Rapid Discovery of Antimicrobial and Antimalarial Agents from Natural Product Fragments
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Microbe Material
2.3. TCM Material
2.4. Malarial Fragments
2.5. Malarial Proteins
2.6. Fermentation, Extraction of the 4 Microbes
2.7. Extraction of the 2 TCMs
2.8. Fragment Isolation
2.9. Biological Assays
2.10. Native MS Experiment
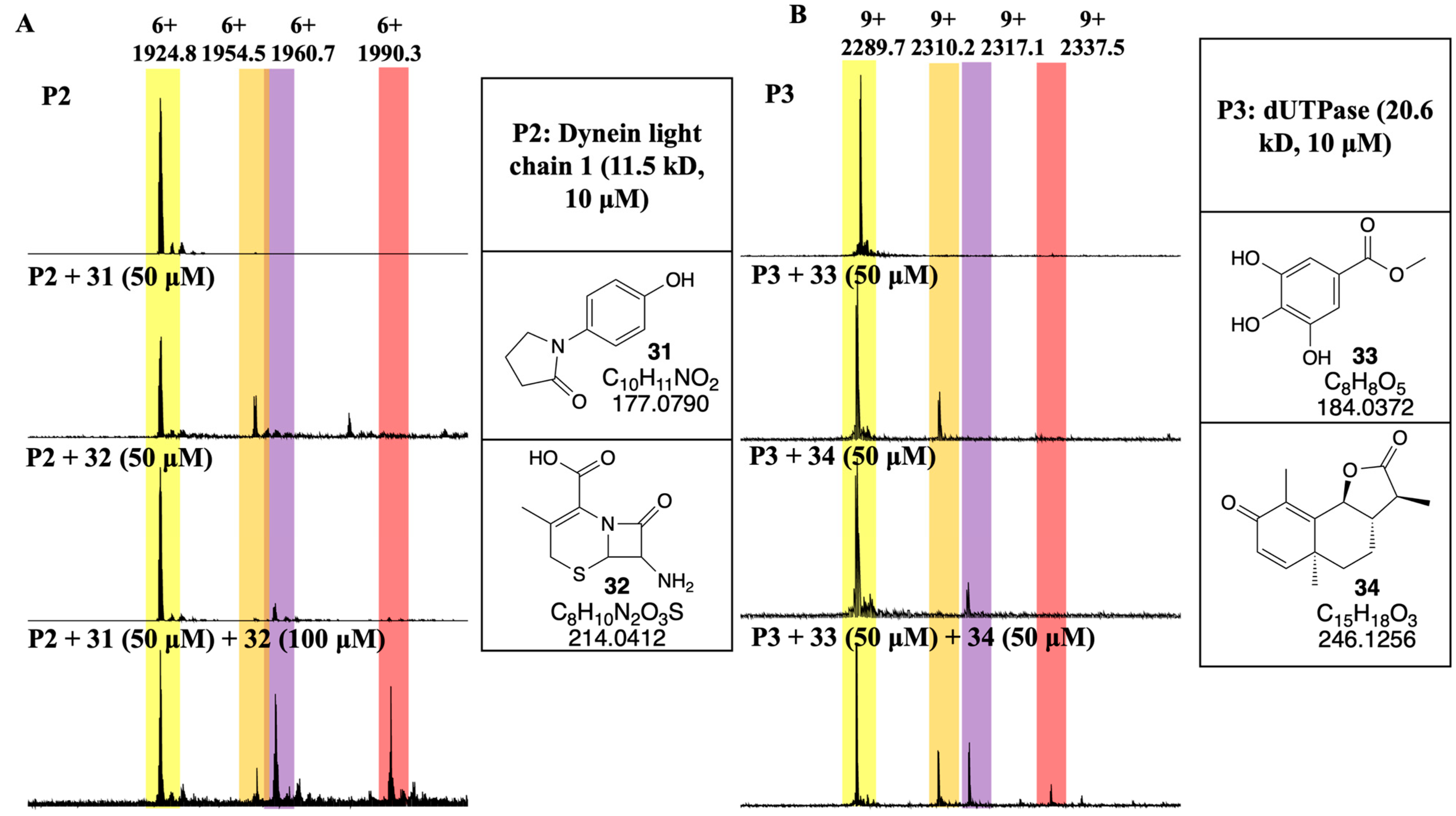
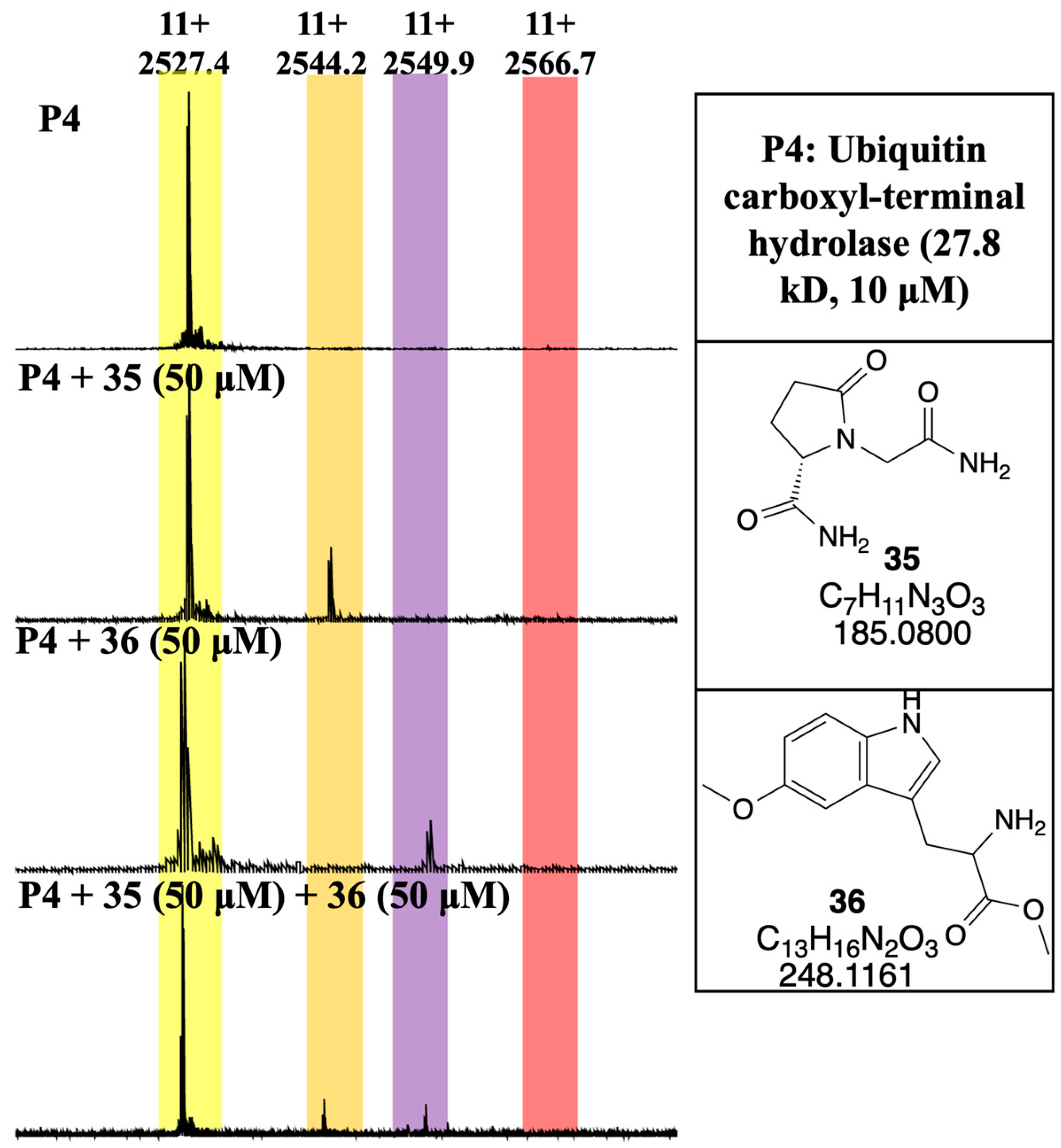
3. Results and Discussion
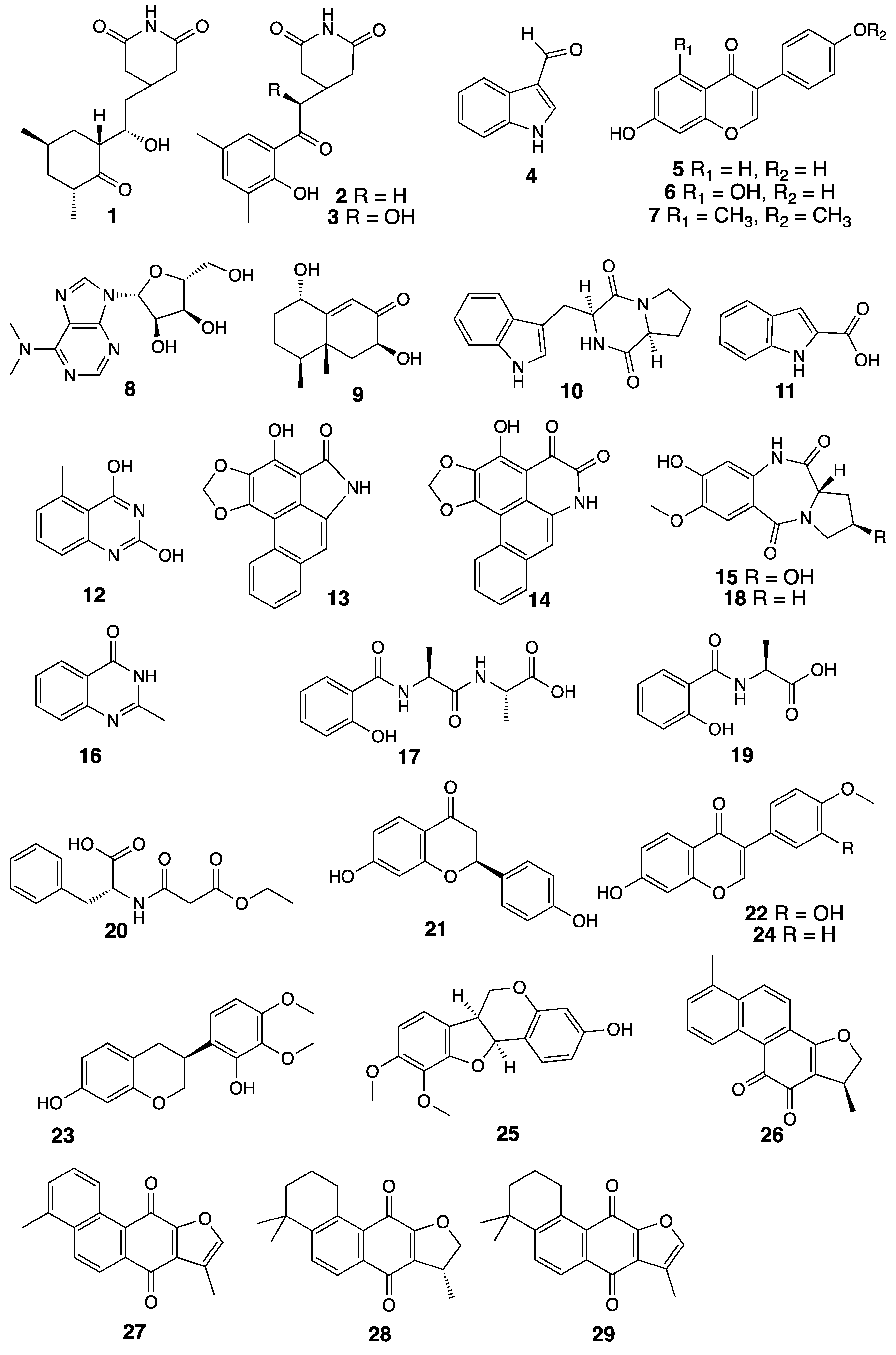
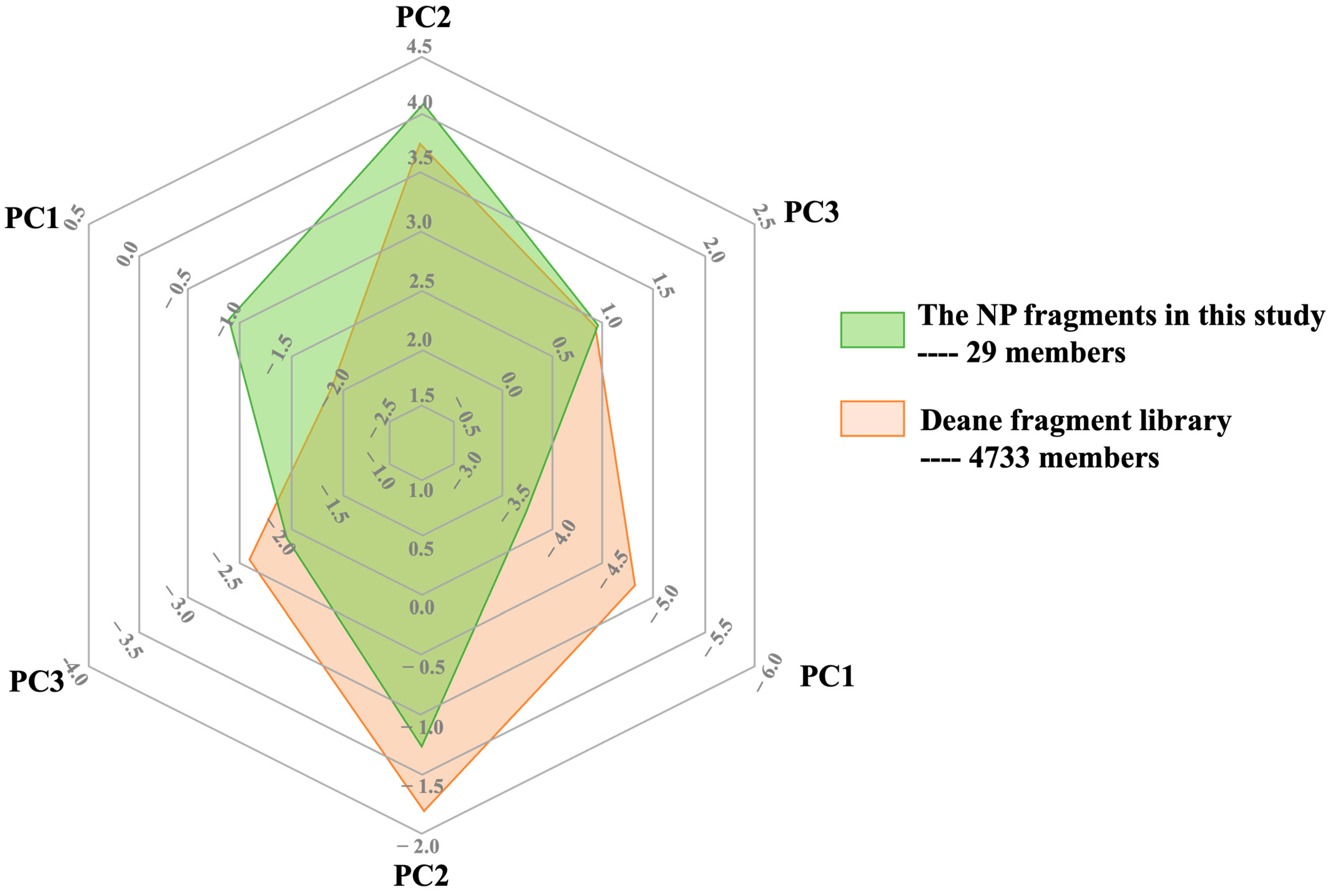
3.1. Developing a Drug-Like Natural Product Fragment Library: Extraction
3.2. Developing a Drug-Like Natural Product Fragment Library: Purification
3.3. Developing a Drug-Like Natural Product Fragment Library: Bioactivity Evaluation
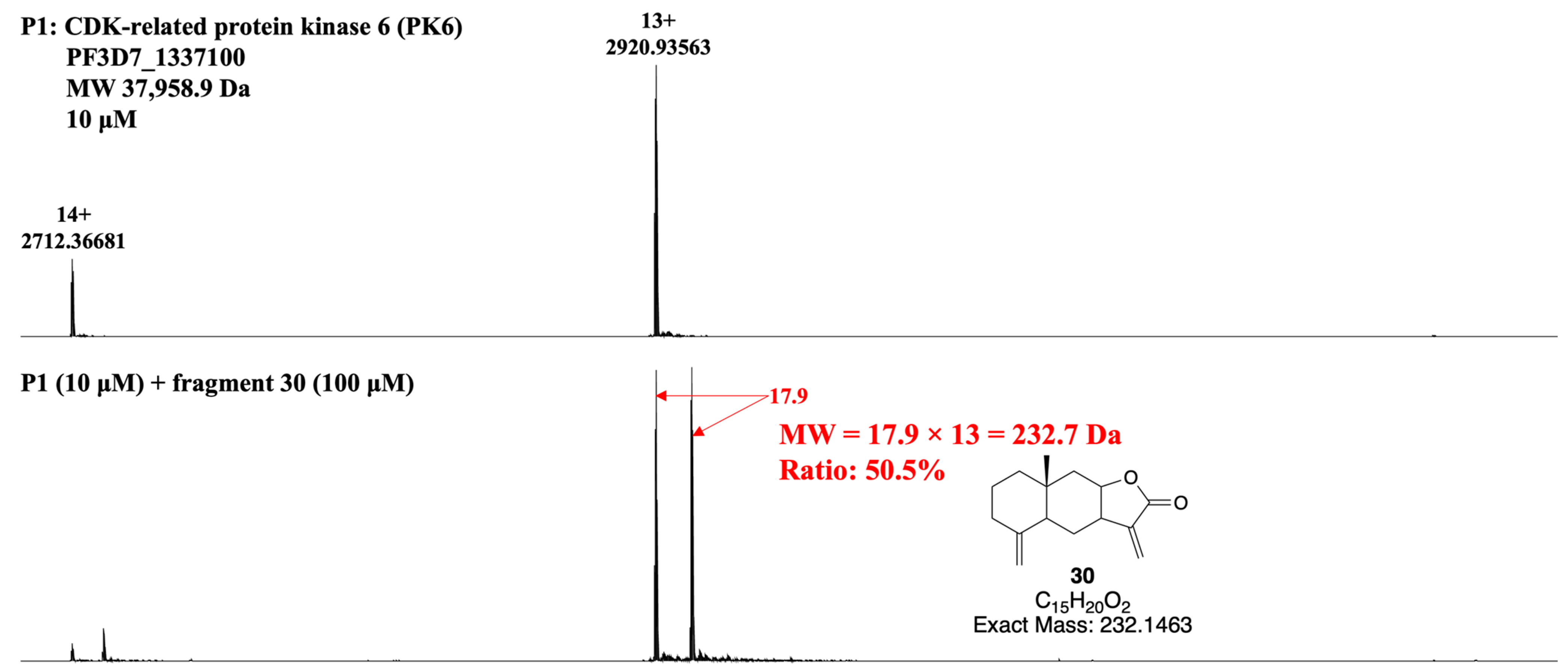
3.4. Developing a Drug-Like Natural Product Fragment Library: Protein Binding
3.5. Developing a Drug-Like Natural Product Fragment Library: Competition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erlanson, D.A.; Fesik, S.W.; Hubbard, R.E.; Jahnke, W.; Jhoti, H. Twenty years on: The impact of fragments on drug discovery. Nat. Rev. Drug Discov. 2016, 15, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.S.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.; Kettle, J.G.; Nowak, T.; Pease, J.E. Expanding medicinal chemistry space. Drug Discov. Today 2013, 18, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Bohacek, R.S.; McMartin, C.; Guida, W.C. The art and practice of structure-based drug design: A molecular modeling perspective. Med. Res. Rev. 1996, 16, 3–50. [Google Scholar] [CrossRef]
