mm, 2.7 μm; Agilent Technologies, Santa Clara, CA, USA). The mobile phase consisted of (A) water with 1% formic acid and (Β) ACN. | Plasma: SPE C18 cartridges (Agilent Technologies, Santa Clara, CA, USA) Urine: filtration with a 0.2 μm Polypropylene syringe filter (Whatman, Maidston, UK). | [171] |
| Uro-A, -B, -C, -D and conjugates | Urine and plasma (men with prostate cancer): consumption of black raspberry products. | HPLC-MS/MS | UPLC system (Acquity UPLC, Waters Corp., Milford, MA, USA) coupled to a triple quadrupole MS (Quattro Ultima, Waters Corp., Beverley, MA, USA). | BEH C18 column (50 × 2.1 mm, 1.7 μm). The mobile phase consisted of (A) water with 1% formic acid and (Β) ACN with 1% formic acid; flow rate 0.75 mL/min. | Urine: samples were treated with β-glucuronidase/sulfatase (S9626, Sigma Chem. Co., St Louis, MO, USA). Extraction with diethyl ether. Plasma: extraction with ACN. | [172] |
4. Current Research on the Bioactivities of Urolithins on Human Health
Recent studies have demonstrated the beneficial effects of Uro-A supplementation in human health and highlighted Uro-A as a promising healthspan promoting and anti-aging compound. Derived from ETs, Uro-A sustains cellular and tissue homeostasis primarily by inducing mitochondrial selective autophagy, known as mitophagy, as demonstrated in both in vitro and in vivo studies using mammalian cells, the nematode Caenorhabditis elegans and mouse models [215]. Mitophagy efficiency declines with age and in several age-associated pathologies, including myopathies, neurodegenerative diseases and autoimmunities, among others [9]. Notably, mitophagy induction restores the age-dependent mitochondrial damage and rejuvenates cellular fitness by promoting mitochondrial activity and energy metabolism, leading eventually to improved muscle function, neuronal homeostasis, healthspan and lifespan extension [9,10,216,217].
Emerging findings from in vivo animal models highlight the potential therapeutic effects of Uro-A supplementation on both tissue-specific diseases, such as neurodegenerative disorders, cardiovascular pathologies, and myopathies, and systemic diseases, including metabolic syndrome and cancer. In preclinical models of cardiac ischemia, atherosclerosis, and diabetic cardiomyopathy, Uro-A treatment has been shown to improve animals’ physiology. Notably, in a mouse model of ischemia-reperfusion injury, pretreatment with Uro-A reduced infarct size and partially preserved ejection fraction. This was accompanied by decreased levels of circulating creatine kinase and lactate dehydrogenase, along with a reduction in apoptotic cells in the heart [218]. Additionally, Uro-A has been found to protect rats from atherosclerosis by lowering plasma lipid levels and reducing aortic lesions [219]. In models of diabetic cardiomyopathy, Uro-A enhanced myocardial contractility, underscoring its protective role in heart health [220].
Several studies have demonstrated the neuroprotective impact of Uro-A on various neurodegenerative conditions across species. Uro-A has been shown to enhance associative learning and memory in transgenic nematodes overexpressing the human amyloid-beta (Aβ1–42) and Tau proteins. It also improved learning, memory retention, neuronal survival, and neurogenesis in the hippocampus of APP/PS1 mice, a model of Alzheimer’s disease (AD). Additionally, Uro-A reduced levels of insoluble Aβ1–42 plaques and phosphorylated Tau, which are significant biomarkers associated with the development and severity of AD [221,222]. In models of ischemic stroke and multiple sclerosis, Uro-A decreased infarct volume and neurological deficits, and diminished the incidence and severity of multiple sclerosis, as well as inflammation and demyelination [223,224]. Furthermore, UA displayed a robust anti-inflammatory effect, resulting in reduced levels of IL-1β, IL-6 and TNFα in brain samples from AD mice [221,222]. Further supporting its anti-inflammatory function, Uro-A enhanced microglial phagocytic activity and inhibited inflammasome activation, thereby regulating neuroinflammation in AD mice [221,225]. Expanding its therapeutic potential against inflammatory diseases, Uro-A administration showed protective effects against inflammatory bowel diseases, such as ulcerative colitis and Crohn’s disease. These pathologies, caused by a deregulated immune system leading to chronic inflammation and microbial dysbiosis, displayed a reduction in colon inflammation markers and improved mucosal integrity in different mouse models [226,227,228,229].
In 2017, the first safety assessment of Uro-A in Wistar rats was reported, where Uro-A did not indicate any target organ toxicities, adverse effects or mortality after repeated oral doses in 28- and 90-day studies. The no-observed-adverse-effect level (NOAEL) was the highest dose tested, corresponding to 5% UA by weight in the diet, or 3451 mg/kg bw/day in males and 3826 mg/kg bw/day in females [230]. When these values are applied to humans, the estimated Uro-A value is approximately 600 mg Uro-A/kg bw, applying a scaling factor of 6.2 for rat-to-human conversion [231]. Moreover, the safety and efficacy of Uro-A were highlighted in its first-in-human clinical trial, establishing favorable bioavailability and no adverse effects at doses ranging from 250 to 2000 mg, which supports Uro-A use as a safe supplement ingredient [232]. In 2018, the U.S. Food and Drug Administration (FDA) officially recognized Uro-A as safe for inclusion in food products and supplements at typical use levels of 250 mg/serving or 500 mg/serving up to a maximum of 500 mg/serving or 1000 mg/serving [233]. Additionally, in a randomized clinical trial in older adults where Uro-A was supplemented at doses of 1000 mg, it was well tolerated and its long-term supplementation benefited muscle endurance and plasma biomarkers [234]. Further supporting the beneficial effects of Uro-A on cellular and tissue homeostasis, a randomized clinical trial showed that middle-aged adults administered with Uro-A at doses of 500 and 1000 mg exhibited improvements in biomarkers related to mitochondrial function, cellular health, and muscle performance, confirming Uro-A’s effectiveness in critical aspects of human physiology [235]. Notably, these trials underscore Uro-A potential for practical dietary interventions. Interestingly, in a study where the levels of Uro-A obtained from dietary supplementation were compared to natural dietary exposure in a healthy population, it was established that in order to achieve the equivalent dosing of 500 mg supplemented Uro-A from dietary exposure via pomegranate juice, an individual would need to drink approximately 1.5 L on average, as that would contain the necessary dietary precursors [181].
In terms of safety for the supplementation of Uro-A’s precursors ETs and EA, limited information is available. As previously mentioned, in an EA subchronic toxicity study conducted on F344 rats, no treatment-related adverse effects or mortality were observed with an estimated no-observed-effect level (NOEL) of 3011 mg/kg bw for male rats and a NOAEL of 3254 mg/kg bw for female rats (5% in the diet) [35]. Importantly, in a study conducted in type 2 diabetic patients supplemented with EA for 8 weeks at doses of 180 mg/day, reduced levels of blood sugar, blood lipids, and insulin resistance were reported with no adverse effects being observed [236]. Regarding ETs, punacalagin was evaluated for its possible toxic effect in Sprague Dawley rats upon the repeated administration of a diet containing 20% pomegranate husk extract with an average of 6% punicalagin for 37 days. The mean oral consumption throughout the study was reported to be 4800 mg punicalagin/kg bw/day and no significant adverse effects were reported apart from a decrease in serum urea and triglyceride values, although these values remained within the normal range [237]. In another study, the acute oral median lethal dose (LD50) of a pomegranate fruit extract containing 30% punicalagins in mice and rats was found to be greater than 5 g/kg body weight and the NOAEL for a subchronic 90-day study in Wistar rats treated with this extract was 600 mg/kg bw/day, which was the highest dose tested, presenting no toxicologically significant changes [238]. As an exception, in a study on ellagitannin metabolism following the consumption of jabuticaba fruit in a non-European population, 63% of subjects reported the occurrence of diarrhea, a side effect that was attributed to the high amount of ellagitannins consumed (1493 mg of ETs, EA and gallic acid in total) [239]. It is worth noting that numerous association studies involving diets and supplements rich in ETs, such as pomegranate and raspberry extracts, or walnuts, have reported health benefits linked to Uros production. For instance, the intake of a standardized oral pomegranate extract containing 75 mg of punicalagins was recently associated with an increase in short-chain fatty acid-producing bacteria in the gut, as well as improvements in the skin microbiome and reduction in skin wrinkles [240,241]. In conclusion, the diverse range of beneficial bioactivities attributed to Uros underscores their significant potential for future research and their promising role in enhancing human health.
