Characterisation of the Volatile Compounds and Key Odourants in Japanese Mandarins by Gas Chromatography–Mass Spectrometry and Gas Chromatography–Olfactometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sample Preparation
2.2. Chemicals and Reagents
2.3. HS-SPME Procedure
2.4. Solvent Extraction
2.5. GC-MS/FID Analysis
2.6. GC-O/MS and AEDA Analysis
2.7. Sensory Profiling
2.8. Data Analysis
3. Results and Discussion
3.1. Extraction of Volatile Compounds in Japanese Mandarin Juices and Peels by HS-SPME
| No. | Compound | LRI I | CAS Number | Abundance (FID Peak Area) | Identification II | |||
|---|---|---|---|---|---|---|---|---|
| Iyokan | Ponkan | Shiranui | Unshiu Mikan | |||||
| 1 | Acetaldehyde 1,2 | 671 | 75-07-0 | 355,475 ± 42,481 c | 1,481,261 ± 126,612 a | 1,189,841 ± 74,013 b | 1,263,030 ± 115,588 b | LRI, MS, STD |
| 2 | Ethyl acetate 1,3,4,5 | 917 | 141-78-6 | - | 2,463,085 ± 156,200 b | 9,149,139 ± 392,714 a | 480,006 ± 22,211 c | LRI, MS, STD |
| 3 | Methyl butanoate 2 | 1001 | 623-42-7 | 119,024 ± 8693 | Trace | - | - | LRI, MS, STD |
| 4 | α-Pinene 1,2,3,4,5,6 | 1038 | 80-56-8 | 177,964 ± 15,502 c | 270,643 ± 16,738 b | 151,267 ± 5409 d | 415,268 ± 4364 a | LRI, MS, STD |
| 5 | α-Thujene 1,3,4 | 1042 | 2867-05-2 | 98,181 ± 2933 a | 43,115 ± 2641 b | 27,773 ± 1533 d | 32,976 ± 1827 c | LRI, MS |
| 6 | Ethyl butanoate 1,2 | 1049 | 105-54-4 | - | 103,026 ± 6719 a | 83,154 ± 6541 b | - | LRI, MS, STD |
| 7 | Hexanal 1,2,3,5 | 1099 | 66-25-1 | 330,073 ± 30,116 b | 606,791 ± 51,092 a | 319,481 ± 25,169 b | 163,403 ± 13,566 c | LRI, MS, STD |
| 8 | β-Pinene 1,3,4,5,6 | 1123 | 127-91-3 | 98,609 ± 7584 b | 125,393 ± 14,584 a | 22,518 ± 479 c | 120,178 ± 5268 a | LRI, MS, STD |
| 9 | Sabinene 1,3,4,5,6 | 1135 | 3387-41-5 | 43,530 ± 4574 b | 120,328 ± 12,367 a | - | 41,604 ± 2843 b | LRI, MS, STD |
| 10 | 1-Penten-3-ol 1,6 | 1165 | 616-25-1 | 55,399 ± 3051 b | - | - | 63,702 ± 3674 a | LRI, MS |
| 11 | Myrcene 1,2,3,4,5,6 | 1175 | 123-35-3 | 236,666 ± 25,932 c | 918,697 ± 14,419 b | 1,219,188 ± 84,847 a | 1,176,388 ± 93,611 a | LRI, MS, STD |
| 12 | α-Phellandrene 1,2,3,4 | 1182 | 99-83-2 | 34,869 ± 3153 d | 300,799 ± 20,009 a | 150,857 ± 8420 b | 119,937 ± 1400 c | LRI, MS, STD |
| 13 | α-Terpinene 1,2,3,4 | 1195 | 99-86-5 | 115,489 ± 11,657 c | 1,296,352 ± 37,576 a | 364,443 ± 32,125 b | 417,156 ± 19,230 b | LRI, MS |
| 14 | Methyl hexanoate 2 | 1197 | 106-70-7 | 75,220 ± 1034 | - | - | - | LRI, MS, STD |
| 15 | Heptanal 1,2,3 | 1198 | 111-71-7 | - | 69,281 ± 4591 a | 43,456 ± 1875 b | - | LRI, MS, STD |
