Paeoniae Radix Alba and Network Pharmacology Approach for Osteoarthritis: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening and Target Prediction of Active Components of PRA
2.2. Collection of PRA-Related Targets
2.3. Screening of Disease-Related Targets
2.4. Protein–Protein Interaction (PPI) Network Construction and Screening of Core Targets
2.5. GO and KEGG Pathway Enrichment Analysis
2.6. Molecular Docking Imitation of Active Compound
3. Network Pharmacology: A Multi-Target and Multi-Component Strategy
4. Extraction and Purification Process of Compounds
5. Analysis of Chemical Compounds
6. Anti-OA Effects
6.1. Crude Extracts
6.2. Pure Compounds
7. Coupling of Network Pharmacology with PRA in OA Diseases
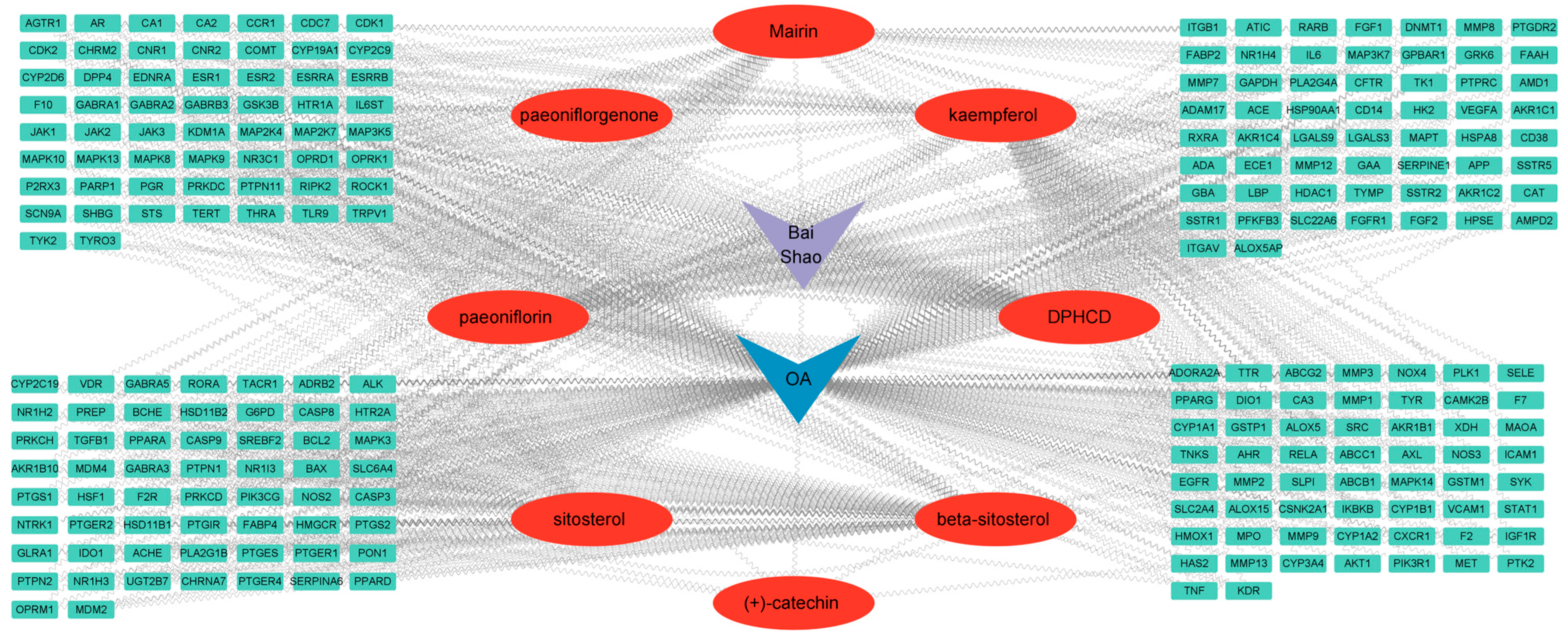
7.1. Screening of Candidate Ingredients of PRA
7.2. Potential Target Genes and the PPI Network Map of PRA Therapy for OA
7.3. Construction and Analysis of the PRA–OA–Potential Target Gene Network
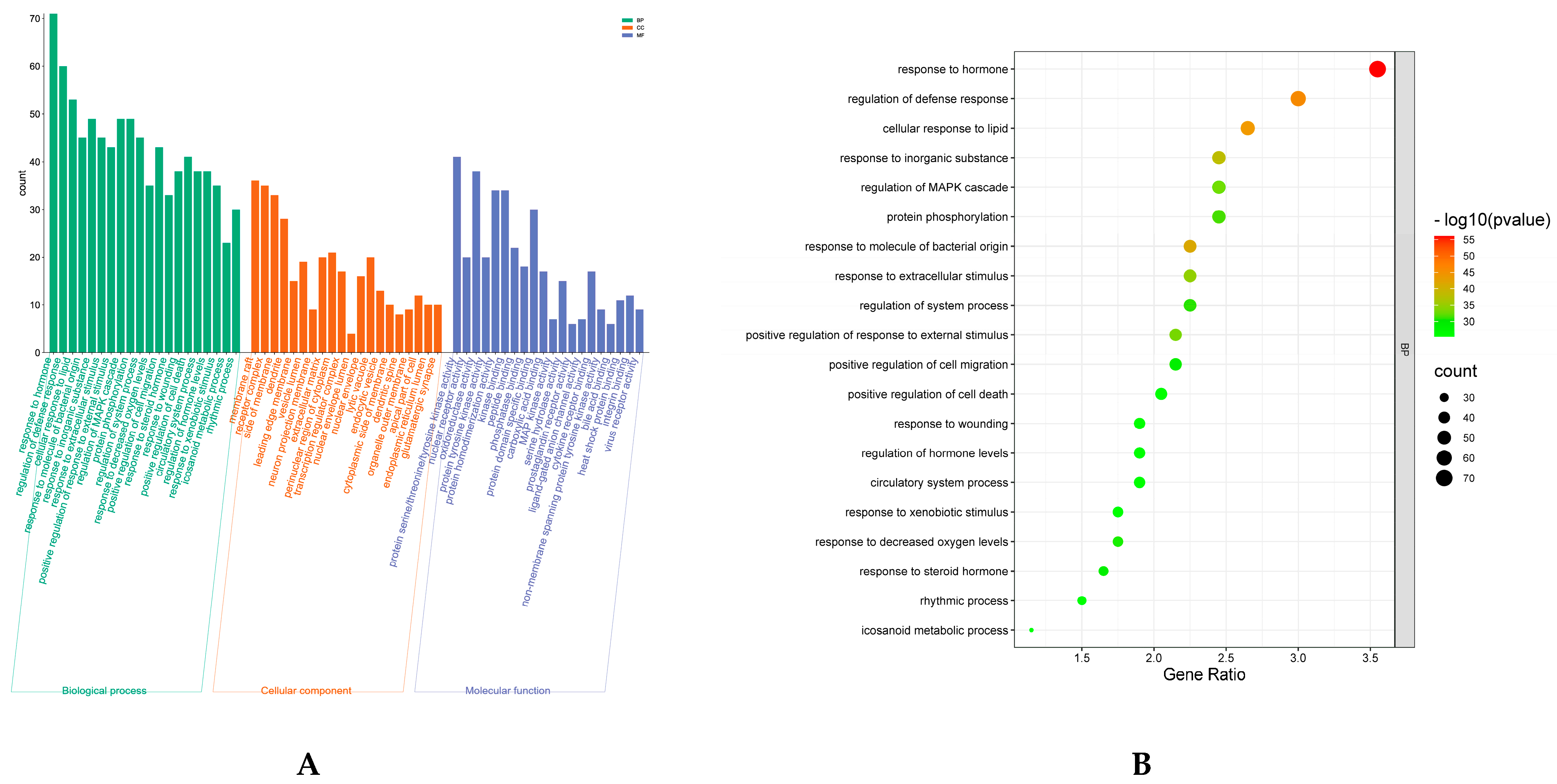
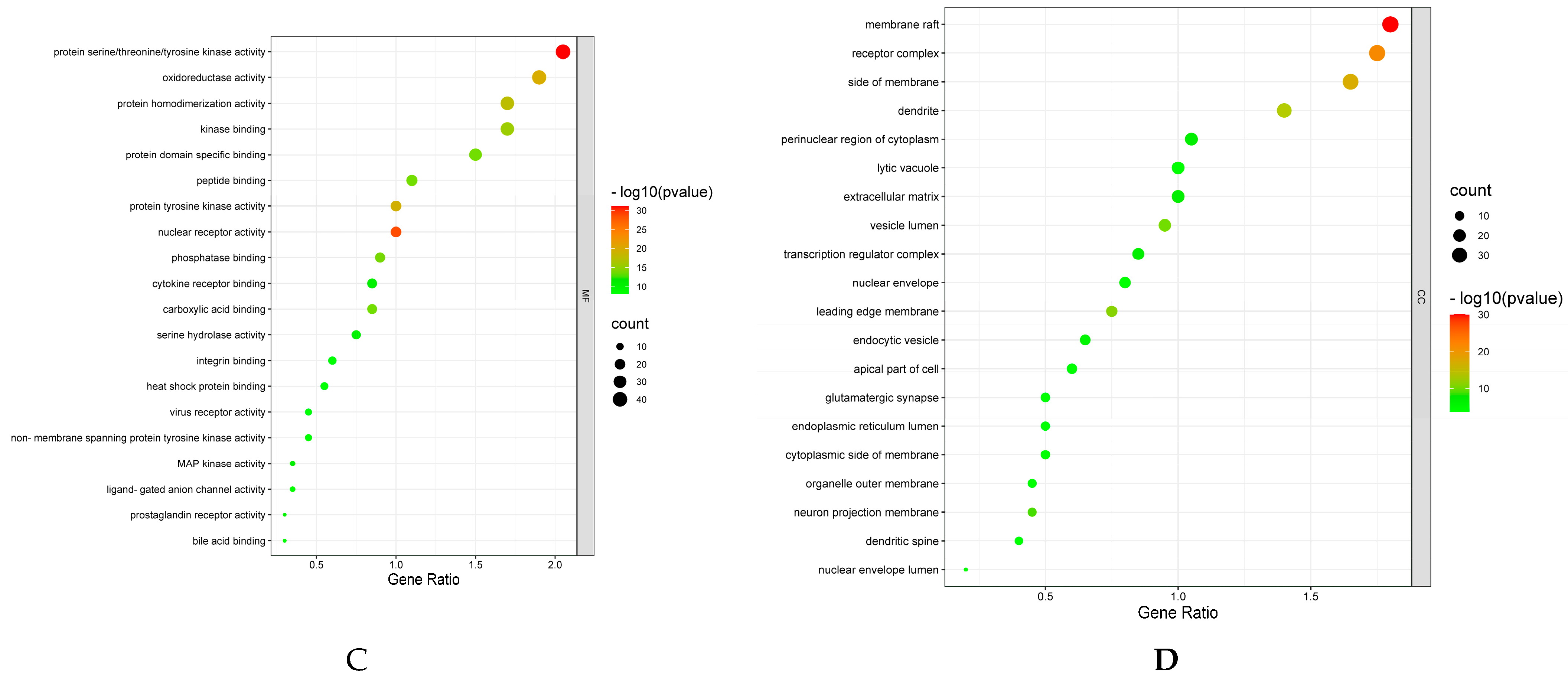
7.4. GO enrichment and KEGG Pathway Analysis
7.5. Analysis of Molecular Docking Results
8. Discussion
9. Conclusions and Future Perspective
10. Study Limitations
- (1)
- The ingredients in TCM are incredibly complex, and the ingredients that play a therapeutic role under the action of metabolic enzymes after absorption by the human body are not all the prototype ingredients of TCM; both OB and DL values of chemical ingredients are used to screen the chemical ingredients of active ingredients, so the tissue distribution of bioavailability of Chinese medicine cannot be quantified objectively.