5. Conclusions
Food and plant components contributing to health protection and promotion are currently of high interest. ETs, as well as EA, which is a product of ETs generated in the stomach and the small intestine, have been documented to offer beneficial health effects to humans. Accumulated data have revealed that these effects are exerted by Uros, which are the metabolic products of EA upon the action of the gut microbiota. As a consequence, analytical methods to study the presence of ETs and EA in plant and food sources are needed, permitting a detailed mapping of these bioactive components in natural sources and in processed foods. In parallel, understanding the generation of Uros within the organism and the ability of each individual to efficiently convert EA in Uros requires reliable and sensitive methods for the determination of Uros in biological samples. Thus, a variety of HPLC and LC-MS methods have been developed, allowing the detection of ETs, EA and Uros in a variety of samples. Due to the pleiotropic activities of EA and Uros, this field of research is expected to be highly active in the coming years.
Author Contributions
Conceptualization, M.G.K.; methodology, C.M., E.K. and M.G.K.; writing—original draft preparation, C.M., E.K., K.P. and M.G.K.; writing—review and editing, P.A.T. and M.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
KP is supported by the Fondation Santé (19656) and the European Union (European Research Council; ERC), under grant agreement “ERC-GA101077374-SynaptoMitophagy”. Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council. Neither the European Union nor the granting authority can be held responsible for them.
Acknowledgments
M.G.K. would like to thank Lóreal-UNESCO for the award “For Women in Science 2023”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Putnik, P.; Kovačević, D.B.; Muchenje, V.; Barba, F.J. Sources, chemistry, and biological potential of ellagitannins and ellagic acid derivatives. Stud. Nat. Prod. Chem. 2019, 60, 189–221. [Google Scholar]
- Zhang, M.; Cui, S.; Mao, B.; Zhang, Q.; Zhao, J.; Zhang, H.; Tang, X.; Chen, W. Ellagic acid and intestinal microflora metabolite urolithin A: A review on its sources, metabolic distribution, health benefits, and biotransformation. Crit. Rev. Food Sci. Nutr. 2023, 63, 6900–6922. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Čižmáriková, M.; Michalková, R.; Mirossay, L.; Mojžišová, G.; Zigová, M.; Bardelčíková, A.; Mojžiš, J. Ellagic acid and cancer hallmarks: Insights from experimental evidence. Biomolecules 2023, 13, 1653. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective potential of ellagic acid: A critical review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, R.; Zhang, S.; Weng, H.; Liu, Y.; Tao, T.; Yu, F.; Li, G.; Wu, J. Promising remedies for cardiovascular disease: Natural polyphenol ellagic acid and its metabolite urolithins. Phytomedicine 2023, 116, 154867. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the natural compound urolithin A on health, disease, and aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- An, L.; Lu, Q.; Wang, K.; Wang, Y. Urolithins: A prospective alternative against brain aging. Nutrients 2023, 15, 3884. [Google Scholar] [CrossRef]
- Available online: https://www.mitopure.com/ (accessed on 3 April 2024).
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural Features and Biological Properties of Ellagitannins in Some Plant Families of the Order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T. Ellagitannins as active constituents of medicinal plants. Planta Med. 1989, 55, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Braconnot, H. Observations sur la préparation et la purification de l’acide gallique, et sur l’existence d’unacide nouveau dans la noix de galle. Ann. Chim. Phys. 1818, 9, 181–189. [Google Scholar]
- Villalba, K.J.O.; Barka, F.V.; Pasos, C.V.; Rodríguez, P.E. Food ellagitannins: Structure, metabolomic fate, and biological properties. In Tannins-Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: Rijeka, Croatia, 2020; pp. 26–46. ISBN 9781789847963. [Google Scholar]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. Changes in phenolic compounds profile and glutathione status in raspberry fruit during storage in ozone-enriched atmosphere. Postharvest Biol. Technol. 2020, 168, 111277. [Google Scholar] [CrossRef]
- Sparzak, B.; Merino-Arevalo, M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Majdan, M.; Fecka, I.; Głód, D.; Bączek, T. HPLC analysis of polyphenols in the fruits of Rubus idaeus L. (Rosaceae). Nat. Prod. Res. 2010, 24, 1811–1822. [Google Scholar] [CrossRef]
- Wada, L.; Ou, B. Antioxidant activity and phenolic content of Oregon caneberries. J. Agric. Food Chem. 2002, 50, 3495–3500. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Mykkänen, H.M.; Heinonen, I.M.; Törrönen, A.R. Ellagic acid content in berries: Influence of domestic processing and storage. Eur. Food Res. Technol. 2000, 212, 75–80. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Potential dietary sources of ellagic acid and other antioxidants among fruits consumed in Brazil: Jabuticaba (Myrciaria jaboticaba (Vell.) Berg). J. Sci. Food Agric. 2012, 92, 1679–1687. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; da Costa, S.C.; Madrona, G.S. Camu-camu bioactive compounds extraction by ecofriendly sequential processes (ultrasound assisted extraction and reverse osmosis). Ultrason. Sonochem. 2020, 64, 105017. [Google Scholar] [CrossRef]
- Jourdes, M.; Michel, J.; Saucier, C.; Quideau, S.; Teissedre, P.L. Identification, amounts, and kinetics of extraction of C-glucosidic ellagitannins during wine aging in oak barrels or in stainless steel tanks with oak chips. Anal. Bioanal. Chem. 2011, 401, 1531–1539. [Google Scholar] [CrossRef]
- Amarowicz, R.; Janiak, M. Hydrolysable tannins. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 337–343. ISBN 9780128140451. [Google Scholar]
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H. Ellagitannins renewed the concept of tannins. In Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; Quideau, S., Ed.; World Scientific: Hackensack, NJ, USA, 2009; pp. 1–54. ISBN 9789812797407. [Google Scholar]
- Jokar, A.; Masoomi, F.; Sadeghpour, O.; Nassiri-Toosi, M.; Hamedi, S. Potential therapeutic applications for Terminalia chebula in Iranian traditional medicine. J. Tradit. Chin. Med. 2016, 36, 250–254. [Google Scholar] [CrossRef]
- Torgbo, S.; Rugthaworn, P.; Sukatta, U.; Sukyai, P. Biological characterization and quantification of Rambutan (Nephelium lappaceum L.) peel extract as a potential source of valuable minerals and ellagitannins for industrial applications. ACS Omega 2022, 7, 34647–34656. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.B.M.; Bennett, R.N.; Quideau, S.; Jacquet, R.; Rosa, E.A.; Ferreira-Cardoso, J.V. Evaluating the potential of chestnut (Castanea sativa Mill.) fruit pericarp and integument as a source of tocopherols, pigments and polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Kaneshima, T.; Myoda, T.; Nakata, M.; Fujimori, T.; Toeda, K.; Nishizawa, M. Antioxidant activity of C-Glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT-Food Sci. Technol. 2016, 69, 76–81. [Google Scholar] [CrossRef]
- Santos, S.A.; Vilela, C.; Domingues, R.M.; Oliveira, C.S.; Villaverde, J.J.; Freire, C.S.; Neto, C.P.; Silvestre, A.J. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crops Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Gordon, M.H.; Pérez-Coello, M.S. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011, 129, 1584–1590. [Google Scholar] [CrossRef]
- Tasaki, M.; Umemura, T.; Maeda, M.; Ishii, Y.; Okamura, T.; Inove, T.; Kuroiwa, Y.; Hirose, M.; Nishikawa, A. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem. Toxicol. 2008, 46, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. Pomegranate Ellagitannins. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 10; ISBN 9781439807132. [Google Scholar]
