Phytochemical Identification and Anti-Oxidative Stress Effects Study of Cimicifugae Rhizoma Extract and Its Major Component Isoferulic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Extract Samples Preparation and Components Identification
2.2.1. HPLC Parameters and Cimicifugae Rhizoma Extract Preparation
2.2.2. UPLC/Q-TOF-MS Parameters and Sample Preparations
2.3. Cell Cultures and Treatment
2.4. MTT Assay on Cell Viability
2.5. Transwell Test for Podocyte Migration
2.6. Podocyte Adhesion Ability Test
2.7. Determination and of ROS Levels
2.8. 4′,6-Diamidino-2-phenylindole (DAPI) Staining and Flow Cytometry Apoptosis
2.9. Enzyme Linked Immunosorbent Assay (ELISA) Assay
2.10. Observation of Actin-Tracker Red-594 Phalloidin Staining Podocyte Cytoskeleton
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
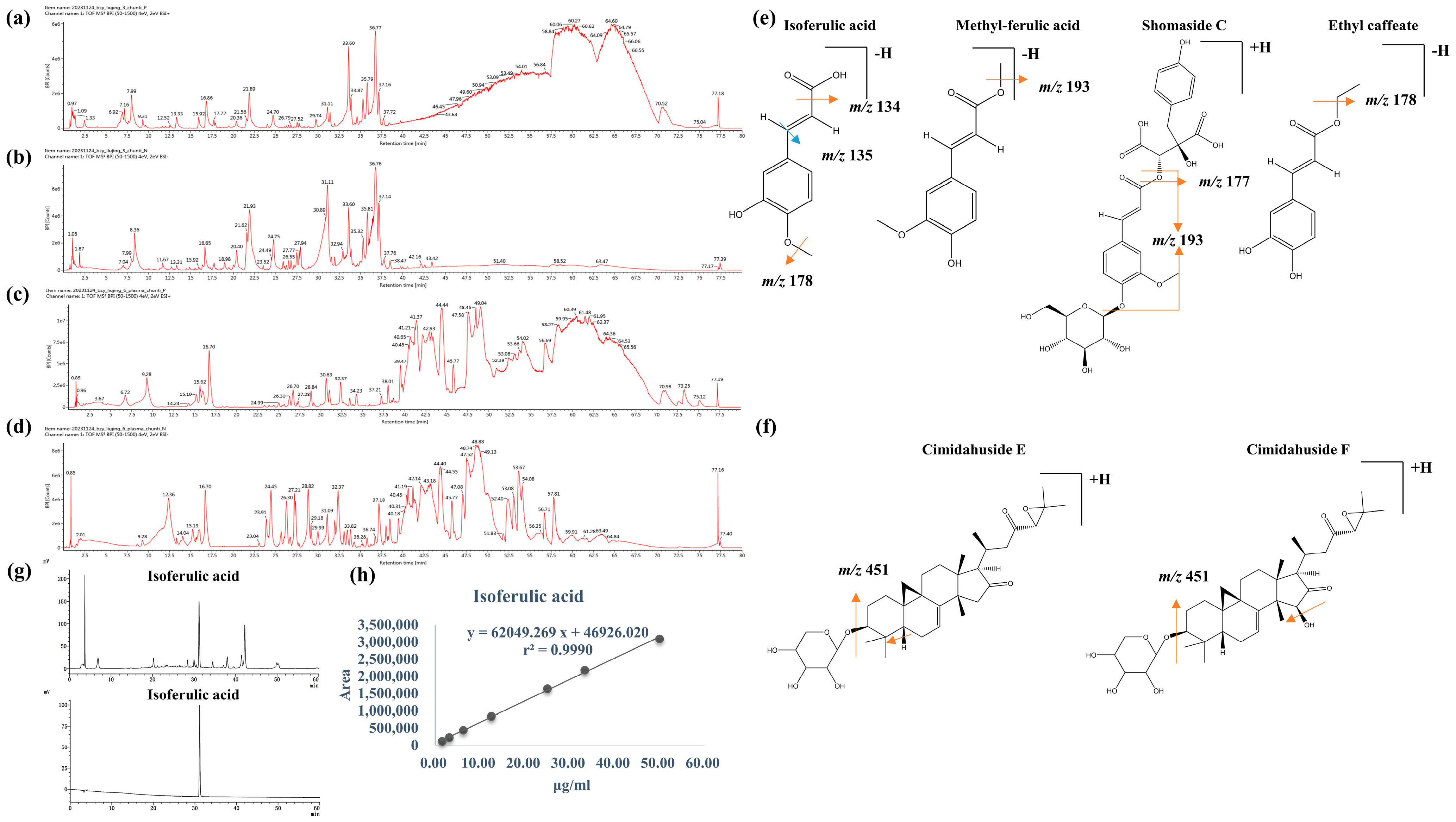
3.1. Identification of the Components in Cimicifugae Rhizoma Extract by UPLC-MS/MS and Quantification of Isoferulic Acid by HPLC
3.1.1. Identification of Phenolic Acids in Cimicifugae Rhizoma Extract
3.1.2. Identification of Triterpene in Cimicifugae Rhizoma Extract
3.1.3. Identification of Isoferulic Acid Content in Cimicifugae Rhizoma Extract by HPLC
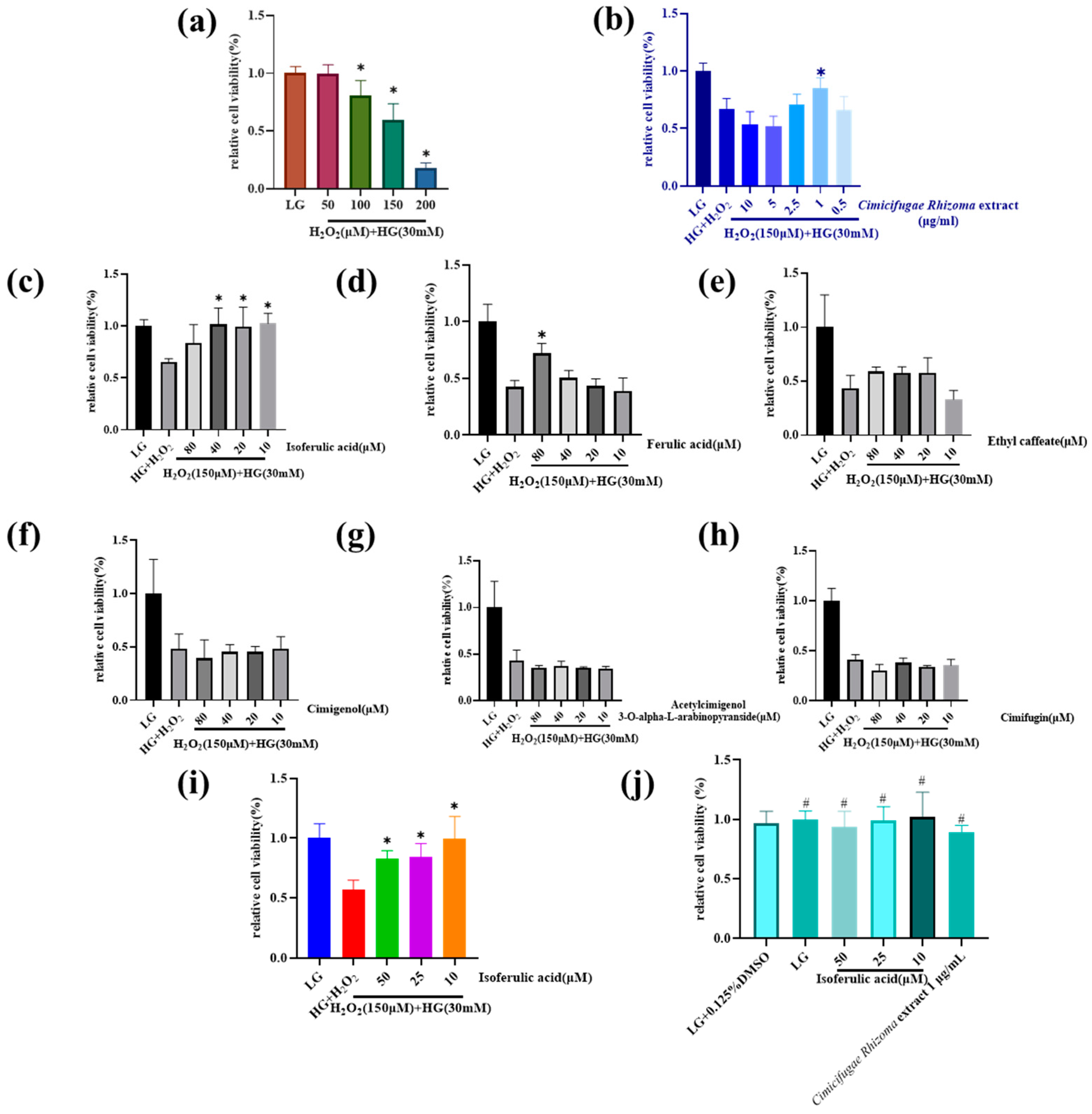
3.2. Cimicifugae Rhizoma Extract and Isoferulic Acid Inhibit H2O2 + HG Cytotoxicity in MPC5 Cells
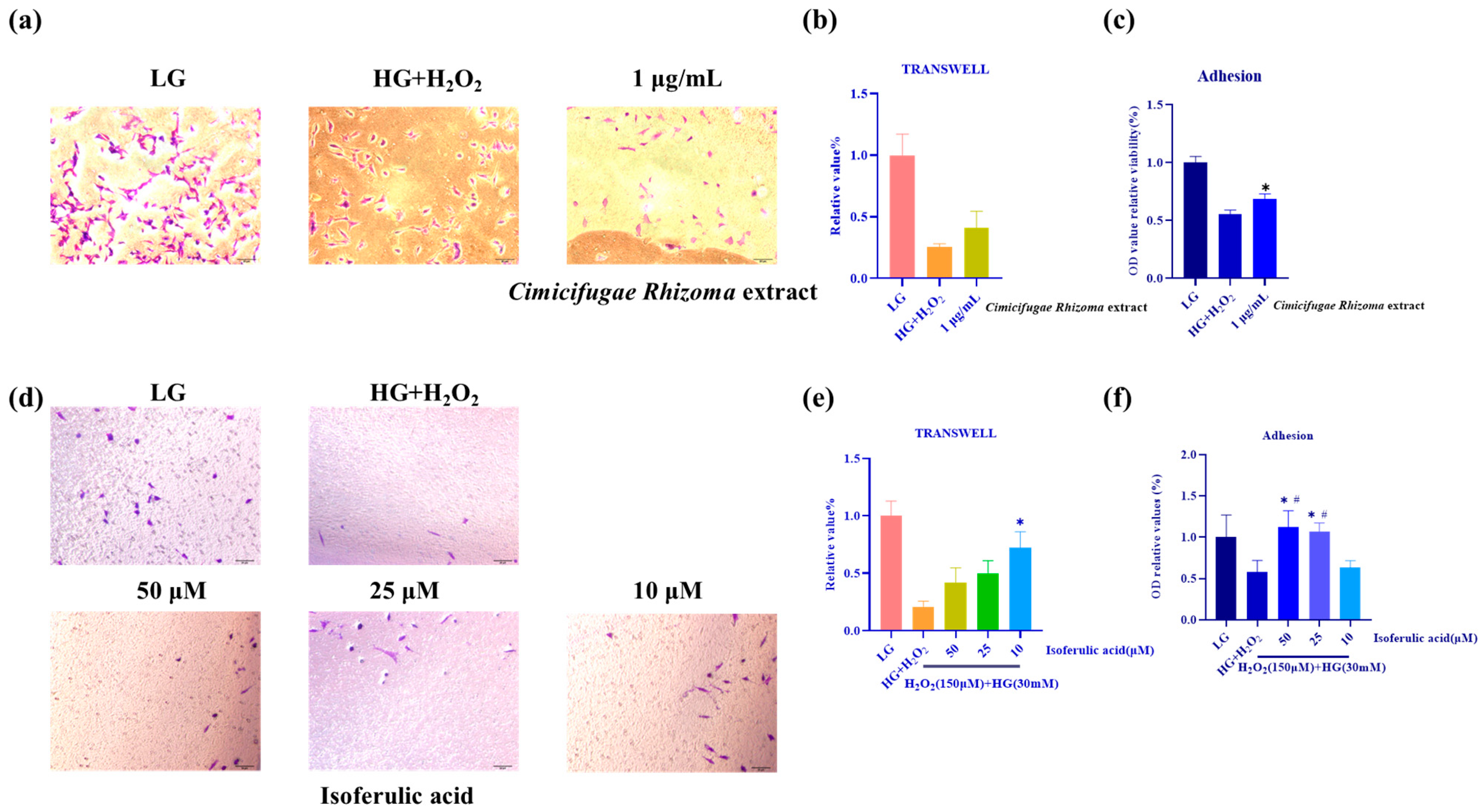
3.3. Isoferulic Acid Shows Potential in Improving MPC5 Cells’ Impaired Mobility and Adhesion
3.4. Isoferulic Acid Inhibits H2O2 + HG-Mediated Cell Apoptosis and Oxidative Stress
3.4.1. Isoferulic Acid Inhibits H2O2 + HG -Mediated Cell Apoptosis
3.4.2. Isoferulic Acid Inhibits H2O2 + HG -Mediated Oxidative Stress and Inflammatory Response in MPC5 Cells
3.5. Effects of Cimicifugae Rhizoma Extract and Isoferulic Acid on the Morphology of the Damaged Podocyte Cytoskeleton
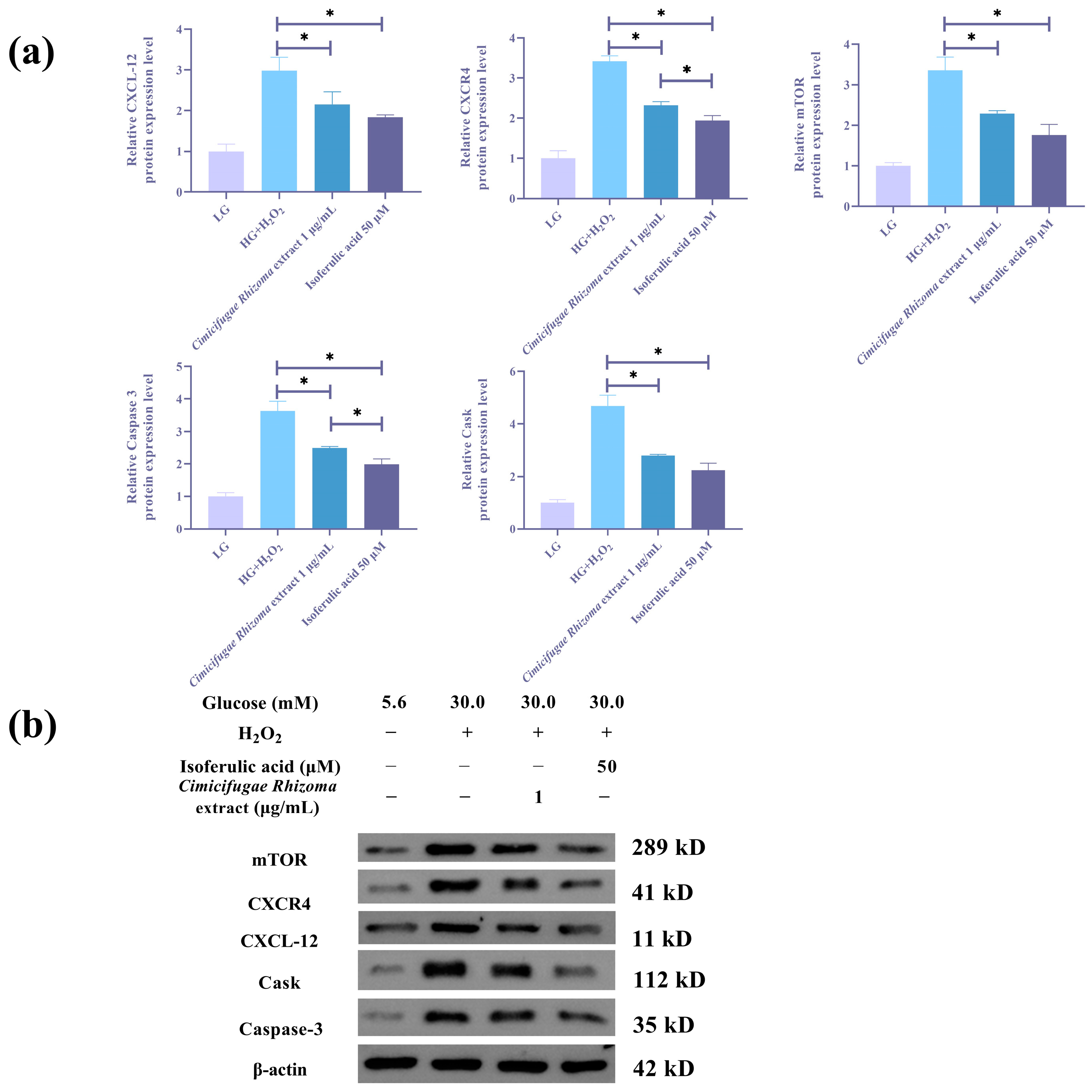
3.6. Cimicifugae Rhizoma Extract and Isoferulic Acid Inhibit H2O2 + HG -Mediated Inflammatory Response and CXCL12/CXCR4 Signaling Pathway in MPC5 Cells