- (2)
- Network pharmacology matches relevant information from public databases, but the existing databases lack a unified standard.
- (3)
- Obtaining disease targets by searching disease-related databases ignores the pathophysiological changes of diseases in the clinical development process and lacks objectivity.
- (4)
- The foundation of the therapeutic effect in Traditional Chinese Medicine does not necessarily lie in a single chemical compound but rather in the collective action of many chemical compounds. Consequently, research focused on the efficacy of chemical drugs targeting single pathways is not fully applicable to the study of the therapeutic effects of Traditional Chinese Medicine.
- (5)
- In this study, the number of components was not considered. However, the impact of both quantity and concentration on therapeutic efficacy cannot be disregarded. Only when a sufficient quantity of the drug reaches the target site can it effectively deliver its therapeutic effect.
- (6)
- In this study, the quantity of active ingredients in PRA was not taken into account, and the effect of the quantity of ingredients on the efficacy of the drug was overlooked.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McAllister, M.; Chemaly, M.; Eakin, A.J.; Gibson, D.S.; McGilligan, V.E. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthr. Cartil. 2018, 26, 612–619. [Google Scholar] [CrossRef]
- Nelson, A.E.; Schwartz, T.A.; Alvarez, C.; Golightly, Y.M. The prevalence of hand osteoarthritis: Comment on the article by Eaton et al. Arthritis Rheumatol. 2022, 74, 1861–1862. [Google Scholar] [CrossRef]
- Sok, D.; Raval, S.; McKinney, J.; Drissi, H.; Mason, A.; Mautner, K.; Kaiser, J.M.; Willett, N.J. NSAIDs Reduce Therapeutic Efficacy of Mesenchymal Stromal Cell Therapy in a Rodent Model of Posttraumatic Osteoarthritis. Am. J. Sports Med. 2022, 50, 1389–1398. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Dou, H.; Liu, Q.; Shu, G.; Lin, J.; Zhang, W.; Peng, G.; Zhong, Z.; Fu, H. Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111352. [Google Scholar] [CrossRef]
- Knoop, J.; Dekker, J.; van Dongen, J.M.; van der Leeden, M.; de Rooij, M.; Peter, W.F.; de Joode, W.; van Bodegom-Vos, L.; Lopuhaä, N.; Bennell, K.L.; et al. Stratified exercise therapy does not improve outcomes compared with usual exercise therapy in people with knee osteoarthritis (OCTOPuS study): A cluster randomised trial. J. Physiother. 2022, 68, 182–190. [Google Scholar] [CrossRef]
- Padilla-Martinez, J.P.; Lewis, W.; Ortega-Martinez, A.; Franco, W. Intrinsic fluorescence and mechanical testing of articular cartilage in human patients with osteoarthritis. J. Biophotonics 2018, 11, e201600269. [Google Scholar] [CrossRef]
- Talaie, R.; Torkian, P.; Clayton, A.; Wallace, S.; Cheung, H.; Chalian, M.; Golzarian, J. Emerging Targets for the Treatment of Osteoarthritis: New Investigational Methods to Identify Neo-Vessels as Possible Targets for Embolization. Diagnostics 2022, 12, 1403. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, X.; Lin, R.; Sun, A.R.; Song, J.; Ye, Z.; Liang, D.; Zhang, M.; Tian, J.; Zhou, X.; et al. Knee Osteoarthritis Therapy: Recent Advances in Intra-Articular Drug Delivery Systems. Drug Des. Devel Ther. 2022, 16, 1311–1347. [Google Scholar] [CrossRef]
- Rai, V.; Khatoon, S.; Bisht, S.; Mehrotra, S. Effect of cadmium on growth, ultramorphology of leaf and secondary metabolites of Phyllanthus amarus Schum. and Thonn. Chemosphere 2005, 61, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Kong, H.; Zhang, M.; Cheng, J.; Wu, J.; Qu, H.; Zhao, Y. Carbon Dots from Paeoniae Radix Alba Carbonisata: Hepatoprotective Effect. Int. J. Nanomed. 2020, 15, 9049–9059. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Q.; Chen, H.-W.; Li, J.; Wu, Q.-J. Efficacy, chemical constituents, and pharmacological actions of Radix Paeoniae Rubra and Radix Paeoniae Alba. Front. Pharmacol. 2020, 11, 1054. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, Y.; Tao, J. Flavonoid-Rich Extract of Paeonia lactiflora Petals Alleviate d-Galactose-Induced Oxidative Stress and Restore Gut Microbiota in ICR Mice. Antioxidants 2021, 10, 1889. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yin, M.; Chu, S.; Yang, M.; Yang, Z.; Zhu, Y.; Huang, L.; Peng, H. Comparative Elucidation of Age, Diameter, and “Pockmarks” in Roots of Paeonia lactiflora Pall. (Shaoyao) by Qualitative and Quantitative Methods. Front. Plant Sci. 2021, 12, 802196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Sheng, Y.; Zhao, D.; Lv, S.; Hu, Y.; Tao, J. Herbaceous peony (Paeonia lactiflora Pall.) as an alternative source of oleanolic and ursolic acids. Int. J. Mol. Sci. 2011, 12, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.F.; Chen, X.; Chen, Y.F.; Liu, S.J.; Xu, C.X.; Chen, L.; Wang, W.B.; Wen, T.T.; Zheng, X.; Liu, J. Aqueous extract of Paeoniae Radix Alba (Paeonia lactiflora Pall.) ameliorates DSS-induced colitis in mice by tunning the intestinal physical barrier, immune responses, and microbiota. J. Ethnopharmacol. 2022, 294, 115365. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, W.; Su, Y.; Yue, Z.; Bao, J. Inhibitory effect of an aqueous extract of Radix Paeoniae Alba on calcium oxalate nephrolithiasis in a rat model. Ren. Fail. 2017, 39, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ruan, A.; Zhou, J.; Zhang, X.; Ma, Y.; Peng, H.; Yang, T.; Wang, Q. Effect of total glucosides of paeony on inflammation and degeneration of chondrocytes in osteoarthritis. Chin. J. Tissue Eng. Res. 2021, 25, 4614. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.Z.; Zheng, C.S.; Xu, X.J.; Wu, M.X.; Liu, X.X. Potential synergistic and multitarget effect of herbal pair Chuanxiong Rhizome-Paeonia Albifora Pall on osteoarthritis disease: A computational pharmacology approach. Chin. J. Integr. Med. 2011, 17, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.X.; Zheng, F.Y.; Huang, G.Z.; Li, S.X.; Liu, F.J.; Man, S.; Wu, J.R.; Li, Y.Y. Anti-arthritic-related metal bioavailability for optimal compatibility ratio of Aconiti Radix Cocta and Paeoniae Radix Alba. Zhongguo Zhong Yao Za Zhi 2020, 45, 5770–5776. [Google Scholar] [CrossRef]
