Abstract
Futibatinib is an excellent fibroblast growth factor receptor 1–4 (FGFR 1–4) inhibitor that exhibits selective anti-tumor activeness against FGFR-deregulated tumors. A new high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) technique for the quantitative analysis of futibatinib in beagle dog plasma was developed, and the effect of Yinchenhao decoction (YCHD) on the pharmacokinetics of futibatinib was evaluated. After processing plasma samples with ethyl acetate extraction in the alkaline condition of sodium carbonate, a C18 column (4.6 mm × 150, 5 μm) was used to accomplish the separation of futibatinib and ripretinib (internal standard, ISTD), with the mobile phase consisting of methanol and 0.1% formic acid in water (60:40). The scanning method adopted a multiple reaction monitoring (MRM) mode with positive ion detection through the triple quadrupole mass spectrometer. The ion transitions for futibatinib and IS were m/z 419.20 → 296.15 and m/z 510.36 → 417.00, respectively. Futibatinib displayed excellent linearity in the range of 1–200 ng/mL. Neither inter-day nor intra-day precision exceeded 6.3%. The %RE values for accuracy ranged from −3.1% to 0.9%. The recovery, stability, and matrix effect of futibatinib also complied with the guidelines for the validation of quantitative analysis methods for biological samples in the 2020 edition of the Chinese Pharmacopoeia. In combination with YCHD, the Cmax of futibatinib increased by 40.84% compared to futibatinib dosage alone., and the AUC(0–t) and AUC(0–∞) of futibatinib increased by 78.06% and 82.71%, respectively. The Vd and CL of futibatinib were reduced by 20.05% and 40.85%, respectively. T1/2 was extended from 3.88 h to 5.26 h. The results indicated that YCHD could affect the pharmacokinetics of futibatinib and increase the plasma exposure of futibatinib. If YCHD is administered along with futibatinib, this study gives a first impression how pharmacokinetics and toxicokinetics would change.
1. Introduction
Cholangiocarcinoma (CCA) is the most common malignant disease in the biliary tract and the second most common primary malignant disease in the liver [1]. Its cells originate from hepatic progenitor cells [2]. Inflammation and bile stasis are important factors in the development of cholangiocarcinoma. Proinflammatory cytokines (i.e., interleukin 6 [IL-6]) activate inducible nitric oxide synthase, leading to an excess of nitric oxide and mediating DNA oxidative damage, inhibiting the expression of DNA repair enzymes and cyclooxygenase 2. Proinflammatory pathways down-regulate the hepatobiliary transit proteins, leading to biliary stasis [3]. Bile acid and oxysterol positively regulate cancer-promoting cellular pathways through a series of pathways [4]. Cholangiocarcinomas are categorized into intrahepatic subtypes (iCCA), perihepatic subtypes (pCCA), and distal subtypes (dCCA) based on anatomical location [5]. According to reports, the most common genetic modifications in cholangiocarcinoma included BRCA1-associated protein 1 (BAP1, 20%), tumor protein P53 (TP53, 20%), isocitrate dehydrogenase 1 (IDH1, 30%), AT-rich interactive domain 1A (ARID1A, 23%), and FGFR2 gene fusions (14%), while the modifications to Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and Erb-b2 receptor tyrosine kinase 2 (ERBB2) were less common [6]. Recently, gene rearrangements leading to the integration of the carcinogenic FGFR 2 had been found in approximately 45% of patients with iCCA [7,8]. The dysregulation of FGFR cell signaling can lead to tumorigenesis in various cancers, all of which with FGFR distortion, such as fusion, point mutation, insertion loss mutation, or expansion [9].
Futibatinib is an excellent FGFR 1–4 inhibitor that exhibits selective anti-tumor activeness against FGFR-deregulated tumors [10]. It is approved and clinically available for patients with previously treated, irresectable, locally advanced, metastatic combined FGFR2 fusion or rearrangement-iCCA.
Yinchenhao Decoction (YCHD) is a classical Chinese herbal formulation composed of three herbs, Yinchen (Artemisiae Scopariae Herba, Artemisia capillaris Thunb), Zhizi (Gardeniae Fructus, Gardenia jasminoides), and Dahuang (Radix Rhei et Rhizoma, Rheum palmatum L) (6:4:2). YCHD has been used by Chinese medicine practitioners for treating jaundice and liver diseases for more than two thousand years, which can eliminate dampness and heat [11]. Yinchen is mainly used to dispel jaundice and eliminate heat and dampness, Zhizi is mainly used to reduce fire and eliminate dampness-heat, and Dahuang is mainly used to remove intestinal heat, detoxify and eliminate dampness [12]. The three herbals work together to prevent stagnation and achieve the efficacy of relieving heat and removing toxins, inducing diuresis and relieving icterus [13]. YCHD is mainly used in modern clinics to treat diseases of the hepatobiliary system, such as acute and chronic viral hepatitis, liver cirrhosis, non-alcoholic fatty liver disease, biliary stasis, etc.., and has demonstrated good results [13]. YCHD has a wide range of applications in the field of liver diseases, so more studies have been conducted to analyze its chemical composition and found more effective compound components, such as 6,7-dimethoxycoumarin, quercetin, gardenia glycosides, rhubarb phenol, rhein, etc. [14]. It is precisely due to the biology of the different components of YCHD that the therapeutic effect of the whole formula is also manifested. For example, 6,7-Dimethoxycoumarin inhibits the progression of liver cancer cells by regulating the p38 mitogen-activated protein kinase (MAPK)/protein kinase B (PKB)/nuclear factor kappa-B (NF-κB) signaling pathway in mice with nonalcoholic fatty liver [15]. Gardenia glycoside is the main active ingredient in gardenia, and Kuo et al. found that gardenia glycoside has a biological ability of detoxification, antioxidant, and anticancer by initiating and enhancing the expressions of Ras/Raf/MEK-1 signaling mediator and inducing glutathione S-transferase (GST) activity as well as the expression of glutathione S-transferase Mu 1 (GSTM1) and GSTM1 through the MAP kinase kinase 2 (MEK-2) pathway [16]. Yang et al. found that rhein showed significant efficacy in non-small cell lung cancer by suppressing the signal transducer and activator of the transcription 3 (STAT3) pathway [17].