3.7. Cimicifugae Rhizoma Extract and Isoferulic Acid Regulates Protein Levels in MPC5 Cells That Were Influenced by HG + H2O2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Precision Investigation
Appendix A.2. Repeatability
Appendix A.3. Stability
Appendix A.4. Linearity Study
Appendix A.5. Sample Recovery Test
Appendix A.6. Optimization of Extraction Methods by Orthogonal Screening
| Factor | Degree of Freedom | Mean Square | F | p-Value |
|---|---|---|---|---|
| A | 2 | 3413.345 | 27.623 | 0.035 |
| B | 2 | 94.831 | 0.767 | 0.566 |
| C | 2 | 52.728 | 0.427 | 0.701 |
| Number | A | B | C | D (Blank) | Isoferulic Acid Content (mg/g) | Isoferulic Acid Extraction Ratio (%) | Rate |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 1 | 30 | 1 | 3.40 | 16.08 | 9.45 |
| 2 | 10 | 2 | 120 | 2 | 3.06 | 30.39 | 14.56 |
| 3 | 10 | 3 | 60 | 3 | 3.01 | 37.58 | 19.89 |
| 4 | 25 | 1 | 120 | 3 | 3.78 | 36.09 | 36.97 |
| 5 | 25 | 2 | 60 | 1 | 3.08 | 54.09 | 36.86 |
| 6 | 25 | 3 | 30 | 2 | 3.62 | 28.06 | 25.60 |
| 7 | 50 | 1 | 60 | 2 | 5.10 | 53.00 | 84.16 |
| 8 | 50 | 2 | 30 | 3 | 4.75 | 70.12 | 91.76 |
| 9 | 50 | 3 | 120 | 1 | 4.03 | 59.15 | 64.29 |
| K1 | 43.90 | 130.58 | 126.81 | 110.60 | |||
| K2 | 99.43 | 143.18 | 140.91 | 124.32 | |||
| K3 | 240.21 | 109.78 | 115.82 | 148.62 | |||
| R | 196.31 | 33.40 | 25.09 | 38.02 |
| Label | Component | Formula | Mass (Da) | m/z (Precursor Ion) | Mass Error (ppm) | Observed RT (min) | Adducts | MS/MS Fragment (Product Ion) |
|---|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid | C9H8O4 | 180.04226 | 179.0351 | 0.7 | 12.56 | −H | 135.0447 |
| 2 | Ferulic acid | C10H10O4 | 194.0579 | 193.051 | 1.8 | 19.17 | −H | 133.0294 |
| 3 | Paeonol | C9H10O3 | 166.063 | 165.0561 | 2.3 | 25.41 | −H | 133.0295 |
| 4 | 4-Hydroxyphenylacetic acid | C8H8O3 | 152.0473 | 151.0401 | 0.3 | 11.46 | −H | 107.0500 |
| 5 | Sinapic acid | C11H12O5 | 224.0685 | 223.0617 | 2.3 | 16.53 | −H | 149.0242 |
| 6 | Methyl caffeate | C10H10O4 | 194.0579 | 193.0507 | 0.4 | 16.22 | −H | 121.0291/93.0340 |
| 7 | Ethyl caffeate | C11H12O4 | 208.0736 | 207.0668 | 2.4 | 22.84 | −H | 178.0826/135.0448/133.0294 |
| 8 | Isoferulic acid | C10H10O4 | 194.0579 | 193.0505 | −0.5 | 16.89 | −H | 178.0286/135.0431/134.0380 |