- Zhang, L.; Han, L.; Wang, X.; Wei, Y.; Zheng, J.; Zhao, L.; Tong, X. Exploring the mechanisms underlying the therapeutic effect of Salvia miltiorrhiza in diabetic nephropathy using network pharmacology and molecular docking. Biosci. Rep. 2021, 41, BSR20203520. [Google Scholar] [CrossRef]
- Shaker, B.; Yu, M.S.; Lee, J.; Lee, Y.; Jung, C.; Na, D. User guide for the discovery of potential drugs via protein structure prediction and ligand docking simulation. J. Microbiol. 2020, 58, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.Y.; Zheng, J.H.; Li, S. TCM network pharmacology: A new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.T.; Lu, Y.; Yan, S.K.; Xiao, X.; Rong, X.L.; Guo, J. Network Pharmacology in Research of Chinese Medicine Formula: Methodology, Application and Prospective. Chin. J. Integr. Med. 2020, 26, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Yu, S.J.; Bai, H.; Ning, K. TCM-Mesh: The database and analytical system for network pharmacology analysis for TCM preparations. Sci. Rep. 2017, 7, 2821. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Shen, M.; Fang, J.; Wei, D.; Qin, L. Advancement in the chemical analysis of Paeoniae Radix (Shaoyao). J. Pharm. Biomed. Anal. 2018, 160, 276–288. [Google Scholar] [CrossRef]
- Deng, H.; Yan, C.; Xiao, T.; Yuan, D.; Xu, J. Total glucosides of Paeonia lactiflora Pall inhibit vascular endothelial growth factor-induced angiogenesis. J. Ethnopharmacol. 2010, 127, 781–785. [Google Scholar] [CrossRef]
- Wei, C.; Wan, L.; Wang, H.; Huang, G. Optimization of Extraction Process for Paeoniae radix Alba by Central Composite Design-Response Surface Methodology. Her. Med. 2016, 175–177. [Google Scholar]
- Shen, M.; Zhang, Q.; Qin, L.; Yan, B. Single Standard Substance for the Simultaneous Determination of Eleven Components in the Extract of Paeoniae Radix Alba (Root of Paeonia lactiflora Pall.). J. Anal. Methods Chem. 2021, 2021, 8860776. [Google Scholar] [CrossRef]
- Jeon, M.H.; Kwon, H.J.; Jeong, J.S.; Lee, Y.M.; Hong, S.P. Detection of albiflorin and paeoniflorin in Paeoniae radix by reversed-phase high-performance liquid chromatography with pulsed amperometric detection. J. Chromatogr. A 2009, 1216, 4568–4573. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Peng, X.; Wang, L.; Tan, B.; Liu, J.; Feng, Y.; Yang, S. Preparative purification of peoniflorin and albiflorin from peony rhizome using macroporous resin and medium-pressure liquid chromatography. J. Sep. Sci. 2012, 35, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhang, S.; Tong, S.; Li, X.; Li, Q.; Yan, J. Elution-extrusion counter-current chromatography for the separation of two pairs of isomeric monoterpenes from Paeoniae Alba Radix. J. Sep. Sci. 2015, 38, 3110–3118. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, Y.; Zhang, L.; Zhou, J.; Yu, Y.; Zhang, S.; Zhou, Y. Green and efficient extraction of total glucosides from Paeonia lactiflora Pall.‘Zhongjiang’ by subcritical water extraction combined with macroporous resin enrichment. Ind. Crops Prod. 2019, 141, 111699. [Google Scholar] [CrossRef]
- Liu, E.H.; Qi, L.W.; Li, B.; Peng, Y.B.; Li, P.; Li, C.Y.; Cao, J. High-speed separation and characterization of major constituents in Radix Paeoniae Rubra by fast high-performance liquid chromatography coupled with diode-array detection and time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2009, 23, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Xu, C.; Sun, S.; Zhou, Q.; Shi, Z.; Li, J.; Chen, T.; Li, Z.; Cui, W. Discrimination and chemical characterization of different Paeonia lactifloras (Radix Paeoniae Alba and Radix Paeoniae Rubra) by infrared macro-fingerprint analysis-through-separation. J. Mol. Struct. 2015, 1099, 68–76. [Google Scholar] [CrossRef]
- Medzhitov, R.; Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 2009, 9, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Lin, S.; Zhan, F. One of the active ingredients in Paeoniae Radix Alba functions as JAK1 inhibitor in rheumatoid arthritis. Front. Pharmacol. 2022, 13, 906763. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Ying, J.; Wan, X.; Han, X.; Shan, Q.; Lyu, Q.; Yang, Q.; Wang, K.; Hao, M.; Liu, E.; et al. Screening of Bioactive Fraction of Radix Paeoniae Alba and Enhancing Anti-Allergic Asthma by Stir-Frying Through Regulating PI3K/AKT Signaling Pathway. Front. Pharmacol. 2022, 13, 863403. [Google Scholar] [CrossRef]
- Li, P.P.; Liu, D.D.; Liu, Y.J.; Song, S.S.; Wang, Q.T.; Chang, Y.; Wu, Y.J.; Chen, J.Y.; Zhao, W.D.; Zhang, L.L.; et al. BAFF/BAFF-R involved in antibodies production of rats with collagen-induced arthritis via PI3K-Akt-mTOR signaling and the regulation of paeoniflorin. J. Ethnopharmacol. 2012, 141, 290–300. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, X.; Kuang, G.; Jiang, R.; Guo, X.; Wu, S.; Wan, J.; Yin, L. Paeoniflorin modulates oxidative stress, inflammation and hepatic stellate cells activation to alleviate CCl4-induced hepatic fibrosis by upregulation of heme oxygenase-1 in mice. J. Pharm. Pharmacol. 2021, 73, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhang, J.; Wang, W.; Wang, T.; Liu, M.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and antibiofilm activities of paeoniflorin against carbapenem-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2020, 128, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.Y.; Chang, H.W.; Lin, C.F.; Liu, C.J.; Hsieh, C.F.; Horng, J.T. Characterization of the anti-influenza activity of the Chinese herbal plant Paeonia lactiflora. Viruses 2014, 6, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, H.; Wang, C.X.; Li, Y.S.; Xie, H.Y.; Luo, J.D.; Zhou, Y. Paeoniflorin inhibits growth of human colorectal carcinoma HT 29 cells in vitro and in vivo. Food Chem. Toxicol. 