- Buenrostro-Figueroa, J.; Mireles, M.; Ascacio-Valdés, J.A.; Aguilera-Carbo, A.; Sepúlveda, L.; Contreras-Esquivel, J.; Rodríguez-Herrera, R.; Aguilar, C.N. Enzymatic biotransformation of pomegranate ellagitannins: Initial approach to reaction conditions. Iran. J. Biotechnol. 2020, 18, 2305. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Yu, Q.; Shen, Y.; Zheng, Z.; Cheng, J.; Zhang, W.; Ye, Y. Exploration of the binding between ellagic acid, a potentially risky food additive, and bovine serum albumin. Food Chem. Toxicol. 2019, 134, 110867. [Google Scholar] [CrossRef] [PubMed]
- Whitley, A.C.; Stoner, G.D.; Darby, M.V.; Walle, T. Intestinal epithelial cell accumulation of the cancer preventive polyphenol ellagic acid--extensive binding to protein and DNA. Biochem. Pharmacol. 2003, 66, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus berries for the control of gastric inflammation: In vitro and in vivo studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Bradish, C.M.; Yousef, G.G.; Ma, G.; Perkins-Veazie, P.; Fernandez, G.E. Anthocyanin, carotenoid, tocopherol, and ellagitannin content of red raspberry cultivars grown under field or high tunnel cultivation in the southeastern United States. J. Amer. Soc. Hort. Sci. 2015, 140, 163–171. [Google Scholar] [CrossRef]
- Mattila, P.; Kumpulainen, J. Determination of free and total phenolic acids in plant-derived foods by HPLC with diode-array detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Sójka, M.; Macierzyński, J.; Zaweracz, W.; Buczek, M. Transfer and mass balance of ellagitannins, anthocyanins, flavan-3-ols, and flavonols during the processing of red raspberries (Rubus idaeus L.) to juice. J. Agric. Food Chem. 2016, 64, 5549–5563. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Sultanbawa, Y. Profiling ellagic acid content: The importance of form and ascorbic acid levels. Int. Food Res. J. 2014, 66, 100–106. [Google Scholar] [CrossRef]
- Diamanti, J.; Mazzoni, L.; Balducci, F.; Cappelletti, R.; Capocasa, F.; Battino, M.; Dobson, G.; Stewart, D.; Mezzetti, B. Use of wild genotypes in breeding program increases strawberry fruit sensorial and nutritional quality. J. Agric. Food Chem. 2014, 62, 3944–3953. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Palmieri, L.; Martinatti, P.; Pojer, E.; Mattivi, F.; Vrhovsek, U. Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp. J. Agric. Food Chem. 2013, 61, 8597–8607. [Google Scholar] [CrossRef]
- Aaby, K.; Wrolstad, R.E.; Ekeberg, D.; Skrede, G. Polyphenol composition and antioxidant activity in strawberry purees; Impact of achenelLevel and storage. J. Agric. Food Chem. 2007, 55, 5156–5166. [Google Scholar] [CrossRef]
- Van de Velde, F.; Pirovani, M.E.; Drago, S.R. Bioaccessibility analysis of anthocyanins and ellagitannins from blackberry at simulated gastrointestinal and colonic levels. J. Food Compos. Anal. 2018, 72, 22–31. [Google Scholar] [CrossRef]
- Bushman, B.S.; Phillips, B.; Isbell, T.; Ou, B.; Crane, J.M.; Knapp, S.J. Chemical composition of caneberry (Rubus spp.) seeds and oils and their antioxidant potential. J. Agric. Food Chem. 2004, 52, 7982–7987. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Çam, M.; Hışıl, Y. Pressurised water extraction of polyphenols from pomegranate peels. Food Chem. 2010, 123, 878–885. [Google Scholar] [CrossRef]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Chaliha, M.; Sultanbawa, Y. Measuring the three forms of ellagic acid: Suitability of extraction solvents. Chem. Pap. 2016, 70, 144–152. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Sharp, J.L.; Wang, X.; You, Y.; Zhang, C. High-performance liquid chromatography–mass spectrometry and evaporative light-scattering detector to compare phenolic profiles of muscadine grapes. J. Chromatogr. A 2012, 1240, 96–103. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 2006, 97, 524–530. [Google Scholar] [CrossRef]
- Malik, N.S.; Perez, J.L.; Lombardini, L.; Cornacchia, R.; Cisneros-Zevallos, L.; Braford, J. Phenolic compounds and fatty acid composition of organic and conventional grown pecan kernels. J. Sci. Food Agric. 2009, 89, 2207–2213. [Google Scholar] [CrossRef]
- Gonçalves, B.; Borges, O.; Costa, H.S.; Bennett, R.; Santos, M.; Silva, A.P. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: Proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010, 122, 154–160. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. LWT-Food Sci. Technol. 2010, 30, 254–259. [Google Scholar] [CrossRef]
- Gong, Y.; Pegg, R.B. Separation of ellagitannin-rich phenolics from US pecans and Chinese hickory nuts using fused-core HPLC columns and their characterization. J. Agric. Food Chem. 2017, 65, 5810–5820. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. J. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef]
- Rojas-Garbanzo, C.; Winter, J.; Montero, M.L.; Zimmermann, B.F.; Schieber, A. Characterization of phytochemicals in Costa Rican guava (Psidium friedrichsthalianum-Nied.) fruit and stability of main compounds during juice processing-(U) HPLC-DAD-ESI-TQD-MSn. J. Food Compos. Anal. 2019, 75, 26–42. [Google Scholar] [CrossRef]
- Dos Santos, W.N.L.; da Silva Sauthier, M.C.; dos Santos, A.M.P.; de Andrade Santana, D.; Azevedo, R.S.A.; da Cruz Caldas, J. Simultaneous determination of 13 phenolic bioactive compounds in guava (Psidium guajava L.) by HPLC-PAD with evaluation using PCA and Neural Network Analysis (NNA). Microchem. J. 2017, 133, 583–592. [Google Scholar] [CrossRef]
- Alezandro, M.R.; Granato, D.; Genovese, M.I. Jaboticaba (Myrciaria jaboticaba (Vell.) Berg), a Brazilian grape-like fruit, improves plasma lipid profile in streptozotocin-mediated oxidative stress in diabetic rats. Int. Food Res. J. 2013, 54, 650–659. [Google Scholar] [CrossRef]
- Silva, R.M.; Pereira, L.D.; Véras, J.H.; do Vale, C.R.; Chen-Chen, L.; da Costa Santos, S. Protective effect and induction of DNA repair by Myrciaria cauliflora seed extract and pedunculagin on cyclophosphamide-induced genotoxicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 810, 40–47. [Google Scholar] [CrossRef]
- Pereira, L.D.; Barbosa, J.M.G.; Ribeiro da Silva, A.J.; Ferri, P.H.; Santos, S.C. Polyphenol and ellagitannin constituents of jabuticaba (Myrciaria cauliflora) and chemical variability at different stages of fruit development. J. Agric. Food Chem. 2017, 65, 1209–1219. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef]
- Santos, S.A.; Freire, C.S.; Domingues, M.R.M.; Silvestre, A.J.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Aoyama, H.; Sakagami, H.; Hatano, T. Three new flavonoids, proanthocyanidin, and accompanying phenolic constituents from Feijoa sellowiana. Biosci. Biotechnol. Biochem. 2018, 82, 31–41. [Google Scholar] [CrossRef]
- Mazzarino, L.; da Silva Pitz, H.; Lorenzen Voytena, A.P.; Dias Trevisan, A.C.; Ribeiro-Do-Valle, R.M.; Maraschin, M. Jaboticaba (Plinia peruviana) extract nanoemulsions: Development, stability, and in vitro antioxidant activity. Drug Dev. Ind. Pharm. 2018, 44, 643–651. [Google Scholar] [CrossRef]
- Karlińska, E.; Masny, A.; Cieślak, M.; Macierzyński, J.; Pecio, Ł.; Stochmal, A.; Kosmala, M. Ellagitannins in roots, leaves, and fruits of strawberry (Fragaria × ananassa Duch.) vary with developmental stage and cultivar. Sci. Hortic. 2021, 275, 109665. [Google Scholar] [CrossRef]
- Fecka, I.; Kucharska, A.Z.; Kowalczyk, A. Quantification of tannins and related polyphenols in commercial products of tormentil (Potentilla tormentilla). PCA 2015, 26, 353–366. [Google Scholar] [CrossRef]