| 16 | Limonene 1,2,3,4,5,6 | 1229 | 138-86-3 | 14,758,307 ± 1,002,304 c | 118,087,550 ± 4,893,815 a | 65,070,290 ± 7,801,430 b | 59,520,248 ± 6,412,533 b | LRI, MS, STD |
| 17 | β-Phellandrene 1,3,4,5,6 | 1233 | 555-10-2 | 98,257 ± 5297 c | 526,885 ± 26,504 a | 277,990 ± 10,190 b | 285,054 ± 16,632 b | LRI, MS |
| 18 | trans-2-Hexenal 1,2,3 | 1239 | 6728-26-3 | 44,549 ± 4045 b | 46,299 ± 3855 b | 261,696 ± 26,963 a | 245,374 ± 12,800 a | LRI, MS, STD |
| 19 | cis-β-Ocimene 1,3,4,5 | 1254 | 3338-55-4 | 46,912 ± 2888 b | 16,193 ± 1296 d | 76,751 ± 2538 a | 26,159 ± 2379 c | LRI, MS, STD |
| 20 | Pentanol 1,3 | 1259 | 71-41-0 | 31,067 ± 2067 b | - | - | 63,300 ± 6002 a | LRI, MS, STD |
| 21 | γ-Terpinene 1,2,3,4,5,6 | 1265 | 99-85-4 | 756,879 ± 37,299 c | 6,955,973 ± 270,481 a | 258,010 ± 26,985 d | 2,637,181 ± 250,490 b | LRI, MS, STD |
| 22 | trans-β-Ocimene 1,3,4,5 | 1267 | 3779-61-1 | 48,591 ± 4735 ab | Trace | 51,405 ± 3879 a | 43,507 ± 2693 b | LRI, MS, STD |
| 23 | p-Mentha-3,8-diene 1 | 1280 | 586-67-4 | - | 34,763 ± 3335 a | 17,783 ± 1703 b | - | LRI, MS |
| 24 | p-Cymene 1,2,3,4,5,6 | 1294 | 99-87-6 | 736,422 ± 54,143 c | 6,257,423 ± 238,852 a | 535,553 ± 15,422 c | 1,754,855 ± 187,714 b | LRI, MS, STD |
| 25 | Terpinolene 1,2,3,4,5,6 | 1304 | 586-62-9 | 196,568 ± 13,512 c | 2,205,629 ± 84,146 a | 737,783 ± 57,846 b | 808,365 ± 121,744 b | LRI, MS, STD |
| 26 | Acetoin 1 | 1305 | 513-86-0 | - | - | - | 756,403 ± 65,559 | LRI, MS, STD |
| 27 | Isoterpinolene 1,4 | 1310 | 586-63-0 | 25,896 ± 1510 b | - | 100,128 ± 9100 a | - | LRI, MS |
| 28 | cis-2-Pentenol 1,6 | 1327 | 1576-95-0 | 19,815 ± 1482 b | 31,352 ± 2402 a | - | - | LRI, MS |
| 29 | trans-2-Heptenal 1 | 1331 | 18829-55-5 | - | 38,634 ± 3120 | - | - | LRI, MS, STD |
| 30 | 6-Methyl-5-hepten-2-one 1,3,4 | 1351 | 110-93-0 | 28,623 ± 864 c | 33,268 ± 1843 b | 25,640 ± 1717 c | 64,389 ± 3462 a | LRI, MS, STD |
| 31 | Hexanol 1,2,3,5,6 | 1357 | 111-27-3 | 164,779 ± 12,132 b | 94,543 ± 4379 c | 47,972 ± 5501 c | 759,679 ± 66,784 a | LRI, MS, STD |
| 32 | cis-Alloocimene 1,4,5 | 1379 | 673-84-7 | 16,464 ± 1658 b | - | 20,509 ± 2266 a | - | LRI, MS, STD |
| 33 | cis-3-Hexenol 1,3,5,6 | 1392 | 928-96-1 | 111,666 ± 2265 b | 44,045 ± 2834 d | 62,168 ± 2074 c | 261,090 ± 9818 a | LRI, MS, STD |
| 34 | Methyl octanoate 1,2 | 1395 | 111-11-5 | 17,354 ± 1571 | - | - | - | LRI, MS, STD |
| 35 | Nonanal 1,2,3,4,5,6 | 1407 | 124-19-6 | - | 38,026 ± 540 a | - | 34,931 ± 459 b | LRI, MS, STD |
