Advances in Therapeutic Peptides Separation and Purification
Abstract
:1. Introduction
2. Reversed-Phase Liquid Chromatography (RPLC)
3. Hydrophilic Interaction Liquid Chromatography (HILIC)
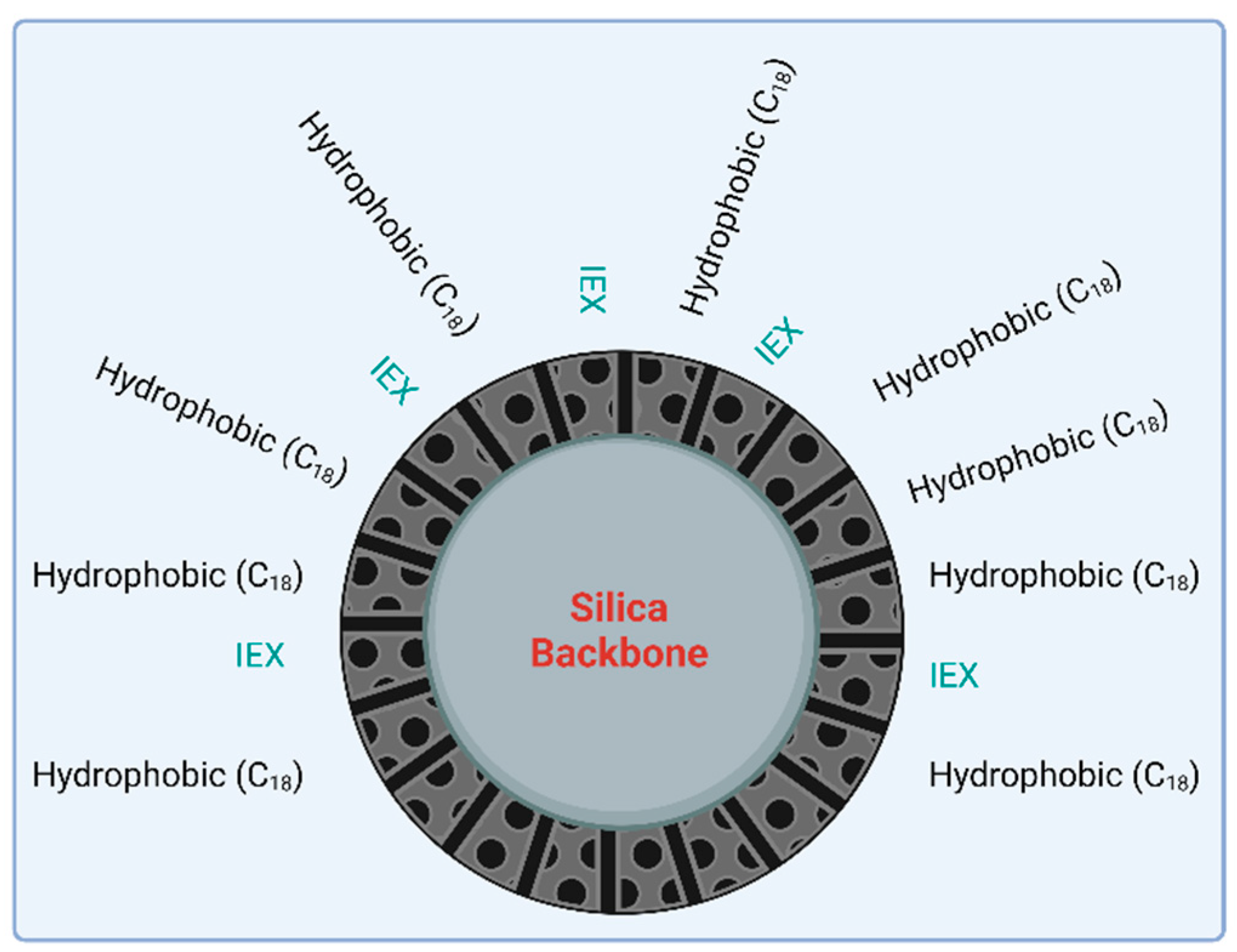
4. Mixed-Mode Chromatography (MMC)
5. Two-Dimensional Separation Methods
6. Sub/Supercritical Fluid Chromatography (SFC)
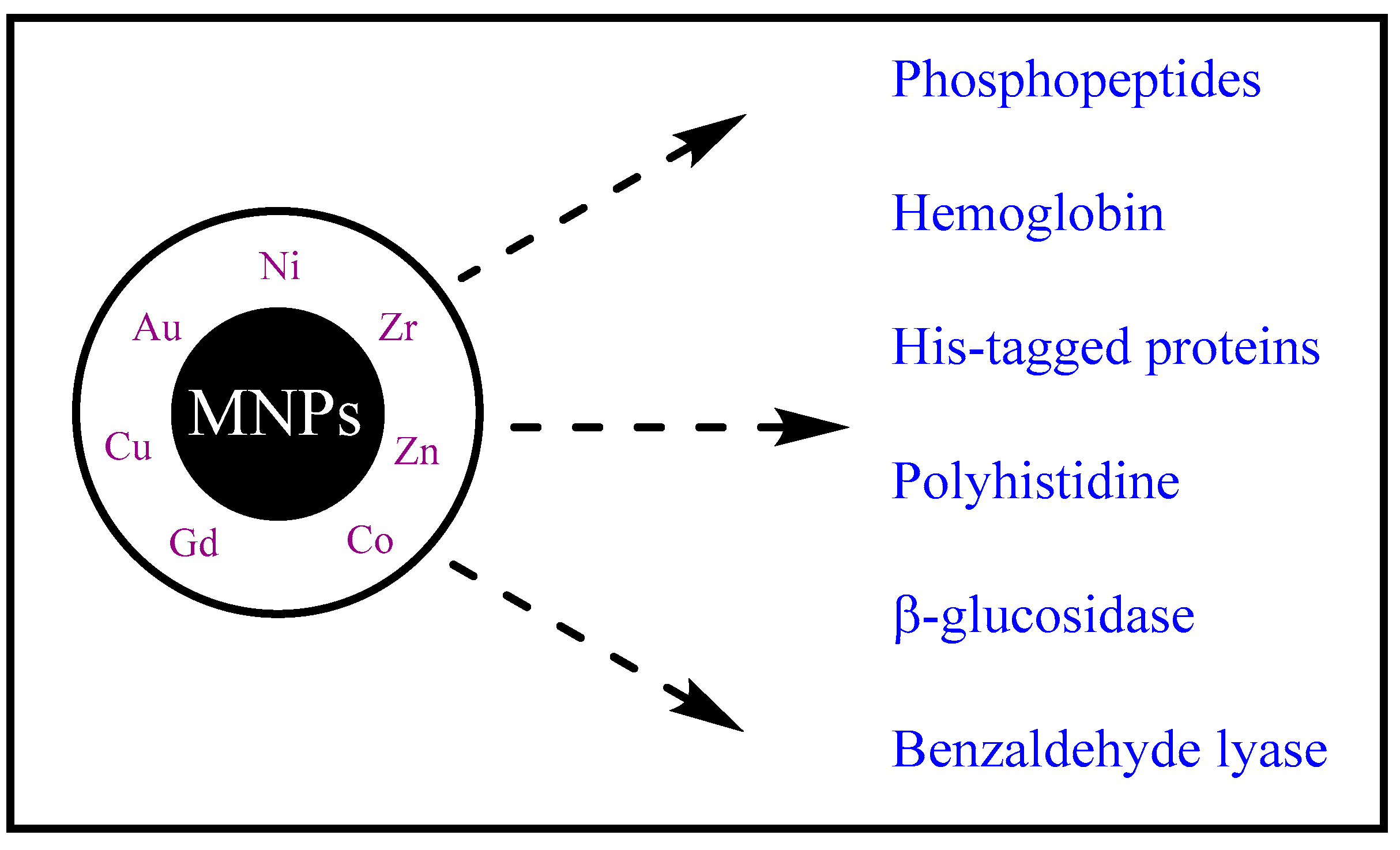
7. Magnetic Nanoparticles (MNPs)
8. Isoelectric Focusing (IEF)
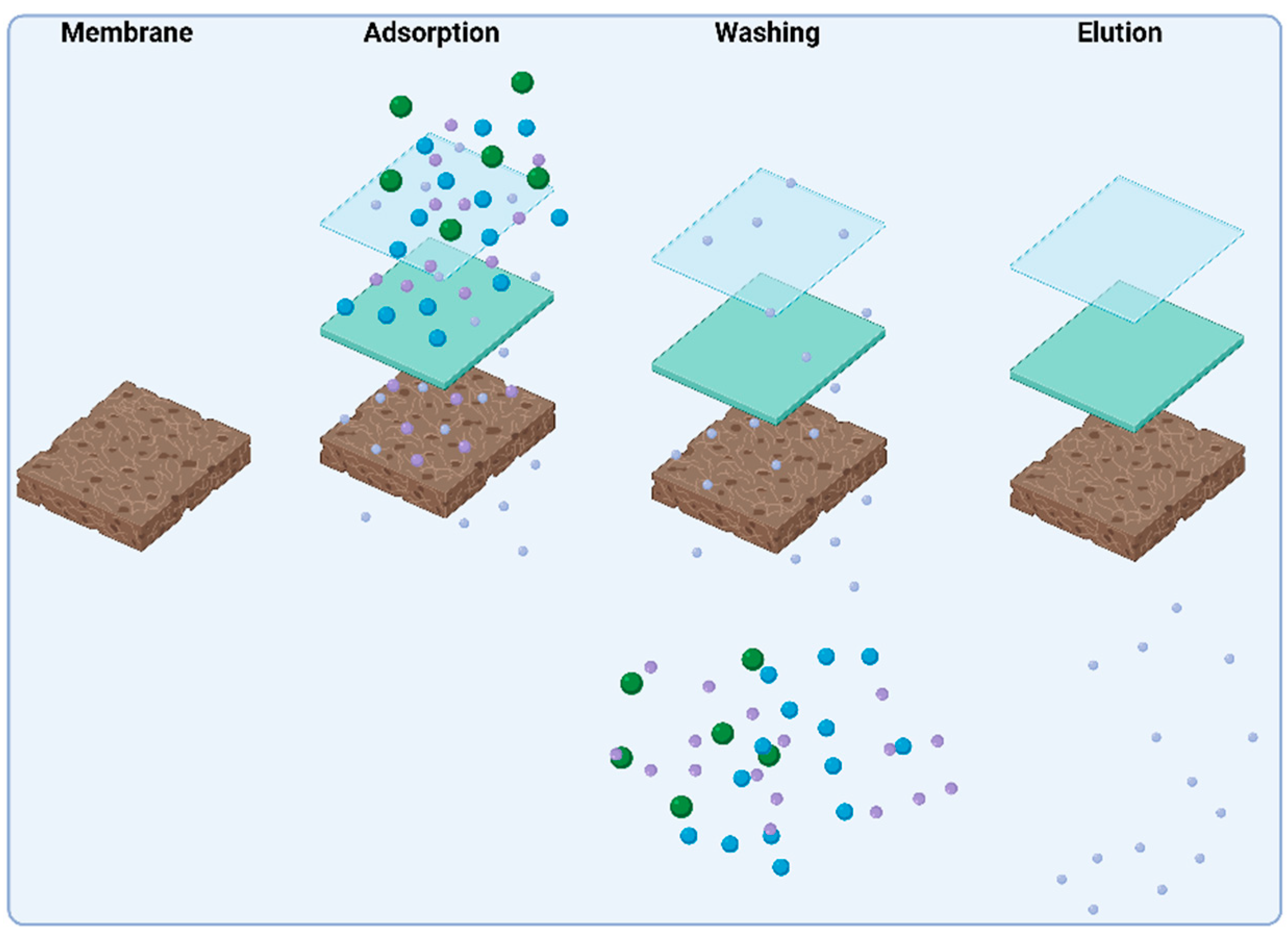
9. Membrane Filtration
10. Sustainable Peptide Separation and Purification
11. Challenges
12. Conclusions and Future Prospect
Funding
Data Availability Statement
Conflicts of Interest
References
- Jensen, S.M.; Potts, G.K.; Ready, D.B.; Patterson, M.J. Specific MHC-I Peptides Are Induced Using PROTACs. Front. Immunol. 2018, 9, 2697. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide-nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wareham, D.W.; Yuan, Y.; Deng, X.; Mata, A.; Azevedo, H.S. Polymyxin B-Triggered Assembly of Peptide Hydrogels for Localized and Sustained Release of Combined Antimicrobial Therapy. Adv. Healthc. Mater. 2021, 10, 2101465. [Google Scholar] [CrossRef] [PubMed]
- Radvar, E.; Azevedo, H.S. Supramolecular Peptide/Polymer Hybrid Hydrogels for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800221. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.T.N.; Huang, L.; Lemos, H.; Mellor, A.; Novakovic, K. Poly(ethylene glycol)-interpenetrated genipin-crosslinked chitosan hydrogels: Structure, pH responsiveness, gelation kinetics, and rheology. J. Appl. Polym. Sci. 2020, 137, 49259. [Google Scholar] [CrossRef]
- Al Musaimi, O. FDA’s stamp of approval: Unveiling peptide breakthroughs in cardiovascular diseases, ACE, HIV, CNS, and beyond. J. Pept. Sci. 2024, e3627. [Google Scholar] [CrossRef] [PubMed]
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2023 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2024, 17, 243. [Google Scholar] [CrossRef]
- Guiochon, G.; Beaver, L.A. Separation science is the key to successful biopharmaceuticals. J. Chromatogr. A 2011, 1218, 8836–8858. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Liu, T.; Li, Z.; Zhang, X.; Chen, X. Peptide-based imaging agents for cancer detection. Adv. Drug Deliv. Rev. 2017, 110, 38–51. [Google Scholar] [CrossRef]
- Deutscher, S.L. Phage display in molecular imaging and diagnosis of cancer. Chem. Rev. 2010, 110, 3196–3211. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Al Shaer, D.; Albericio, F.; Torre, B.G.D.L. 2022 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2023, 16, 336. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, A.; de la Torre, B.G.; Albericio, F. Liquid-Phase Peptide Synthesis (LPPS): A Third Wave for the Preparation of Peptides. Chem. Rev. 2022, 122, 13516–13546. [Google Scholar] [CrossRef] [PubMed]
- Pfister, D.; Nicoud, L.; Morbidelli, M. Continuous Biopharmaceutical Processes: Chromatography, Bioconjugation, and Protein Stability; Cambridge Series in Chemical Engineering; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- De Luca, C.; Felletti, S.; Lievore, G.; Chenet, T.; Morbidelli, M.; Sponchioni, M.; Cavazzini, A.; Catani, M. Modern trends in downstream processing of biotherapeutics through continuous chromatography: The potential of Multicolumn Countercurrent Solvent Gradient Purification. Trends. Analyt. Chem. 2020, 132, 116051. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Catani, M.; Cavazzini, A.; Martelli, G.; Corbisiero, D.; Cantelmi, P.; Fantoni, T.; Mattellone, A.; De Luca, C.; Felletti, S.; et al. Sustainability in peptide chemistry: Current synthesis and purification technologies and future challenges. Green Chem. 2022, 24, 975–1020. [Google Scholar] [CrossRef]
- Hanke, A.T.; Ottens, M. Purifying biopharmaceuticals: Knowledge-based chromatographic process development. Trends Biotechnol. 2014, 32, 210–220. [Google Scholar] [CrossRef]