Futibatinib is used to treat iCCA patients with FGFR2 integration or rearrangement. Meanwhile, YCHD is effective in the treatment of hepatobiliary diseases and has positive effects on cancer and cholangiocarcinoma [11]. However, there is currently no study that indicates the effect of YCHD on the pharmacokinetics of futibatinib. Only a limited number of analytical methods have been recorded for the quantification of futibatinib [18]. The objective of this study was to create and optimize a cost-effective and concise method for the extraction and quantification of futibatinib in beagle dog plasma. The newly developed HPLC-MS/MS method offered several advantages over the previous method, including excellent linearity, smaller sample volume, weaker matrix effect, and less solvent usage. Meanwhile, the study of the herb–drug interaction could provide a theory for the combination therapy of these drugs. The method was used to evaluate the effect of YCHD on the pharmacokinetics of futibatinib and to provide an experimental basis for the combined use of these drugs in the clinic.
2. Materials and Methods
2.1. Chemical Materials and Reagents
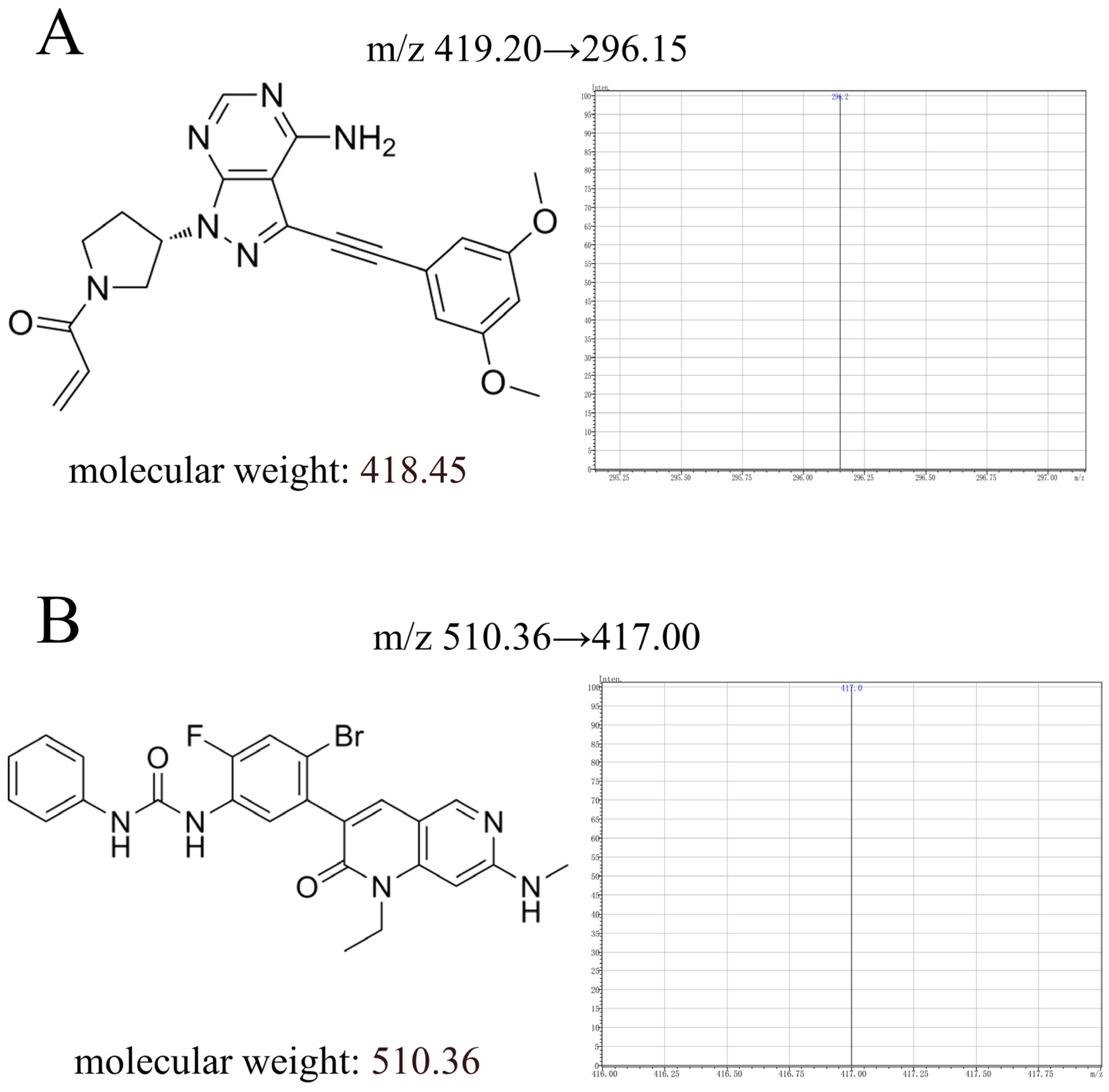
Yinchenhao decoction (production lot number:10059655245743) was purchased from Xishitang Nourishing and Nourishing Specialty Store (Beijing, China). Futibatinib (Figure 1A, purity ≥ 98%, CAS:1448169-71-8) and ripretinib (internal standard, Figure 1B, purity ≥ 98%, CAS:1442472-39-0) were bought from Shanghai Perfemiker Chemical Technology Co., Ltd. (Shanghai, China). Analytical methanol, 0.1% formic acid and ethyl acetate were bought from Tianjin Komel Chemical Agents Development Center. (Tianjin, China). EP tubes were bought from Beijing Labgic Technology Co., Ltd. (Beijing, China). HPLC vials were bought from Shimadzu (China) Co., Ltd. (Shanghai, China). Ultrapure water was produced through the ultrapure water unit.
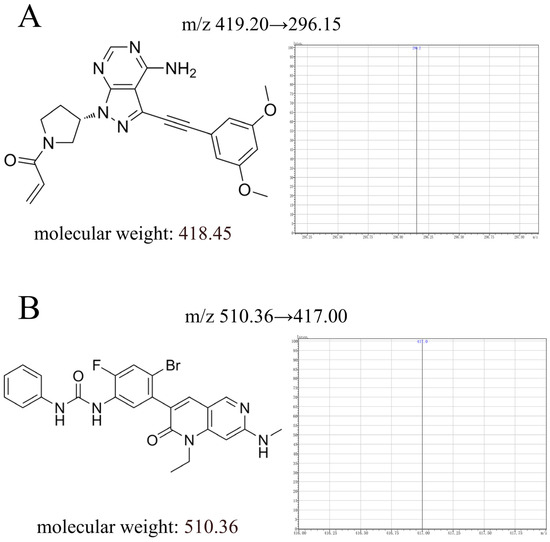
Figure 1.
The chemical structure, molecular weight, and daughter ion of futibatinib (A) and ripretinib (B) in this study.