- Kashchenko, N.I.; Olennikov, D.N. Phenolome of asian agrimony tea (Agrimonia asiatica Juz., Rosaceae): LC-MS profile, α-glucosidase inhibitory potential and stability. Foods 2020, 9, 1348. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pimentel-Moral, S.; Arráez-Román, D.; Segura-Carretero, A. HPLC-DAD-Q-ToF-MS profiling of phenolic compounds from mango (Mangifera indica L.) seed kernel of different cultivars and maturation stages as a preliminary approach to determine functional and nutraceutical value. Food Chem. 2021, 337, 127764. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Sánchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Int. Food Res. J. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Lestario, L.N.; Howard, L.R.; Brownmiller, C.; Stebbins, N.B.; Liyanage, R.; Lay, J.O. Changes in polyphenolics during maturation of Java plum (Syzygium cumini Lam.). Int. Food Res. J. 2017, 100, 385–391. [Google Scholar] [CrossRef]
- de Oliveira, L.M.; Porte, A.; de Oliveira Godoy, R.L.; da Costa Souza, M.; Pacheco, S.; de Araujo Santiago, M.C.P.; Gouvêa, A.C.M.S.; da Silva de Mattos do Nascimento, L.; Borguini, R.G. Chemical characterization of Myrciaria floribunda (H. West ex Willd) fruit. Food Chem. 2018, 248, 247–252. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Arráez-Román, D.; Segura-Carretero, A.; Ferranti, P.; Nicoletti, R.; Perrotta, G.M.; Gómez-Caravaca, A.M. Establishment of pressurized-liquid extraction by response surface methodology approach coupled to HPLC-DAD-TOF-MS for the determination of phenolic compounds of myrtle leaves. Anal. Bioanal. Chem. 2018, 410, 3547–3557. [Google Scholar] [CrossRef]
- Gajera, H.P.; Gevariya, S.N.; Hirpara, D.G.; Patel, S.V.; Golakiya, B.A. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cumini L.) landraces. J. Food Technol. 2017, 54, 3180–3191. [Google Scholar] [CrossRef]
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and quantification of the major phenolic constituents in Juglans regia L. peeled kernels and pellicles, using HPLC-MS/MS. Food Chem. 2021, 352, 129404. [Google Scholar] [CrossRef]
- Konczak, I.; Maillot, F.; Dalar, A. Phytochemical divergence in 45 accessions of Terminalia ferdinandiana (Kakadu plum). Food Chem. 2014, 151, 248–256. [Google Scholar] [CrossRef]
- Glabasnia, A.; Hofmann, T. Sensory-directed identification of taste-active ellagitannins in American (Quercus alba L.) and European oak wood (Quercus robur L.) and quantitative analysis in bourbon whiskey and oak-matured red wines. J. Agric. Food Chem. 2006, 54, 3380–3390. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Tu Anh, T.T.; Mai Van, T.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef]
- Pellati, F.; Bruni, R.; Righi, D.; Grandini, A.; Tognolini, M.; Pio Prencipe, F.; Poli, F.; Benvenuti, S.; Del Rio, D.; Rossi, D. Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharmacol. 2013, 147, 277–285. [Google Scholar] [CrossRef]
- Alagan, A.; Jantan, I.; Kumolosasi, E.; Ogawa, S.; Abdullah, M.A.; Azmi, N. Protective effects of Phyllanthus amarus against lipopolysaccharide-induced neuroinflammation and cognitive impairment in rats. Front. Pharmacol. 2019, 10, 632. [Google Scholar] [CrossRef]
- Odubanjo, V.O.; Ibukun, E.O.; Oboh, G.; Adefegha, S.A. Aqueous extracts of two tropical ethnobotanicals (Tetrapleura tetraptera and Quassia undulata) improved spatial and non-spatial working memories in scopolamine-induced amnesic rats: Influence of neuronal cholinergic and antioxidant systems. Biomed. Pharmacother. 2018, 99, 198–204. [Google Scholar] [CrossRef]
- Siraj, M.A.; Shilpi, J.A.; Hossain, M.G.; Uddin, S.J.; Islam, M.K.; Jahan, I.A.; Hossain, H. Anti-inflammatory and antioxidant activity of Acalypha hispida leaf and analysis of its major bioactive polyphenols by HPLC. Adv. Pharm. Bull. 2016, 6, 275–283. [Google Scholar] [CrossRef]
- Jaramillo-García, V.; Trindade, C.; Lima, E.; Guecheva, T.N.; Villela, I.; Martinez-Lopez, W.; Corrêa, D.S.; Ferraz, A.d.B.F.; Moura, S.; Sosa, M.Q.; et al. Chemical characterization and cytotoxic, genotoxic, and mutagenic properties of Baccharis trinervis (Lam, Persoon) from Colombia and Brazil. J. Ethnopharmacol. 2018, 213, 210–220. [Google Scholar] [CrossRef]
- Hafsa, J.; Hammi, K.M.; Khedher, M.R.B.; Smach, M.A.; Charfeddine, B.; Limem, K.; Majdoub, H. Inhibition of protein glycation, antioxidant and antiproliferative activities of Carpobrotus edulis extracts. Biomed. Pharmacother. 2016, 84, 1496–1503. [Google Scholar] [CrossRef]
- Karimi, E.; Ghorbani Nohooji, M.; Habibi, M.; Ebrahimi, M.; Mehrafarin, A.; Khalighi-Sigaroodi, F. Antioxidant potential assessment of phenolic and flavonoid rich fractions of Clematis orientalis and Clematis ispahanica (Ranunculaceae). Nat. Prod. Res. 2018, 32, 1991–1995. [Google Scholar] [CrossRef]
- Cho, C.H.; Jang, H.; Lee, M.; Kang, H.; Heo, H.J.; Kim, D.O. Sea buckthorn (Hippophae rhamnoides L.) leaf extracts protect neuronal PC-12 cells from oxidative stress. J. Microbiol. Biotechnol. 2017, 27, 1257–1265. [Google Scholar] [CrossRef]
- Vieira, G.S.; Marques, A.S.; Machado, M.T.; Silva, V.M.; Hubinger, M.D. Determination of anthocyanins and non-anthocyanin polyphenols by ultra performance liquid chromatography/electrospray ionization mass spectrometry (UPLC/ESI–MS) in jussara (Euterpe edulis) extracts. J. Food Sci. Technol. 2017, 54, 2135–2144. [Google Scholar] [CrossRef]
- Vu, D.C.; Vo, P.H.; Coggeshall, M.V.; Lin, C.H. Identification and characterization of phenolic compounds in black walnut kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef]
- de Britto Policarpi, P.; Turcatto, L.; Demoliner, F.; Ferrari, R.A.; Bascuñan, V.L.A.F.; Ramos, J.C.; Jachmanián, J.; Vitali, L.; Micke, G.A.; Block, J.M. Nutritional potential, chemical profile and antioxidant activity of Chichá (Sterculia striata) nuts and its by-products. Food Res. Int. 2018, 106, 736–744. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Mullen, W.; Crozier, A. Comparison of the polyphenolic composition and antioxidant activity of European commercial fruit juices. Food Funct. 2010, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Vegara, S.; Martí, N.; Lorente, J.; Coll, L.; Streitenberger, S.; Valero, M.; Saura, D. Chemical guide parameters for Punica granatum cv.‘Mollar’fruit juices processed at industrial scale. Food Chem. 2014, 147, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Inada, K.O.P.; Duarte, P.A.; Lapa, J.; Miguel, M.A.L.; Monteiro, M. Jabuticaba (Myrciaria jaboticaba) juice obtained by steam-extraction: Phenolic compound profile, antioxidant capacity, microbiological stability, and sensory acceptability. J. Food Sci. Technol. 2018, 55, 52–61. [Google Scholar] [CrossRef]
- Teixeira, L.L.; Costa, G.R.; Dörr, F.A.; Ong, T.P.; Pinto, E.; Lajolo, F.M.; Hassimotto, N.M.A. Potential Antiproliferative Activity of Polyphenol Metabolites against Human Breast Cancer Cells and Their Urine Excretion Pattern in Healthy Subjects Following Acute Intake of a Polyphenol-Rich Juice of Grumixama (Eugenia brasiliensis Lam.). Food Funct. 2017, 8, 2266–2274. [Google Scholar] [CrossRef]
- Lee, J.H.; Talcott, S.T. Ellagic acid and ellagitannins affect on sedimentation in muscadine juice and wine. J. Agric. Food Chem. 2002, 50, 3971–3976. [Google Scholar] [CrossRef]
- Garcia-Estevez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Validation of a mass spectrometry method to quantify oak ellagitannins in wine samples. J. Agric. Food Chem. 2012, 60, 1373–1379. [Google Scholar] [CrossRef]