- Al Musaimi, O.; Valenzo, O.M.M.; Williams, D.R. Prediction of peptides retention behavior in reversed-phase liquid chromatography based on their hydrophobicity. J. Sep. Sci. 2023, 46, 2200743. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; Mercado-Valenzo, O.M.; Williams, D.R. Factors Influencing the Prediction Accuracy of Model Peptides in Reversed-Phase Liquid Chromatography. Separations 2023, 10, 81. [Google Scholar] [CrossRef]
- Gavva, V.; Al Musaimi, O.; Bent, C.; Williams, D.R. Determining the Hydrophobicity Index of Protected Amino Acids and Common Protecting Groups. Separations 2023, 10, 456. [Google Scholar] [CrossRef]
- Yoshida, T.; Okada, T.; Hobo, T.; Chiba, R. Calculation of amino acid hydrophilicity indices for retention of peptides on amide, diol and silica columns in normal-phase liquid chromatography. Chromatographia 2000, 52, 418–424. [Google Scholar] [CrossRef]
- Yoshida, T. Calculation of peptide retention coefficients in normal-phase liquid chromatography. J. Chromatogr. A 1998, 808, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gilar, M.; Jaworski, A. Retention behavior of peptides in hydrophilic-interaction chromatography. J. Chromatogr. A 2011, 1218, 8890–8896. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K.; Euerby, M.R.; Petersson, P. Investigation into reversed phase chromatography peptide separation systems part III: Establishing a column characterisation database. J. Chromatogr. A 2020, 1622, 461093. [Google Scholar] [CrossRef]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography, 3rd ed.; Wiley-Interscience: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Stanton, P. Ion-Exchange Chromatography. In HPLC of Peptides and Proteins: Methods and Protocols; Aguilar, M.-I., Ed.; Springer: New York, NY, USA; Totowa, NJ, USA, 2004; pp. 23–43. [Google Scholar]
- Lin, C.-W.; Haeuptle, M.A.; Aebi, M. Supercharging Reagent for Enhanced Liquid Chromatographic Separation and Charging of Sialylated and High-Molecular-Weight Glycopeptides for NanoHPLC–ESI-MS/MS Analysis. Anal. Chem. 2016, 88, 8484–8494. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Canonica, F.; Wüthrich, S.; Fettelschoss-Gabriel, A.; Schlapbach, R.; Nanni, P. m-nitrobenzyl alcohol supercharging reagent enhances the chromatographic separation and the charging of disulfide bond linked and His-tag peptides. J. Chromatogr. A 2024, 1722, 464828. [Google Scholar] [CrossRef]
- Ma, Z.; Shang, P.; Liu, D.; Nie, Y.; Liu, Y.; Guo, X.; Wei, B.; Bai, L.; Qiao, X. Preparation and chromatographic performance of chiral peptide-based stationary phases for enantiomeric separation. Chirality 2023, 35, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K.; Euerby, M.R.; Lau, J.; Thøgersen, H.; Petersson, P. Investigation into reversed phase chromatography peptide separation systems part I: Development of a protocol for column characterisation. J. Chromatogr. A 2019, 1603, 113–129. [Google Scholar] [CrossRef]
- Field, J.K.; Euerby, M.R.; Petersson, P. Investigation into reversed phase chromatography peptide separation systems part II: An evaluation of the robustness of a protocol for column characterisation. J. Chromatogr. A 2019, 1603, 102–112. [Google Scholar] [CrossRef]
- Petersson, P.; Buckenmaier, S.; Euerby, M.R.; Stoll, D.R. A strategy for assessing peak purity of pharmaceutical peptides in reversed-phase chromatography methods using two-dimensional liquid chromatography coupled to mass spectrometry. Part I: Selection of columns and mobile phases. J. Chromatogr. A 2023, 1693, 463874. [Google Scholar] [CrossRef]
- Field, J.K.; Euerby, M.R.; Haselmann, K.F.; Petersson, P. Investigation into reversed-phase chromatography peptide separation systems Part IV: Characterisation of mobile phase selectivity differences. J. Chromatogr. A 2021, 1641, 461986. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Bruce, J.; Euerby, M.R.; Field, J.K.; Petersson, P. Investigation into reversed-phase chromatography peptide separation systems part V: Establishment of a screening strategy for development of methods for assessment of pharmaceutical peptides’ purity. J. Chromatogr. A 2022, 1668, 462888. [Google Scholar] [CrossRef]