2.2. Animal Experiments
Six healthy adult beagle dogs (8~10 kg, 3 males and 3 females) were purchased from Hubei Yi zhicheng bio-technology Co. (Hubei, China). The production license number was SCXK2021(HUBEI)2021-0020. This study was approved by the Ethics Committee of the Animal Laboratory of Henan University of Science and Technology (code number: 202307001) and the animals were also cared for following China’s Guidelines for Ethical Review of Laboratory Animals Welfare (GB/T 35892-2018). The animals in the study were raised under the same environment (25~27 °C, 12 h light/dark cycle, 40~60% relative humidity). Each beagle dog was marked with a different colored collar.
A two-cycle self-controlled design was adopted for this animal experiment. The day before the experiment, six healthy adult beagle dogs were fasted for at least 12 h and kept drinking water freely. In the 1st period, they were orally given futibatinib (0.67 mg/kg) and blood was drawn at successive times. The blood samples (approximately 2.0 mL) were obtained from the vein of the hind limb and forelimb with intravenous needles at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h and stored in a heparin-containing EP tube. After a 1-week washout period, in the 2nd period, YCHD (1.2 g/kg) was dosed orally to the same six beagle dogs every morning until the 14th day. Half an hour after feeding YCHD on day 14, futibatinib (0.67 mg/kg) was then administered orally. The blood collecting points were the same in both periods. All samples were centrifuged at 9500× g for 11 min at 4 °C. The obtained supernatant was stored at −20 °C prior to analysis.
2.3. Instrumentations
The instrument used for chromatographic analysis was a high-performance liquid chromatograph (LC-20A) including a DGU-20A degasser, LC-20AD pump, CTO-20A column heater, CBM-20A signal receiver, SPD-20A detector, SIL-20A autosampler, and Shimadzu chemistry workstation (Shimadzu, Japan).
The mass spectrometry analysis was performed using a Shimadzu LCMS-8045 triple quadrupole mass spectrometer (Kyoto, Japan). Other instruments included ultrapure water unit (UPR-II-5/10T, Sichuan Youpu Ultrapure Technology Co., Ltd. Sichuan, China), vortex mixer (SG-VM-01, Shanghai Shangdao Instrument Manufacturing Co., Ltd. Shanghai, China), and electronic analytical balance (FA1004B, Shanghai Youke Instrumentation Co., Ltd. Shanghai, China).
2.4. Analytical Conditions
The chromatographic column was a SHIMADZU VP-ODS C18 column (4.6 mm × 150, 5 μm), and the column temperature was 40 °C. The mobile phase consisted of methanol and 0.1% formic acid (60:40) with a flow rate of 0.3 mL/min, and the injection volume was 5 μL. The run time for the entire analysis was 8 min.
Under positive ion conditions of electrospray ionization (ESI) source, mass spectrometry was conducted in multiple reaction monitoring mode (MRM). The ionic transitions of futibatinib and IS were m/z 419.20→296.15 and m/z 510.36→417.00, respectively. The atomizing gas flow rate was 3.0 L/min. The heating gas flow rate and drying gas flow rate were both 10.0 L/min. The interface and detector voltages were 4000 V and 1720 V. The interface and desolventization temperatures were 300 °C and 526 °C, respectively. Labsolutions LCMS Workstation Software completed data collection and control.
2.5. Preparation of Standard and Quality Control (QC) Samples
The futibatinib (1.0 mg/mL) and ripretinib (1.0 mg/mL) stock solutions were obtained in methanol. The stock solutions were continuously diluted with methanol to obtain a futibatinib working solution (1, 10, and 100 μg/mL) and ripretinib working solution (1 μg/mL). The blank plasma was added with different concentrations and volumes of the working solution to obtain a plasma standard curve. The final concentrations of the standard curve for futibatinib were 1, 2, 5, 10, 20, 80, 160, and 200 ng/mL. Similarly, the same operation was used to obtain QC samples (2, 20, and 160 ng/mL). All solution samples were stored at 4 °C.
2.6. Sample Preparation
A quantity of 100 μL of plasma sample, 20 μL of IS working solution, and 200 μL of 10% sodium carbonate solution were sequentially added to a 1.5 mL EP tube and mixed thoroughly. After that, 1 mL of ethyl acetate was added. The mixture was vortexed for 1.0 min and then centrifuged at 8150× for 10 min at 4 °C to obtain a supernatant. Afterwards, 800 μL of the upper organic phase was transferred to another 1.5 mL EP tube and blown dry with airflow at 55 °C, the remnant was dissolved with 100 µL of the mobile phase. A total of 5 µL was sampled into the HPLC-MS/MS system for analysis.
2.7. Method Validation
According to the “Guidelines for the Validation of quantitative analysis methods for biological samples” in the 2020 edition of the Chinese Pharmacopoeia, the selectivity, linearity, precision, accuracy, lower limit of quantification (LLOQ), recovery, matrix effect (ME), and stability of this method were thoroughly validated [19].
2.7.1. Selectivity
The selectivity of this proposed method was determined based on a comparative analysis of blank plasma, blank plasma containing futibatinib and ISTD, and plasma samples collected after an oral dose of 0.67 mg/kg futibatinib to observe the presence of endogenous substances or other interfering signals in the region where the peak of drug appeared. All plasma samples were collected from six beagle dogs (n = 6).
2.7.2. Linearity and the LLOQ (Lower Limit of Quantification)
The standard curve was obtained through evaluating the response of the instrument to futibatinib over a defined range of concentrations (1, 2, 5, 10, 20, 50, 100, and 200 ng/mL). The linear regression of futibatinib was carried out by the least square method on the peak area ratio (y, futibatinib/ISTD) versus the corresponding concentrations (x), where the y-axis was the ratio of the peak area of futibatinib (As) to the peak area of ISTD (Ai). The smallest value of the standard curve was LLOQ.
2.7.3. Precision and Accuracy
Six QC samples of futibatinib (1, 2, 20, 160 ng/mL) were prepared with various volumes of working solution and blank plasma and processed according to the “plasma sample processing method”. Intra-day precision was assessed on the same day, while inter-day precision was gained through three consecutive days.
2.7.4. Recovery and Matrix Effect (ME)
Six QC (Quality Control) samples of futibatinib (2, 20, 160 ng/mL) were formulated. The extraction recovery was calculated by the following formula: Recovery = A/B × 100%. A represents the peak area of the spiked samples prior to extraction, and B represents the peak area of the spiked pure solution. The ME was calculated by the following formula: ME = C/D × 100%. C represents the peak area of the spiked samples after extraction, and D represents the peak area of the spiked pure solution.