| 9 | Methyl ferulate | C11H12O4 | 208.0736 | 207.0667 | 2.0 | 23.51 | −H | 133.0293 |
References
- Ma, Z.; Liu, M.; Liu, G.; Zhou, Z.; Ren, X.; Sun, L.; Wang, M. A comprehensive quality evaluation of Cimicifugae Rhizoma using UPLC-Q-Orbitrap-MS/MS coupled with multivariate chemometric methods. J. AOAC Int. 2023, 106, 1313–1322. [Google Scholar] [CrossRef]
- Niu, X.N.; Qin, R.L.; Zhao, Y.D.; Han, L.; Lu, J.C.; Lv, C.N. Simultaneous determination of 19 constituents in Cimicifugae Rhizoma by HPLC-DAD and screening for antioxidants through DPPH free radical scavenging assay. Biomed. Chromatogr. 2019, 33, e4624. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Chen, D. Evaluation of antioxidant activity of isoferulic acid in vitro. Nat. Prod. Commun. 2011, 6, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, W.J.; Yang, S.J.; Huh, J.W.; Choi, J.; Hong, H.N.; Hwang, O.; Cho, S.W. Inhibitory effects of Cimicifuga heracleifolia extract on glutamate formation and glutamate dehydrogenase activity in cultured islets. Mol. Cells 2004, 17, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, S.; Li, F.; Zhang, B.; Qu, Y.; Sun, T.; Luo, T.; Li, D. Investigation of antioxidant interactions between Radix Astragali and Cimicifuga foetida and identification of synergistic antioxidant compounds. PLoS ONE 2014, 9, e87221. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J. Enzym. Inhib. Med. Chem. 2009, 24, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Meeprom, A.; Sompong, W.; Chan, C.B.; Adisakwattana, S. Isoferulic Acid, a New Anti-Glycation Agent, Inhibits Fructose- and Glucose-Mediated Protein Glycation in Vitro. Molecules 2013, 18, 6439–6454. [Google Scholar] [CrossRef] [PubMed]
- Meeprom, A.; Chan, C.B.; Sompong, W.; Adisakwattana, S. Isoferulic acid attenuates methylglyoxal-induced apoptosis in INS-1 rat pancreatic beta-cell through mitochondrial survival pathways and increasing glyoxalase-1 activity. Biomed. Pharmacother. 2018, 101, 777–785. [Google Scholar] [CrossRef]