2012, 50, 1560–1567. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, C.; Fan, Y.; Shi, D.; Jian, W. Total glucosides of paeony (TGP) extracted from Radix Paeoniae Alba exerts neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the cAMP/PKA/CREB signaling pathway. J. Ethnopharmacol. 2019, 245, 112182. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Feng, J.; Du, Q.; Ai, J.; Lv, Z. Paeoniflorin Attenuates Myocardial Fibrosis in Isoprenaline-induced Chronic Heart Failure Rats via Inhibiting P38 MAPK Pathway. Curr. Med. Sci. 2020, 40, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, Y.; Hou, X.; Xu, J.; Mu, X.; Chen, W. Influence of Paeonia lactiflora roots extract on cAMP-phosphodiesterase activity and related anti-inflammatory action. J. Ethnopharmacol. 2011, 137, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.; Kang, J.; Ma, Z.; Jie, L.; Feng, M.; Liu, D.; Mao, J.; Wang, P.; Xing, R. Total glucosides of white paeony capsule alleviate articular cartilage degeneration and aberrant subchondral bone remodeling in knee osteoarthritis. Phytother. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.F.; Sun, F.F.; Jiang, L.F.; Bao, J.P.; Wu, L.D. Paeoniflorin inhibits IL-1β-induced MMP secretion via the NF-κB pathway in chondrocytes. Exp. Ther. Med. 2018, 16, 1513–1519. [Google Scholar] [CrossRef]
- Hu, P.F.; Chen, W.P.; Bao, J.P.; Wu, L.D. Paeoniflorin inhibits IL-1β-induced chondrocyte apoptosis by regulating the Bax/Bcl-2/caspase-3 signaling pathway. Mol. Med. Rep. 2018, 17, 6194–6200. [Google Scholar] [CrossRef]
- Wu, L.; Tang, R.; Xiong, W.; Song, S.; Guo, Q.; Zhang, Q. Paeoniflorin shows chondroprotective effects under IL-1β stress by regulating circ-PREX1/miR-140-3p/WNT5B axis. J. Orthop. Surg. Res. 2023, 18, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chang, Q.; Huang, T.; Huang, C. Paeoniflorin inhibits IL-1β-induced expression of inflammatory mediators in human osteoarthritic chondrocyte. Mol. Med. Rep. 2018, 17, 3306–3311. [Google Scholar] [CrossRef] [PubMed]
- Chao Wei, C.; Yi Shan, D.; Fei Yue, L.; Tian You, F. Total Glucosides of Peony Alleviate Joint Destruction in Rabbits with Osteoarthritis. Nat. Prod. Commun. 2023, 18, 1934578X231192211. [Google Scholar] [CrossRef]
- Ahmad, M.; Malik, K.; Tariq, A.; Zhang, G.; Yaseen, G.; Rashid, N.; Sultana, S.; Zafar, M.; Ullah, K.; Khan, M.P.Z. Botany, ethnomedicines, phytochemistry and pharmacology of Himalayan paeony (Paeonia emodi Royle.). J. Ethnopharmacol. 2018, 220, 197–219. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Jin, D.E.; Yang, G.Y.; Zhang, Y.Z.; Wang, J.M.; Kong, W.P.; Tao, Q.W. Total glucosides of paeony for rheumatoid arthritis: A systematic review of randomized controlled trials. Complement. Ther. Med. 2017, 34, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 13, 16072. [Google Scholar] [CrossRef] [PubMed]
- Krustev, E.; Rioux, D.; McDougall, J.J. Mechanisms and mediators that drive arthritis pain. Curr. Osteoporos. Rep. 2015, 13, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lou, Z.; Zheng, S.; Wu, J.; Yao, Q.; Chen, R.; Kou, L.; Chen, D. Intra-articular drug delivery systems for osteoarthritis therapy: Shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 2022, 29, 767–791. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Chen, J.; Li, Y.; Liu, M.; Yang, S. Efficacy and safety of traditional Chinese medicine rehabilitation program in the treatment of knee osteoarthritis: A randomized controlled trial protocol. Ann. Palliat. Med. 2021, 10, 6909–6918. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Zhang, W.; Li, P.; Song, D.; Niu, H.; Shi, S.; Wang, S.; Duan, J. Structural characterization and biological activities of two α-glucans from Radix Paeoniae Alba. Glycoconj. J. 2016, 33, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yin, Q.; Tian, J.; Gao, X.; Qin, X.; Du, G.; Zhou, Y. Studies on the Changes of Pharmacokinetics Behaviors of Phytochemicals and the Influence on Endogenous Metabolites After the Combination of Radix Bupleuri and Radix Paeoniae Alba Based on Multi-Component Pharmacokinetics and Metabolomics. Front. Pharmacol. 2021, 12, 630970. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, S. Network-based relating pharmacological and genomic spaces for drug target identification. PLoS ONE 2010, 5, e11764. [Google Scholar] [CrossRef]
- Lee, W.S.; Lee, E.G.; Sung, M.S.; Yoo, W.H. Kaempferol inhibits IL-1β-stimulated, RANKL-mediated osteoclastogenesis via downregulation of MAPKs, c-Fos, and NFATc1. Inflammation 2014, 37, 1221–1230. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ye, G.; Huang, B. Kaempferol alleviates the interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2017, 23, 3925. [Google Scholar] [CrossRef]
- Jiang, R.; Hao, P.; Yu, G.; Liu, C.; Yu, C.; Huang, Y.; Wang, Y. Kaempferol protects chondrogenic ATDC5 cells against inflammatory injury triggered by lipopolysaccharide through down-regulating miR-146a. Int. Immunopharmacol. 2019, 69, 373–381. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.-C.; Jiao, P.; Hong, M.; Nam, J.-B.; Brownell, L.; Hyun, E.; Jia, Q. UP1306, a botanical composition with analgesic and anti-inflammatory effect. Pharmacogn. Res. 2016, 8, 186. [Google Scholar] [CrossRef]
- Du, W.; Liang, X.; Wang, S.; Lee, P.; Zhang, Y. The underlying mechanism of Paeonia lactiflora Pall. in Parkinson’s disease based on a network pharmacology approach. Front. Pharmacol. 2020, 11, 581984. [Google Scholar] [CrossRef]
- Fang, H.; Beier, F. Mouse models of osteoarthritis: Modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 2014, 10, 413–421. [Google Scholar] [CrossRef]