- Congreve, M.; Carr, R.; Murray, C.; Jhoti, H. A rule of three for fragment-based lead discovery? Drug Discov. Today 2003, 8, 876–877. [Google Scholar] [CrossRef]
- Doak, B.C.; Morton, C.J.; Simpson, J.S.; Scanlon, M.J. Design and evaluation of the performance of an NMR screening fragment library. Aust. J. Chem. 2013, 66, 1465–1472. [Google Scholar] [CrossRef]
- Ruddigkeit, L.; van Deursen, R.; Blum, L.C.; Reymond, J.L. Enumeration of 166 billion organic small molecules in the chemical universe database GDB-17. J. Chem. Inf. Model. 2012, 52, 2864–2875. [Google Scholar] [CrossRef]
- Ertl, P. Cheminformatics analysis of organic substituents: Identification of the most common substituents, calculation of substituent properties, and automatic identification of drug-like bioisosteric groups. J. Chem. Inf. Comput. Sci. 2003, 43, 374–380. [Google Scholar] [CrossRef]
- Leeson, P.D.; St-Gallay, S.A. The influence of the ‘organizational factor’ on compound quality in drug discovery. Nat. Rev. Drug Discov. 2011, 10, 749–765. [Google Scholar] [CrossRef]
- Hann, M.M.; Leach, A.R.; Harper, G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comput. Sci. 2001, 41, 856–864. [Google Scholar] [CrossRef]
- Leach, A.R.; Hann, M.M. Molecular complexity and fragment-based drug discovery: Ten years on. Curr. Opin. Chem. Biol. 2011, 15, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Weissman, K.J.; Leadlay, P.F. Combinatorial biosynthesis of reduced polyketides. Nat. Rev. Microbiol. 2005, 3, 925–936. [Google Scholar] [CrossRef]