| 36 | trans-2-Hexenol 1,2,3,6 | 1410 | 928-95-0 | 27,407 ± 2534 b | - | 35,615 ± 2096 b | 125,055 ± 8855 a | LRI, MS, STD |
| 37 | p-Mentha-1,3,8-triene 1,3,4 | 1413 | 18368-95-1 | - | 29,461 ± 922 | - | - | LRI, MS |
| 38 | trans-2-Octenal 1 | 1433 | 2548-87-0 | - | 80,771 ± 8385 | - | - | LRI, MS, STD |
| 39 | Ethyl octanoate 1,2,5 | 1438 | 106-32-1 | 11,923 ± 1142 | - | - | - | LRI, MS, STD |
| 40 | p-Cymenene 3 | 1457 | 1195-32-0 | 54,839 ± 1755 d | 326,135 ± 15,218 a | 181,567 ± 4767 b | 106,258 ± 6924 c | LRI, MS |
| 41 | Heptanol 1,2,3,6 | 1460 | 111-70-6 | 32,725 ± 2726 b | 50,075 ± 5515 a | - | 53,777 ± 601 a | LRI, MS, STD |
| 42 | Acetic acid 1,3,4,5,6 | 1465 | 64-19-7 | - | - | 21,066 ± 2144 b | 38,735 ± 2687 a | LRI, MS, STD |
| 43 | cis-Limonene oxide 1,3,4,6 | 1468 | 13837-75-7 | - | Trace | - | - | LRI, MS |
| 44 | α-Cubebene 1,2,3,5 | 1473 | 17699-14-8 | - | - | - | 24,606 ± 668 | LRI, MS |
| 45 | trans-Limonene oxide 3,4 | 1481 | 4959-35-7 | - | 45,880 ± 2321 | - | - | LRI, MS |
| 46 | δ-Elemene 1,3,5,6 | 1487 | 20307-84-0 | 18,083 ± 526 b | - | 36,304 ± 2783 a | - | LRI, MS |
| 47 | 2-Ethylhexanol 3 | 1500 | 104-76-7 | 29,728 ± 3204 b | 35,732 ± 1743 a | 31,297 ± 2398 b | - | LRI, MS, STD |
| 48 | α-Copaene 1,2,3,4,5,6 | 1513 | 3856-25-5 | - | - | 16,648 ± 1234 | - | LRI, MS |
| 49 | trans-2-Heptenol 1 | 1520 | 33467-76-4 | 11,027 ± 740 | - | - | - | LRI, MS |
| 50 | Ethyl nonanoate 1 | 1544 | 123-29-5 | - | - | - | 22,281 ± 2145 | LRI, MS, STD |
| 51 | Linalool 1,2,3,4,5,6 | 1552 | 78-70-6 | 168,557 ± 9898 a | 66,715 ± 855 b | 32,790 ± 2499 c | 42,386 ± 2325 c | LRI, MS, STD |
| 52 | Octanol 1,2,3,4,5,6 | 1567 | 111-87-5 | 30,638 ± 3224 b | 54,564 ± 1669 a | 56,588 ± 1463 a | 33,413 ± 530 b | LRI, MS, STD |
| 53 | Methylthymol 3,4 | 1602 | 1076-56-8 | - | 178,301 ± 5016 | - | - | LRI, MS |
| 54 | β-Elemene 1,3,4,5,6 | 1611 | 515-13-9 | - | - | 29,338 ± 2679 b | 88,288 ± 5858 a | LRI, MS |
| 55 | Terpinen-4-ol 1,2,3,4,6 | 1618 | 562-74-3 | - | 106,146 ± 5719 a | 35,464 ± 3323 b | 22,148 ± 2571 c | LRI, MS, STD |
| 56 | β-Caryophyllene 1,3,4,5 | 1624 | 87-44-5 | - | - | 49,205 ± 4560 | - | LRI, MS, STD |
| 57 | p-Menth-1-en-9-al 3 | 1635 | 29548-14-9 | - | 17,407 ± 2037 | - | - | LRI, MS |
| 58 | Nonanol 1,3,4,5,6 | 1662 | 143-08-8 | 19,302 ± 1450 b | 34,318 ± 3526 a | 22,458 ± 2048 b | 35,654 ± 2020 a | LRI, MS, STD |
| 59 | Alloaromadendrene 3,4 | 1671 | 25246-27-9 | - | - | 82,652 ± 8966 a | 32,436 ± 2132 b | LRI, MS |