- Gu, R.; Liu, N.; Luo, S.; Huang, W.; Zha, Z.; Yang, J. MicroRNA-9 regulates the development of knee osteoarthritis through the NF-kappaB1 pathway in chondrocytes. Medicine 2016, 95, e4315. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, H.; Pan, J.; Hu, Z.; Liu, L.; Liu, Y.; Yu, X.; Bai, X.; Cai, D.; Zhang, H. Fargesin ameliorates osteoarthritis via macrophage reprogramming by downregulating MAPK and NF-κB pathways. Arthritis Res. Ther. 2021, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Jza, B.; Jwa, B.; Quan, C.; Wd, D.; Ffa, B.; Hya, B.; Sya, B.; Hjab, C.; Ptab, E. Loganin ameliorates cartilage degeneration and osteoarthritis development in an osteoarthritis mouse model through inhibition of NF-κB activity and pyroptosis in chondrocytes. J. Ethnopharmacol. 2020, 247, 112261. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Peng, L.; Li, C.; Ji, Q.; Li, P. Salidroside Alleviates Cartilage Degeneration Through NF-κB Pathway in Osteoarthritis Rats. Drug Des. Dev. Ther. 2020, 14, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Nakamura, H.; Masukohongo, K.; Kato, T.; Nishioka, K. Catabolic stress induces expression of hypoxiainducible factor (HIF)-1α in articular chondrocytes: Involvement of HIF-1α in the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2005, 7, 225. [Google Scholar] [CrossRef]
- Pf, D.; Swoboda, B.; Cramer, T. Commentary The role of HIF-1α in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res. Ther. 2006, 8, 104. [Google Scholar] [CrossRef]
- Chang, J.; Jackson, S.G.; Wardale, J.; Jones, S.W. Hypoxia Modulates the Phenotype of Osteoblasts Isolated From Knee Osteoarthritis Patients, Leading to Undermineralized Bone Nodule Formation. Arthritis Rheum. 2014, 66, 1789–1799. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, C.; Ni, L.; Huang, C.; Chen, D.; Shi, K.; Jin, H.; Zhang, K.; Li, Y.; Xie, L. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020, 11, 481. [Google Scholar] [CrossRef]
- Hamilton, J.L.; Nagao, M.; Levine, B.R.; Chen, D.; Olsen, B.R.; Im, H.J. Targeting VEGF and Its Receptors for the Treatment of Osteoarthritis and Associated Pain. J. Bone Min. Res. 2016, 31, 911–924. [Google Scholar] [CrossRef]
- Qian, J.J.; Xu, Q.; Xu, W.M.; Cai, R.; Huang, G.C. Expression of VEGF-A Signaling Pathway in Cartilage of ACLT-induced Osteoarthritis Mouse Model. J. Orthop. Surg. Res. 2021, 16, 379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Kuo, S.J.; Liu, S.C.; Wang, S.W.; Tsai, C.H.; Fong, Y.C.; Tang, C.H. Apelin Affects the Progression of Osteoarthritis by Regulating VEGF-Dependent Angiogenesis and miR-150-5p Expression in Human Synovial Fibroblasts. Cells 2020, 9, 594. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, Y.; Bao, Y.; Liu, X.; Xu, H. LncRNA MEG3 promotes recovery of knee osteoarthritis in rats through regulating VEGF expression. Panminerva Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Suzek, T.O.; Zhang, J.; Wang, J.; Bryant, S.H. PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009, 37, W623–W633. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, D514–D517. [Google Scholar] [CrossRef]
- Qiu, J. Traditional medicine: A culture in the balance. Nature 2007, 448, 126–128. [Google Scholar] [CrossRef]


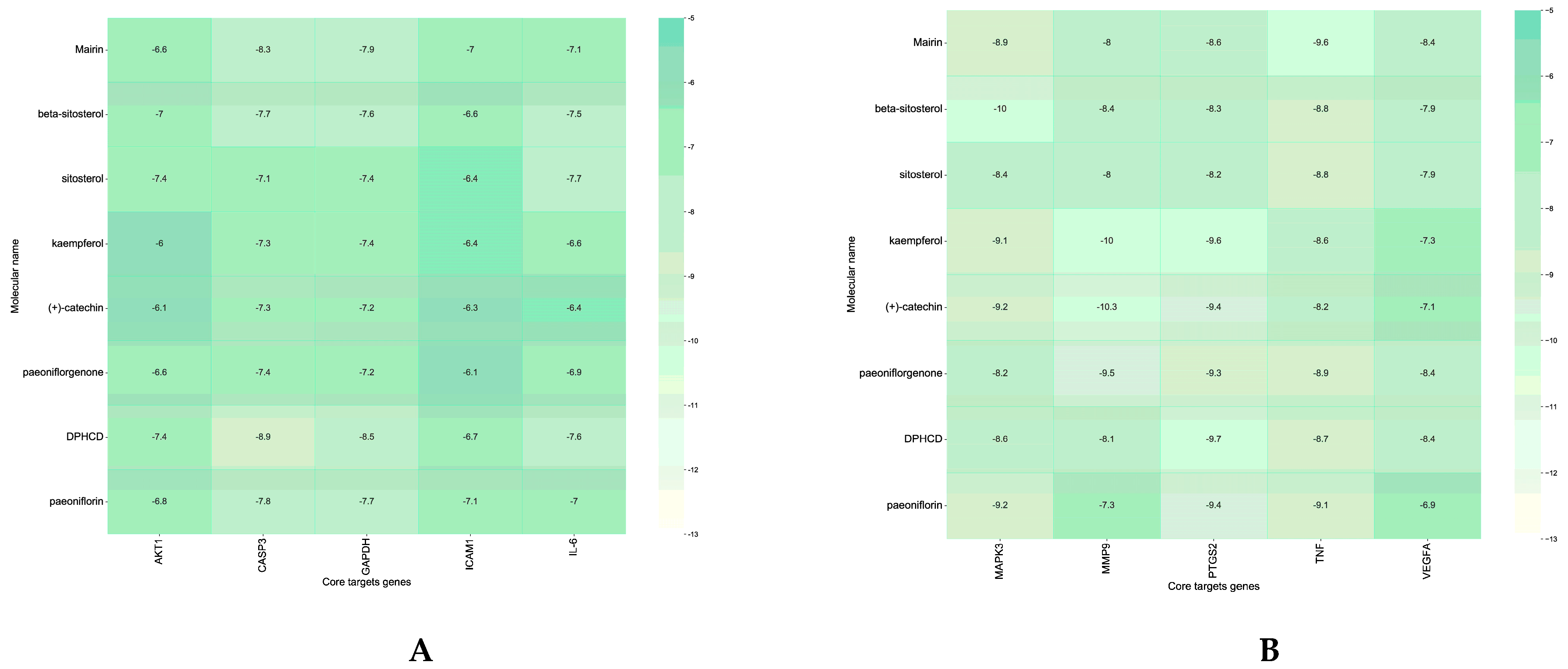

| No | Mol ID | Compound | Molecular Structure | Molecular Weight | AlogP | Hdon | Hacc | OB (%) | DL | HL |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MOL001910 | 11α,12α-epoxy-3ꞵ-23-dihydroxy-30-norolean-20-en-28,12ꞵ-olide | 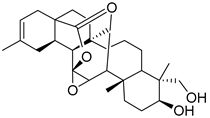 | 470.71 | 3.91 | 2 | 5 | 64.77 | 0.4 | 2.62 |
| 2 | MOL001918 | Paeoniflorgenone | 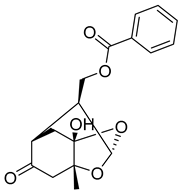 | 318.35 | 0.79 | 1 | 6 | 87.59 | 0.4 | 7.45 |
| 3 | MOL001919 | DPHCD | 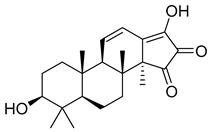 | 358.52 | 2.69 | 2 | 4 | 43.56 | 0.5 | 4.34 |
| 4 | MOL001921 | Lactiflorin | 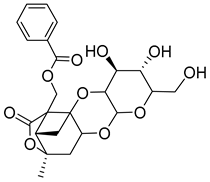 | 462.49 | −0.57 | 3 | 10 | 49.12 | 0.8 | 7.26 |
| 5 | MOL001924 | Paeoniflorin | 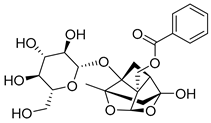 | 480.51 | −1.28 | 5 | 11 | 53.87 | 0.8 | 13.9 |
| 6 | MOL001925 | paeoniflorin_qt |  | 318.35 | 0.46 | 2 | 6 | 68.18 | 0.4 | 8.81 |
| 7 | MOL001928 | albiflorin_qt |  | 318.35 | 0.42 | 2 | 6 | 66.64 | 0.3 | 6.54 |
| 8 | MOL001930 | benzoyl paeoniflorin | 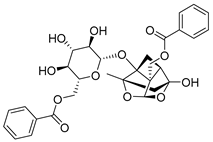 | 584.62 | 0.81 | 4 | 12 | 31.27 | 0.8 | −1.9 |
| 9 | MOL000211 | Mairin | 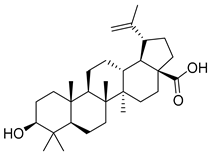 | 456.78 | 6.52 | 2 | 3 | 55.38 | 0.8 | 8.87 |
| 10 | MOL000358 | beta-sitosterol | 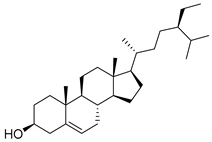 | 414.79 | 8.08 | 1 | 1 | 36.91 | 0.8 | 5.36 |
| 11 | MOL000359 | Sitosterol | 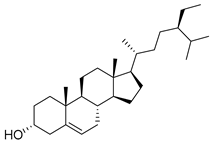 | 414.79 | 8.08 | 1 | 1 | 36.91 | 0.8 | 5.37 |
| 12 | MOL000422 | Kaempferol | 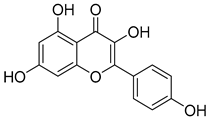 | 286.25 | 1.77 | 4 | 6 | 41.88 | 0.2 | 14.7 |
| 13 | MOL000492 | (+)-catechin | 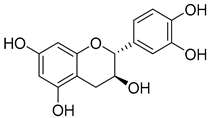 | 290.29 | 1.92 | 5 | 6 | 54.83 | 0.2 | 0.61 |
| NO | Target | Symbol | NO | Target | Symbol |
|---|---|---|---|---|---|
| 1 | Gamma-aminobutyric acid receptor subunit alpha-1 | GABRA1 | 117 | Insulin-like growth factor I receptor | IGF1R |
| 2 | Progesterone receptor | PGR | 118 | Tyrosine-protein kinase SRC | SRC |
| 3 | Androgen Receptor | AR | 119 | Vascular endothelial growth factor receptor 2 | KDR |
| 4 | Cytochrome P450 19A1 | CYP19A1 | 120 | MAP kinase p38 alpha | MAPK14 |
| 5 | Testis-specific androgen-binding protein | SHBG | 121 | Peroxisome proliferator-activated receptor gamma | PPARG |
| 6 | Glucocorticoid receptor | NR3C1 | 122 | Thrombin | F2 |
| 7 | Estrogen receptor alpha | ESR1 | 123 | Nitric-oxide synthase, endothelial | NOS3 |
| 8 | Tyrosine-protein kinase JAK1 | JAK1 | 124 | Coagulation factor VII | F7 |