- Lenčo, J.; Jadeja, S.; Naplekov, D.K.; Krokhin, O.V.; Khalikova, M.A.; Chocholouš, P.; Urban, J.; Broeckhoven, K.; Nováková, L.; Švec, F. Reversed-Phase Liquid Chromatography of Peptides for Bottom-Up Proteomics: A Tutorial. J. Proteome Res. 2022, 21, 2846–2892. [Google Scholar] [CrossRef]
- Alpert, A.J. Hydrophilic-interaction chromatography for the separation of peptides, nucleic acids and other polar compounds. J. Chromatogr. A 1990, 499, 177–196. [Google Scholar] [CrossRef]
- Di Palma, S.; Boersema, P.J.; Heck, A.J.R.; Mohammed, S. Zwitterionic Hydrophilic Interaction Liquid Chromatography (ZIC-HILIC and ZIC-cHILIC) Provide High Resolution Separation and Increase Sensitivity in Proteome Analysis. Anal. Chem. 2011, 83, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- Alpert, A.J. Effect of salts on retention in hydrophilic interaction chromatography. J. Chromatogr. A 2018, 1538, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T. Hydrophilic interaction chromatography for the analysis of biopharmaceutical drugs and therapeutic peptides: A review based on the separation characteristics of the hydrophilic interaction chromatography phases. J. Sep. Sci. 2019, 42, 130–213. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Janás, P. Recent advances in stationary phases and understanding of retention in hydrophilic interaction chromatography. A review. Anal. Chim. Acta 2017, 967, 12–32. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J. Pharm. Biomed. Anal. 2016, 128, 73–88. [Google Scholar] [CrossRef]
- Nogueira, R.; Lämmerhofer, M.; Lindner, W. Alternative high-performance liquid chromatographic peptide separation and purification concept using a new mixed-mode reversed-phase/weak anion-exchange type stationary phase. J. Chromatogr. A 2005, 1089, 158–169. [Google Scholar] [CrossRef]
- Washburn, M.P.; Wolters, D.; Yates, J.R., 3rd. Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 2001, 19, 242–247. [Google Scholar] [CrossRef]
- Gritti, F.; Guiochon, G. Separation of peptides and intact proteins by electrostatic repulsion reversed phase liquid chromatography. J. Chromatogr. A 2014, 1374, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccanti, G.; Gasparrini, F.; Calcaterra, A.; Villani, C.; Ciogli, A. Static vs. Dynamic Electrostatic Repulsion Reversed Phase Liquid Chromatography: Solutions for Pharmaceutical and Biopharmaceutical Basic Compounds. Separations 2021, 8, 59. [Google Scholar] [CrossRef]
- Galea, C.; Mangelings, D.; Vander Heyden, Y. Characterization and classification of stationary phases in HPLC and SFC—A review. Anal. Chim. Acta 2015, 886, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Poole, C.F. Chromatographic test methods for characterizing alkylsiloxane-bonded silica columns for reversed-phase liquid chromatography. J. Chromatogr. B 2018, 1092, 207–219. [Google Scholar] [CrossRef]
- Pedersen, J.S. The Nature of Amyloid-like Glucagon Fibrils. J. Diabetes Sci. Technol. 2010, 4, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccanti, G.; Manetto, S.; Bassan, M.; Foschini, A.; Orlandin, A.; Ricci, A.; Cabri, W.; Ismail, O.H.; Catani, M.; Cavazzini, A.; et al. Boosting basic-peptide separation through dynamic electrostatic-repulsion reversed-phase (d-ERRP) liquid chromatography. RSC Adv. 2020, 10, 12604–12610. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccanti, G.; Manetto, S.; Bassan, M.; Macis, M.; Cabri, W.; Ciogli, A.; Ricci, A.; Gasparrini, F. Assessing the performance of new chromatographic technologies for the separation of peptide epimeric impurities: The case of Icatibant. Eur. J. Pharm. Sci. 2024, 193, 106682. [Google Scholar] [CrossRef]
- Alpert, A.J. Electrostatic Repulsion Hydrophilic Interaction Chromatography for Isocratic Separation of Charged Solutes and Selective Isolation of Phosphopeptides. Anal. Chem. 2008, 80, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Ren, Y.; Dutta, B.; Sze, S.K. Comparative evaluation of electrostatic repulsion–hydrophilic interaction chromatography (ERLIC) and high-pH reversed phase (Hp-RP) chromatography in profiling of rat kidney proteome. J. Proteom. 2013, 82, 254–262. [Google Scholar] [CrossRef]
