Characterization of Alginates of Sargassum from the Archipelago of Guadeloupe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of Alginates
2.2. Fourier Transform Infrared Spectroscopy (FT-IR Spectroscopy)
2.3. Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy)
2.4. SEM/EDX Characterization and Elemental Composition
2.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. In Vitro Cellular Assay of Oxidative Stress
3. Results
3.1. Yield, Visual Aspect, and Solubility of Extracts
3.2. Spectroscopic Analyses
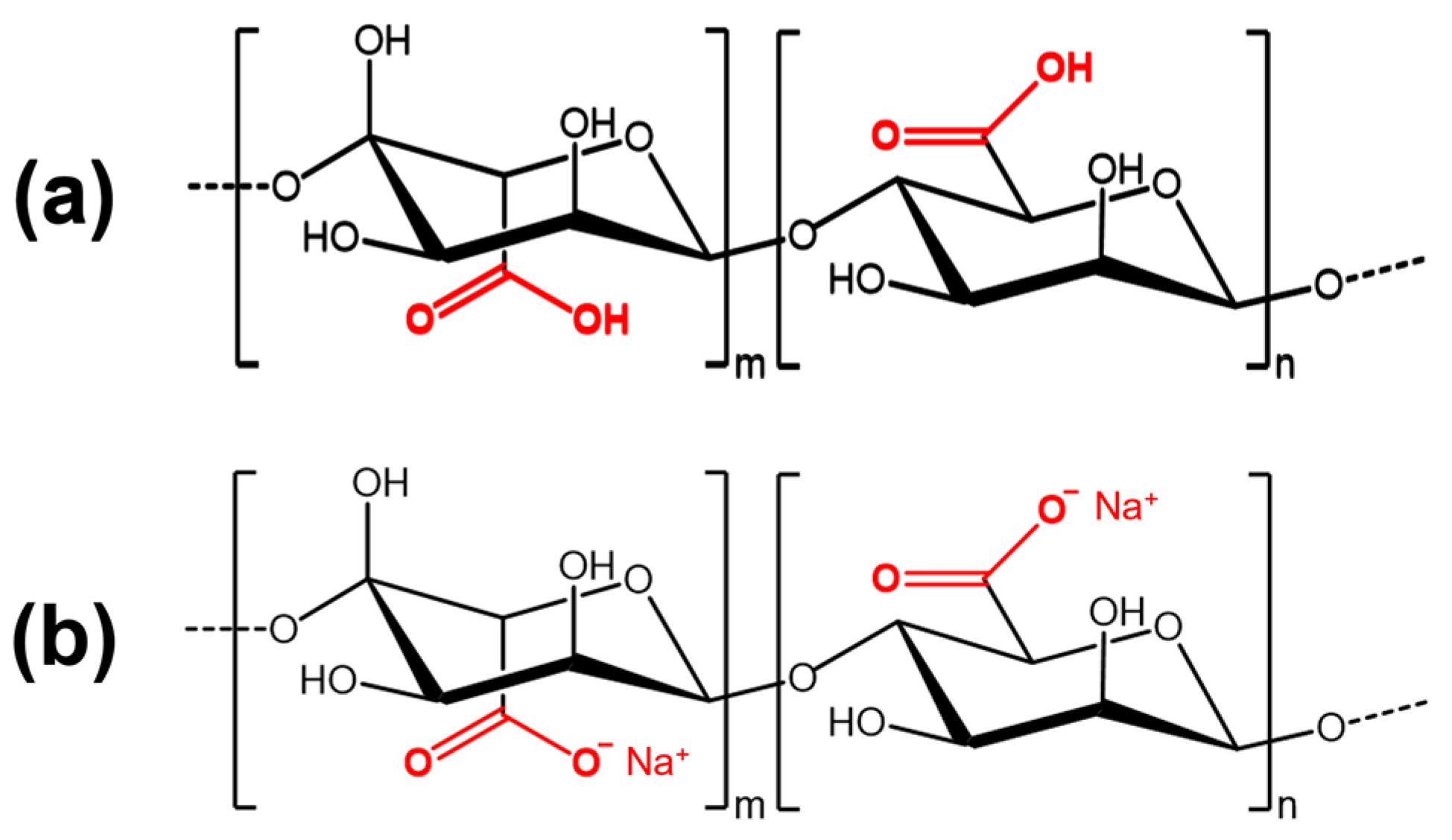
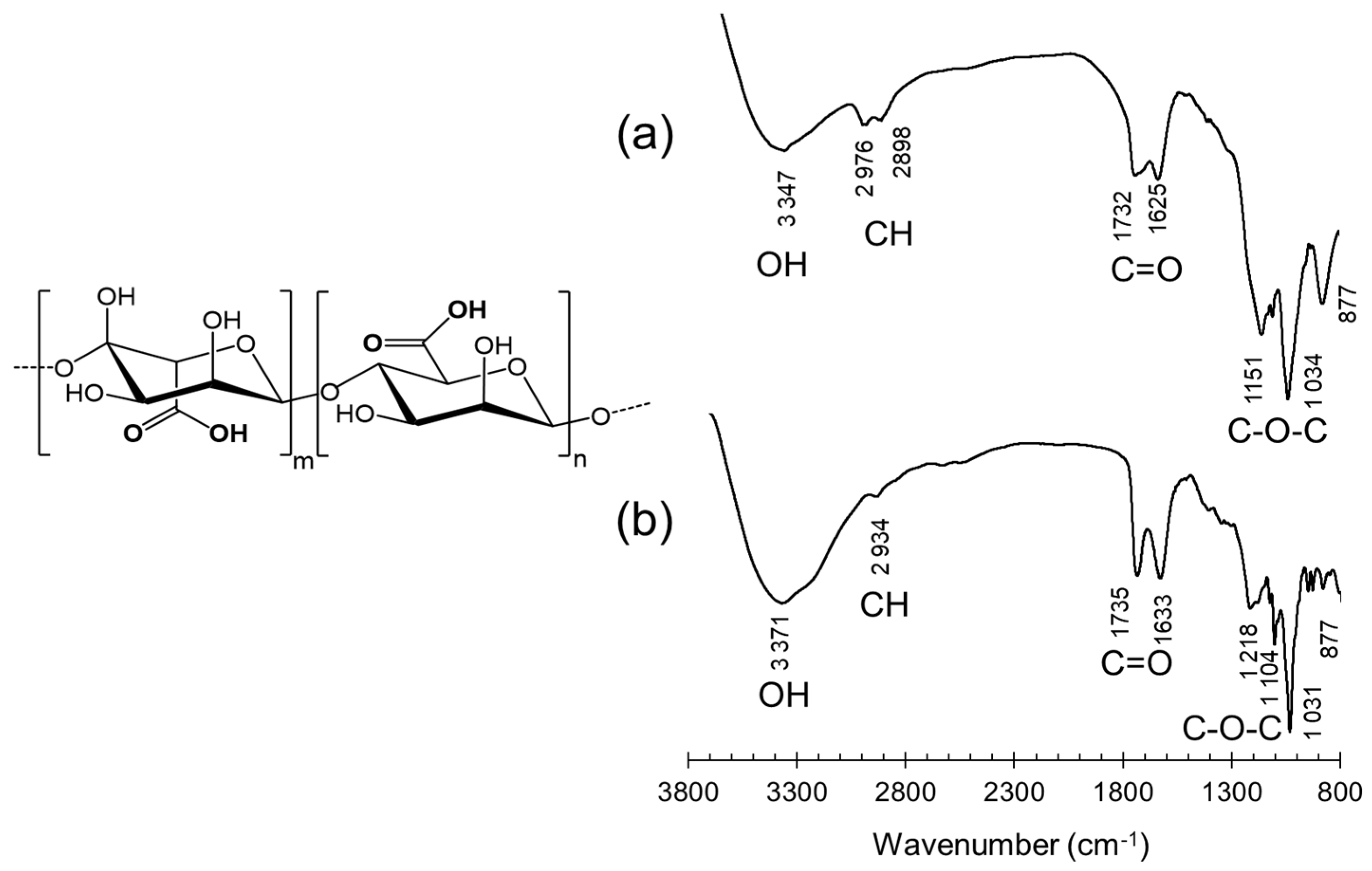
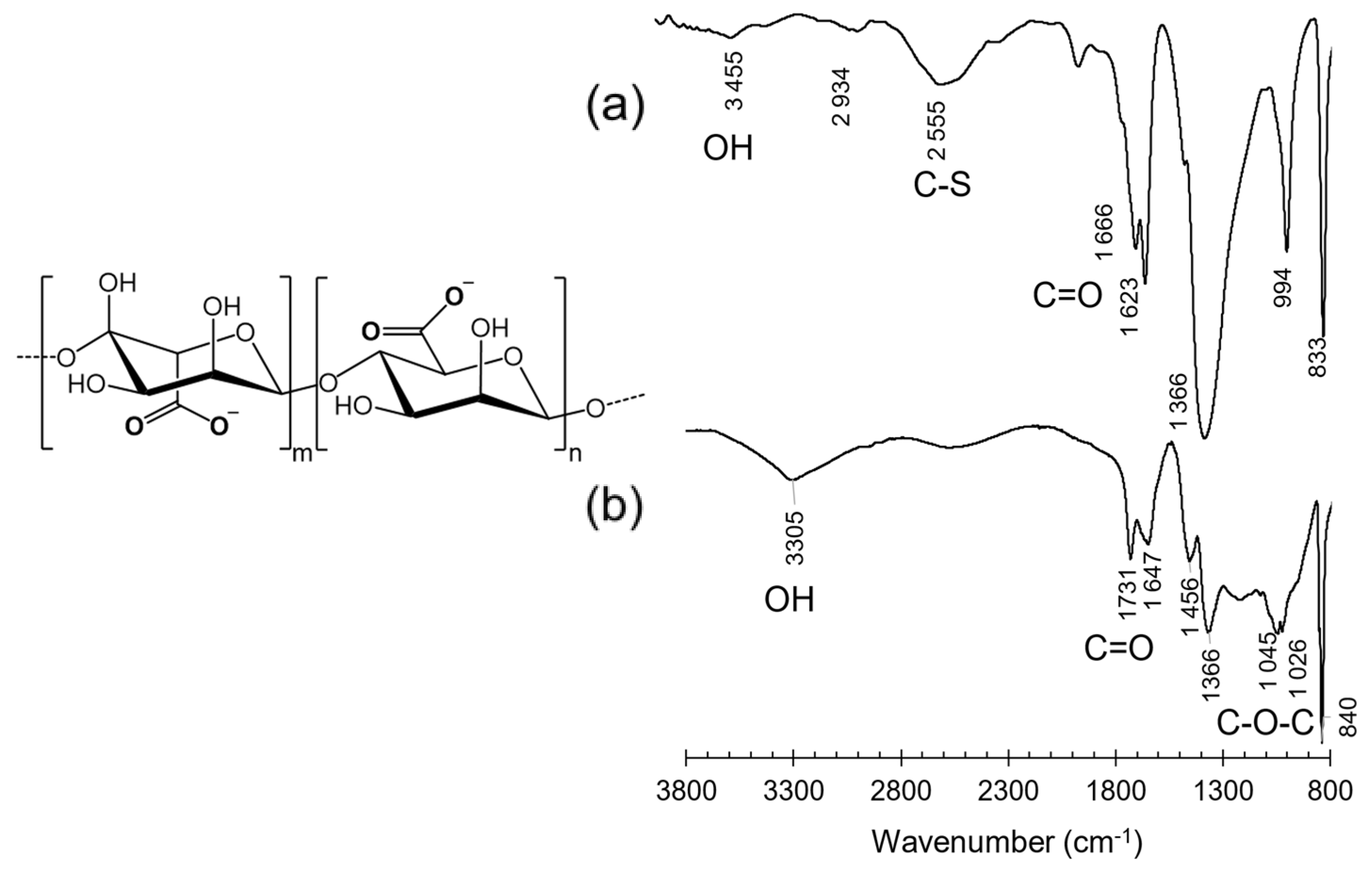
3.2.1. Infrared Spectroscopy
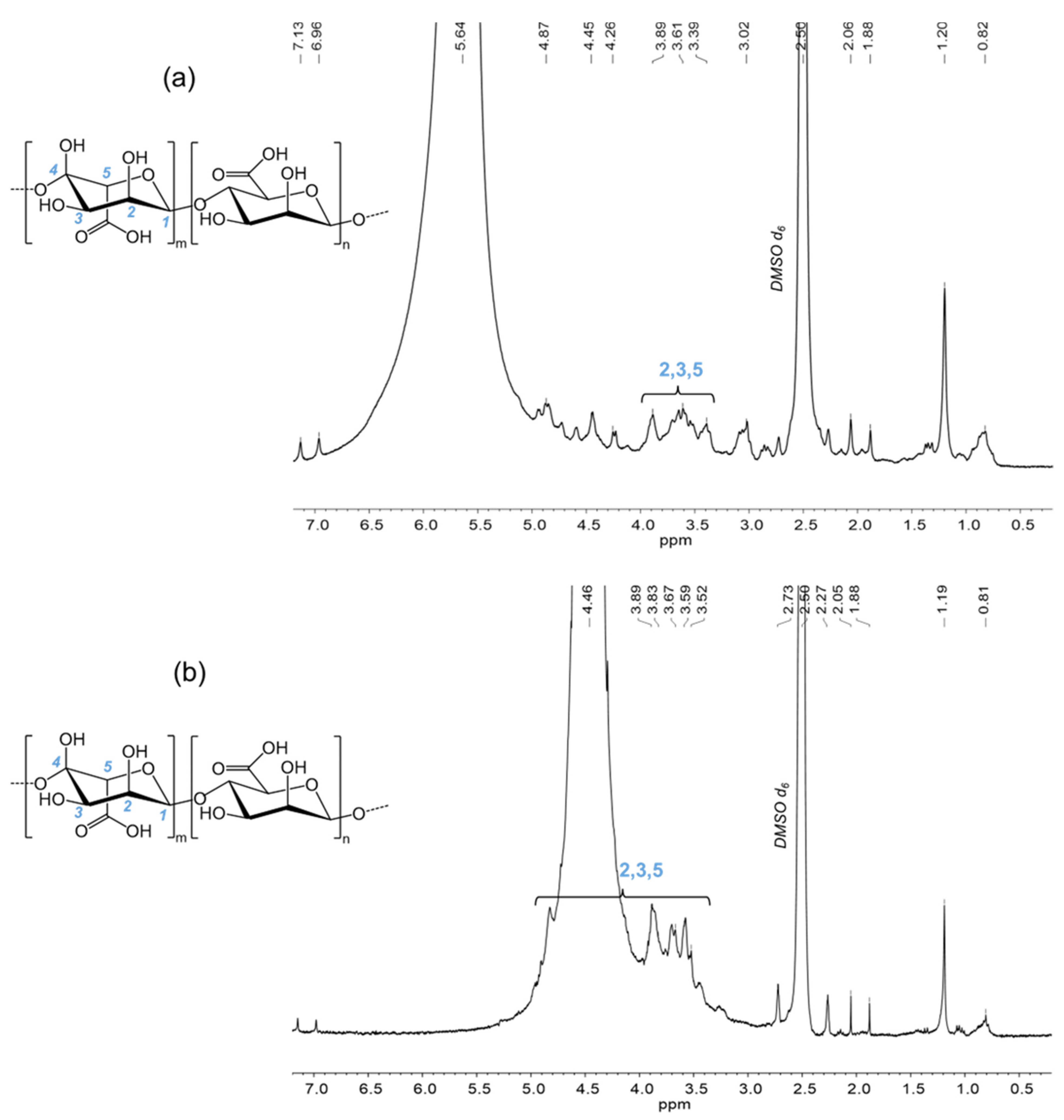
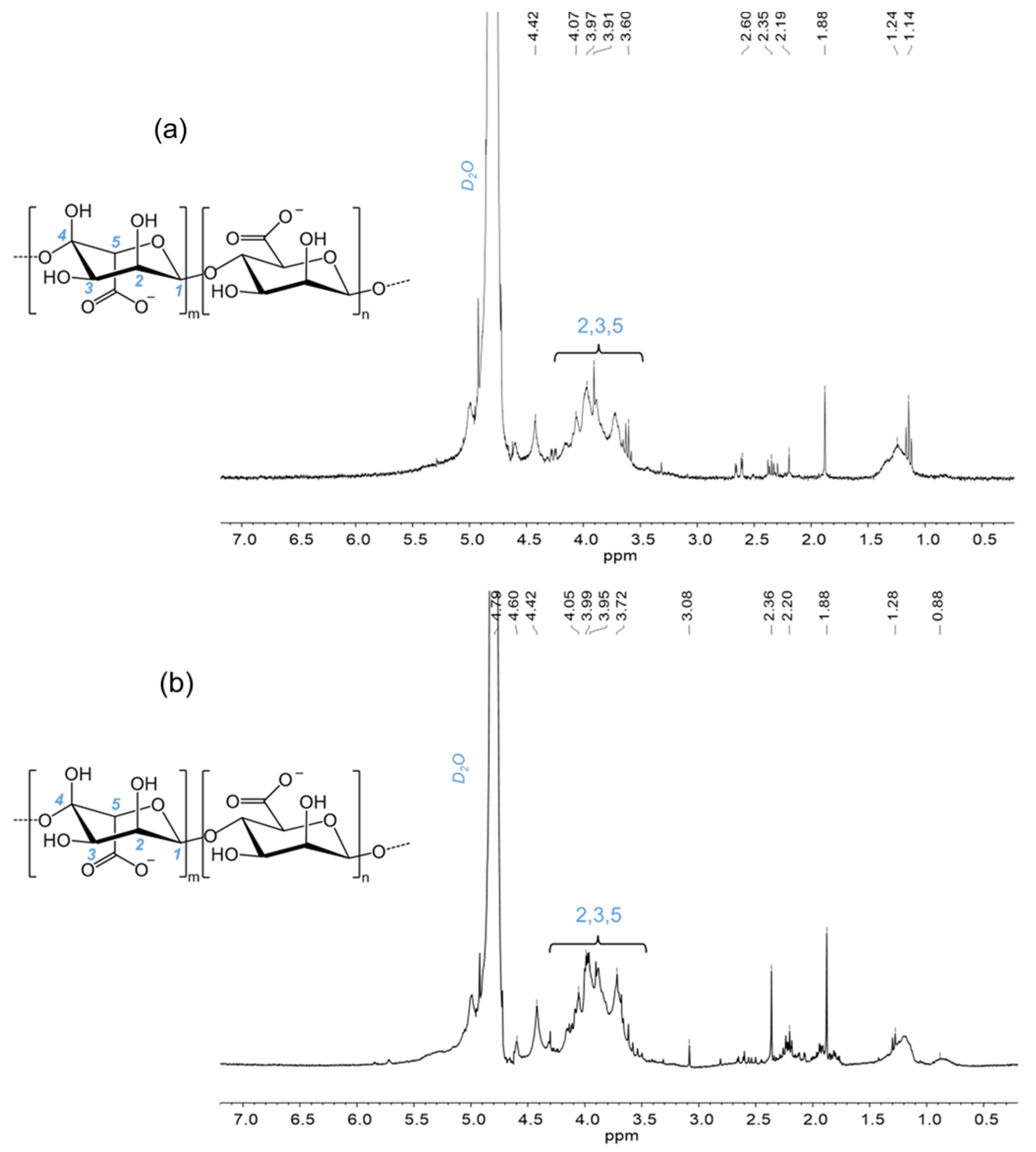
3.2.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
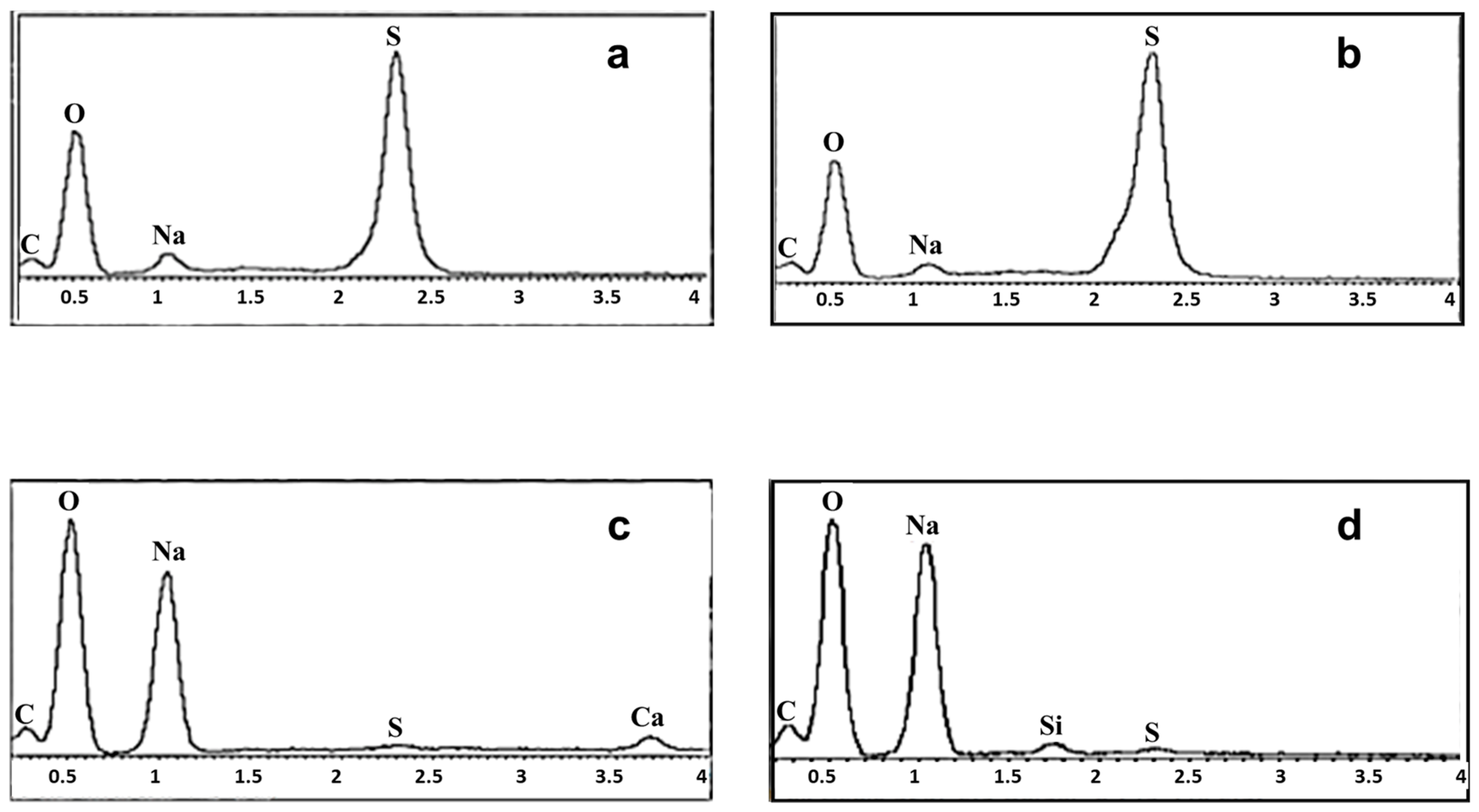
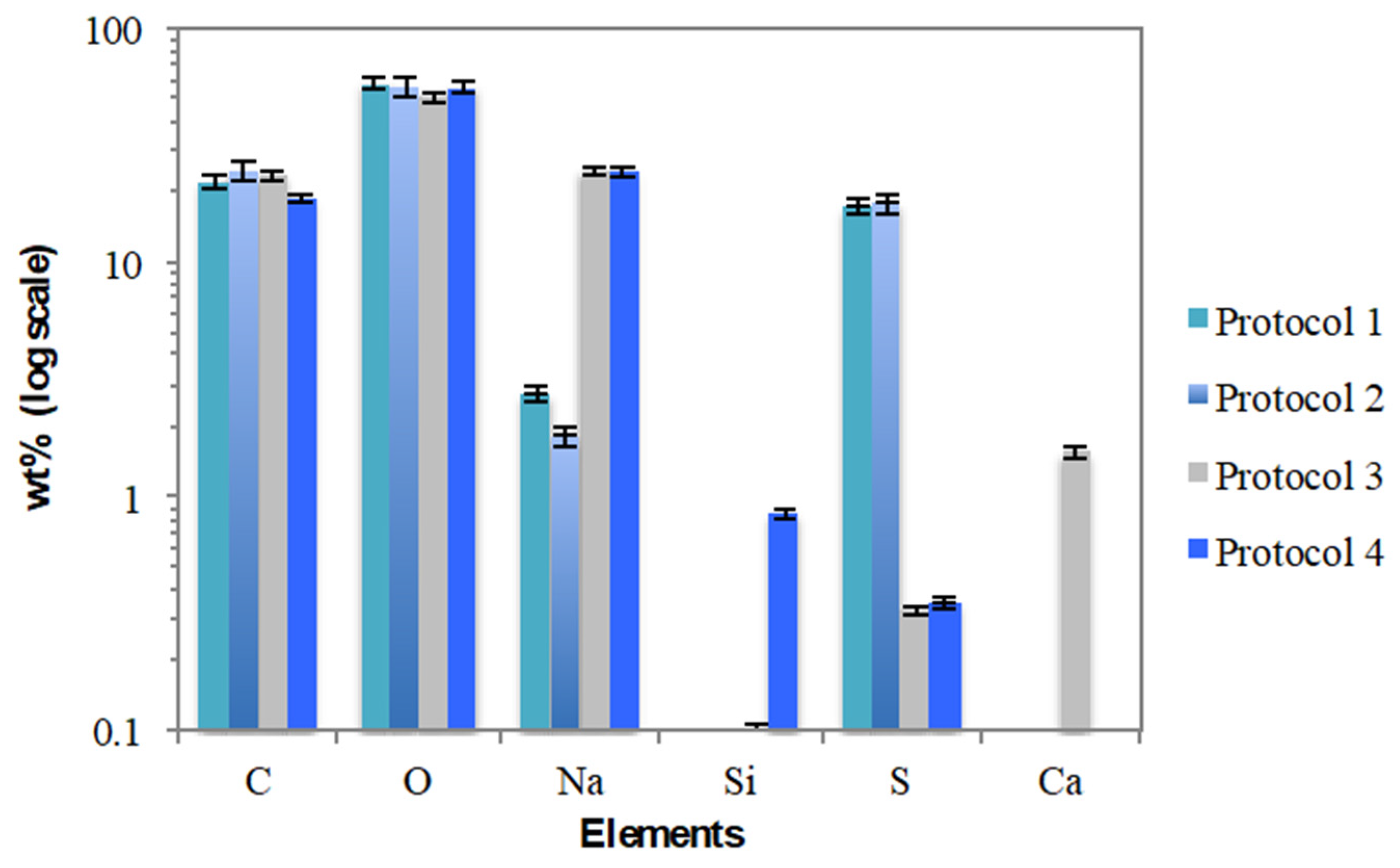
3.3. Morphological Analysis (SEM/EDX) and Elemental Composition
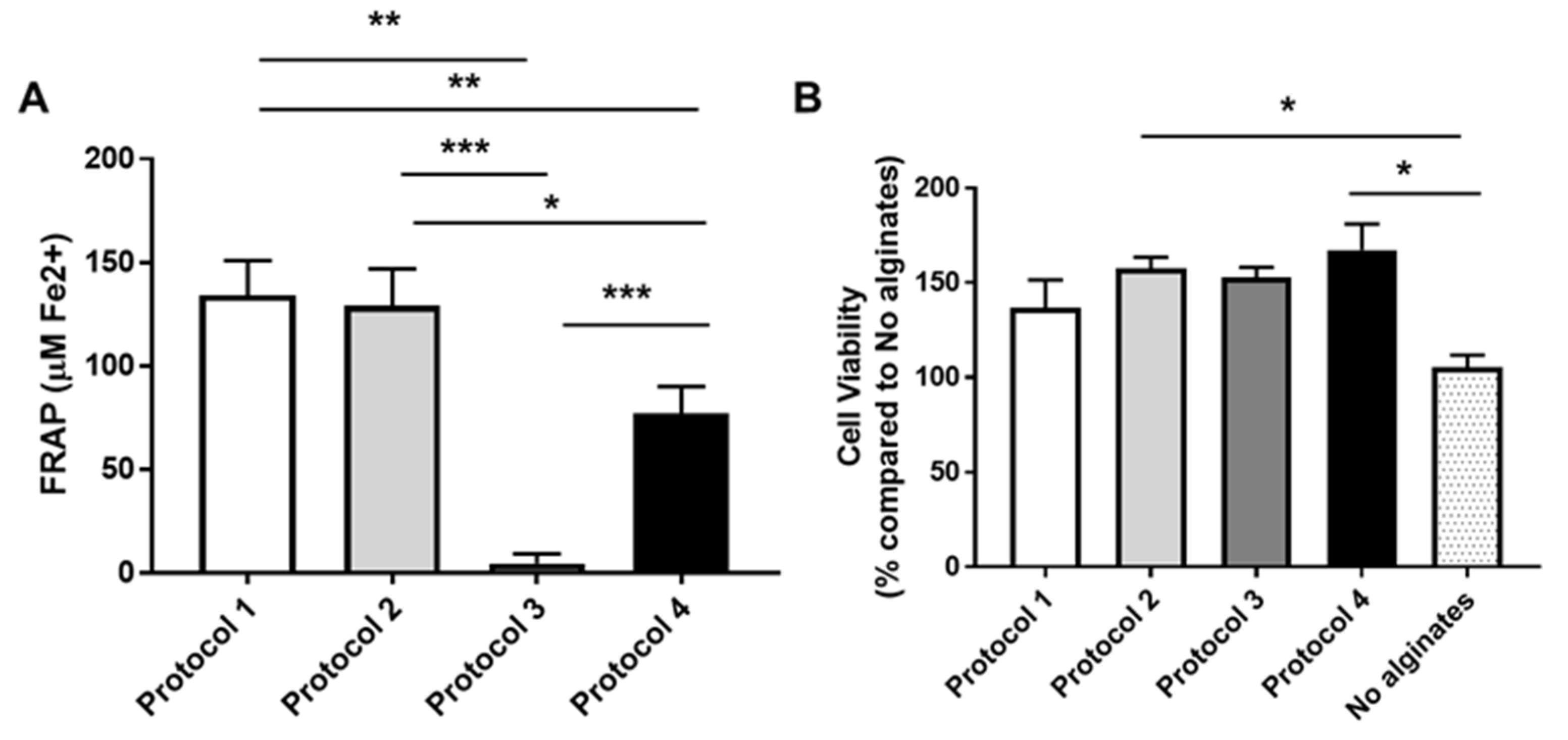
3.4. Antioxidant Capacity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Espinosa, L.A.; Freile-Pelegrín, Y.; Hernández-Nuñez, E.; Robledo, D. A comparative study of Sargassum species from the Yucatan Peninsula coast: Morphological and chemical characterization. Phycologia 2020, 59, 261–271. [Google Scholar] [CrossRef]