| 60 | Citronellyl acetate 1,3,4,5,6 | 1671 | 150-84-5 | - | - | 35,537 ± 3362 | - | LRI, MS, STD |
| 61 | α-Humulene 3,4,5,6 | 1699 | 6753-98-6 | - | - | 18,615 ± 1885 b | 34,186 ± 3438 a | LRI, MS |
| 62 | α-Terpineol 1,2,3,4,5,6 | 1710 | 98-55-5 | 86,918 ± 2007 a | 29,838 ± 1710 b | 18,280 ± 1213 c | - | LRI, MS, STD |
| 63 | trans-trans-2,4-Nonadienal 1,3 | 1718 | 5910-87-2 | 26,636 ± 1626 | - | - | - | LRI, MS |
| 64 | Dodecanal 3,4 | 1723 | 112-54-9 | 25,070 ± 642 | - | - | - | LRI, MS, STD |
| 65 | β-Selinene 1,4,5,6 | 1725 | 17066-67-0 | - | - | 75,720 ± 4007 a | 72,054 ± 5407 a | LRI, MS |
| 66 | Germacrene D 1,3,4,5,6 | 1739 | 23986-74-5 | - | - | - | 16,146 ± 627 | LRI, MS, STD |
| 67 | Neryl acetate 1,3,4,6 | 1741 | 141-12-8 | 21,898 ± 1699 | - | - | Trace | LRI, MS, STD |
| 68 | Valencene 1,2,3,4 | 1744 | 4630-07-3 | - | - | 1,814,673 ± 135,772 a | 816,674 ± 34,213 b | LRI, MS, STD |
| 69 | α-Selinene 1,5 | 1753 | 473-13-2 | - | - | 90,580 ± 5318 a | 45,375 ± 4649 b | LRI, MS |
| 70 | α-Farnesene 1,3,4,5,6 | 1761 | 502-61-4 | - | - | 25,118 ± 1716 a | 22,744 ± 1662 a | LRI, MS, STD |
| 71 | Decanol 3,4 | 1767 | 112-30-1 | Trace | 24,327 ± 1835 b | 55,265 ± 4087 a | - | LRI, MS, STD |
| 72 | Citronellol 1,2,3,4,5,6 | 1768 | 106-22-9 | - | 15,891 ± 1034 b | 24,504 ± 2087 a | - | LRI, MS, STD |
| 73 | δ-Cadinene 1,3,4,6 | 1780 | 483-76-1 | 22,324 ± 1343 c | - | 33,587 ± 822 a | 30,342 ± 991 b | LRI, MS |
| 74 | trans-trans-2,4-Decadienal 1,3,5,6 | 1833 | 25152-84-5 | 10,661 ± 987 b | 13,234 ± 917 a | - | - | LRI, MS, STD |
| 75 | Hexanoic acid 1,3,4,6 | 1855 | 142-62-1 | - | - | - | 15,390 ± 989 | LRI, MS, STD |
| 76 | Geranyl acetone 1,6 | 1871 | 3796-70-1 | 29,679 ± 2190 c | 45,827 ± 3877 b | 38,750 ± 1213 b | 65,161 ± 5630 a | LRI, MS, STD |
| 77 | Heptanoic acid 1 | 1964 | 111-14-8 | - | - | - | 15,899 ± 406 | LRI, MS, STD |
| 78 | β-Ionone 1,2,3 | 1965 | 14901-07-6 | 13,113 ± 1145 b | 15,134 ± 1570 b | - | 42,300 ± 2247 a | LRI, MS, STD |
| 79 | Dodecanol 3 | 1972 | 112-53-8 | - | 8952 ± 455 | - | - | LRI, MS, STD |
| 80 | Octanoic acid 1,3,4,5,6 | 2064 | 124-07-2 | 16,593 ± 252 d | 19,187 ± 532 c | 42,068 ± 2523 b | 82,148 ± 4344 a | LRI, MS, STD |
| 81 | Nonanoic acid 1,3,4,5,6 | 2171 | 112-05-0 | Trace | Trace | 26,792 ± 2109 b | 89,453 ± 7917 a | LRI, MS, STD |
| 82 | Thymol 3 | 2177 | 89-83-8 | - | 11,779 ± 342 | - | Trace | LRI, MS, STD |
| 83 | p-Menth-8-ene-1,2-diol 3,5 | 2288 | 1946-00-5 | Trace | 45,352 ± 1248 | Trace | Trace | LRI, MS |