- Imbernon, J.R.; Jacquemard, C.; Bret, G.; Marcou, G.; Kellenberger, E. Comprehensive analysis of commercial fragment libraries. RSC Med. Chem. 2022, 13, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.H.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method—A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Bajsa, J.; McCluskey, A.; Gordon, C.P.; Stewart, S.G.; Hill, T.A.; Sahu, R.; Duke, S.O.; Tekwani, B.L. The antiplasmodial activity of norcantharidin analogs. Bioorg. Med. Chem. Lett. 2010, 20, 6688–6695. [Google Scholar] [CrossRef]
- Suberu, J.O.; Gorka, A.P.; Jacobs, L.; Roepe, P.D.; Sullivan, N.; Barker, G.C.; Lapkin, A.A. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract—Possible synergistic and resistance mechanisms. PLoS ONE 2013, 8, e80790. [Google Scholar] [CrossRef]
- Camp, D.; Davis, R.A.; Campitelli, M.; Ebdon, J.; Quinn, R.J. Drug-like properties: Guiding principles for the design of natural product libraries. J. Nat. Prod. 2012, 75, 72–81. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Liu, M.M.; Abdel-Mageed, W.M.; Ren, B.; He, W.N.; Huang, P.; Li, X.L.; Bolla, K.; Guo, H.; Chen, C.X.; Song, F.H.; et al. Endophytic Streptomyces sp. Y3111 from traditional Chinese medicine produced antitubercular pluramycins. Appl. Microbiol. Biotechnol. 2014, 98, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.M.; Grkovic, T.; Liu, X.T.; Han, J.Y.; Zhang, L.X.; Quinn, R.J. A systems approach using OSMAC, Log P and NMR fingerprinting: An approach to novelty. Synth. Syst. Biotechnol. 2017, 2, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.H.; Huang, L.F.; Zheng, S.H.; Wang, D.M.; Chen, S.L.; Zhang, H.T.; Yang, S.H. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus Huangqi. Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.D.; Cao, Y.F.; Che, Y.Y.; Li, J.; Shang, Z.P.; Zhao, W.J.; Qiao, Y.J.; Zhang, J.Y. Danshen: A phytochemical and pharmacological overview. Chin. J. Nat. Med. 2019, 17, 59–80. [Google Scholar]