- Rossignolo, J.A.; Felicio Peres Duran, A.J.; Bueno, C.; Martinelli Filho, J.E.; Savastano Junior, H.; Tonin, F.G. Algae application in civil construction: A review with focus on the potential uses of the pelagic Sargassum spp. Biomass. J. Environ. Manag. 2022, 303, 114258. [Google Scholar] [CrossRef]
- Marsh, R.; Kliris, N.S.; Tompkins, E.L.; Dash, J.; Dominguez Almela, V.; Tonon, T.; Oxenford, H.A.; Webber, M. Climate-sargassum interactions across scales in the tropical Atlantic. PLoS Clim. 2023, 2, e0000253. [Google Scholar] [CrossRef]
- Van Tussenbroek, B.I.; Arana, H.A.H.; Rodríguez-Martínez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; Gonzalez-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Resiere, D.; Valentino, R.; Nevière, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabié, A.; Lebrun, T.; Mégarbane, B.; Guerrier, G.; et al. Sargassum seaweed on Caribbean islands: An international public health concern. Lancet 2018, 392, 2691. [Google Scholar] [CrossRef]
- Sissini, M.N.; de Barros Barreto, M.B.B.; Szechy, M.T.M.; de Lucena, M.B.; Oliveira, M.C.; Gower, J.; Liu, G.; de Oliveira Bastos, E.; Milstein, D.; Gusmao, F.; et al. The floating Sargassum (Phaeophyceae) of the South Atlantic Ocean—Likely scenarios. Phycologia 2017, 56, 321–328. [Google Scholar] [CrossRef]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. Sargassum Seaweed as a Source of Anti-Inflammatory Substances and the Potential Insight of the Tropical Species: A Review. Mar. Drugs 2019, 17, 590. [Google Scholar] [CrossRef] [PubMed]