| 9 | Tyrosine-protein kinase JAK2 | JAK2 | 125 | Transcription factor p65 | RELA |
| 10 | Vanilloid receptor | TRPV1 | 126 | Inhibitor of nuclear factor kappa-B kinase subunit beta | IKBKB |
| 11 | Cytochrome P450 2D6 | CYP2D6 | 127 | RAC-alpha serine/threonine-protein kinase | AKT1 |
| 12 | Cytochrome P450 2C9 | CYP2C9 | 128 | Tumor necrosis factor | TNF |
| 13 | Tyrosine-protein kinase JAK3 | JAK3 | 129 | Xanthine dehydrogenase/oxidase | XDH |
| 14 | Tyrosine-protein kinase TYK2 | TYK2 | 130 | Interstitial collagenase | MMP1 |
| 15 | Delta opioid receptor (by homology) | OPRD1 | 131 | Signal transducer and activator of transcription 1-alpha/beta | STAT1 |
| 16 | Kappa Opioid receptor (by homology) | OPRK1 | 132 | Heme oxygenase 1 | HMOX1 |
| 17 | Serotonin 1a (5-HT1a) receptor | HTR1A | 133 | Cytochrome P450 3A4 | CYP3A4 |
| 18 | Steryl-sulfatase | STS | 134 | CYP1A2 | CYP1A2 |
| 19 | Poly [ADP-ribose] polymerase-1 | PARP1 | 135 | Cytochrome P450 1A1 | CYP1A1 |
| 20 | c-Jun N-terminal kinase 1 | MAPK8 | 136 | Intercellular adhesion molecule 1 | ICAM1 |
| 21 | Glycogen synthase kinase-3 beta | GSK3B | 137 | E-selectin | SELE |
| 22 | c-Jun N-terminal kinase 3 | MAPK10 | 138 | Vascular cell adhesion protein 1 | VCAM1 |
| 23 | Dual specificity mitogen-activated protein kinase kinase 4 | MAP2K4 | 139 | Cytochrome P450 1B1 | CYP1B1 |
| 24 | MAP kinase p38 delta | MAPK13 | 140 | Arachidonate 5-lipoxygenase | ALOX5 |
| 25 | Cyclin-dependent kinase 2/cyclin A | CDK2 | 141 | Hyaluronan synthase 2 | HAS2 |
| 26 | Dual specificity mitogen-activated protein kinase kinase 7 | MAP2K7 | 142 | Glutathione S-transferase P | GSTP1 |
| 27 | c-Jun N-terminal kinase 2 | MAPK9 | 143 | Aryl hydrocarbon receptor | AHR |
| 28 | CDC7 | CDC7 | 144 | Solute carrier family 2, facilitated glucose transporter member 4 | SLC2A4 |
| 29 | Interleukin-6 receptor subunit beta | IL6ST | 145 | Type I iodothyronine deiodinase | DIO1 |
| 30 | Estrogen receptor beta | ESR2 | 146 | Glutathione S-transferase Mu 1 | GSTM1 |
| 31 | Catechol O-methyltransferase | COMT | 147 | Antileukoproteinase | SLPI |
| 32 | C-C chemokine receptor type 1 | CCR1 | 148 | NADPH oxidase 4 | NOX4 |
| 33 | Estrogen-related receptor alpha | ESRRA | 149 | Aldose reductase (by homology) | AKR1B1 |
| 34 | Estrogen-related receptor beta | ESRRB | 150 | Tyrosinase | TYR |
| 35 | Serine/threonine-protein kinase RIPK2 | RIPK2 | 151 | Multidrug resistance-associated protein 1 | ABCC1 |
| 36 | Muscarinic acetylcholine receptor M2 | CHRM2 | 152 | P-glycoprotein 1 | ABCB1 |
| 37 | Thyroid hormone receptor alpha | THRA | 153 | ATP-binding cassette sub-family G member 2 | ABCG2 |
| 38 | Dipeptidyl peptidase IV | DPP4 | 154 | Monoamine oxidase A | MAOA |
| 39 | Endothelin receptor ET-A (by homology) | EDNRA | 155 | Tyrosine-protein kinase SYK | SYK |
| 40 | Thrombin and coagulation factor X | F10 | 156 | Matrix metalloproteinase 9 | MMP9 |
| 41 | Cyclin-dependent kinase 1 | CDK1 | 157 | Matrix metalloproteinase 2 | MMP2 |
| 42 | Carbonic anhydrase II | CA2 | 158 | Arachidonate 15-lipoxygenase | ALOX15 |
| 43 | Carbonic anhydrase I | CA1 | 159 | Adenosine A2a receptor (by homology) | ADORA2A |
| 44 | P2X purinoceptor 3 | P2RX3 | 160 | Transthyretin | TTR |
| 45 | GABA-A receptor; alpha-3/beta-3/gamma-2 | GABRB3 | 161 | Tankyrase-2 | TNKS |
| 46 | GABA-A receptor; alpha-2/beta-3/gamma-2 | GABRA2 | 162 | Casein kinase II alpha | CSNK2A1 |
| 47 | Mitogen-activated protein kinase kinase kinase 5 | MAP3K5 | 163 | Epidermal growth factor receptor erbB1 | EGFR |
| 48 | Cannabinoid receptor 2 | CNR2 | 164 | Myeloperoxidase | MPO |
| 49 | TYRO3 | TYRO3 | 165 | PI3-kinase p85-alpha subunit | PIK3R1 |
| 50 | Type-1 angiotensin II receptor (by homology) | AGTR1 | 166 | Focal adhesion kinase 1 | PTK2 |
| 51 | DNA-dependent protein kinase | PRKDC | 167 | Matrix metalloproteinase 13 | MMP13 |
| 52 | Cannabinoid receptor 1 | CNR1 | 168 | Matrix metalloproteinase 3 | MMP3 |
| 53 | Rho-associated protein kinase 1 | ROCK1 | 169 | Carbonic anhydrase III | CA3 |
| 54 | Sodium channel protein type I× alpha subunit | SCN9A | 170 | Serine/threonine-protein kinase PLK1 | PLK1 |
| 55 | Lysine-specific histone demethylase 1 | KDM1A | 171 | Hepatocyte growth factor receptor | MET |
| 56 | Telomerase reverse transcriptase | TERT | 172 | Interleukin-8 receptor A | CXCR1 |
| 57 | Protein-tyrosine phosphatase 2C | PTPN11 | 173 | CaM kinase II beta | CAMK2B |
| 58 | Toll-like receptor (TLR7/TLR9) | TLR9 | 174 | Tyrosine-protein kinase receptor UFO | AXL |
| 59 | Heat shock factor protein 1 | HSF1 | 175 | Aldo-keto reductase family 1 member C2 (by homology) | AKR1C2 |
| 60 | Nerve growth factor receptor Trk-A | NTRK1 | 176 | Aldo-keto reductase family 1 member C1 (by homology) | AKR1C1 |
| 61 | Protein kinase C delta | PRKCD | 177 | Aldo-keto reductase family 1 member C4 (by homology) | AKR1C4 |
| 62 | Prostaglandin G/H synthase 1 | PTGS1 | 178 | Beta-amyloid A4 protein | APP |
| 63 | Prostaglandin G/H synthase 2 | PTGS2 | 179 | Matrix metalloproteinase 12 | MMP12 |
| 64 | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit, gamma isoform | PIK3CG | 180 | CD38 | CD38 |
| 65 | 5-hydroxytryptamine 2A receptor | HTR2A | 181 | Cystic fibrosis transmembrane conductance regulator | CFTR |
| 66 | Gamma-aminobutyric-acid receptor alpha-5 subunit | GABRA5 | 182 | 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 | PFKFB3 |
| 67 | Gamma-aminobutyric-acid receptor alpha-3 subunit | GABRA3 | 183 | G protein-coupled receptor kinase 6 | GRK6 |
| 68 | Beta-2 adrenergic receptor | ADRB2 | 184 | Microtubule-associated protein tau | MAPT |
| 69 | Sodium-dependent serotonin transporter | SLC6A4 | 185 | CAT | CAT |
| 70 | Mu-type opioid receptor | OPRM1 | 186 | Bile acid receptor FXR | NR1H4 |
| 71 | Neuronal acetylcholine receptor protein, alpha-7 chain | CHRNA7 | 187 | G-protein coupled bile acid receptor 1 | GPBAR1 |