- Khalaf, R.; Forrer, N.; Buffolino, G.; Gétaz, D.; Bernardi, S.; Butté, A.; Morbidelli, M. Doping reversed-phase media for improved peptide purification. J. Chromatogr. A 2015, 1397, 11–18. [Google Scholar] [CrossRef]
- Kadlecová, Z.; Boudová, H.; Kalíková, K. The benefits of mixed-mode chromatography columns for separation of peptides and protein digests. Monatsh. Chem. 2023, 154, 993–1002. [Google Scholar] [CrossRef]
- Mant, C.T.; Hodges, R.S. Mixed-mode hydrophilic interaction/cation-exchange chromatography (HILIC/CEX) of peptides and proteins. J. Sep. Sci. 2008, 31, 2754–2773. [Google Scholar] [CrossRef] [PubMed]
- Sereda, T.J.; Mant, C.T.; Sönnichsen, F.D.; Hodges, R.S. Reversed-phase chromatography of synthetic amphipathic α-helical peptides as a model for ligand/receptor interactions Effect of changing hydrophobic environment on the relative hydrophilicity/hydrophobicity of amino acid side-chains. J. Chromatogr. A 1994, 676, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Hörth, P.; Miller, C.A.; Preckel, T.; Wenz, C. Efficient fractionation and improved protein identification by peptide OFFGEL electrophoresis. Mol. Cell Proteom. 2006, 5, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Schirle, M.; Heurtier, M.A.; Kuster, B. Profiling core proteomes of human cell lines by one-dimensional PAGE and liquid chromatography-tandem mass spectrometry. Mol. Cell Proteom. 2003, 2, 1297–1305. [Google Scholar] [CrossRef]
- Olivieri, A.C.; Escandar, G.M. Chapter 3—Experimental Three-Way/Second-Order Data. In Practical Three-Way Calibration; Olivieri, A.C., Escandar, G.M., Eds.; Elsevier: Boston, MA, USA, 2014; pp. 27–45. [Google Scholar]
- Aebischer, M.K.; Chapel, S.; Guillarme, D.; Heinisch, S. Theoretical and practical guidelines for solvent dilution between the two dimensions in online comprehensive two-dimensional liquid chromatography. J. Chromatogr. A 2024, 1718, 464725. [Google Scholar] [CrossRef] [PubMed]
- Guillarme, D.; Rouvière, F.; Heinisch, S. Theoretical and practical comparison of RPLC and RPLC × RPLC: How to consider dilution effects and sensitivity in addition to separation power? Anal. Bioanal. Chem. 2023, 415, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Stoll, D.R.; Sylvester, M.; Euerby, M.R.; Buckenmaier, S.M.C.; Petersson, P. A Strategy for assessing peak purity of pharmaceutical peptides in reversed-phase chromatography methods using two-dimensional liquid chromatography coupled to mass spectrometry. Part II: Development of second-dimension gradient conditions. J. Chromatogr. A 2023, 1693, 463873. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef]
- Saito, M. History of supercritical fluid chromatography: Instrumental development. J. Biosci. Bioeng. 2013, 115, 590–599. [Google Scholar] [CrossRef]
- Schiavone, N.M.; Bennett, R.; Hicks, M.B.; Pirrone, G.F.; Regalado, E.L.; Mangion, I.; Makarov, A.A. Evaluation of global conformational changes in peptides and proteins following purification by supercritical fluid chromatography. J. Chromatogr. B 2019, 1110, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, D.; Tsukamoto, A.; Caldwell, J.; Caldwell, W. Rapid Peptide Separation by Supercritical Fluid Chromatography. Bioanalysis 2010, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M. Advantageous use of SFC for separation of crude therapeutic peptides and peptide libraries. J. Pharm. Biomed. Anal. 2020, 185, 113227. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Makarov, A.A.; Bennett, R.; Haidar Ahmad, I.A.; DaSilva, J.; Reibarkh, M.; Mangion, I.; Mann, B.F.; Regalado, E.L. Chaotropic Effects in Sub/Supercritical Fluid Chromatography via Ammonium Hydroxide in Water-Rich Modifiers: Enabling Separation of Peptides and Highly Polar Pharmaceuticals at the Preparative Scale. Anal. Chem. 2019, 91, 13907–13915. [Google Scholar] [CrossRef] [PubMed]