- Sanz, M.; de Simón, B.F.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Hernández, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Ascacio-Valdés, J.A.; Gironés-Vilaplana, A.; Del Rio, D.; Moreno, D.A.; García-Viguera, C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly) phenolic compounds. Food Chem. 2014, 145, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Papapostolou, H.; Vlysidis, A.; Gardeli, C.; Komaitis, M.; Papanikolaou, S.; Koutinas, A.A. Extraction of phenolic compounds and succinic acid production from spent sulphite liquor. Chem. Technol. Biotechnol. 2016, 91, 2751–2760. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viskelis, P.; Bobinas, Č.; Mieželienė, A.; Alenčikienė, G.; Venskutonis, P.R. Raspberry marc extracts increase antioxidative potential, ellagic acid, ellagitannin and anthocyanin concentrations in fruit purees. LWT-Food Sci. Technol. 2016, 66, 460–467. [Google Scholar] [CrossRef]
- Sójka, M.; Klimczak, E.; Macierzyński, J.; Kołodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur. Food Res. Technol. 2013, 237, 995–1007. [Google Scholar] [CrossRef]
- Da Silva Pinto, M.; Lajolo, F.M.; Genovese, M.I. Bioactive compounds and antioxidant capacity of strawberry jams. Plant Foods Hum. Nutr. 2007, 62, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Widyawati, P.S.; Dwi, T.; Budianta, W.; Kusuma, F.A.; Wijaya, E.L. Difference of Solvent Polarity to Phytochemical Content and Antioxidant Activity of Pluchea indicia Less Leaves Extracts. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 850–855. [Google Scholar]
- Arapitsas, P. Hydrolyzable tannin analysis in food. Food Chem. 2012, 135, 1708–1717. [Google Scholar] [CrossRef]
- Theocharis, G.; Andlauer, W. Innovative microwave-assisted hydrolysis of ellagitannins and quantification as ellagic acid equivalents. Food Chem. 2013, 138, 2430–2434. [Google Scholar] [CrossRef]
- Abdulla, R.; Mansur, S.; Lai, H.; Ubul, A.; Sun, G.; Huang, G.; Aisa, H.A. Qualitative analysis of polyphenols in macroporous resin pretreated pomegranate husk extract by HPLC-QTOF-MS. Phytochem. Anal. 2017, 28, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Dong, X.; Guo, M. Phenolic profiling of Duchesnea Indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, O.D.; Kulkarni, Y.A. Mini-review of analytical methods used in quantification of ellagic acid. Rev. Anal. Chem. 2020, 39, 31–44. [Google Scholar] [CrossRef]
- Furuuchi, R.; Yokoyama, T.; Watanabe, Y.; Hirayama, M. Identification and quantification of short oligomeric proanthocyanidins and other polyphenols in boysenberry seeds and juice. J. Agric. Food Chem. 2011, 59, 3738–3746. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Groothuis, S.F.; Prencipe, F.P.; Amir, R.; Benvenuti, S.; Pellati, F. Metabolite fingerprinting of Punica granatum L. (pomegranate) polyphenols by means of high-performance liquid chromatography with diode array and electrospray ionization-mass spectrometry detection. J. Chromatogr. A 2017, 1480, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the major phenolic compounds in pomegranate juices by HPLC–DAD–ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Kontoudakis, N.; Canals, J.M.; García-Romero, E.; Gómez-Alonso, S.; Zamora, F.; Hermosín-Gutiérrez, I. Improved method for the extraction and chromatographic analysis on a fused-core column of ellagitannins found in oak-aged wine. Food Chem. 2017, 226, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Godiyal, S.; Laddha, K. Validated high-performance thin-layer chromatographic method for quantification of gallic acid and ellagic acid in fruits of Terminalia chebula, Phyllanthus emblica, and Quercus infectoria. J. Sep. Sci. 2023, 46, 2200991. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated method for the characterization and quantification of extractable and nonextractable ellagitannins after acid hydrolysis in pomegranate fruits, juices, and extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Topalović, A.; Knežević, M.; Gačnik, S.; Mikulic-Petkovsek, M. Detailed chemical composition of juice from autochthonous pomegranate genotypes (Punica granatum L.) grown in different locations in Montenegro. Food Chem. 2020, 330, 127261. [Google Scholar] [CrossRef]
- Da Silva Pinto, M.; Lajolo, F.M.; Genovese, M.I. Bioactive compounds and quantification of total ellagic acid in strawberries (Fragaria × Ananassa Duch.). Food Chem. 2008, 107, 1629–1635. [Google Scholar] [CrossRef]
- Hejduk, A.; Sójka, M.; Klewicki, R. Stability of ellagitannins in processing products of selected Fragaria fruit during 12 months of storage. Food Sci. Nutr. 2023, 11, 1354–1366. [Google Scholar] [CrossRef] [PubMed]
- Gasperotti, M.; Masuero, D.; Vrhovsek, U.; Guella, G.; Mattivi, F. Profiling and accurate quantification of Rubus ellagitannins and ellagic acid conjugates using direct UPLC-Q-TOF HDMS and HPLC-DAD analysis. J. Agric. Food Chem. 2010, 58, 4602–4616. [Google Scholar] [CrossRef] [PubMed]
- Gudej, J.; Tomczyk, M. Determination of flavonoids, tannins and ellagic acid in leaves from Rubus L. species. Arch. Pharm. Res. 2004, 27, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Richard-Dazeur, C.; Jacolot, P.; Niquet-Léridon, C.; Goethals, L.; Barbezier, N.; Anton, P.M. HPLC-DAD Optimization of quantification of vescalagin, gallic and ellagic acid in chestnut tannins. Heliyon 2023, 9, e18993. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, G.; Demirbag, C.; Bahsi, I.; Ozgul, L.; Alkaya, D.B.; Onurlu, H.I.; Seyhan, S.A. Determination of Ellagic Acid in the Wastes of Walnut, Chestnut, and Pomegranate Grown in Turkey. ACS Symposium Series; Jayaprakasha, G.K., Patil, B.S., Gattuso, G., Eds.; American Chemical Society: Washington, DC, USA, 2018, 2018; Volume 1286, pp. 81–103. ISBN 9780841232969. [Google Scholar]
- Kumar, N.; Pratibha; Neeraj; Sami, R.; Khojah, E.; Aljahani, A.H.; Al-Mushhin, A.A.M. Effects of drying methods and solvent extraction on quantification of major bioactive compounds in pomegranate peel waste using HPLC. Sci. Rep. 2022, 12, 8000. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria × Ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef]
- Michel, J.; Jourdes, M.; Silva, M.A.; Giordanengo, T.; Mourey, N.; Teissedre, P.-L. Impact of concentration of ellagitannins in oak wood on their levels and organoleptic influence in red wine. J. Agric. Food Chem. 2011, 59, 5677–5683. [Google Scholar] [CrossRef]
- Kool, M.M.; Comeskey, D.J.; Cooney, J.M.; McGhie, T.K. Structural identification of the main ellagitannins of a boysenberry (Rubus loganbaccus × baileyanus Britt.) extract by LC–ESI-MS/MS, MALDI-TOF-MS and NMR spectroscopy. Food Chem. 2010, 119, 1535–1543. [Google Scholar] [CrossRef]
- Akter, S.; Hong, H.; Netzel, M.; Tinggi, U.; Fletcher, M.; Osborne, S.; Sultanbawa, Y. Determination of ellagic acid, punicalagin, and castalagin from Terminalia ferdinandiana (Kakadu Plum) by a validated UHPLC-PDA-MS/MS methodology. Food Anal. Methods 2021, 14, 2534–2544. [Google Scholar] [CrossRef]
- Kula, M.; Majdan, M.; Głód, D.; Krauze-Baranowska, M. Phenolic composition of fruits from different cultivars of red and black raspberries grown in poland. J. Food Compos. Anal. 2016, 52, 74–82. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, J.; Yang, Z.; Gao, F.; Qi, P.; Li, X.; Zhu, F.; Li, J.; Zhang, J. Determination of ellagic acid in wine by solid-phase extraction–ultra-high performance liquid chromatography–tandem mass spectrometry. Food Anal. Methods 2019, 12, 1103–1110. [Google Scholar] [CrossRef]
- Shahzad, M.N.; Ahmad, S.; Tousif, M.I.; Ahmad, I.; Rao, H.; Ahmad, B.; Basit, A. Profiling of phytochemicals from aerial parts of Terminalia Neotaliala using LC-ESI-MS2 and determination of antioxidant and enzyme inhibition activities. PLoS ONE 2022, 17, e0266094. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.; Campo, M.; Bellumori, M.; Cecchi, L.; Vignolini, P.; Innocenti, M.; Mulinacci, N. Tannins from different parts of the chestnut trunk (Castanea sativa Mill.): A green and effective extraction method and their profiling by high-performance liquid chromatography-diode array detector-mass spectrometry. ACS Food Sci. Technol. 2023, 3, 1903–1912. [Google Scholar] [CrossRef]