| Total peak area | 19,489,768 ± 1,099,266 c | 143,464,378 ± 5,751,127 a | 83,195,275 ± 7,855,946 b | 73,527,094 ± 7,195,905 b | ||||
3.2. Extraction of Volatile Compounds in Japanese Mandarin Peels by Solvent Extraction
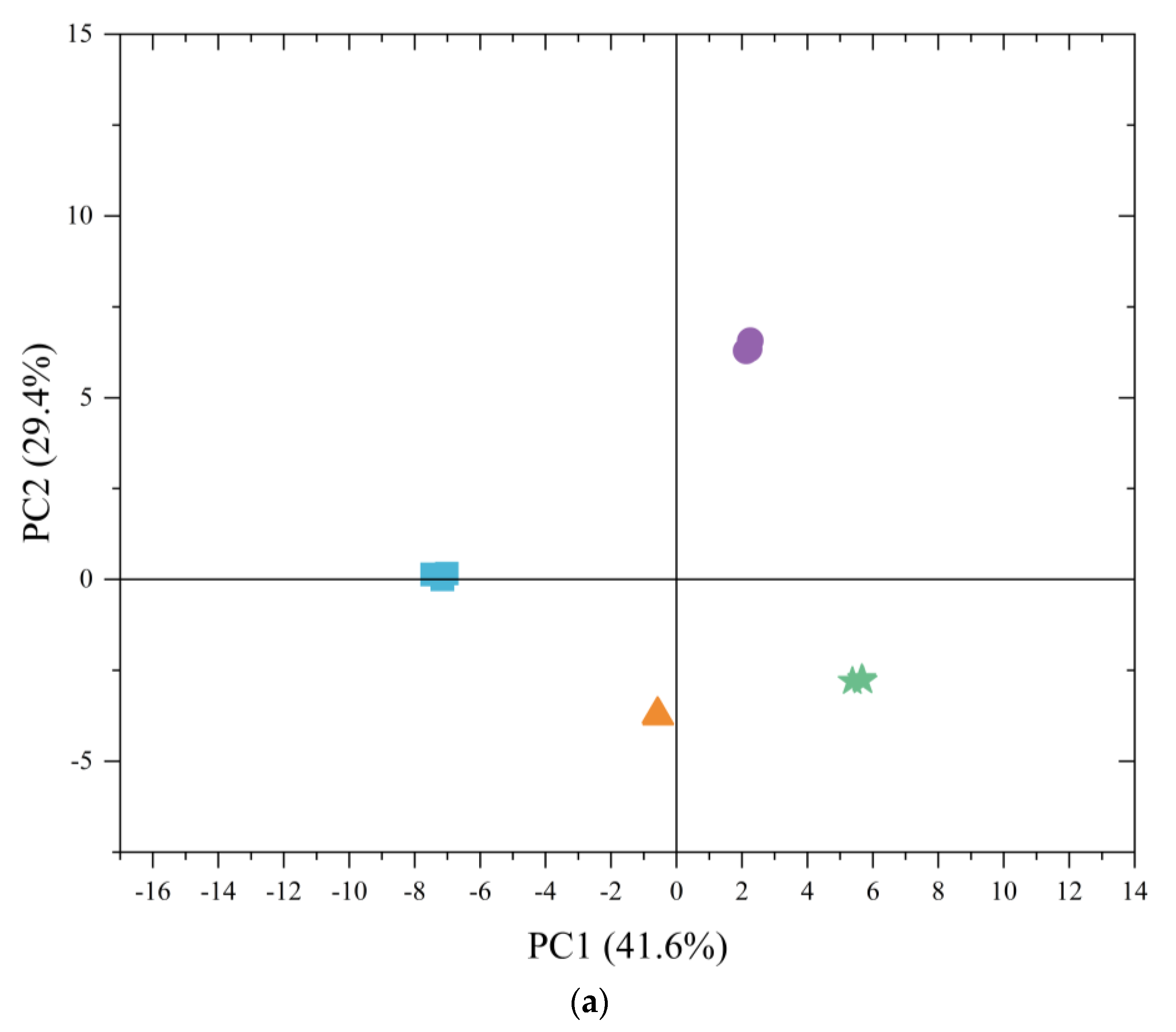
3.3. PCA of Japanese Mandarin
3.4. Key Odourants of Japanese Mandarin Peel Extracts and Heatmap Analysis
3.5. Sensory Evaluation of Japanese Mandarin Peel Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.; Han, L.; Huang, L.; Tan, X.; Wu, H.; Li, G. Association between flavor composition and sensory profile in thermally processed mandarin juices by multidimensional gas chromatography and multivariate statistical analysis. Food Chem. 2023, 419, 136026. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Fabroni, S.; Feng, S.; Rapisarda, P.; Rouseff, R. Chemistry of citrus flavor. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 447–470. [Google Scholar] [CrossRef]
- Wang, L.; He, F.; Huang, Y.; He, J.; Yang, S.; Zeng, J.; Deng, C.; Jiang, X.; Fang, Y.; Wen, S.; et al. Genome of Wild Mandarin and Domestication History of Mandarin. Mol. Plant 2018, 11, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Barry, G.H.; Caruso, M.; Gmitter, F.G. Commercial scion varieties. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 83–104. [Google Scholar] [CrossRef]
- Eom, H.J.; Lee, D.; Lee, S.; Noh, H.J.; Hyun, J.W.; Yi, P.H.; Kang, K.S.; Kim, K.H. Flavonoids and a Limonoid from the Fruits of Citrus unshiu and Their Biological Activity. J. Agric. Food Chem. 2016, 64, 7171–7178. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, L.; Yaniv, Y.; Porat, R.; Carmi, N. Mandarin fruit quality: A review. J. Sci. Food Agric. 2018, 98, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wu, Q.; Niu, Y.; Wu, M.; Zhu, J.; Zhou, X.; Chen, X.; Wang, H.; Li, J.; Kong, J. Characterization of the Key Aroma Compounds in Five Varieties of Mandarins by Gas Chromatography-Olfactometry, Odor Activity Values, Aroma Recombination, and Omission Analysis. J. Agric. Food Chem. 2017, 65, 8392–8401. [Google Scholar] [CrossRef] [PubMed]
- Yazici, K.; Balijagic, J.; Goksu, B.; Bilgin, O.F.; Ercisli, S. Comparison of Some Fruit Quality Parameters of Selected 12 Mandarin Genotypes from Black Sea Region in Turkey. ACS Omega 2023, 8, 19719–19727. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, M.; Thi Minh Tu, N.; Onishi, Y.; Ogawa, E.; Choi, H.S. Characteristic odor components of Citrus reticulata Blanco (ponkan) cold-pressed oil. Biosci. Biotechnol. Biochem. 