2.7.5. Stability
The stability of futibatinib was tested with six copies of the QC sample (2, 20, 160 ng/mL) under the following four conditions. At first, the samples were left at room temperature for 6 h to test their short-term stability. Also, the samples were stored at −20 °C for 4 weeks to assess their long-term stability. In addition, samples taken from the autosampler (4 °C) were kept for 24 h to determine the stability of the samples in the autosampler. Finally, the samples were freeze–thawed three times (−20 to 25 °C) to test the freeze–thaw stability of their analytes in the samples. The RSD of each concentration should be within ±15% by comparing the measured concentration to the labeled concentration.
2.8. Statistics and Analysis
The established HPLC-MS/MS method was utilized to detect the concentration of futibatinib in beagle dog plasma. Then, DAS (Drug And Statistics, version 2.0) was utilized to obtain the main pharmacokinetic parameters of futibatinib alone and combined with YCHD medication by the statistical moment model. The main pharmacokinetic parameters of futibatinib were as follows: Cmax, Tmax, t1/2, CL, Vd, MRT, and AUC. Also, the pharmacokinetic parameters of the two groups were compared with SPSS 25.0 using the nonparametric independent sample t-test. p < 0.05 means the difference is significant.
3. Results
3.1. Method Validation
3.1.1. Selectivity
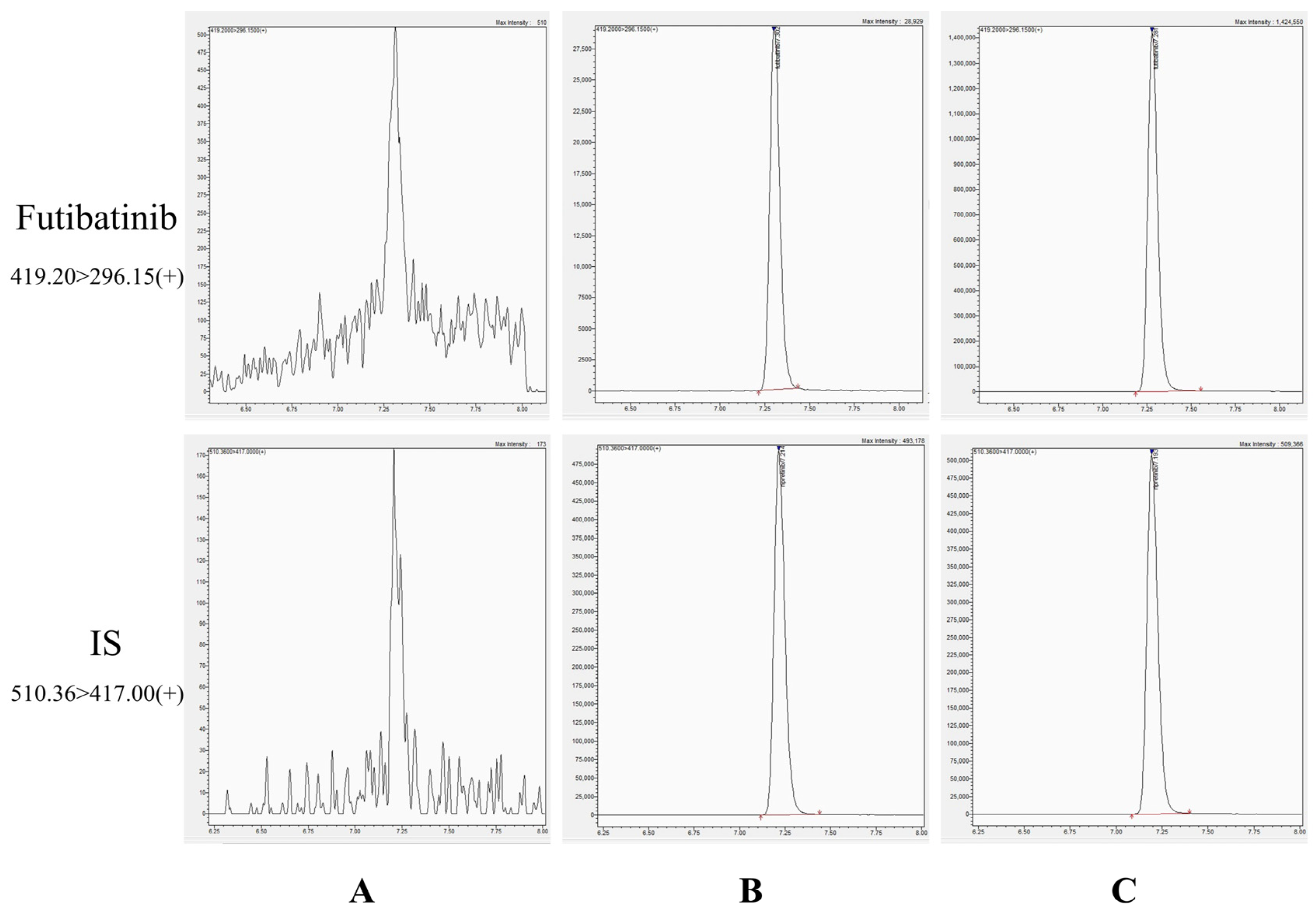
As shown in Figure 2, the chromatographic peak shapes of futibatinib and ISTD in plasma were excellently defined and not interfered with by endogenous substances in plasma. The retention times of futibatinib and ISTD were 7.30 min and 7.21 min, respectively. The good peak shape and stable baseline of the chromatogram indicate the superior selectivity of the method.
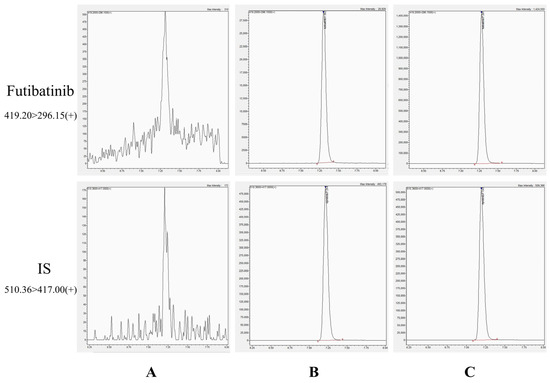
Figure 2.
Chromatograms of futibatinib and ISTD. (A) blank plasma; (B) a blank plasma spiked with futibatinib (1 ng/mL) and ISTD (200 ng/mL); (C) a plasma sample (46.08 ng/mL) 1.5 h after giving futibatinib.