- Liu, I.M.; Hsu, F.L.; Chen, C.F.; Cheng, J.T. Antihyperglycemic action of isoferulic acid in streptozotocin-induced diabetic rats. Br. J. Pharmacol. 2000, 129, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.M.; Chen, W.C.; Cheng, J.T. Mediation of beta-endorphin by isoferulic acid to lower plasma glucose in streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 2003, 307, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Elmarakby, A.A.; Sullivan, J.C. Relationship between Oxidative Stress and Inflammatory Cytokines in Diabetic Nephropathy. Cardiovasc. Ther. 2012, 30, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, N.; Haruna, Y.; Kondeti, V.K.; Kanwar, Y.S. Oxidative stress in diabetic nephropathy. Curr. Med. Chem. 2010, 17, 4256–4269. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, W.Y.; Pan, D.B.; Shi, D.F.; Pang, Q.Q.; Li, H.B.; Yao, X.J.; Yao, Z.H.; Yu, Y.; Yao, X.S. Phenolic acids and their glycosides from the rhizomes of Cimicifuga dahurica. Fitoterapia 2019, 134, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; Wang, Q.; Han, Y.-Q.; Jiang, M.; Gao, J.; Miao, Y.; Bai, G. Bioactivity-based UPLC/Q-TOF/MS strategy for screening of anti-inflammatory components from Cimicifugae Rhizoma. Chin. Chem. Lett. 2017, 28, 476–481. [Google Scholar] [CrossRef]
- Lim, J.O.; Song, K.H.; Lee, I.S.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Shin, I.S.; Kim, T. Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-κB Phosphorylation in Ovalbumin-Induced Asthma. Antioxidants 2021, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, S.; Wen, J.; Wang, R.; Lu, J.; Muhammad-Biu, A.; Wang, S.; Du, K.; Wei, W.; Tian, X.; et al. A comprehensive strategy integrating metabolomics with DNA barcoding for discovery of combinatorial discriminatory quality markers: A case of Cimicifuga foetida and Cimicifuga dahurica. Arab. J. Chem. 2024, 17, 105613. [Google Scholar] [CrossRef]
- Fan, M.; Qin, K.; Ding, F.; Huang, Y.; Wang, X.; Cai, B. Identification and differentiation of major components in three different “Sheng-ma” crude drug species by UPLC/Q-TOF-MS. Acta Pharm. Sin. B 2017, 7, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; You, L.; Yang, C.; Sai, N.; Wu, H.; Sun, M.; Cai, M.; Peng, H.; Liang, X.; Yin, X.; et al. Phytochemical identification of Xiaoer Huanglong Granule and pharmacokinetic study in the rat using its seven major bioactive components. J. Sep. Sci. 2022, 45, 2804–2818. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.F.; Song, X.J.; Nagai, T.; Yamamoto, M.; Dai, Y.; He, L.L.; Kiyohara, H.; Yao, X.S.; Yao, Z.H. Chemical profile of Cimicifuga heracleifolia Kom. And immunomodulatory effect of its representative bioavailable component, cimigenoside on Poly(I: C)-induced airway inflammation. J. Ethnopharmacol. 2021, 267, 113615. [Google Scholar] [CrossRef] [PubMed]
- Dan, C.; Zhou, Y.; Ye, D.; Peng, S.; Ding, L.; Gross, M.L.; Qiu, S.X. Cimicifugadine from Cimicifuga foetida, a new class of triterpene alklaoids with novel reactivity. Org. Lett. 2007, 9, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Alagpulinsa, D.A.; Cao, J.J.L.; Sobell, D.; Poznansky, M.C. Harnessing CXCL12 signaling to protect and preserve functional beta-cell mass and for cell replacement in type 1 diabetes. Pharmacol. Ther. 2019, 193, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, F.S.; Chen, L.H.; Advani, S.L.; Thai, K.; Batchu, S.N.; Alghamdi, T.A.; White, K.E.; Sood, M.M.; Gibson, I.W.; Connelly, K.A.; et al. CXCR4 promotes renal tubular cell survival in male diabetic rats: Implications for ligand inactivation in the human kidney. Endocrinology 2015, 156, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Fujita, H.; Fujishima, H.; Shimizu, T.; Sato, T.; Morii, T.; Tsukiyama, K.; Narita, T.; Takahashi, T.; Drucker, D.J.; et al. Stromal cell-derived factor-1 is upregulated by dipeptidyl peptidase-4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int. 2016, 90, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Lee, K.H.; Kim, M.Y.; Choi, K.H. Caspase-3-mediated cleavage of the NF-kappa B subunit p65 at the NH2 terminus potentiates naphthoquinone analog-induced apoptosis. J. Biol. Chem. 2001, 276, 24638–24644. [Google Scholar] [CrossRef] [PubMed]