- Seyhan, S.; Yalcin, G.; Seyhan, S.A. The extraction and determination of ellagic acid and resveratrol in blueberry species by HPLC-DAD and LC-MS/MS. J. Res. Pharm. 2023, 27, 311–320. [Google Scholar] [CrossRef]
- De Andrade Neves, N.; César Stringheta, P.; Ferreira Da Silva, I.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Identification and quantification of phenolic composition from different species of Jabuticaba (Plinia Spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021, 355, 129605. [Google Scholar] [CrossRef] [PubMed]
- Oracz, J.; Żyżelewicz, D.; Pacholczyk-Sienicka, B. UHPLC-DAD-ESI-HRMS/MS profile of phenolic compounds in northern red oak (Quercus rubra L., Syn. Q. Borealis F. Michx) seeds and its transformation during thermal processing. Ind. Crops Prod. 2022, 189, 115860. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Karkanis, A.; Fernandes, Â.; Petropoulos, S.A.; Calhelha, R.; Petrović, J.; Soković, M.; Rosa, E.; Barros, L.; Ferreira, I.C.F.R. Bioactive properties of Sanguisorba minor L. cultivated in central Greece under different fertilization regimes. Food Chem. 2020, 327, 127043. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Z.; Li, X.; Zhang, J.; Hu, X. Analysis of ellagic acid in pomegranate rinds by capillary electrophoresis and high-performance liquid chromatography. Phytochem. Anal. 2008, 19, 86–89. [Google Scholar] [CrossRef]
- Costa, E.V.; Lima, D.L.D.; Evtyugin, D.V.; Esteves, V.I. Development and application of a capillary electrophoresis method for the determination of ellagic acid in E. Globulus wood and in filtrates from E. Globulus kraft pulp. Wood Sci. Technol. 2014, 48, 99–108. [Google Scholar] [CrossRef]
- Spisso, A.; Gomez, F.J.V.; Fernanda Silva, M. Determination of ellagic acid by capillary electrophoresis in Argentinian wines. Electrophoresis 2018, 39, 1621–1627. [Google Scholar] [CrossRef]
- Wojciechowska, O.; Kujawska, M. Urolithin A in health and diseases: Prospects for Parkinson’s disease management. Antioxidants 2023, 12, 1479. [Google Scholar] [CrossRef] [PubMed]
- Raimundo, A.F.; Ferreira, S.; Tomás-Barberán, F.A.; Santos, C.N.; Menezes, R. Urolithins: Diet-derived bioavailable metabolites to tackle diabetes. Nutrients 2021, 13, 4285. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2023, 89, 101109. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef]
- García-Villalba, R.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Identification of novel urolithin metabolites in human feces and urine after the intake of a pomegranate extract. J. Agric. Food Chem. 2019, 67, 11099–11107. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Romo-Vaquero, M.; García-Villalba, R.; González-Sarrías, A.; Beltrán, D.; Tomás-Barberán, F.A.; Espín, J.C.; Selma, M.V. Interindividual variability in the human metabolism of ellagic acid: Contribution of Gordonibacter to urolithin production. J. Funct. Foods 2015, 17, 785–791. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; González-Sarrías, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramírez-de-Molina, A.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Aguirre, C.E.; García-Villalba, R.; Beltrán, D.; Frutos-Lisón, M.D.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Gut bacteria involved in ellagic acid metabolism to yield human urolithin metabotypes revealed. J. Agric. Food Chem. 2023, 71, 4029–4035. [Google Scholar] [CrossRef]
- Beltrán, D.; Frutos-Lisón, M.D.; García-Villalba, R.; Yuste, J.E.; García, V.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. NMR spectroscopic identification of urolithin G, a novel trihydroxy urolithin produced by human intestinal Enterocloster Species. J. Agric. Food Chem. 2023, 71, 11921–11928. [Google Scholar] [CrossRef]
- Milala, J.; Kosmala, M.; Karlińska, E.; Juśkiewicz, J.; Zduńczyk, Z.; Fotschki, B. Ellagitannins from strawberries with different degrees of polymerization showed different metabolism through gastrointestinal tract of rats. J. Agric. Food Chem. 2017, 65, 10738–10748. [Google Scholar] [CrossRef]
- Żary-Sikorska, E.; Kosmala, M.; Milala, J.; Fotschki, B.; Ognik, K.; Juśkiewicz, J. Concentrations of blood serum and urinal ellagitannin metabolites depend largely on the post-intake time and duration of strawberry phenolics ingestion in rats. Pol. J. Food Nutr. Sci. 2019, 69, 379–386. [Google Scholar] [CrossRef]
- Lin, I.-C.; Wu, J.-Y.; Fang, C.-Y.; Wang, S.-C.; Liu, Y.-W.; Ho, S.-T. Absorption and metabolism of urolithin A and ellagic acid in mice and their cytotoxicity in human colorectal cancer cells. Evid. Based Complement. Alternat. Med. 2023, 2023, 8264716. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aronson, W.J.; Zhang, Y.; Henning, S.M.; Moro, A.; Lee, R.; Sartippour, M.; Harris, D.M.; Rettig, M.; Suchard, M.A.; et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007, 55, 7732–7737. [Google Scholar] [CrossRef]
- González-Barrio, R.; Truchado, P.; Ito, H.; Espín, J.C.; Tomás-Barberán, F.A. UV and MS Identification of urolithins and nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals. J. Agric. Food Chem. 2011, 59, 1152–1162. [Google Scholar] [CrossRef]
- González-Barrio, R.; Truchado, P.; García-Villalba, R.; Hervás, G.; Frutos, P.; Espín, J.C.; Tomás-Barberán, F.A. Metabolism of oak leaf ellagitannins and urolithin production in beef cattle. J. Agric. Food Chem. 2012, 60, 3068–3077. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Barrio, R.; Cerdá, B.; López-Bote, C.; Rey, A.I.; Tomás-Barberán, F.A. Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J. Agric. Food Chem. 2007, 55, 10476–10485. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, J.; Xiao, D.; Edirisinghe, I.; Burton-Freeman, B.M.; Sandhu, A.K. Pharmacokinetic evaluation of red raspberry (poly)phenols from two doses and association with metabolic indices in adults with prediabetes and insulin resistance. J. Agric. Food Chem. 2021, 69, 9238–9248. [Google Scholar] [CrossRef]
- Moreno Uclés, R.; González-Sarrías, A.; Espín, J.C.; Tomás-Barberán, F.A.; Janes, M.; Cheng, H.; Finley, J.; Greenway, F.; Losso, J.N. Effects of red raspberry polyphenols and metabolites on the biomarkers of inflammation and insulin resistance in type 2 diabetes: A pilot study. Food Funct. 2022, 13, 5166–5176. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, A.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Functional deficits in gut microbiome of young and middle-aged adults with prediabetes apparent in metabolizing bioactive (poly)phenols. Nutrients 2020, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Grainger, E.M.; Thomas-Ahner, J.M.; Hinton, A.; Gu, J.; Riedl, K.; Vodovotz, Y.; Abaza, R.; Schwartz, S.J.; Clinton, S.K. Dose-dependent increases in ellagitannin metabolites as biomarkers of intake in humans consuming standardized black raspberry food products designed for clinical trials. Mol. Nutr. Food Res. 2020, 64, 1900800. [Google Scholar] [CrossRef]
- Henning, S.M.; Seeram, N.P.; Zhang, Y.; Li, L.; Gao, K.; Lee, R.-P.; Wang, D.C.; Zerlin, A.; Karp, H.; Thames, G.; et al. Strawberry consumption is associated with increased antioxidant capacity in serum. J. Med. Food 2010, 13, 116–122. [Google Scholar] [CrossRef]
- Sandhu, A.K.; Miller, M.G.; Thangthaeng, N.; Scott, T.M.; Shukitt-Hale, B.; Edirisinghe, I.; Burton-Freeman, B. Metabolic fate of strawberry polyphenols after chronic intake in healthy older adults. Food Funct. 2018, 9, 96–106. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Álvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A.; et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef]