3.1.2. Linearity and LLOQ
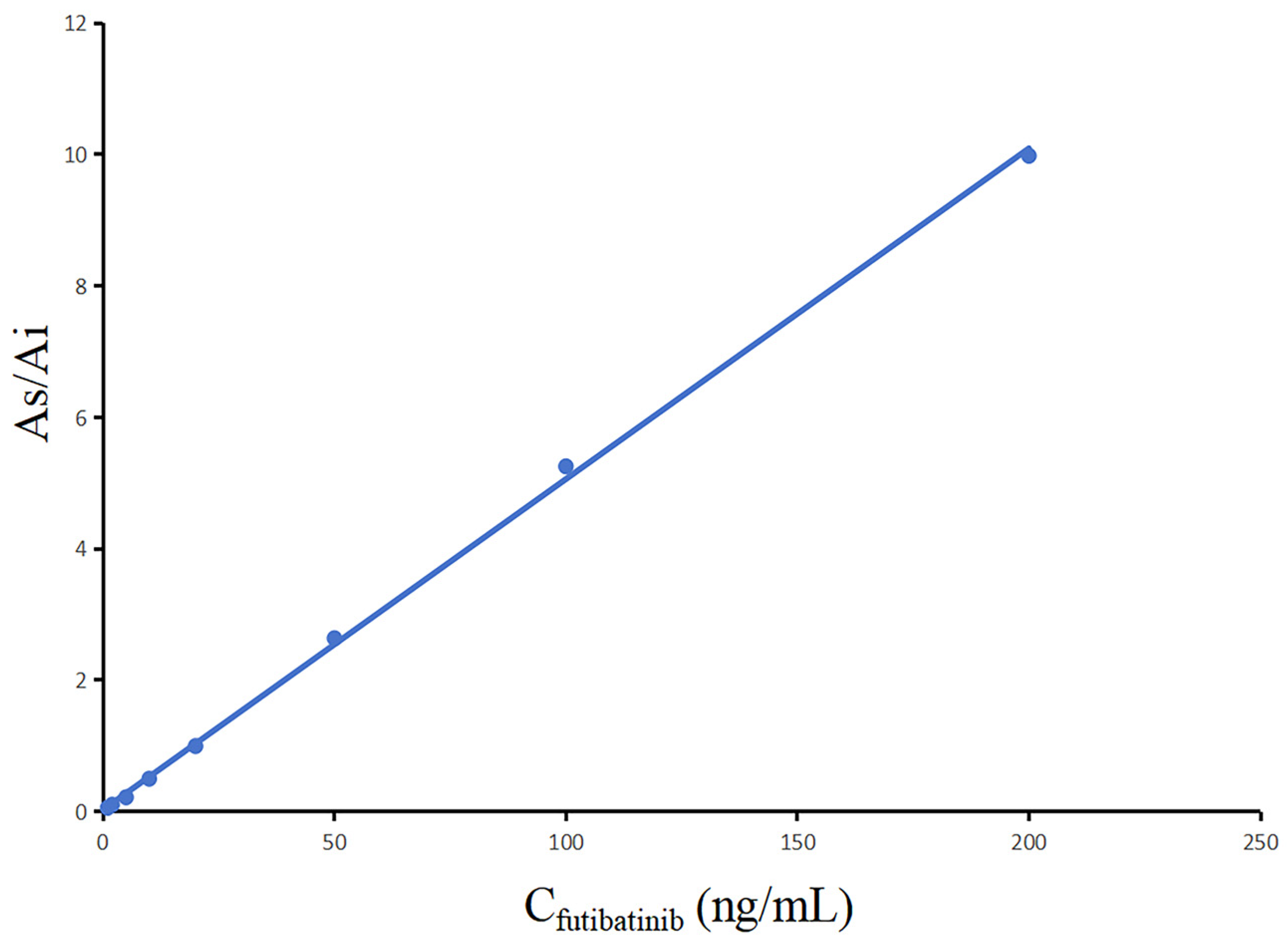
As indicated in Figure 3, the calibration curve of futibatinib was clearly linear in the measuring range of 1~200 ng/mL. The standard curve regression equation of futibatinib was y = 5.03 × 10−2 x +2.66 × 10−2, r2 = 0.9992. In addition, LLOQ was the smallest value of the standard curve (1.0 ng/mL).
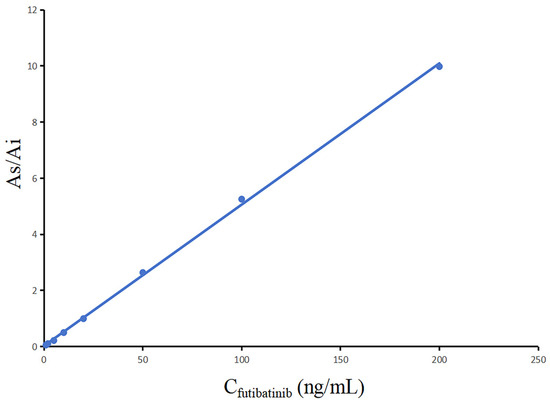
Figure 3.
Calibration curve of futibatinib.
3.1.3. Precision and Accuracy
In Table 1, the precision and accuracy of futibatinib were determined by repeating the tests at the LLOQ and QC levels for the three concentrations. All of these values were within the guideline requirements (within ±15%).

Table 1.
The intra-day and inter-day precision and accuracy of futibatinib in beagle dog plasma (n = 6, Mean ± SD).
3.1.4. Extraction Recovery and Matrix Effect (ME)
Table 2 showed the recoveries and ME of futibatinib (2, 20, 160 ng/mL). The recoveries of futibatinib (2, 20, 160 ng/mL) were more than 84.54% (RSD < 15%, n = 6). Meanwhile, the ME values of futibatinib (2, 20, 160 ng/mL) ranged from 99.01% to 101.87%, indicating that ME was negligible in the detection of futibatinib in the plasma of beagle dogs.

Table 2.
Recovery and matrix effects of futibatinib in beagle dog plasma (n = 6, Mean ± SD).
3.1.5. Stability
The stability of futibatinib in four different conditions of storage and temperature is shown in Table 3, and the %RE values ranged from −5.7% to 1.5%. The results showed that the measurements were stable and there was no significant degradation under all four storage conditions mentioned above.

Table 3.
Stability of futibatinib in beagle dog plasma subjected to various conditions (n = 6, Mean ± SD).