- Wier, E.M.; Fu, K.; Hodgson, A.; Sun, X.; Wan, F. Caspase-3 cleaved p65 fragment dampens NF-kappaB-mediated anti-apoptotic transcription by interfering with the p65/RPS3 interaction. FEBS Lett. 2015, 589, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Ford, B.M.; Bhandary, B.; Cavaglieri, R.D.; Block, K.; Barnes, J.L.; Gorin, Y.; Choudhury, G.G.; Abboud, H.E. Mammalian Target of Rapamycin Regulates Nox4-Mediated Podocyte Depletion in Diabetic Renal Injury. Diabetes 2013, 62, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, Y.; Hao, M.; Li, C.; Wang, J.; Shu, F.; Du, L.; Zhu, X.; Zhang, Q.; Yin, X. The mTOR promotes oxidative stress-induced apoptosis of mesangial cells in diabetic nephropathy. Mol. Cell. Endocrinol. 2018, 473, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Hu, K.; Ye, M.; Wu, D.; He, Q. Rapamycin Reduces Podocyte Apoptosis and is Involved in Autophagy and mTOR/P70S6K/4EBP1 Signaling. Cell. Physiol. Biochem. 2018, 48, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, S.; Lehtonen, E.; Kudlicka, K.; Holthöfer, H.; Farquhar, M.G. Nephrin Forms a Complex with Adherens Junction Proteins and CASK in Podocytes and in Madin-Darby Canine Kidney Cells Expressing Nephrin. Am. J. Pathol. 2004, 165, 923–936. [Google Scholar] [CrossRef] [PubMed]





| Label | Component | Formula | Mass (Da) | m/z (Precursor Ion) | Mass Error (ppm) | Observed RT (min) | Adducts | MS/MS Fragment (Product Ion) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12β-O-Acetyl-Liposide B | C37H58O11 | 678.3979 | 677.3907 | 0.0 | 36.76 | −H | 617.3701 | [19] |
| 2 | 12β-O-Acetylcimifugoside A | C37H56O11 | 676.3823 | 677.3870 | −3.7 | 27.88 | +H | 541.3164/195.1206/181.1043 | [19] |
| 3 | 12β-Hydroxystigmasterolxyloside | C35H56O10 | 636.3874 | 635.3801 | 0.0 | 31.04 | −H | 577.3384 | [19] |
| 4 | 15α-HydroxycimifugosideH2 | C35H54O11 | 650.3666 | 649.3609 | 2.4 | 25.09 | −H | 531.2990 | [22] |
| 5 | 24-O-Acetyl-7,8-didehydroclerosterolxyloside | C37H58O11 | 678.3979 | 677.3901 | −0.8 | 33.58 | −H | [20] | |
| 6 | 24-O-Acetyl-Cresolxyloside | C37H60O11 | 680.4136 | 679.4059 | −0.5 | 37.16 | −H | [20] | |
| 7 | 7,8-Didehydroclerosterolxyloside | C35H54O9 | 618.3768 | 619.3830 | −1.6 | 31.06 | +H | 469.3325/451.3226/433.3109 | [20] |