| 72 | Apoptosis regulator Bcl-2 | BCL2 | 188 | 5-lipoxygenase activating protein | ALOX5AP |
| 73 | BAX | BAX | 189 | Fatty acid binding protein intestinal | FABP2 |
| 74 | Caspase-9 | CASP9 | 190 | Anandamide amidohydrolase | FAAH |
| 75 | Caspase-3 | CASP3 | 191 | Solute carrier family 22 member 6 (by homology) | SLC22A6 |
| 76 | Caspase-8 | CASP8 | 192 | Angiotensin-converting enzyme | ACE |
| 77 | Transforming growth factor beta-1 | TGFB1 | 193 | Retinoic acid receptor beta | RARB |
| 78 | Serum paraoxonase/arylesterase 1 | PON1 | 194 | Retinoid X receptor alpha | RXRA |
| 79 | HMG-CoA reductase | HMGCR | 195 | Cytosolic phospholipase A2 | PLA2G4A |
| 80 | LXR-alpha | NR1H3 | 196 | Integrin alpha-4/beta-1 | ITGB1 |
| 81 | Sterol regulatory element-binding protein 2 | SREBF2 | 197 | G protein-coupled receptor 44 | PTGDR2 |
| 82 | Cytochrome P450 2C19 | CYP2C19 | 198 | Integrin alpha-V/beta-3 | ITGAV |
| 83 | Butyrylcholinesterase | BCHE | 199 | Leukocyte common antigen | PTPRC |
| 84 | Nuclear receptor ROR-alpha | RORA | 200 | Endothelin-converting enzyme 1 | ECE1 |
| 85 | Protein-tyrosine phosphatase 1B | PTPN1 | 201 | AMP deaminase 2 | AMPD2 |
| 86 | Corticosteroid binding globulin | SERPINA6 | 202 | Interleukin-6 | IL6 |
| 87 | Glucose-6-phosphate 1-dehydrogenase | G6PD | 203 | Monocyte differentiation antigen CD14 | CD14 |
| 88 | Nuclear receptor subfamily 1 group I member 3 (by homology) | NR1I3 | 204 | Lipopolysaccharide-binding protein | LBP |
| 89 | Acetylcholinesterase | ACHE | 205 | Galectin-3 | LGALS3 |
| 90 | Vitamin D receptor | VDR | 206 | Galectin-9 | LGALS9 |
| 91 | LXR-beta | NR1H2 | 207 | Heat shock protein HSP 90-alpha | HSP90AA1 |
| 92 | Prostanoid EP1 receptor (by homology) | PTGER1 | 208 | Somatostatin receptor 2 | SSTR2 |
| 93 | Prostanoid EP2 receptor (by homology) | PTGER2 | 209 | Somatostatin receptor 1 | SSTR1 |
| 94 | 11-beta-hydroxysteroid dehydrogenase 1 | HSD11B1 | 210 | Vascular endothelial growth factor A | VEGFA |
| 95 | Glycine receptor subunit alpha-1 | GLRA1 | 211 | Acidic fibroblast growth factor | FGF1 |
| 96 | Peroxisome proliferator-activated receptor delta | PPARD | 212 | Basic fibroblast growth factor | FGF2 |
| 97 | Nitric oxide synthase, inducible (by homology) | NOS2 | 213 | Heparanase | HPSE |
| 98 | UDP-glucuronosyltransferase 2B7 | UGT2B7 | 214 | Plasminogen activator inhibitor-1 | SERPINE1 |
| 99 | 11-beta-hydroxysteroid dehydrogenase 2 | HSD11B2 | 215 | Matrix metalloproteinase 7 | MMP7 |
| 100 | Prolyl endopeptidase | PREP | 216 | Matrix metalloproteinase 8 | MMP8 |
| 101 | Prostanoid EP4 receptor (by homology) | PTGER4 | 217 | Beta-glucocerebrosidase | GBA |
| 102 | Indoleamine 2,3-dioxygenase | IDO1 | 218 | Hexokinase type II | HK2 |
| 103 | Protein Mdm4 | MDM4 | 219 | Histone deacetylase 1 | HDAC1 |
| 104 | p53-binding protein Mdm-2 | MDM2 | 220 | ADAM17 | ADAM17 |
| 105 | Prostanoid IP receptor | PTGIR | 221 | Somatostatin receptor 5 | SSTR5 |
| 106 | Fatty acid binding protein adipocyte | FABP4 | 222 | Fibroblast growth factor receptor 1 | FGFR1 |
| 107 | Prostaglandin E synthase | PTGES | 223 | Glyceraldehyde-3-phosphate dehydrogenase liver | GAPDH |
| 108 | T-cell protein-tyrosine phosphatase | PTPN2 | 224 | Lysosomal alpha-glucosidase | GAA |
| 109 | MAP kinase ERK1 | MAPK3 | 225 | Heat shock cognate 71 kDa protein | HSPA8 |
| 110 | Aldo-keto reductase family 1 member B10 | AKR1B10 | 226 | DNA (cytosine-5)-methyltransferase 1 | DNMT1 |
| 111 | Phospholipase A2 group 1B | PLA2G1B | 227 | Adenosine deaminase | ADA |
| 112 | Protein kinase C eta | PRKCH | 228 | Mitogen-activated protein kinase kinase kinase 7 | MAP3K7 |
| 113 | Neurokinin 1 receptor (by homology) | TACR1 | 229 | Thymidine kinase, cytosolic | TK1 |
| 114 | Peroxisome proliferator-activated receptor alpha | PPARA | 230 | S-adenosylmethionine decarboxylase 1 | AMD1 |
| 115 | Proteinase-activated receptor 1 | F2R | 231 | Thymidine phosphorylase | TYMP |
| 116 | ALK tyrosine kinase receptor | ALK | 232 | AICAR transformylase | ATIC |
| NO | Target | Symbol | Betweenness Centrality | Closeness Centrality | Degree |
|---|---|---|---|---|---|
| 1 | Progesterone receptor | PGR | 0.000649864 | 0.503131524 | 6 |
| 2 | Androgen Receptor | AR | 0.000649864 | 0.503131524 | 6 |
| 3 | Cytochrome P450 19A1 | CYP19A1 | 0.000649864 | 0.503131524 | 6 |
| 4 | Estrogen receptor alpha | ESR1 | 0.002190625 | 0.503131524 | 6 |
| 5 | Estrogen receptor beta | ESR2 | 0.000649864 | 0.503131524 | 6 |
| 6 | Type-1 angiotensin II receptor (by homology) | AGTR1 | 0.000766102 | 0.503131524 | 6 |
| 7 | Prostaglandin G/H synthase 2 | PTGS2 | 0.001790972 | 0.501039501 | 6 |
| 8 | Testis-specific androgen-binding protein | SHBG | 0.000434223 | 0.501039501 | 5 |
| 9 | Glucocorticoid receptor | NR3C1 | 0.000434223 | 0.501039501 | 5 |
| 10 | Muscarinic acetylcholine receptor M2 | CHRM2 | 0.00046555 | 0.501039501 | 5 |
| 11 | Gamma-aminobutyric-acid receptor alpha-2 subunit | GABRA2 | 0.000461864 | 0.501039501 | 5 |
| 12 | Protein-tyrosine phosphatase 2C | PTPN11 | 0.000434223 | 0.501039501 | 5 |
| 13 | Prostaglandin G/H synthase 1 | PTGS1 | 0.001790972 | 0.501039501 | 5 |
| 14 | Protein-tyrosine phosphatase 1B | PTPN1 | 0.000519994 | 0.501039501 | 5 |
| 15 | Nitric oxide synthase, inducible (by homology) | NOS2 | 0.000414611 | 0.501039501 | 5 |
| 16 | Aldo-keto reductase family 1 member B10 | AKR1B10 | 0.000414611 | 0.501039501 | 5 |
| 17 | Phospholipase A2 group 1B | PLA2G1B | 0.000414611 | 0.501039501 | 5 |
| 18 | Tyrosine-protein kinase SRC | SRC | 0.000542448 | 0.501039501 | 5 |
| 19 | Vascular endothelial growth factor receptor 2 | KDR | 0.000542448 | 0.501039501 | 5 |
| Pathway Name | Symbol |
|---|---|