- Puhari, S.S.M.; Yuvaraj, S.; Vasudevan, V.; Ramprasath, T.; Arunkumar, K.; Amutha, C.; Selvam, G.S. Fucoidan from Sargassum wightii reduces oxidative stress through upregulating Nrf2/HO-1 signaling pathway in alloxan-induced diabetic cardiomyopathy rats. Mol. Biol. Rep. 2023, 50, 8855–8866. [Google Scholar] [CrossRef] [PubMed]
- Falkeborg, M.; Cheong, L.-Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Xue, C.; Sun, L. Effect of molecular weight on the antioxidant property of low molecular weight alginate from Laminaria japonica. J. Appl. Phycol. 2012, 24, 295–300. [Google Scholar] [CrossRef]
- Borazjani, N.J.; Tabarsa, M.; You, S.; Rezaei, M. Effects of extraction methods on molecular characteristics, antioxidant properties and immunomodulation of alginates from Sargassum angustifolium. Int. J. Biol. Macromol. 2017, 101, 703–711. [Google Scholar] [CrossRef]
- Nava-Jiménez, I.A.; Tejeda-Vega, S.; Cortina-Ramírez, G.E.; Zarazúa-Ortega, G.; Berriozabal-Islas, C.; Sánchez-Hernández, H. Macro and microelement analysis of Sargassum fluitans and Sargassum natans arriving in the coastal zone of Cancun, Quintana Roo, Mexico: Análisis de macro y microelementos de Sargassum fluitans y Sargassum natans que arriban a la zona costera de Cancún, Quintana Roo, México. Rev. Biol. Mar. Oceanogr. 2022, 57, 2022. [Google Scholar] [CrossRef]