- Chen, Z.J.; Liu, L.J.; Gao, C.F.; Chen, W.J.; Vong, C.T.; Yao, P.F.; Yang, Y.H.; Li, X.Z.; Tang, X.D.; Wang, S.P.; et al. Astragali Radix (Huangqi): A promising edible immunomodulatory herbal medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef]
- Chang, X.N.; Chen, X.F.; Guo, Y.X.; Gong, P.; Pei, S.Y.; Wang, D.N.; Wang, P.P.; Wang, M.R.; Chen, F.X. Advances in chemical composition, extraction techniques, analytical methods, and biological activity of Astragali radix. Molecules 2022, 27, 1058. [Google Scholar] [CrossRef] [PubMed]
- Poncet, J.; Jouin, P.; Castro, B.; Nicolas, L.; Boutar, M.; Gaudemer, A. Tetramic acid chemistry. 1. reinvestigation of racemization during the synthesis of tetramic acids via dieckmann cyclization. J. Chem. Soc.-Perkin Trans. 1990, 1, 611–616. [Google Scholar] [CrossRef]
- Wang, L.L.; Ma, R.F.; Liu, C.Y.; Liu, H.X.; Zhu, R.Y.; Guo, S.Z.; Tang, M.K.; Li, Y.; Niu, J.Z.; Fu, M.; et al. Salvia miltiorrhiza: A potential red light to the development of cardiovascular diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef]
- Ren, J.; Fu, L.; Nile, S.H.; Zhang, J.; Kai, G.Y. Salvia miltiorrhiza in treating cardiovascular diseases: A review on Its pharmacological and clinical applications. Front. Pharmacol. 2019, 10, 753. [Google Scholar] [CrossRef]
- Li, Z.M.; Xu, S.W.; Liu, P.Q. Salvia miltiorrhiza Burge (Danshen): A golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 2018, 39, 802–824. [Google Scholar] [CrossRef]