| Pathways in cancer | AGTR1, AKT1, ALK, AR, BAX, BCL2, CAMK2B, CASP3, CASP8, CASP9, CDK2, EDNRA, EGFR, ESR1, ESR2, F2, F2R, FGF1, FGF2, FGFR1, GSK3B, GSTM1, GSTP1, HDAC1, HMOX1, HSP90AA1, IGF1R, IKBKB, IL6, IL6ST, ITGAV, ITGB1, JAK1, JAK2, JAK3, MDM2, MET, MMP1, MMP2, MMP9, NOS2, NTRK1, PIK3R1, PPARD, PPARG, MAPK3, MAPK8, MAPK9, MAPK10, PTGER1, PTGER2, PTGER4, PTGS2, PTK2, RARB, RELA, ROCK1, RXRA, STAT1, TERT, TGFB1, VEGFA |
| Lipid and atherosclerosis | AKT1, BAX, BCL2, CAMK2B, CASP3, CASP8, CASP9, CD14, MAPK14, CYP1A1, CYP2C9, GSK3B, HSPA8, HSP90AA1, ICAM1, IKBKB, IL6, JAK2, LBP, MAP3K5, MMP1, MMP3, MMP9, NOS3, PIK3R1, PPARG, MAPK3, MAPK8, MAPK9, MAPK10, MAPK13, MAP2K7, PTK2, RELA, RXRA, SELE, MAP2K4, SRC, MAP3K7, TNF, VCAM1, GSTM1, GSTP1, HMOX1, ITGAV, KDR, MMP2, VEGFA, AGTR1, SERPINE1, PRKCD, STAT1, TGFB1, NOX4, CCR1, FGF2, IL6ST, JAK1, PIK3CG, PTGS2, SYK, TYK2, CDK2, JAK3, AHR, CAT, AKR1C4, CYP1A2, CYP1B1, AKR1C1, AKR1C2, EGFR, MET, PTPN1, PTPN11, ALOX5, ITGB1, NOS2, VDR, RIPK2, TLR9, HDAC1, MDM2, CSNK2A1, MMP13, CYP2D6, ESR1, ESR2, IGF1R, RORA, NTRK1, ACE, PPARA, HK2, ROCK1, GAPDH, PTPRC, NR1H3, F2, ADAM17, F2R, SLC2A4, NR1H2, PARP1, G6PD, ALOX5AP, PLA2G4A, CXCR1, HTR2A, PRKCH, PTGER2, PTGER4, TRPV1, ABCC1, OPRD1, ALK, CDK1, APP, CHRNA7, MAPT, SSTR1, SSTR2, SSTR5, MMP7, TERT, COMT, MAOA |
| Chemical carcinogenesis—receptor activation | ADRB2, AHR, AKT1, AR, BCL2, CHRNA7, CYP1A1, CYP1A2, CYP1B1, CYP3A4, EGFR, ESR1, ESR2, FGF2, GSTM1, HSP90AA1, JAK2, PGR, PIK3R1, PPARA, MAPK3, RELA, RXRA, SRC, UGT2B7, VDR, VEGFA, NR1I3, HSPA8, MMP2, MMP9, NOS3, OPRM1, PRKCD |
| Neuroactive ligand receptor interaction | ADORA2A, ADRB2, AGTR1, CHRM2, CHRNA7, CNR1, CNR2, EDNRA, F2, F2R, GABRA1, GABRA2, GABRA3, GABRA5, GABRB3, GLRA1, NR3C1, HTR1A, HTR2A, OPRD1, OPRK1, OPRM1, P2RX3, PTGER1, PTGER2, PTGER4, PTGIR, SSTR1, SSTR2, SSTR5, TACR1, THRA, TRPV1, CAMK2B, CD38, EGFR, FGF1, FGF2, FGFR1, KDR, MET, NOS2, NOS3, NTRK1, VEGFA |
| Proteoglycans in cancer | AKT1, CAMK2B, CASP3, MAPK14, EGFR, ESR1, FGF2, FGFR1, IGF1R, ITGAV, ITGB1, KDR, MDM2, MET, MMP2, MMP9, PIK3R1, MAPK3, MAPK13, PTK2, PTPN11, ROCK1, SRC, TGFB1, TNF, VEGFA, HPSE, CD14, FGF1, HSPA8, IKBKB, MAPT, MAP3K5, NTRK1, PLA2G4A, MAPK8, MAPK9, MAPK10, MAP2K7, RELA, MAP2K4, MAP3K7, AXL, BAX, BCL2, GSK3B, IL6, JAK1, JAK2, PLA2G1B, ADORA2A, CNR1, F2R |
| HIF-1 signaling pathway | AKT1, BCL2, CAMK2B, EGFR, GAPDH, HK2, HMOX1, IGF1R, IL6, NOS2, NOS3, SERPINE1, PFKFB3, PIK3R1, MAPK3, RELA, VEGFA |
| FoxO signaling pathway | AKT1, CAT, CDK2, MAPK14, EGFR, IGF1R, IKBKB, IL6, MDM2, PIK3R1, PLK1, MAPK3, MAPK8, MAPK9, MAPK10, MAPK13, SLC2A4, TGFB1, CDK1, HSP90AA1, PGR, SERPINE1, RELA, AR, CAMK2B |
| cAMP signaling pathway | ADORA2A, ADRB2, AKT1, CAMK2B, CFTR, CHRM2, EDNRA, F2R, HTR1A, PIK3R1, PPARA, MAPK3, MAPK8, MAPK9, MAPK10, PTGER2, RELA, ROCK1, SSTR1, SSTR2, SSTR5 |
| Serotonergic synapse | ALOX5, ALOX15, APP, CASP3, CYP2C19, CYP2C9, CYP2D6, GABRB3, HTR1A, HTR2A, MAOA, PLA2G4A, MAPK3, PTGS1, PTGS2, SLC6A4 |
| VEGF signaling pathway | AKT1, CASP9, MAPK14, KDR, NOS3, PIK3R1, PLA2G4A, MAPK3, MAPK13, PTGS2, PTK2, SRC, VEGFA, F2, F2R, ITGB1, PIK3CG, PTGIR, PTGS1, ROCK1, SYK, AGTR1, EGFR, CXCR1, PTPN11, ACHE, BCL2, CAMK2B, CHRM2, CHRNA7, JAK2, HDAC1, MPO, RELA, MAP3K7, CD38, ADRB2, EDNRA, OPRD1, NOS2, SERPINE1 |
| Steroid hormone biosynthesis | STS, AKR1C4, COMT, CYP1A1, CYP1A2, CYP1B1, CYP3A4, CYP19A1, AKR1C1, AKR1C2, HSD11B1, HSD11B2, UGT2B7, CYP2C19, CYP2C9, GSTM1, GSTP1, PTGS2, CYP2D6, MAOA, CAT, IDO1 |
| Necroptosis | PARP1, ALOX15, BAX, BCL2, CAMK2B, CASP8, HSP90AA1, JAK1, JAK2, JAK3, PLA2G4A, MAPK8, MAPK9, MAPK10, STAT1, TNF, TYK2, CASP3, CASP9, CSNK2A1, GSK3B, MMP7, PPARD, MAP3K7, CFTR, ITGB1, MAP3K5, MAP2K7, ROCK1, SRC, ADORA2A, MAOA, MAPT, HSPA8, RELA, HDAC1, PPARG |
| Gastric cancer | AKT1, BAX, BCL2, CDK2, EGFR, FGF1, FGF2, GSK3B, MET, ABCB1, PIK3R1, MAPK3, RARB, RXRA, TERT, TGFB1, FGFR1, IGF1R, MDM2, ALK, CASP9, JAK3, ESR1, ESR2, PGR, HDAC1, IKBKB, PTPN11, RELA, GSTM1, GSTP1, HMOX1, G6PD, HK2, NTRK1, CD14, MPO, PPARD, CAMK2B, VEGFA, SYK, PLA2G4A, PRKCD, PTPRC, TNF, HSD11B2 |
| Chemokine signaling pathway | AKT1, CCR1, GRK6, GSK3B, IKBKB, CXCR1, JAK2, JAK3, PIK3CG, PIK3R1, PRKCD, MAPK3, PTK2, RELA, ROCK1, SRC, STAT1, CASP9, DIO1, ESR1, HDAC1, ITGAV, MDM2, RXRA, THRA |
| Platinum drug resistance | AKT1, BAX, BCL2, CASP3, CASP8, CASP9, GSTM1, GSTP1, MDM2, MAP3K5, PIK3R1, MAPK3, CDK1, CDK2, HDAC1, IL6ST, JAK1, JAK3, RELA, SRC, SYK, MDM4, SERPINE1 |
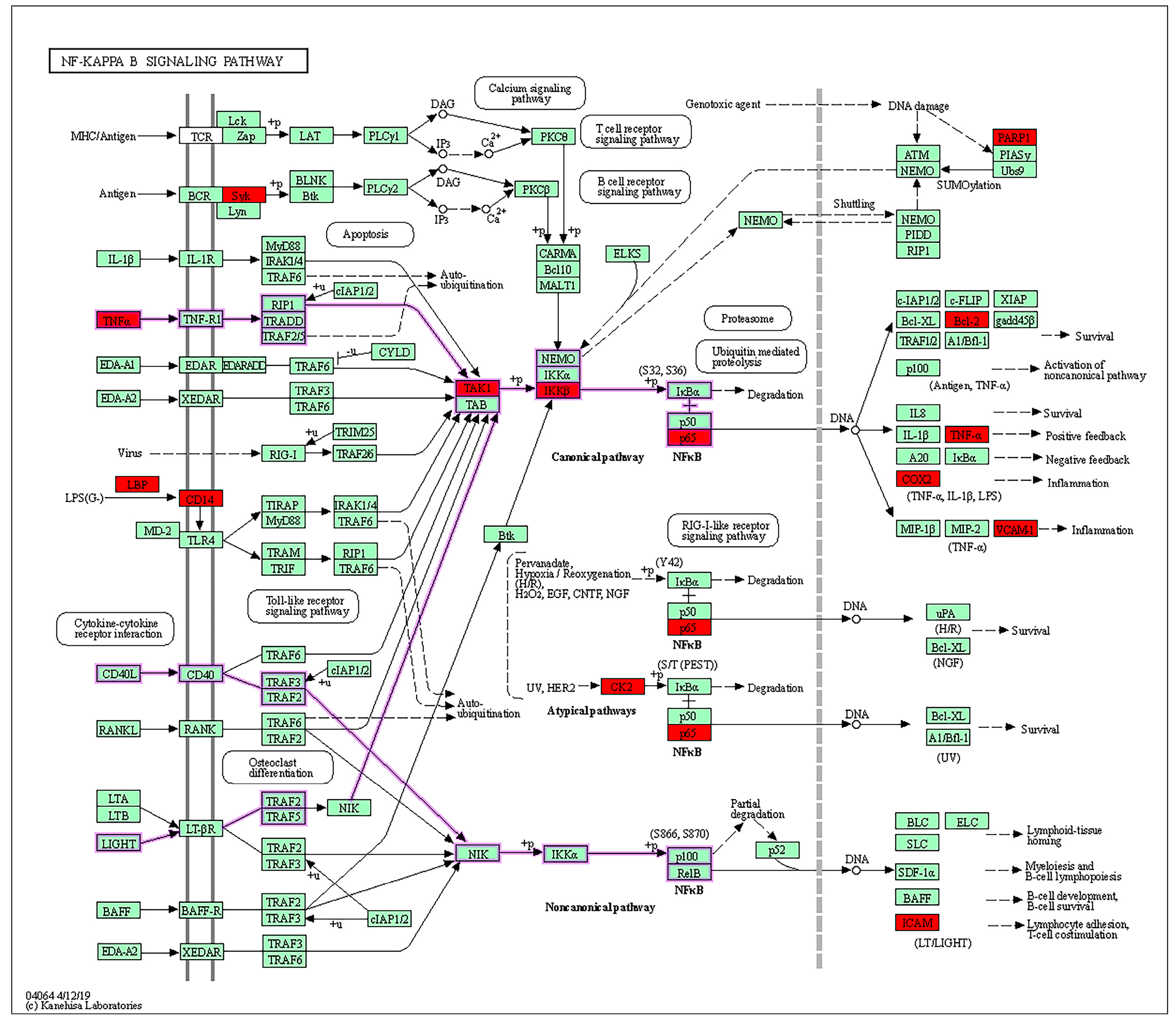
| NF-kappa B signaling pathway | PARP1, BCL2, CD14, CSNK2A1, ICAM1, IKBKB, LBP, PTGS2, RELA, SYK, MAP3K7, TNF, VCAM1 |
| MicroRNAs in cancer | BCL2, CASP3, CYP1B1, DNMT1, EGFR, HDAC1, HMOX1, IKBKB, MDM2, MDM4, MET, MMP9, ABCC1, ABCB1, PIK3R1, MAPK3, PTGS2, ROCK1, VEGFA |
| Signaling pathways regulating pluripotency of stem cells | AKT1, MAPK14, ESRRB, FGF2, FGFR1, GSK3B, IGF1R, IL6ST, JAK1, JAK2, JAK3, PIK3R1, MAPK3, MAPK13, BCL2, EGFR, IL6, PTPN2, PTPN11, STAT1, TYK2 |
| Retrograde endocannabinoid signaling | CNR1, MAPK14, FAAH, GABRA1, GABRA2, GABRA3, GABRA5, GABRB3, MAPK3, MAPK8, MAPK9, MAPK10, MAPK13, PTGS2, CHRNA7, HTR1A, P2RX3, SCN9A, GRK6, OPRM1, SRC |
| Bladder cancer | TYMP, EGFR, MDM2, MMP1, MMP2, MMP9, MAPK3, SRC, VEGFA, CSNK2A1, FGFR1, IGF1R, MET, PTPN1, MAP3K7, CDK1, HTR2A, GRK6, HSPA8, CXCR1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Bai, C.; Zhang, Y. Paeoniae Radix Alba and Network Pharmacology Approach for Osteoarthritis: A Review. Separations 2024, 11, 184. https://doi.org/10.3390/separations11060184
Wang B, Bai C, Zhang Y. Paeoniae Radix Alba and Network Pharmacology Approach for Osteoarthritis: A Review. Separations. 2024; 11(6):184. https://doi.org/10.3390/separations11060184
Chicago/Turabian StyleWang, Bo, Changcai Bai, and Yuanyuan Zhang. 2024. "Paeoniae Radix Alba and Network Pharmacology Approach for Osteoarthritis: A Review" Separations 11, no. 6: 184. https://doi.org/10.3390/separations11060184