- Govender, K.; Naicker, T.; Baijnath, S.; Chuturgoon, A.A.; Abdul, N.S.; Docrat, T.; Kruger, H.G.; Govender, T. Sub/supercritical fluid chromatography employing water-rich modifier enables the purification of biosynthesized human insulin. J. Chromatogr. B 2020, 1155, 122126. [Google Scholar] [CrossRef]
- Roy, D.; Wahab, M.F.; Berger, T.A.; Armstrong, D.W. Ramifications and Insights on the Role of Water in Chiral Sub/Supercritical Fluid Chromatography. Anal. Chem. 2019, 91, 14672–14680. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Regalado, E.L.; Mergelsberg, I.; Welch, C.J. Extending the range of supercritical fluid chromatography by use of water-rich modifiers. Org. Biomol. Chem. 2013, 11, 4925–4929. [Google Scholar] [CrossRef] [PubMed]
- Govender, K.; Naicker, T.; Baijnath, S.; Kruger, H.G.; Govender, T. The development of a sub/supercritical fluid chromatography based purification method for peptides. J. Pharm. Biomed. Anal. 2020, 190, 113539. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Schmidtsdorff, S.; Schmidt, A.H.; Parr, M.K. Retention modeling of therapeutic peptides in sub-/supercritical fluid chromatography. Sep. Sci. Plus 2024, 7, 2300239. [Google Scholar] [CrossRef]
- Neumann, J.; Schmidtsdorff, S.; Schmidt, A.H.; Parr, M.K. Controlling the elution order of insulin and its analogs in sub-/supercritical fluid chromatography using methanesulfonic acid and 18-crown-6 as mobile phase additives. J. Sep. Sci. 2023, 46, 2300520. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahreinizad, H.; Amiri, Z.; Aliabadi, H.A.M.; Salimi-Bani, M.; Nakisa, A.; Davoodi, F.; Tahmasebi, B.; Ahmadpour, F.; Radinekiyan, F.; et al. Functionalized magnetic nanoparticles for the separation and purification of proteins and peptides. TrAC Trends Anal. Chem. 2021, 141, 116291. [Google Scholar] [CrossRef]
- Asgharnasl, S.; Eivazzadeh-Keihan, R.; Radinekiyan, F.; Maleki, A. Preparation of a novel magnetic bionanocomposite based on factionalized chitosan by creatine and its application in the synthesis of polyhydroquinoline, 1,4-dyhdropyridine and 1,8-dioxo-decahydroacridine derivatives. Int. J. Biol. Macromol. 2020, 144, 29–46. [Google Scholar] [CrossRef]
- Bani, M.S.; Hatamie, S.; Haghpanahi, M.; Bahreinizad, H.; Alavijeh, M.H.S.; Eivazzadeh-Keihan, R.; Wei, Z.H. Casein-Coated Iron Oxide Nanoparticles for in vitro Hyperthermia for Cancer Therapy. SPIN 2019, 09, 1940003. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S.M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A.; et al. Metal-based nanoparticles for bone tissue engineering. J. Tissue Eng. Regen. Med. 2020, 14, 1687–1714. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Maleki, A.; Salimi Bani, M.; Azizi, M. A new generation of star polymer: Magnetic aromatic polyamides with unique microscopic flower morphology and in vitro hyperthermia of cancer therapy. J. Mater. Sci. 2020, 55, 319–336. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Radinekiyan, F.; Maleki, A.; Salimi Bani, M.; Hajizadeh, Z.; Asgharnasl, S. A novel biocompatible core-shell magnetic nanocomposite based on cross-linked chitosan hydrogels for in vitro hyperthermia of cancer therapy. Int. J. Biol. Macromol. 2019, 140, 407–414. [Google Scholar] [CrossRef]
- Maleki, A.; Panahzadeh, M.; Eivazzadeh-keihan, R. Agar: A natural and environmentally-friendly support composed of copper oxide nanoparticles for the green synthesis of 1,2,3–triazoles. Green Chem. Lett. Rev. 2019, 12, 395–406. [Google Scholar] [CrossRef]
- Chen, X.; Rao, J.; Wang, J.; Gooding, J.J.; Zou, G.; Zhang, Q. A facile enantioseparation for amino acids enantiomers using β-cyclodextrins functionalized Fe3O4 nanospheres. Chem. Commun. 2011, 47, 10317–10319. [Google Scholar] [CrossRef]
- Tarhan, T.; Tural, B.; Tural, S.; Topal, G. Enantioseparation of Mandelic Acid Enantiomers With Magnetic Nano-Sorbent Modified by a Chiral Selector. Chirality 2015, 27, 835–842. [Google Scholar] [CrossRef]