3.2. Pharmacokinetics of Herb–Drug Interaction
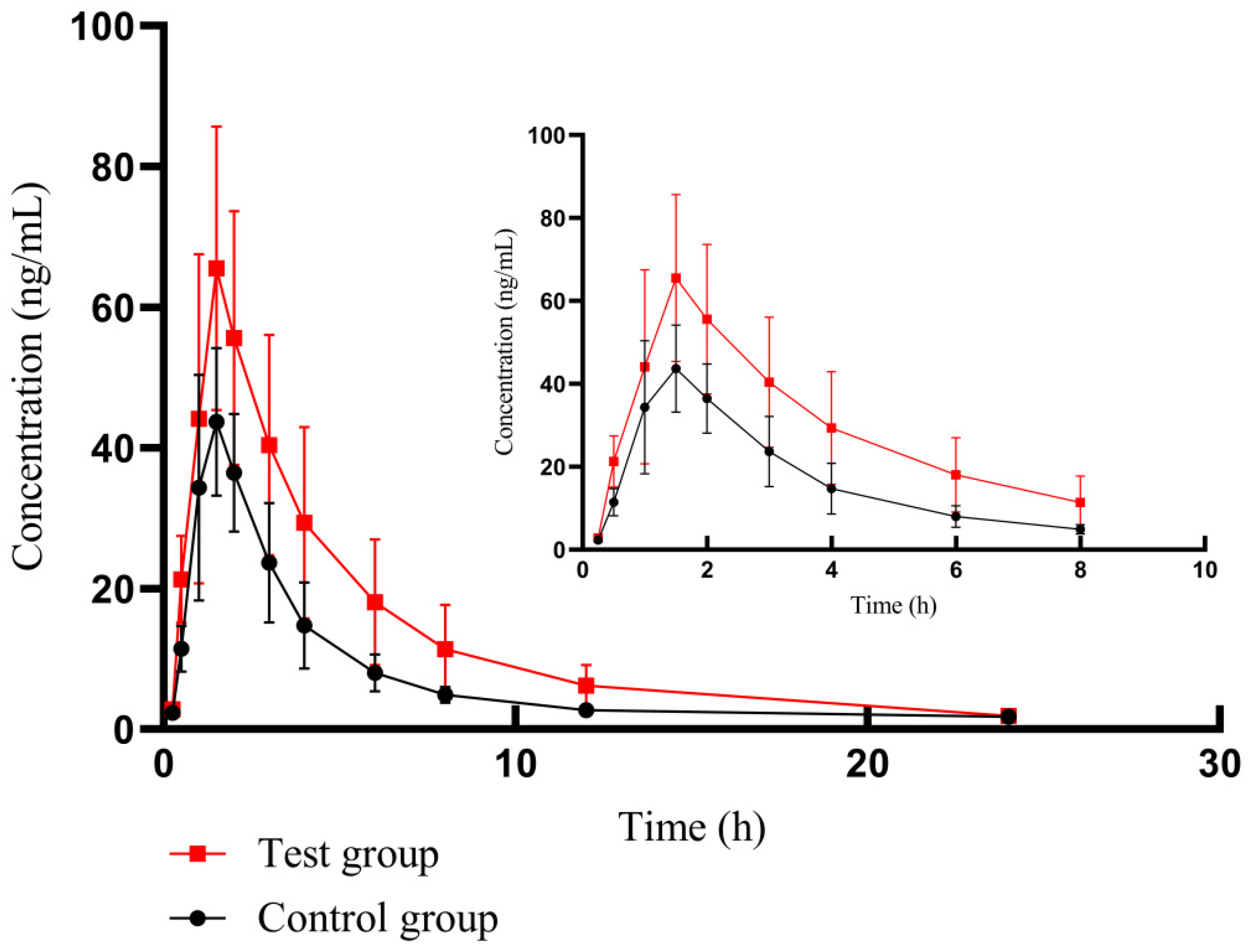
By using the bioassay developed based on HPLC-MS/MS technology, we successfully assayed the plasma concentration of futibatinib in beagle dogs and obtained the pharmacokinetics of different groups in Table 4. The mean plasma drug concentration–time profiles of futibatinib alone and futibatinib combined with YCHD are shown in Figure 4.

Table 4.
Pharmacokinetic parameters of futibatinib in beagles. Control group: futibatinib alone administration; experimental group: futibatinib combined with YCHD (n = 6, Mean ± SD).
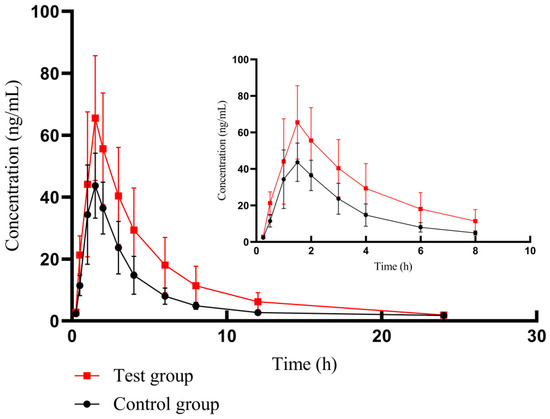
Figure 4.
The mean plasma drug concentration–time curve of futibatinib in two groups. The control group: futibatinib alone administration; the experimental group: futibatinib co-administration with YCHD.
After co-administration with YCHD, the Cmax of futibatinib increased by 40.85%, and the AUC(0–t) (area under curve) and AUC(0–∞) of futibatinib increased by 78.06% and 82.71% compared to futibatinib dosage alone. The Vd and CL of futibatinib were reduced by 20.05% and 40.85%, respectively. T1/2 was extended from 3.88 h to 5.26 h. YCHD significantly altered the pharmacokinetic parameters of futibatinib in beagle dogs. These changes suggested that YCHD enhanced the plasma exposure of futibatinib in beagle dogs.
4. Discussion
4.1. Method Development and Optimization
An HPLC-MS/MS method was established for the detection of futibatinib concentration in plasma. The method was fully compliant with bioanalytical standards, with superior sensitivity and recovery, greatly improving the standardization and reliability of the assay. Because futibatinib and ripretinib had excellent peak shapes, as well as no intervention by endogenous substances, ripretinib was selected as ISTD. The new method offered several advantages over the previous method, including excellent linearity, smaller sample volume (100 μL), weaker matrix effect, and less solvent usage.