- Rosen, J.; Lovgren, A.; Kogej, T.; Muresan, S.; Gottfries, J.; Backlund, A. ChemGPS-NP(Web): Chemical space navigation online. J. Comput. Aided Mol. Des. 2009, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.; Gottfries, J.; Muresan, S.; Backlund, A. ChemGPS-NP: Tuned for navigation in biologically relevant chemical space. J. Nat. Prod. 2007, 70, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Carbery, A.; Skyner, R.; von Delft, F.; Deane, C.M. Fragment Libraries Designed to Be Functionally Diverse Recover Protein Binding Information More Efficiently Than Standard Structurally Diverse Libraries. J. Med. Chem. 2022, 65, 11404–11413. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, P.; Hartman, A.M.; Hirsch, A.K.H.; Empting, M. Concepts and core principles of fragment-based drug design. Molecules 2019, 24, 4309. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Howard, S.; Cons, B.D. Fragment-based drug discovery and its application to challenging drug targets. Struct. -Based Drug Des. Insights Acad. Ind. 2017, 61, 475–484. [Google Scholar]
- Maveyraud, L.; Mourey, L. Protein X-ray crystallography and drug discovery. Molecules 2020, 25, 1030. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.; Walser, R. Applied biophysical methods in fragment-based drug discovery. SLAS Discov. 2020, 25, 471–490. [Google Scholar] [CrossRef]
- Liu, M.; Mirza, A.; McAndrew, P.C.; Thapaliya, A.; Pierrat, O.A.; Stubbs, M.; Hahner, T.; Chessum, N.E.A.; Innocenti, P.; Caldwell, J.; et al. Determination of ligand-binding affinity (Kd) using transverse relaxation rate (R2) in the ligand-observed 1H NMR experiment and applications to fragment-based drug discovery. J. Med. Chem. 2023, 66, 10617–10627. [Google Scholar] [CrossRef]