- Darko, C.N.S.; Premarathna, A.D.; Humayun, S.; Agyei-Tuffour, B.; Goosen, N.J.; Tuvikene, R. Physico- and biochemical properties of alginates extracted from Ecklonia maxima and Sargassum fluitans using a simple cascade process. J. Appl. Phycol. 2023, 36, 661–674. [Google Scholar] [CrossRef]
- Mohammed, A.; Bissoon, R.; Bajnath, E.; Mohammed, K.; Lee, T.; Bissram, M.; John, M.N.; Jalsa, N.K.; Lee, K.Y. Ward Multistage extraction and purification of waste Sargassum natans to produce sodium alginate: An optimization approach. Carbohydr. Polym. 2018, 198, 109–118. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, R.E.; Roy, P.D.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Carrillo-Domínguez, S.; Collado-Vides, L.; García-Sánchez, M.; van Tussenbroek, B.I. Element concentrations in pelagic Sargassum along the Mexican Caribbean coast in 2018–2019. PeerJ 2020, 8, e8667. [Google Scholar] [CrossRef]
- Draget, K.I.; Bræk, G.S.; Smidsrød, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; McHugh, D.J.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E. Pilot plant scale extraction of alginate from Macrocystis pyrifera. 1. Effect of pre-extraction treatments on yield and quality of alginate. J. Appl. Phycol. 1998, 10, 507–513. [Google Scholar] [CrossRef]
- Lorbeer, A.J.; Lahnstein, J.; Bulone, V.; Nguyen, T.; Zhang, W. Multiple-response optimization of the acidic treatment of the brown alga Ecklonia radiata for the sequential extraction of fucoidan and alginate. Bioresour. Technol. 2015, 197, 302–309. [Google Scholar] [CrossRef]