2004, 68, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Lan Phi, N.T.; Park, Y.-H.; Sawamura, M. Volatile Profiles in Cold-Pressed Peel Oil from Korean and Japanese Shiranui (Citrus unshiu Marcov. × C. sinensis Osbeck × C. reticulata Blanco). Biosci. Biotechnol. Biochem. 2006, 70, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, M. Citrus Essential Oils: Flavor and Fragrance; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Augusto, F.; Leite e Lopes, A.; Zini, C.A. Sampling and sample preparation for analysis of aromas and fragrances. TrAC Trends Anal. Chem. 2003, 22, 160–169. [Google Scholar] [CrossRef]
- Goh, R.M.V.; Lau, H.; Liu, S.Q.; Lassabliere, B.; Guervilly, R.; Sun, J.; Bian, Y.; Yu, B. Comparative analysis of pomelo volatiles using headspace-solid phase micro-extraction and solvent assisted flavour evaporation. LWT Food Sci. Technol. 2019, 99, 328–345. [Google Scholar] [CrossRef]
- Hou, J.; Liang, L.; Wang, Y. Volatile composition changes in navel orange at different growth stages by HS-SPME–GC–MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, K.; Aoki, H.; Oikawa, T.; Sakurai, K.; Kasahara, Y.; Kawakami, Y. Characteristic volatile components of Japanese sour citrus fruits: Yuzu, Sudachi and Kabosu. Flavour Fragr. J. 2012, 27, 341–355. [Google Scholar] [CrossRef]
- Park, M.K.; Cha, J.Y.; Kang, M.C.; Jang, H.W.; Choi, Y.S. The effects of different extraction methods on essential oils from orange and tangor: From the peel to the essential oil. Food Sci. Nutr. 2024, 12, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Barboni, T.; Luro, F.; Chiaramonti, N.; Desjobert, J.-M.; Muselli, A.; Costa, J. Volatile composition of hybrids Citrus juices by headspace solid-phase micro extraction/gas chromatography/mass spectrometry. Food Chem. 2009, 116, 382–390. [Google Scholar] [CrossRef]
- Cheong, M.W.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012, 135, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.R.; Jayaprakasha, G.K.; Patil, B.S. Headspace and Solid-Phase Microextraction Methods for the Identification of Volatile Flavor Compounds in Citrus Fruits. In Instrumental Methods for the Analysis and Identification of Bioactive Molecules, ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1185, pp. 243–256. [Google Scholar] [CrossRef]
- Bures, M.S.; Maslov Bandic, L.; Vlahovicek-Kahlina, K. Determination of Bioactive Components in Mandarin Fruits: A Review. Crit. Rev. Anal. Chem. 2023, 53, 1489–1514. [Google Scholar] [CrossRef] [PubMed]