- Bahrami, A.; Hejazi, P. Electrostatic immobilization of pectinase on negatively charged AOT-Fe3O4 nanoparticles. J. Mol. Catal. B Enzym. 2013, 93, 1–7. [Google Scholar] [CrossRef]
- Saravanakumar, T.; Palvannan, T.; Kim, D.-H.; Park, S.-M. Optimized immobilization of peracetic acid producing recombinant acetyl xylan esterase on chitosan coated-Fe3O4 magnetic nanoparticles. Process Biochem. 2014, 49, 1920–1928. [Google Scholar] [CrossRef]
- Sui, Y.; Cui, Y.; Nie, Y.; Xia, G.-M.; Sun, G.-X.; Han, J.-T. Surface modification of magnetite nanoparticles using gluconic acid and their application in immobilized lipase. Colloids Surf. B Biointerfaces 2012, 93, 24–28. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Yang, Y.; Liu, X.; Lei, L.; Zhou, L.; Pan, F. Facile synthesis of amino-silane modified superparamagnetic Fe3O4 nanoparticles and application for lipase immobilization. J. Biotechnol. 2010, 150, 171–174. [Google Scholar] [CrossRef]
- Xu, X.; Chen, H.; Cao, Y.; Lin, Y.; Liu, J.A. A Novel Fluorescent Nanoparticle for Sensitive Detection of Cry1Ab Protein In Vitro and In Vivo. J. Fluoresc. 2018, 28, 863–869. [Google Scholar] [CrossRef]
- Salimi, K.; Usta, D.D.; Koçer, İ.; Çelik, E.; Tuncel, A. Protein A and protein A/G coupled magnetic SiO(2) microspheres for affinity purification of immunoglobulin G. Int. J. Biol. Macromol. 2018, 111, 178–185. [Google Scholar] [CrossRef]
- Mirahmadi-Zare, S.Z.; Aboutalebi, F.; Allafchian, M.; Pirjamali, L.; Nasr-Esfahani, M.H. Layer by layer coating of NH(2)-silicate/polycarboxylic acid polymer saturated by Ni(2+) onto the super magnetic NiFe(2)O(4) nanoparticles for sensitive and bio-valuable separation of His-tagged proteins. Protein Expr. Purif. 2018, 143, 71–76. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yang, X.; Yin, M.; Ren, J.; Qu, X. The use of multifunctional magnetic mesoporous core/shell heteronanostructures in a biomolecule separation system. Biomaterials 2011, 32, 4683–4690. [Google Scholar] [CrossRef]
- Peng, M.; Zhou, Y.; Wang, Y.; Yi, Z.; Li, S.; Wan, C. Identified Small Open Reading Frame-Encoded Peptides in Human Serum with Nanoparticle Protein Coronas. J. Proteome Res. 2024, 23, 368–376. [Google Scholar] [CrossRef]
- Zhang, X.; Hua, S.; Feng, Q.; Ding, C.-F.; Wu, Y.; Yan, Y. A novel hydrophilic polymer-coated magnetic nanomaterial based on the HILIC strategy for fast separation of glycopeptides and glycosylated exosomes. Anal. Bioanal. Chem. 2023, 415, 5755–5767. [Google Scholar] [CrossRef]
- Powell, C.D.; Atkinson, A.J.; Ma, Y.; Marcos-Hernandez, M.; Villagran, D.; Westerhoff, P.; Wong, M.S. Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J. Nanopart. Res. 2020, 22, 48. [Google Scholar] [CrossRef]
- Le, T.-D.; Suttikhana, I.; Ashaolu, T.J. State of the art on the separation and purification of proteins by magnetic nanoparticles. J. Nanobiotechnol. 2023, 21, 363. [Google Scholar] [CrossRef]
- Wang, J.; Han, Q.; Wang, K.; Li, S.; Luo, W.; Liang, Q.; Zhong, J.; Ding, M. Recent advances in development of functional magnetic adsorbents for selective separation of proteins/peptides. Talanta 2023, 253, 123919. [Google Scholar] [CrossRef]
- Büyükköroğlu, G.; Dora, D.D.; Özdemir, F.; Hızel, C. Chapter 15—Techniques for Protein Analysis. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 317–351. [Google Scholar]
- Farmerie, L.; Rustandi, R.R.; Loughney, J.W.; Dawod, M. Recent advances in isoelectric focusing of proteins and peptides. J. Chromatogr. A. 2021, 1651, 462274. [Google Scholar] [CrossRef]
- Šalplachta, J.; Horká, M.; Šlais, K. Preparative isoelectric focusing in a cellulose-based separation medium. J. Sep. Sci. 2017, 40, 2498–2505. [Google Scholar] [CrossRef]
- Adamec, J.; Bodzon-Kułakowska, A.; Boone, C.; Burns, A.; Ciborowski, P.; Drabik, A.; Frederick, K.; Havlícek, V.; McMillan, J.; Meza, J.; et al. Proteomic Profiling and Analytical Chemistry: The Crossroads, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Available online: https://www.sciencedirect.com/book/9780444636881/proteomic-profiling-and-analytical-chemistry#book-description (accessed on 28 June 2024).