- Perez, R.; Kaas, R.; Campello, F.; Arbault, S.; Barbaroux, O. La Culture des Algues Marines Dans le Monde; Institut Français de Recherche pour l’Exploitation de la Mer, Ed.; IFREMER: Plouzané, France, 1992. [Google Scholar]
- Calumpong, H.P.; Maypa, A.P.; Magbanua, M. Population and alginate yield and quality assessment of four Sargassum species in Negros Island, central Philippines. In Proceedings of the Sixteenth International Seaweed Symposium, Cebu City, Philippines, 12–17 April 1998; Kain, J.M., Brown, M.T., Lahaye, M., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 211–215. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and characterization of an alginate from the brown seaweed Sargassum turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Mian, A.J.; Percival, E. Carbohydrates of the brown seaweeds himanthalia lorea, bifurcaria bifurcata, and Padina pavonia. Carbohydr. Res. 1973, 26, 133–146. [Google Scholar] [CrossRef]
- Souchet, N. Etude de L’influence de la Periode de Recolte de L’algue Brune Laminaria longicruris sur sa Composition en Polysaccharides Bioactifs. Ph.D. Thesis, Université Laval, Quebec City, QC, Canada, 2004. [Google Scholar]
- Rioux, L.-E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Hu, H.; Xie, Y.; Li, Y.; Li, L.; Tian, N.; Zhu, W.; Yan, G.; Wu, C. Development of high drug-loading nanomicelles targeting steroids to the brain. IJN 2013, 9, 55. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; de Vaca, R.P.-C.; Quintal-Novelo, C.; Olivera-Castillo, L.; Moo-Puc, R. Hepatoprotective effect of a fucoidan extract from Sargassum fluitans Borgesen against CCl4-induced toxicity in rats. Int. J. Biol. Macromol. 2020, 145, 500–509. [Google Scholar] [CrossRef]
- Ptak, S.H.; Sanchez, L.; Fretté, X.; Kurouski, D. Complementarity of Raman and Infrared spectroscopy for rapid characterization of fucoidan extracts. Plant Methods 2021, 17, 130. [Google Scholar] [CrossRef] [PubMed]
- Soto-Vásquez, M.R.; Alvarado-García, P.A.A.; Youssef, F.S.; Ashour, M.L.; Bogari, H.A.; Elhady, S.S. FTIR Characterization of Sulfated Polysaccharides Obtained from Macrocystis integrifolia Algae and Verification of Their Antiangiogenic and Immunomodulatory Potency In Vitro and In Vivo. Mar. Drugs 2023, 21, 36. [Google Scholar] [CrossRef]
- Leal, D.; Matsuhiro, B.; Rossi, M.; Caruso, F. FT-IR spectra of alginic acid block fractions in three species of brown seaweeds. Carbohydr. Res. 2008, 343, 308–316. [Google Scholar] [CrossRef]
- Jauregui-Rincon, J.; Antonio, J.; Medina-Ramirez, I. Zimm-Bragg Model Applied to Sorption of Dyes by Biopolymers: Alginic Acid and Xanthan. In Biotechnology of Biopolymers; Elnashar, M., Ed.; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Samuelsson, B.; Sjövall, J.; Munch-Petersen, J. The Solubility of Alginate at Low pH. Acta Chem. Scand. 1963, 17, 1653–1662. [Google Scholar] [CrossRef]