In the MS analysis, futibatinib and ISTD had a more powerful and stable response under positive ion conditions, which was eventually selected. The ionic transitions of futibatinib and IS were m/z 419.20 → 296.15 and m/z 510.36 → 417.00, respectively. Meanwhile, the conditions of atomizing gas flow rate, drying gas flow rate, heating gas flow rate, interface voltage, detector voltage, interface temperature, and desolventization temperature were all optimized.
4.2. Herb–Drug Interaction of YCHD and Futibatinib
Drug combinations are very common in clinical treatment. With the extensive use of herbs around the world, the combination of herbs with drugs has become increasingly popular. However, the interaction between herbs and drugs is a double-edged sword, which may reduce the undesirable effects of drugs and improve clinical efficacy or may produce serious adverse effects. For example, the combination of Bushen Jianpi formula and sorafenib may further reduce the survival of hepatocellular carcinoma cell lines by promoting apoptosis and obstructing the cell cycle [20]. Spontaneous bleeding has been reported when danshen or ginkgo is combined with warfarin due to the inhibitory effect of its active ingredients on warfarin metabolism [21]. Therefore, the study of herb–drug interactions is essential for drug combination.
The most common adverse reactions of futibatinib were hyperphosphatemia, diarrhea, and nausea. The safety profile of futibatinib was manageable, and adverse reactions were well controlled clinically, with very few instances leading to treatment interruption [22]. Futibatinib demonstrated sustained objective responses and favorable tolerability, significantly maintaining patients’ quality of life [23]. Consequently, the combination of YCHD and futibatinib in clinical settings has the potential to mitigate futibatinib-related adverse reactions and enhance therapeutic outcomes.
The results of our previous study showed that Chaihu Shugan pills can induce the metabolism of duloxetine and decrease plasma exposure of it and its metabolites in beagle dogs [24]. Both silybinin and compound glycyrrhizin significantly inhibited the metabolism of pexidartinib by suppressing the expression of CYP3A4 and CYP2C9 in rats [25]. In this experiment, YCHD was administered to beagles for 14 successive days, which was adequate to achieve stable blood levels and to impact hepatic pharmacological enzymes. The Cmax, t1/2, CL, AUC(0–t) and AUC(0–∞) of futibatinib were changed significantly after the co-administration of both drugs, with Cmax and AUC(0–∞) having higher responses than the other parameters (p < 0.01). As Vd decreased, the plasma concentration of futibatinib was elevated, resulting in an intensified and prolonged therapeutic response. Consequently, when futibatinib is administered along with YCHD, an appropriate dosage adjustment of futibatinib is imperative to optimize both therapeutic efficacy and safety.
Yamamiya et al. found that futibatinib was metabolized primarily through the oxidative pathway, which was mediated by CYP. Among them, CYP3A4, CYP2C9, CYP2D6, and CYP3A contributed 70.5%, 10.5%, 11.2%, and 78.3% to CYP enzyme-associated metabolism of futibatinib, respectively [26]. Meanwhile, mass balance analysis showed that fecal excretion was the main elimination pathway, and metabolites were mainly present in plasma and feces [27]. In recent years, several studies have revealed that the active components in YCHD can impact the activity of CYP. Himanshu Rastogi et al. revealed that quercetin had a powerful inhibition effect on CYP3A4 and CYP2D6 [28]. Yitong Liu et al. identified that emodin effectively inhibited CYP1A2 and CYP3A4 activities, and rhein effectively inhibited the activities of CYP2C9 and CYP2C19 [29]. Li-Na Gao et al. revealed that genipin had a significant inhibitory effect on CYP3A4, which was not only manifested in mRNAs and protein expression but also in enzymatic activity [30]. Therefore, we hypothesize that certain active ingredients in YCHD might inhibit the metabolism of futibatinib through affecting the activities of CYP3A4, CYP2C9, or CYP2D6. However, whether these changes are caused by the effect of enzyme activity needs to be verified by further experiments. Meanwhile, this experiment only studied the effect of YCHD on the pharmacokinetics of futibatinib, without sifting specific compounds. In the next experiment, the effects of different concentrations of quercetin, emodin, and rhein on the pharmacokinetics of futibatinib could be studied to provide further experimental evidence for clinical dose adjustment.
In summary, we infer that YCHD could affect the pharmacokinetics of futibatinib by inhibiting CYP3A4, CYP2D6, or CYP2C9 activity. This study may provide foundational data for the combination of YCHD and futibatinib.
5. Conclusions
The HPLC-MS/MS method that was established for the determination of futibatinib in beagle dog plasma was rapid, highly sensitive, stable, and met the pharmacokinetic guidelines. The method was well-validated and offered significant advantages in excellent linearity, smaller sample volume, and weaker matrix effect in comparison with published methods. The results indicated that YCHD affects the pharmacokinetics of futibatinib and increased the plasma exposure of futibatinib. Based on this study, the dosage of futibatinib should be adjusted when the two drugs are used in clinical combination.