- Shen, Y.; Berger, S.J.; Anderson, G.A.; Smith, R.D. High-Efficiency Capillary Isoelectric Focusing of Peptides. Anal. Chem. 2000, 72, 2154–2159. [Google Scholar] [CrossRef]
- Chingin, K.; Astorga-Wells, J.; Pirmoradian Najafabadi, M.; Lavold, T.; Zubarev, R.A. Separation of Polypeptides by Isoelectric Point Focusing in Electrospray-Friendly Solution Using a Multiple-Junction Capillary Fractionator. Anal. Chem. 2012, 84, 6856–6862. [Google Scholar] [CrossRef]
- Pirmoradian, M.; Zhang, B.; Chingin, K.; Astorga-Wells, J.; Zubarev, R.A. Membrane-Assisted Isoelectric Focusing Device As a Micropreparative Fractionator for Two-Dimensional Shotgun Proteomics. Anal. Chem. 2014, 86, 5728–5732. [Google Scholar] [CrossRef]
- Roland, T.J.; Strauss, G.L.; Bushra, N.; Muschol, M.; Koria, P. Isoelectric point (pI)-based phase separation (pI-BPS) purification of elastin-like polypeptides (ELPs) containing charged, biologically active fusion proteins (ELP-FPs). Biotechnol. Prog. 2023, 39, e3381. [Google Scholar] [CrossRef]
- Frolov, A.I.; Chankeshwara, S.V.; Abdulkarim, Z.; Ghiandoni, G.M. pIChemiSt ─ Free Tool for the Calculation of Isoelectric Points of Modified Peptides. J. Chem. Inf. Model. 2023, 63, 187–196. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane transport theory. In Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Fundamentals of Pressure-Driven Membrane Separation Processes. In Membrane Technology; Cui, Z.F., Muralidhara, H.S., Eds.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 1–18. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Purification and fractionation of bioactive peptides through membrane filtration: A critical and application review. Trends Food Sci. Technol. 2023, 131, 118–128. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Kadel, S.; Pellerin, G.; Thibodeau, J.; Perreault, V.; Lainé, C.; Bazinet, L. How Molecular Weight Cut-Offs and Physicochemical Properties of Polyether Sulfone Membranes Affect Peptide Migration and Selectivity during Electrodialysis with Filtration Membranes. Membranes 2019, 9, 153. [Google Scholar] [CrossRef]
- Persico, M.; Bazinet, L. Fouling prevention of peptides from a tryptic whey hydrolysate during electromembrane processes by use of monovalent ion permselective membranes. J. Membr. Sci. 2018, 549, 486–494. [Google Scholar] [CrossRef]
- Beaubier, S.; Przybylski, R.; Bodin, A.; Nedjar, N.; Dhulster, P.; Kapel, R. Ultrafiltration Fractionation of Bovine Hemoglobin Hydrolysates: Prediction of Separation Performances for Optimal Enrichment in Antimicrobial Peptide. Membranes 2021, 11, 73. [Google Scholar] [CrossRef]
- Doyen, A.; Beaulieu, L.; Saucier, L.; Pouliot, Y.; Bazinet, L. Demonstration of in vitro anticancer properties of peptide fractions from a snow crab by-products hydrolysate after separation by electrodialysis with ultrafiltration membranes. Sep. Purif. Technol. 2011, 78, 321–329. [Google Scholar] [CrossRef]
- Lapointe, J.-F.; Gauthier, S.F.; Pouliot, Y.; Bouchard, C. Selective separation of cationic peptides from a tryptic hydrolysate of β-lactoglobulin by electrofiltration. Biotechnol. Bioeng. 2006, 94, 223–233. [Google Scholar] [CrossRef]
- Quaisie, J.; Ma, H.; Guo, Y.; Tuly, J.A.; Igbokwe, C.J.; Ekumah, J.-N.; Akpabli-Tsigbe, N.D.K.; Yanhua, D.; Liu, D. Highly stable, antihypertensive, and antioxidative peptide production from Apostichopus japonicus by integrated enzymatic membrane reactor and nanofilter-purification mechanism. Food Funct. 2022, 13, 2306–2322. [Google Scholar] [CrossRef]
- Uluko, H.; Zhang, S.; Liu, L.; Tsakama, M.; Lu, J.; Lv, J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods 2015, 18, 1138–1146. [Google Scholar] [CrossRef]
- Roblet, C.; Akhtar, M.J.; Mikhaylin, S.; Pilon, G.; Gill, T.; Marette, A.; Bazinet, L. Enhancement of glucose uptake in muscular cell by peptide fractions separated by electrodialysis with filtration membrane from salmon frame protein hydrolysate. J. Funct. Foods 2016, 22, 337–346. [Google Scholar] [CrossRef]