- Pfundstein, B.; Haubner, R.; Würtele, G.; Gehres, N.; Ulrich, C.M.; Owen, R.W. Pilot walnut intervention study of urolithin bioavailability in human volunteers. J. Agric. Food Chem. 2014, 62, 10264–10273. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; García-Villalba, R.; García-Mantrana, I.; Rodríguez-Varela, A.; Romo-Vaquero, M.; Collado, M.C.; Tomás-Barberán, F.A.; Espín, J.C.; Selma, M.V. Urolithins in human breast milk after walnut intake and kinetics of Gordonibacter colonization in newly born: The role of mothers’ urolithin metabotypes. J. Agric. Food Chem. 2020, 68, 12606–12616. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A. Chromatographic and spectroscopic characterization of urolithins for their determination in biological samples after the intake of foods containing ellagitannins and ellagic acid. J. Chromatogr. A 2016, 1428, 162–175. [Google Scholar] [CrossRef] [PubMed]
- García-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espín, J.C.; Selma, M.V.; Collado, M.C. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals by tracking walnuts consumption over three days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hooft, J.J.J.; De Vos, R.C.H.; Mihaleva, V.; Bino, R.J.; Ridder, L.; De Roo, N.; Jacobs, D.M.; Van Duynhoven, J.P.M.; Vervoort, J. Structural elucidation and quantification of phenolic conjugates present in human urine after tea intake. Anal. Chem. 2012, 84, 7263–7271. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Dunngalvin, G.; Kern, T.; Blanco-Bose, W.; Auwerx, J.; Aebischer, P.; Rinsch, C. Direct supplementation with urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr. 2022, 76, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Iglesias-Aguirre, C.E.; Meoro, A.; Selma, M.V.; Espín, J.C. Pharmacological therapy determines the gut microbiota modulation by a pomegranate extract nutraceutical in metabolic syndrome: A randomized clinical trial. Mol. Nutr. Food Res. 2021, 65, 2001048. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 h. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Lee, R.-P.; Huang, J.; Thames, G.; Korn, M.; Ben-Nissan, D.; Heber, D.; Li, Z. Pomegranate juice alters the microbiota in breast milk and infant stool: A pilot study. Food Funct. 2022, 13, 5680–5689. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Jilma-Stohlawetz, P.; Rios, J.; Hingorani, L.; Derendorf, H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J. Agric. Food Chem. 2006, 54, 8956–8961. [Google Scholar] [CrossRef]
- Seeram, N.P.; Zhang, Y.; McKeever, R.; Henning, S.M.; Lee, R.; Suchard, M.A.; Li, Z.; Chen, S.; Thames, G.; Zerlin, A.; et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J. Med. Food 2008, 11, 390–394. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, C.; Hernández, L.; Pérez, A.; Vaillant, F. Diversity of urinary excretion patterns of main ellagitannins’ colonic metabolites after ingestion of tropical highland blackberry (Rubus adenotrichus) Juice. Food Res. Inter. 2014, 55, 161–169. [Google Scholar] [CrossRef]
- Gu, J.; Thomas-Ahner, J.M.; Riedl, K.M.; Bailey, M.T.; Vodovotz, Y.; Schwartz, S.J.; Clinton, S.K. Dietary black raspberries impact the colonic microbiome and phytochemical metabolites in mice. Mol. Nutr. Food Res. 2019, 63, 1800636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, P.-K.; Wong, H.-C.; Zhao, D. Oral supplementation of Gordonibacter Urolithinfaciens promotes ellagic acid metabolism and urolithin bioavailability in mice. Food Chem. 2024, 437, 137953. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Jourdes, M.; Kurpik, M.; Szulc, M.; Szaefer, H.; Chmielarz, P.; Kreiner, G.; Krajka-Kuźniak, V.; Mikołajczak, P.Ł.; Teissedre, P.-L.; et al. Neuroprotective effects of pomegranate juice against Parkinson’s disease and presence of ellagitannins-derived metabolite—Urolithin A—In the brain. Int. J. Mol. Sci. 2019, 21, 202. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.M.; Grainger, E.M.; Thomas-Ahner, J.M.; Hinton, A.; Gu, J.; Riedl, K.M.; Vodovotz, Y.; Abaza, R.; Schwartz, S.J.; Clinton, S.K. Application of a low polyphenol or low ellagitannin dietary intervention and its impact on ellagitannin metabolism in men. Mol. Nutr. Food Res. 2017, 61, 1600224. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Urpi-Sarda, M.; García-Villalba, R.; Rabassa, M.; López-Uriarte, P.; Bulló, M.; Jáuregui, O.; Tomás-Barberán, F.; Salas-Salvadó, J.; Espín, J.C.; et al. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J. Agric. Food Chem. 2012, 60, 8930–8940. [Google Scholar] [CrossRef] [PubMed]
- Jurgoński, A.; Juśkiewicz, J.; Fotschki, B.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Grzelak-Błaszczyk, K.; Markiewicz, L. Metabolism of strawberry mono- and dimeric ellagitannins in rats fed a diet containing fructo-oligosaccharides. Eur. J. Nutr. 2017, 56, 853–864. [Google Scholar] [CrossRef]
- Kosmala, M.; Jurgoński, A.; Juśkiewicz, J.; Karlińska, E.; Macierzyński, J.; Rój, E.; Zduńczyk, Z. Chemical composition of blackberry press cake, polyphenolic extract, and defatted seeds, and their effects on cecal fermentation, bacterial metabolites, and blood lipid profile in rats. J. Agric. Food Chem. 2017, 65, 5470–5479. [Google Scholar] [CrossRef]
- Mosele, J.I.; Gosalbes, M.; Macià, A.; Rubió, L.; Vázquez-Castellanos, J.F.; Jiménez Hernández, N.; Moya, A.; Latorre, A.; Motilva, M. Effect of daily intake of pomegranate juice on fecal microbiota and feces metabolites from healthy volunteers. Mol. Nutr. Food Res. 2015, 59, 1942–1953. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Lee, R.-P.; Lu, Q.-Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.-H.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef]
- Aichinger, G.; Stevanoska, M.; Beekmann, K.; Sturla, S.J. Physiologically-based pharmacokinetic modeling of the postbiotic supplement urolithin A predicts its bioavailability is orders of magnitude lower than concentrations that induce toxicity, but also neuroprotective effects. Mol. Nutr. Food Res. 2023, 67, 2300009. [Google Scholar] [CrossRef] [PubMed]
- Dacrema, M.; Sommella, E.; Santarcangelo, C.; Bruno, B.; Marano, M.G.; Insolia, V.; Saviano, A.; Campiglia, P.; Stornaiuolo, M.; Daglia, M. Metabolic profiling, in vitro bioaccessibility and in vivo bioavailability of a commercial bioactive Epilobium angustifolium L. extract. Biomed. Pharmacother. 2020, 131, 110670. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Mondal, G.; Khan, W.; Gurley, B.J.; Yates, C.R. Development of a liquid chromatography-tandem mass spectrometry (LC–MS/MS) method for characterizing pomegranate extract pharmacokinetics in humans. J. Pharm. Biomed. Anal. 2023, 233, 115477. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, K.; Zhang, H.; Li, Y.; Han, L.; Liu, H.; Wang, M. The profile of buckwheat tannins based on widely targeted metabolome analysis and pharmacokinetic study of ellagitannin metabolite urolithin A. LWT-Food Sci. Technol. 2022, 156, 113069. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, S.; Ye, Q.; Hou, X.; Yang, G.; Lu, J.; Hai, Y.; Shen, J.; Fang, Y. A novel streptococcus thermophilus FUA329 isolated from human breast milk capable of producing urolithin A from ellagic acid. Foods 2022, 11, 3280. [Google Scholar] [CrossRef]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Rodrigues, R.F.; Maróstica Junior, M.R.; Fonseca, B.D.S.; De Menezes, C.R.; et al. Bioaccessibility and catabolism of phenolic compounds from Jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2020, 65, 103714. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time course production of urolithins from ellagic acid by human gut microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Frutos, M.D.; Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Metabolism of different dietary phenolic compounds by the urolithin-producing human-gut bacteria Gordonibacter urolithinfaciens and Ellagibacter isourolithinifaciens. Food Funct. 2020, 11, 7012–7022. [Google Scholar] [CrossRef]