Author Contributions
X.Q. proposed the experimental design. C.W. conducted the experiment and wrote this paper. S.L., J.X., Y.Z. and J.C. conducted partial experiments and processed the preliminary data. All authors were involved in analyzing the data, writing or editing the article, approving future versions, and agreeing to take responsibility for all elements of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Animal Laboratory of Henan University of Science and Technology (202307001) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Everhart, J.E.; Ruhl, C.E. Burden of Digestive Diseases in the United States Part III: Liver, Biliary Tract, and Pancreas. Gastroenterology 2009, 136, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Raggi, C.; Invernizzi, P.; Andersen, J.B. Impact of microenvironment and stem-like plasticity in cholangiocarcinoma: Molecular networks and biological concepts. J. Hepatol. 2015, 62, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Kosters, A.; Karpen, S.J. The Role of Inflammation in Cholestasis: Clinical and Basic Aspects. Semin. Liver Dis. 2010, 30, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2017, 15, 95–111. [Google Scholar]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Borad, M.J.; Gores, G.J.; Roberts, L.R. Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr. Opin. Gastroenterol. 2015, 31, 264–268. [Google Scholar] [CrossRef]
- Sia, D.; Losic, B.; Moeini, A.; Cabellos, L.; Hao, K.; Revill, K.; Bonal, D.; Miltiadous, O.; Zhang, Z.; Hoshida, Y.; et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat. Commun. 2015, 6, 6087. [Google Scholar] [CrossRef]
- De, S.K. Futibatinib: A Potent and Irreversible Inhibitor of Fibroblast Growth Factor Receptors for Treatment of the Bile Duct Cancer. Curr. Med. Chem. 2024, 31, 666–670. [Google Scholar] [CrossRef]
- Sootome, H.; Fujita, H.; Ito, K.; Ochiiwa, H.; Fujioka, Y.; Ito, K.; Miura, A.; Sagara, T.; Ito, S.; Ohsawa, H.; et al. Futibatinib Is a Novel Irreversible FGFR 1-4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res. 2020, 80, 4986–4997. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, T.; Liao, X.; Li, Z.; Lin, R.; Qi, X.; Chen, G.; Sun, L.; Lin, L. Network pharmacology based research into the effect and mechanism of Yinchenhao Decoction against Cholangiocarcinoma. Chin. Med. 2021, 16, 13. [Google Scholar] [CrossRef]
- Qin, C.; Qiu, Q.; Li, P.; Mo, H.; Shi, J.; Liu, X.; Gu, B. Pharmacological Action and Clinical Application of Virgate Wormwood Decoction. Henan Tradit. Chin. Med. 2023, 43, 984–991. (In Chinese) [Google Scholar]
- Guo, Y.; Sun, F.; Li, X.; Wang, A. Clinical Application of Yinchenhao Decoction in Hepatobiliary Disease. J. Liaoning Univ. Tradit. Chin. Med. 2020, 22, 57–60. (In Chinese) [Google Scholar]
- Meng, S.; Xing, S. A classical prescription-a chemical study of Yinchenhao Decoction. Asia-Pac. Tradit. Med. 2009, 5, 173–176. (In Chinese) [Google Scholar]
- Ye, M.; Liu, C.; Liu, J.; Lu, F.; Xue, J.; Li, F.; Tang, Y. Scoparone inhibits the development of hepatocellular carcinoma by modulating the p38 MAPK/Akt/NF-κB signaling in nonalcoholic fatty liver disease mice. Environ. Toxicol. 2024, 39, 551–561. [Google Scholar] [CrossRef]
- Kuo, W.H.; Chou, F.P.; Young, S.C.; Chang, Y.C.; Wang, C.J. Geniposide activates GSH S-transferase by the induction of GST M1 and GST M2 subunits involving the transcription and phosphorylation of MEK-1 signaling in rat hepatocytes. Toxicol. Appl. Pharmacol. 2005, 208, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, J.; Xu, L.; Lin, S.; Xiang, Y.; Dai, X.; Liang, G.; Huang, X.; Zhu, J.; Zhao, C. Rhein shows potent efficacy against non-small-cell lung cancer through inhibiting the STAT3 pathway. Cancer Manag. Res. 2019, 11, 1167–1176. [Google Scholar] [CrossRef]
- Bhikshapathi, D.; Jogeswararao, P. Development of Stability Indicating Method for the Quantification of Futibatinib in K2EDTA Human Plasma by LC-MS/MS. Int. J. Pharm. Qual. Assur. 2023, 14, 927–932. [Google Scholar]
- Zhou, Z.; Liu, X.; Wu, T.; Que, Z.; Wu, Z.; Wu, W.; Fu, S.; Zhang, S.; Yang, Y.; Jiang, H.; et al. Herbal formula of Bushen Jianpi combined with sorafenib inhibits hepatocellular carcinoma growth by promoting cell apoptosis and blocking the cell cycle. J. Tradit. Chin. Med. 2021, 41, 194–202. [Google Scholar]
- Chua, Y.T.; Ang, X.L.; Zhong, X.M.; Khoo, K.S. Interaction between warfarin and Chinese herbal medicines. Singap. Med. J. 2015, 56, 11–18. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Bahleda, R.; Hierro, C.; Sanson, M.; Bridgewater, J.; Arkenau, H.T.; Tran, B.; Kelley, R.K.; Park, J.O.; Javle, M.; et al. Futibatinib, an Irreversible FGFR1-4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022, 12, 402–415. [Google Scholar] [CrossRef]
- Javle, M.; King, G.; Spencer, K.; Borad, M.J. Futibatinib, an Irreversible FGFR1-4 Inhibitor for the Treatment of FGFR-Aberrant Tumors. Oncologist 2023, 28, 928–943. [Google Scholar] [CrossRef]
- Bi, Y.T.; Kang, Y.R.; Woshur, G.; Ding, H.Z.; Wang, S.S.; Qiu, X.J. Effect of Chaihu Shugan Pills on the Pharmacokinetics of Duloxetine and its Metabolite 4-Hydroxyduloxetine in Beagle Dogs: A Herb-Drug Interaction Study. Evid. Based Complement. Alternat. Med. 2022, 2022, 2350560. [Google Scholar] [CrossRef]
- Su, Y.; Wei, X.; Cheng, Q.; Qi, H.; Chen, J.; Qiu, X.-J. Exploring the Effect of Compound Glycyrrhizin and Silybinin on the Metabolism of Pexidartinib in Rats Based on CYP3A4 and CYP2C9. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 6737062. [Google Scholar] [CrossRef]
- Yamamiya, I.; Hunt, A.; Takenaka, T.; Sonnichsen, D.; Mina, M.; He, Y.; Benhadji, K.A.; Gao, L. Evaluation of the Cytochrome P450 3A and P-glycoprotein Drug-Drug Interaction Potential of Futibatinib. Clin. Pharmacol. Drug Dev. 2023, 12, 966–978. [Google Scholar] [CrossRef]
- Yamamiya, I.; Hunt, A.; Yamashita, F.; Sonnichsen, D.; Muto, T.; He, Y.; Benhadji, K.A. Evaluation of the Mass Balance and Metabolic Profile of Futibatinib in Healthy Participants. Clin. Pharmacol. Drug Dev. 2023, 12, 927–939. [Google Scholar] [CrossRef]
- Rastogi, H.; Jana, S. Evaluation of inhibitory effects of caffeic acid and quercetin on human liver cytochrome p450 activities. Phytother. Res. 2014, 28, 1873–1878. [Google Scholar] [CrossRef]
- Liu, Y.; Mapa, M.S.T.; Sprando, R.L. Anthraquinones inhibit cytochromes P450 enzyme activity in silico and in vitro. J. Appl. Toxicol. 2021, 41, 1438–1445. [Google Scholar] [CrossRef]
- Gao, L.N.; Zhang, Y.; Cui, Y.L.; Yan, K. Evaluation of genipin on human cytochrome P450 isoenzymes and P-glycoprotein in vitro. Fitoterapia 2014, 98, 130–136. [Google Scholar] [CrossRef]
- Song, W. Interpretation of the Guiding Principles of Analytical Method Validation Based on the Chinese Pharmacopoeia 2020 Version. Shandong Chem. Ind. 2021, 50, 95–96. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).