| 8 | Acetylcimigenol 3-O-alpha-L-arabinopyranside | C37H58O10 | 662.4030 | 663.4090 | −1.9 | 37.16 | +H | 623.3551 | [22] |
| 9 | 25-Anhydrocimigenol-xyloside | C35H54O8 | 602.3819 | 603.3875 | −2.8 | 33.68 | +H, +Na | 583.3613 | [22] |
| 10 | 12β-Hydroxycitrinol | C30H48O6 | 504.3451 | 503.3370 | −1.6 | 36.73 | −H | 503.3370 | [19] |
| 11 | Arcotoxin | C37H56O11 | 676.3823 | 677.3888 | −1.1 | 26.69 | +H | 131.0894 | |
| 12 | Cimigenol xyloside | C35H56O9 | 620.3924 | 643.3838 | 3.4 | 28.02 | +Na | 435.3249 | |
| 13 | Cimiside A | C35H56O10 | 636.3874 | 635.3808 | 1.2 | 28.16 | −H | 445.8311 | [19] |
| 14 | Cimiside C | C43H70O16 | 842.4664 | 841.4598 | 0.8 | 31.41 | −H | 637.3959/447.3112 | |
| 15 | Cimiside H1 | C35H52O9 | 616.3611 | 615.3545 | 1.1 | 36.33 | −H | 251.2021 | [19] |
| 16 | Cimiside H2 | C35H54O10 | 634.3717 | 633.3642 | −0.3 | 27.75 | −H | 501.2875/369.2438 | [19] |
| 17 | Cimicifugine | C35H51NO8 | 613.3615 | 614.3693 | 0.9 | 22.45 | +H | 446.3062/184.0776 | [23] |
| 18 | Cimaroside V | C47H76O19 | 944.4981 | 943.4898 | −1.0 | 68.58 | −H | 303.2335 | [22] |
| 19 | Cimaroside I | C36H58O11 | 666.3979 | 665.3910 | 0.5 | 33.69 | −H | [20] | |
| 20 | Cimiacerin A | C35H54O9 | 618.3768 | 619.3831 | −1.5 | 35.79 | +H, +Na | 451.3225 | [22] |
| 21 | Cimidahuside E | C35H52O8 | 600.3662 | 601.3728 | −1.2 | 35.77 | +H | 451.3195 | [19] |
| 22 | Cimidahuside F | C35H52O9 | 616.3611 | 617.3687 | 0.5 | 27.77 | +H | 451.3328 | [22] |
| 23 | Cimidahuside G | C35H56O9 | 620.3924 | 621.3972 | −4.0 | 31.51 | +H | 453.3379 | |
| 24 | Cimidahuside H | C35H54O9 | 618.3768 | 641.3644 | −2.5 | 35.29 | +Na | 451.3223/433.3122 | [22] |
| 25 | Cimicidanol | C30H44O5 | 484.3189 | 485.327 | 1.6 | 27.76 | +H | 449.3901 | [22] |
| 26 | Isodahurinol/Cimigenol | C30H48O5 | 488.3502 | 489.3563 | −2.4 | 31.51 | +H | 453.3379 | [22] |
| 27 | Ferulic acid | C10H10O4 | 194.0579 | 193.0495 | −5.9 | 19.52 | −H | standards data | |
| 28 | Cimidahurinine | C14H20O8 | 316.1158 | 315.1069 | −5.3 | 8.11 | −H | 153.9128/149.2179 | |
| 29 | Ethyl caffeate | C11H12O4 | 208.0736 | 207.0664 | 0.8 | 22.80 | −H | standards data | |