- Goh, R.M.V.; Pua, A.; Liu, S.Q.; Lassabliere, B.; Leong, K.-C.; Sun, J.; Tan, L.P.; Yu, B. Characterisation of Volatile Compounds in Kumquat and Calamansi Peel Oil Extracts. J. Essent. Oil Bear. Plants 2020, 23, 953–969. [Google Scholar] [CrossRef]
- Tietel, Z.; Plotto, A.; Fallik, E.; Lewinsohn, E.; Porat, R. Taste and aroma of fresh and stored mandarins. J. Sci. Food Agric. 2011, 91, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Suh, J.H.; Gmitter, F.G.; Wang, Y. Differentiation between Flavors of Sweet Orange (Citrus sinensis) and Mandarin (Citrus reticulata). J. Agric. Food Chem. 2018, 66, 203–211. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drug Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, M.; Zhu, K.; Liu, Y.; Wen, H.; Kong, J.; Chen, M.; Cao, L.; Ye, J.; Zhang, H.; et al. Multiomics integrated with sensory evaluations to identify characteristic aromas and key genes in a novel brown navel orange (Citrus sinensis). Food Chem. 2024, 444, 138613. [Google Scholar] [CrossRef]
- Goh, R.M.V.; Pua, A.; Ee, K.H.; Huang, Y.; Liu, S.Q.; Lassabliere, B.; Yu, B. Investigation of changes in non-traditional indices of maturation in Navel orange peel and juice using GC-MS and LC-QTOF/MS. Food Res. Int. 2021, 148, 110607. [Google Scholar] [CrossRef]
- Barnes, B.B.; Wilson, M.B.; Carr, P.W.; Vitha, M.F.; Broeckling, C.D.; Heuberger, A.L.; Prenni, J.; Janis, G.C.; Corcoran, H.; Snow, N.H.; et al. “Retention Projection” Enables Reliable Use of Shared Gas Chromatographic Retention Data Across Laboratories, Instruments, and Methods. Anal. Chem. 2013, 85, 11650–11657. [Google Scholar] [CrossRef] [PubMed]
- Pua, A.; Lau, H.; Liu, S.Q.; Tan, L.P.; Goh, R.M.V.; Lassabliere, B.; Leong, K.C.; Sun, J.; Cornuz, M.; Yu, B. Improved detection of key odourants in Arabica coffee using gas chromatography-olfactometry in combination with low energy electron ionisation gas chromatography-quadrupole time-of-flight mass spectrometry. Food Chem. 2020, 302, 125370. [Google Scholar] [CrossRef] [PubMed]
- Ohata, M.; Zhou, L.; Ando, S.; Kaneko, S.; Osada, K.; Yada, Y. Application of integrative physiological approach to evaluate human physiological responses to the inhalation of essential oils of Japanese citrus fruits iyokan (Citrus iyo) and yuzu (Citrus junos). Biosci. Biotechnol. Biochem. 2021, 86, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Omura, M.; Shimada, T. Citrus breeding, genetics and genomics in Japan. Breed. Sci. 2016, 66, 3–17. [Google Scholar] [CrossRef]