- Quinn, R.J.; Mak, T.; Littler, D.R.; Rossjohn, J.; Liu, M.M. Discovery of Anti-SARS-CoV-2 Nsp9 Binders from Natural Products by a Native Mass Spectrometry Approach. J. Nat. Prod. 2023, 86, 2630–2637. [Google Scholar] [CrossRef]
- Liu, M.; Littler, D.R.; Rossjohn, J.; Quinn, R.J. Binding studies of the prodrug HAO472 to SARS-Cov-2 nsp9 and variants. ACS Omega 2022, 7, 7327–7332. [Google Scholar] [CrossRef]
- Littler, D.R.; Liu, M.; McAuley, J.L.; Lowery, S.A.; Illing, P.T.; Gully, B.S.; Purcell, A.W.; Chandrashekaran, I.R.; Perlman, S.; Purcell, D.F.J.; et al. A natural product compound inhibits coronaviral replication in vitro by binding to the conserved Nsp9 SARS-CoV-2 protein. J. Biol. Chem. 2021, 297, 101362. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.; Pedro, L.; Mak, T.; McCormick, B.; Rowley, J.; Liu, M.M.; Di Capua, A.; Williams-Noonan, B.; Pham, N.B.; Pouwer, R.; et al. Fragment-based screening of a natural product library against 62 potential malaria drug targets employing native mass spectrometry. ACS Infect. Dis. 2018, 4, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Bancet, A.; Raingeval, C.; Lomberget, T.; Le Borgne, M.; Guichou, J.F.; Krimm, I. Fragment linking strategies for structure-based drug design. J. Med. Chem. 2020, 63, 11420–11435. [Google Scholar] [CrossRef] [PubMed]
- Daher, W.; Pierrot, C.; Kalamou, H.; Pinder, J.C.; Margos, G.; Dive, D.; Franke-Fayard, B.; Janse, C.J.; Khalife, J. Plasmodium falciparum Dynein light chain 1 interacts with actin/myosin during blood stage development. J. Biol. Chem. 2010, 285, 20180–20191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kehrer, J.; Singer, M.; Reinig, M.; Santos, J.M.; Mair, G.R.; Frischknecht, F. Functional