- Zhang, M.; Cui, S.; Mao, B.; Zhang, Q.; Zhao, J.; Tang, X.; Chen, W. Urolithin A produced by novel microbial fermentation possesses anti-aging effects by improving mitophagy and reducing reactive oxygen species in Caenorhabditis elegans. J. Agric. Food Chem. 2023, 71, 6348–6357. [Google Scholar] [CrossRef]
- Liu, Q.; Hua, Z.; Chen, M.; Liu, S.; Ahmed, S.; Hou, X.; Yang, G.; Fang, Y. Changes in polyphenols and antioxidant properties of pomegranate peels fermented by urolithin A-producing Streptococcus thermophilus FUA329. ACS Food Sci. Technol. 2023, 3, 1383–1392. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; Quifer-Rada, P.; Marhuenda-Muñoz, M.; Arancibia-Riveros, C.; Vallverdú-Queralt, A.; Tresserra-Rimbau, A.; Ruiz-León, A.M.; Casas, R.; Estruch, R.; Bodega, P.; et al. Microbial phenolic metabolites in urine are inversely linked to certain features of metabolic syndrome in spanish adolescents. Antioxidants 2022, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B.M. Plasma and urinary (poly)phenolic profiles after 4-week red raspberry (Rubus idaeus L.) intake with or without fructo-oligosaccharide supplementation. Molecules 2020, 25, 4777. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Leoni, G.; Maldini, M.; Natarelli, L.; Comitato, R.; Schonlau, F.; Virgili, F.; Canali, R. Absorption, metabolism, and effects at transcriptome level of a standardized french oak wood extract, robuvit, in healthy volunteers: Pilot study. J. Agric. Food Chem. 2014, 62, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Yang, J.; Cui, J.; Fan, Y.; Li, N.; Wang, C.; Liu, Y.; Dong, Y. Stability and mechanism of phenolic compounds from raspberry extract under in vitro gastrointestinal digestion. LWT-Food Sci. Technol. 2021, 139, 110552. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Mattivi, F.; Vrhovsek, U. Development of a targeted method for twenty-three metabolites related to polyphenol gut microbial metabolism in biological samples, using SPE and UHPLC–ESI-MS/MS. Talanta 2014, 128, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Bayle, M.; Roques, C.; Marion, B.; Audran, M.; Oiry, C.; Bressolle-Gomeni, F.M.M.; Cros, G. Development and validation of a liquid chromatography-electrospray ionization-tandem mass spectrometry method for the determination of urolithin C in rat plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2016, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.M.; Toribio, L.; García-Villalba, R.; Villalgordo, J.M.; Althobaiti, Y.; Tomás-Barberán, F.A.; Bernal, J. Separation of isomeric forms of urolithin glucuronides using supercritical fluid chromatography. J. Agric. Food Chem. 2023, 71, 3033–3039. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef]
- Jiménez-Loygorri, J.I.; Villarejo-Zori, B.; Viedma-Poyatos, Á.; Zapata-Muñoz, J.; Benítez-Fernández, R.; Frutos-Lisón, M.D.; Tomás-Barberán, F.A.; Espín, J.C.; Area-Gómez, E.; Gomez-Duran, A.; et al. Mitophagy curtails cytosolic mtDNA-dependent activation of cGAS/STING inflammation during aging. Nat. Commun. 2024, 15, 830. [Google Scholar] [CrossRef]
- Qin, X.; Li, H.; Zhao, H.; Fang, L.; Wang, X. Enhancing healthy aging with small molecules: A mitochondrial perspective. Med. Res. Rev, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Tang, L.; Mo, Y.; Li, Y.; Zhong, Y.; He, S.; Zhang, Y.; Tang, Y.; Fu, S.; Wang, X.; Chen, A. Urolithin A alleviates myocardial ischemia/reperfusion injury via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2017, 486, 774–780. [Google Scholar] [CrossRef]
- Cui, G.H.; Chen, W.Q.; Shen, Z.Y. Urolithin A shows anti-atherosclerotic activity via activation of class B scavenger receptor and activation of Nef2 signaling pathway. Pharmacol. Rep. 2018, 70, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Savi, M.; Bocchi, L.; Mena, P.; Dall’Asta, M.; Crozier, A.; Brighenti, F.; Stilli, D.; Del Rio, D. In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflamm. 2019, 16, 62. [Google Scholar] [CrossRef]
- Shen, P.X.; Li, X.; Deng, S.Y.; Zhao, L.; Zhang, Y.Y.; Deng, X.; Han, B.; Yu, J.; Li, Y.; Wang, Z.Z.; et al. Urolithin A ameliorates experimental autoimmune encephalomyelitis by targeting aryl hydrocarbon receptor. EBioMedicine 2021, 64, 103227. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, A.; Zheng, Y.R.; Wu, X.L.; Tang, W.D.; Liu, M.R.; Ma, S.J.; Jiang, L.; Hu, W.W.; Zhang, X.N.; Chen, Z. Urolithin A-activated autophagy but not mitophagy protects against ischemic neuronal injury by inhibiting ER stress in vitro and in vivo. CNS Neurosci. Ther. 2019, 25, 976–986. [Google Scholar] [CrossRef]
- Madsen, H.B.; Park, J.H.; Chu, X.; Hou, Y.; Li, Z.; Rasmussen, L.J.; Croteau, D.L.; Bohr, V.A.; Akbari, M. The cGAS-STING signaling pathway is modulated by urolithin A. Mech. Ageing Dev. 2024, 217, 111897. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on inflammatory bowel disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, S.; Zhang, H.; Jo, Y.; Kang, J.S.; Ha, K.T.; Joo, J.; Lee, H.J.; Ryu, D. Gut microbiota-generated metabolites: Missing puzzles to hosts’ health, diseases, and aging. BMB Rep. 2024, 6194. [Google Scholar] [CrossRef] [PubMed]
- Heilman, J.; Andreux, P.; Tran, N.; Rinsch, C.; Blanco-Bose, W. Safety assessment of urolithin A, a metabolite produced by the human gut microbiota upon dietary intake of plant derived ellagitannins and ellagic acid. Food Chem. Toxicol. 2017, 108, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharma. 2016, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Andreux, P.A.; Blanco-Bose, W.; Ryu, D.; Burdet, F.; Ibberson, M.; Aebischer, P.; Auwerx, J.; Singh, A.; Rinsch, C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat. Metab. 2019, 1, 595–603. [Google Scholar] [CrossRef] [PubMed]
- FDA. GRAS Notice GRN No. 791: Urolithin A; US Food and Drug Administration: Silver Spring, MD, USA, 2018. [Google Scholar]
- Liu, S.; D’Amico, D.; Shankland, E.; Bhayana, S.; Garcia, J.M.; Aebischer, P.; Rinsch, C.; Singh, A.; Marcinek, D.J. Effect of urolithin A supplementation on muscle endurance and mitochondrial health in older adults: A randomized clinical trial. JAMA Netw. Open 2022, 5, e2144279. [Google Scholar] [CrossRef]
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A improves muscle strength, exercise performance, and biomarkers of mitochondrial health in a randomized trial in middle-aged adults. Cell Rep. Med. 2022, 3, 100633. [Google Scholar] [CrossRef]
- Ghadimi, M.; Foroughi, F.; Hashemipour, S.; Rashidi Nooshabadi, M.; Ahmadi, M.H.; Ahadi Nezhad, B.; Khadem Haghighian, H. Randomized double-blind clinical trial examining the ellagic acid effects on glycemic status, insulin resistance, antioxidant, and inflammatory factors in patients with type 2 diabetes. Phytother. Res. 2021, 35, 1023–1032. [Google Scholar] [CrossRef]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- Patel, C.; Dadhaniya, P.; Hingorani, L.; Soni, M.G. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2008, 46, 2728–2735. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Metabolism of ellagitannins from Jabuticaba (Myrciaria jaboticaba) in normoweight, overweight and obese Brazilians: Unexpected laxative effects influence urolithins urinary excretion and metabotype distribution. J. Funct. Foods 2019, 57, 299–308. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Chakkalakal, M.; Pan, A.; Nadora, D.; Min, M.; Dumont, A.; Burney, W.A.; Chambers, C.J. Prospective randomized, double-blind, placebo-controlled study of a standardized oral pomegranate extract on the gut microbiome and short-chain fatty acids. Foods 2023, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, M.; Nadora, D.; Gahoonia, N.; Dumont, A.; Burney, W.; Pan, A.; Chambers, C.J.; Sivamani, R.K. Prospective randomized double-blind placebo-controlled study of oral pomegranate extract on skin wrinkles, biophysical features, and the gut-skin axis. J. Clin. Med. 2022, 11, 6724. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).