| 30 | Cimicifugic acid E | C21H20O10 | 432.1057 | 431.0983 | −0.1 | 21.93 | −H | 165.0557 | [22] |
| 31 | Isocimicifugamide | C25H31NO10 | 505.1948 | 504.1861 | −2.8 | 21.81 | −H | 343.9951 | [22] |
| 32 | Cimicifugaside B | C33H40O18 | 724.3962 | 723.3952 | −3.6 | 33.62 | −H | 723.3952 | [16] |
| 33 | Shomaside C | C27H30O15 | 594.3390 | 617.3677 | −3.0 | 27.73 | +Na, +H | 193.9128/177.0620 | [19] |
| 34 | Methyl-ferulic acid | C11H12O4 | 208.0679 | 207.0673 | −6.8 | 22.93 | −H | 194.1196/193.0924 | |
| 35 | Caffeicacid-β-D-glucopyranosylester | C15H18O9 | 342.0951 | 341.0893 | 4.5 | 10.54 | −H | ||
| 36 | Cimicifugamide | C25H31NO10 | 505.1948 | 504.1860 | −3.1 | 17.76 | −H | [22] | |
| 37 | Cimidahurinine | C14H20O8 | 316.1158 | 315.1077 | −2.7 | 9.09 | −H | 153.0564 | |
| 38 | Isoferulic acid | C10H10O4 | 194.0579 | 193.0505 | −0.5 | 16.89 | −H | standards data | |
| 39 | Cimifugin | C16H18O6 | 306.2462 | 305.2379 | 2.1 | 53.65 | −H | 235.9243 | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chang, A.; Peng, H.; Yin, X.; Dong, X.; Qu, C.; Ni, J. Phytochemical Identification and Anti-Oxidative Stress Effects Study of Cimicifugae Rhizoma Extract and Its Major Component Isoferulic Acid. Separations 2024, 11, 175. https://doi.org/10.3390/separations11060175
Liu J, Chang A, Peng H, Yin X, Dong X, Qu C, Ni J. Phytochemical Identification and Anti-Oxidative Stress Effects Study of Cimicifugae Rhizoma Extract and Its Major Component Isoferulic Acid. Separations. 2024; 11(6):175. https://doi.org/10.3390/separations11060175
Chicago/Turabian StyleLiu, Jing, Aqian Chang, Hulinyue Peng, Xingbin Yin, Xiaoxv Dong, Changhai Qu, and Jian Ni. 2024. "Phytochemical Identification and Anti-Oxidative Stress Effects Study of Cimicifugae Rhizoma Extract and Its Major Component Isoferulic Acid" Separations 11, no. 6: 175. https://doi.org/10.3390/separations11060175
APA StyleLiu, J., Chang, A., Peng, H., Yin, X., Dong, X., Qu, C., & Ni, J. (2024). Phytochemical Identification and Anti-Oxidative Stress Effects Study of Cimicifugae Rhizoma Extract and Its Major Component Isoferulic Acid. Separations, 11(6), 175. https://doi.org/10.3390/separations11060175