genetic evaluation of DNA house-cleaning enzymes in the malaria parasite: dUTPase and Ap4AH are essential in Plasmodium berghei but ITPase and NDH are dispensable. Expert Opin. Ther. Targets 2019, 23, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Moreno, G.; Sánchez-Carrasco, P.; Ruiz-Pérez, L.M.; Johansson, N.G.; Müller, S.; Baragana, B.; Hampton, S.E.; Gilbert, I.H.; Kaiser, M.; Sarkar, S.; et al. Validation of Plasmodium falciparum dUTPase as the target of 5′-tritylated deoxyuridine analogues with anti-malarial activity. Malar. J. 2019, 18, 392. [Google Scholar] [CrossRef]
- Imhoff, R.D.; Rosenthal, M.R.; Ashraf, K.; Bhanot, P.; Ng, C.L.; Flaherty, D.P. Identification of covalent fragment inhibitors for Plasmodium falciparum UCHL3 with anti-malarial efficacy. Bioorganic Med. Chem. Lett. 2023, 94, 129458. [Google Scholar] [CrossRef]
- Artavanis-Tsakonas, K.; Weihofen, W.A.; Antos, J.M.; Coleman, B.I.; Comeaux, C.A.; Duraisingh, M.T.; Gaudet, R.; Ploegh, H.L. Characterization and structural studies of the ubiquitin and nedd8 hydrolase UCHL3. J. Biol. Chem. 2010, 285, 6857–6866. [Google Scholar] [CrossRef]
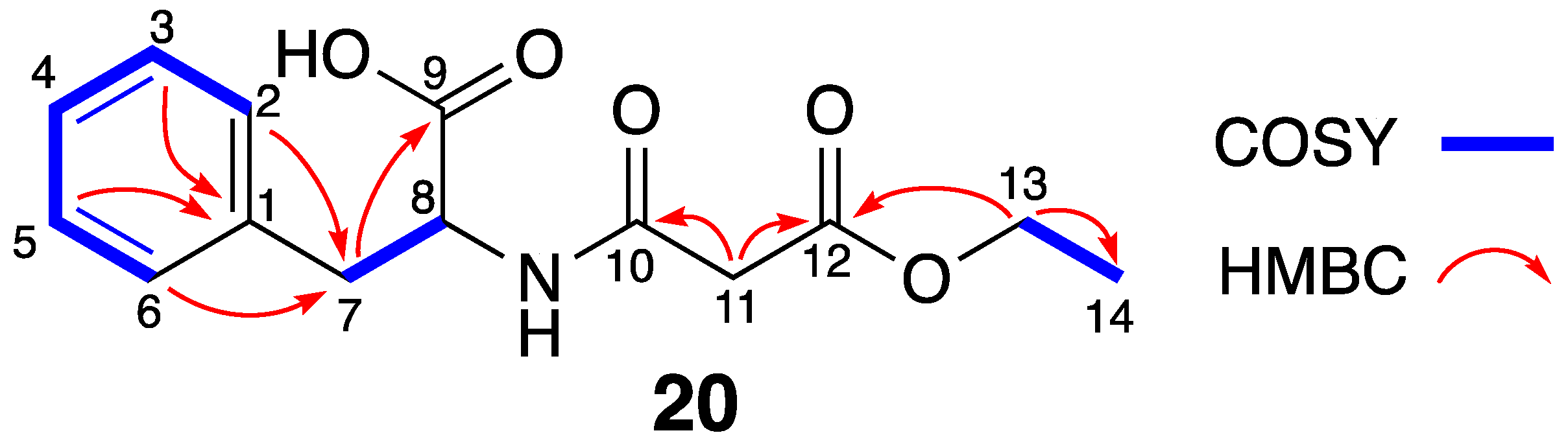
| Pos. | δC, Type | δH, mult (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 134.8, C | |||
| 2 | 129.5, CH | 7.26, d (7.6) | 3 | 4, 6, 7 |
| 3 | 128.6, CH | 7.34, t (7.6) | 2, 4 | 1, 5 |
| 4 | 127.3, CH | 7.29, t (7.6) | 3, 5 | 2, 6 |
| 5 | 128.6, CH | 7.34, t (7.6) | 4, 6 | 1, 3 |
| 6 | 129.5, CH | 7.26, d (7.6) | 5 | 2, 4, 7 |
| 7 | 35.9, CH2 | 3.09, t (6.1) | 8 | 1, 2, 6, 8, 9 |
| 8 | 53.2, CH | 4.19, m | 7 | |
| 9 | 170.5, C | |||
| 10 | 168.1, C | |||