- Dibdiakova, J.; Matic, J.; Wubshet, S.G.; Uhl, W.; Manamperuma, L.D.; Rusten, B.; Vik, E.A. Membrane Separation of Chicken Byproduct Hydrolysate for Up-Concentration of Bioactive Peptides. Membranes 2024, 14, 28. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Kenworthy, M.N.; Mukherjee, S.; Kopach, M.E.; Wegner, K.; Gallou, F.; Smith, A.G.; Roschangar, F. Sustainability Challenges in Peptide Synthesis and Purification: From R&D to Production. J. Org. Chem. 2019, 84, 4615–4628. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, F.; Jankowski, V.; Günthner, T.; Salem, S.; Nierhaus, M.; Schulz, A.; Zidek, W.; Jankowski, J. Replacement of acetonitrile by ethanol as solvent in reversed phase chromatography of biomolecules. J. Chromatogr. B 2010, 878, 763–768. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The Chemistry of Dimethyl Carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Greening Fmoc/tBu solid-phase peptide synthesis. Green Chem. 2020, 22, 996–1018. [Google Scholar] [CrossRef]
- Bozza, D.; De Luca, C.; Felletti, S.; Spedicato, M.; Presini, F.; Giovannini, P.P.; Carraro, M.; Macis, M.; Cavazzini, A.; Catani, M.; et al. Dimethyl carbonate as a green alternative to acetonitrile in reversed-phase liquid chromatography. Part II: Purification of a therapeutic peptide. J. Chromatogr. A 2024, 1713, 464530. [Google Scholar] [CrossRef]
- Peyrin, E.; Lipka, E. Preparative supercritical fluid chromatography as green purification methodology. TrAC, Trends Anal. Chem. 2024, 171, 117505. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. Poly ionic liquid cryogel of polyethyleneimine: Synthesis, characterization, and testing in absorption studies. J. Appl. Polym. Sci. 2016, 133, 43478. [Google Scholar] [CrossRef]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprocess. Technol. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Zhou, N.E.; Mant, C.T.; Hodges, R.S. Effect of preferred binding domains on peptide retention behavior in reversed-phase chromatography: Amphipathic alpha-helices. Pept. Res. 1990, 3, 8–20. [Google Scholar] [PubMed]
- Lau, S.Y.M.; Taneja, A.K.; Hodges, R.S. Effects of high-performance liquid chromatographic solvents and hydrophobic matrices on the secondary and quarternary structure of a model protein: Reversed-phase and size-exclusion high-performance liquid chromatography. J. Chromatogr. A 1984, 317, 129–140. [Google Scholar] [CrossRef]
- Busnel, J.-M.; Lion, N.; Girault, H.H. Capillary Electrophoresis as a Second Dimension to Isoelectric Focusing for Peptide Separation. Anal. Chem. 2007, 79, 5949–5955. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, D.M.M.; Al Musaimi, O.; Albericio, F. Advances in solid-phase peptide synthesis in aqueous media (ASPPS). Green Chem. 2022, 24, 6360–6372. [Google Scholar] [CrossRef]
| # | Technology | Applications |
|---|---|---|
| 1 | RPLC | Separation, identification, and purification |
| 2 | HILIC | Separation, identification, and purification |
| 3 | MMC | Separation, identification, and purification |
| 4 | 2D | Separation, identification, and purification |
| 5 | SFC | Separation, identification, and purification |
| 6 | MNPs | Separation and purification |
| 7 | IEF | Separation and purification |
| 8 | Membrane filtration | Purification |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Musaimi, O.; Jaradat, D.M.M. Advances in Therapeutic Peptides Separation and Purification. Separations 2024, 11, 233. https://doi.org/10.3390/separations11080233
Al Musaimi O, Jaradat DMM. Advances in Therapeutic Peptides Separation and Purification. Separations. 2024; 11(8):233. https://doi.org/10.3390/separations11080233
Chicago/Turabian StyleAl Musaimi, Othman, and Da’san M. M. Jaradat. 2024. "Advances in Therapeutic Peptides Separation and Purification" Separations 11, no. 8: 233. https://doi.org/10.3390/separations11080233
APA StyleAl Musaimi, O., & Jaradat, D. M. M. (2024). Advances in Therapeutic Peptides Separation and Purification. Separations, 11(8), 233. https://doi.org/10.3390/separations11080233