- Myklestad, S.; Haug, A. Studies on the solubility of alginic acid from Ascophyllum Nodosum at low pH. In Proceedings of the Fifth International Seaweed Symposium, Halifax, UK, 25–28 August 1965; Elsevier: Amsterdam, The Netherlands, 1966; pp. 297–303. [Google Scholar] [CrossRef]
- Awad, H.; Aboul-Enein, H.Y. A Validated HPLC Assay Method for the Determination of Sodium Alginate in Pharmaceutical Formulations. J. Chromatogr. Sci. 2013, 51, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Hanjabam, M.D.; Kumar, A.; Tejpal, C.S.; Krishnamoorthy, E.; Kishore, P.; Ashok Kumar, K. Isolation of crude fucoidan from Sargassum wightii using conventional and ultra-sonication extraction methods. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100200. [Google Scholar] [CrossRef]
- Yuan, Y.; Macquarrie, D. Microwave assisted extraction of sulfated polysaccharides (fucoidan) from Ascophyllum nodosum and its antioxidant activity. Carbohydr. Polym. 2015, 129, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Saji, S.; Hebden, A.; Goswami, P.; Du, C. A Brief Review on the Development of Alginate Extraction Process and Its Sustainability. Sustainability 2022, 14, 5181. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; FAO Fisheries Technical Paper, No. 441; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcin, N.; Thesnor, V.; Duvauchelle, V.; Ponce-Mora, A.; Gimeno-Mallench, L.; Narayanin-Richenapin, S.; Brelle, L.; Bejarano, E.; Yacou, C.; Sylvestre, M.; et al. Characterization of Alginates of Sargassum from the Archipelago of Guadeloupe. Separations 2024, 11, 226. https://doi.org/10.3390/separations11080226
Marcin N, Thesnor V, Duvauchelle V, Ponce-Mora A, Gimeno-Mallench L, Narayanin-Richenapin S, Brelle L, Bejarano E, Yacou C, Sylvestre M, et al. Characterization of Alginates of Sargassum from the Archipelago of Guadeloupe. Separations. 2024; 11(8):226. https://doi.org/10.3390/separations11080226
Chicago/Turabian StyleMarcin, Naika, Valendy Thesnor, Valentin Duvauchelle, Alejandro Ponce-Mora, Lucia Gimeno-Mallench, Stacy Narayanin-Richenapin, Laura Brelle, Eloy Bejarano, Christelle Yacou, Muriel Sylvestre, and et al. 2024. "Characterization of Alginates of Sargassum from the Archipelago of Guadeloupe" Separations 11, no. 8: 226. https://doi.org/10.3390/separations11080226
APA StyleMarcin, N., Thesnor, V., Duvauchelle, V., Ponce-Mora, A., Gimeno-Mallench, L., Narayanin-Richenapin, S., Brelle, L., Bejarano, E., Yacou, C., Sylvestre, M., Onésippe-Potiron, C., Meffre, P., Benfodda, Z., & Cebrian-Torrejon, G. (2024). Characterization of Alginates of Sargassum from the Archipelago of Guadeloupe. Separations, 11(8), 226. https://doi.org/10.3390/separations11080226