| 11 | 41.6, CH2 | 3.35, s | 10, 12 | |
| 12 | 166.9, C | |||
| 13 | 60.7, CH2 | 4.09, q (7.1) | 14 | 12, 14 |
| 14 | 14.0, CH3 | 1.19, t (7.1) | 13 | 13 |
| Organism (Strain) | Minimum Inhibitory Concentration (μg/mL) | ||||
|---|---|---|---|---|---|
| 3 | 10 | 13 | 14 | Control | |
| Bacillus Calmette-Guérin (Pasteur 1173P2, BCG) | 100 | 4 | 8 | 8 | 0.37 [a] |
| Staphylococcus aureus (ATCC 6538) | NA # | 8 | 8 | 8 | 0.7 [b] |
| Methicillin-resistant S. aureus (Clinical strain of Chaoyang hospital) | NA | 8 | 8 | NA | 0.7 [b] |
| Bacillus subtilis (ATCC 6633) | NA | NA | NA | NA | 0.35 [b] |
| Pseudomonas aeruginosa (PAO1) | NA | NA | NA | NA | 3 [c] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Liu, X.; Zhang, L.; Van Voorhis, W.C.; Quinn, R.J.; Liu, M. Rapid Discovery of Antimicrobial and Antimalarial Agents from Natural Product Fragments. Separations 2024, 11, 194. https://doi.org/10.3390/separations11070194
Han J, Liu X, Zhang L, Van Voorhis WC, Quinn RJ, Liu M. Rapid Discovery of Antimicrobial and Antimalarial Agents from Natural Product Fragments. Separations. 2024; 11(7):194. https://doi.org/10.3390/separations11070194
Chicago/Turabian StyleHan, Jianying, Xueting Liu, Lixin Zhang, Wesley C. Van Voorhis, Ronald J. Quinn, and Miaomiao Liu. 2024. "Rapid Discovery of Antimicrobial and Antimalarial Agents from Natural Product Fragments" Separations 11, no. 7: 194. https://doi.org/10.3390/separations11070194
APA StyleHan, J., Liu, X., Zhang, L., Van Voorhis, W. C., Quinn, R. J., & Liu, M. (2024). Rapid Discovery of Antimicrobial and Antimalarial Agents from Natural Product Fragments. Separations, 11(7), 194. https://doi.org/10.3390/separations11070194