- Obenland, D.; Arpaia, M.L. Managing Postharvest Storage Issues in ‘Shiranui’ Mandarin. Horttechnology 2023, 33, 118–124. [Google Scholar] [CrossRef]
- Umano, K.; Hagi, Y.; Shibamoto, T. Volatile chemicals identified in extracts from newly hybrid citrus, dekopon (Shiranuhi mandarin Suppl. J.). J. Agric. Food Chem. 2002, 50, 5355–5359. [Google Scholar] [CrossRef]
- Shimizu, T.; Tanizawa, Y.; Mochizuki, T.; Nagasaki, H.; Yoshioka, T.; Toyoda, A.; Fujiyama, A.; Kaminuma, E.; Nakamura, Y. Draft Sequencing of the Heterozygous Diploid Genome of Satsuma (Citrus unshiu Marc.) Using a Hybrid Assembly Approach. Front. Genet. 2017, 8, 180. [Google Scholar] [CrossRef]
- Barboni, T.; Paolini, J.; Tomi, P.; Luro, F.; Muselli, A.; Costa, J. Characterization and Comparison of Volatile Constituents of Juice and Peel from Clementine, Mandarin and their Hybrids. Nat. Prod. Commun. 2011, 6, 1495–1498. [Google Scholar] [CrossRef]
- Jia, X.; Ren, J.; Fan, G.; Reineccius, G.A.; Li, X.; Zhang, N.; An, Q.; Wang, Q.; Pan, S. Citrus juice off-flavor during different processing and storage: Review of odorants, formation pathways, and analytical techniques. Crit. Rev. Food Sci. Nutr. 2022, 64, 3018–3043. [Google Scholar] [CrossRef]
- Miyazaki, T.; Plotto, A.; Baldwin, E.A.; Reyes-De-Corcuera, J.I.; Gmitter, F.G., Jr. Aroma characterization of tangerine hybrids by gas-chromatography-olfactometry and sensory evaluation. J. Sci. Food Agric. 2012, 92, 727–735. [Google Scholar] [CrossRef]
- Choi, H.-S. Character Impact Odorants of Citrus Hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] Cold-Pressed Peel Oil. J. Agric. Food Chem. 2003, 51, 2687–2692. [Google Scholar] [CrossRef]
- Sun, R.; Xing, R.; Zhang, J.; Wei, L.; Ge, Y.; Deng, T.; Zhang, W.; Chen, Y. Authentication and quality evaluation of not from concentrate and from concentrate orange juice by HS-SPME-GC-MS coupled with chemometrics. LWT Food Sci. Technol. 2022, 162, 113504. [Google Scholar] [CrossRef]
- Uehara, A.; Baldovini, N. Volatile constituents of yuzu (Citrus junos Sieb. ex Tanaka) peel oil: A review. Flavour Fragr. J. 2021, 36, 292–318. [Google Scholar] [CrossRef]
- B’Chir, F.; Arnaud, M.J. Chemical profile and extraction yield of essential oils from peel of Citrus limon, Citrus aurantium, and Citrus limetta: A review. In Studies in Natural Products Chemistry; Atta Ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 79, pp. 135–204. [Google Scholar] [CrossRef]
- Cheong, M.W.; Chong, Z.S.; Liu, S.Q.; Zhou, W.; Curran, P.; Bin, Y. Characterisation of calamansi (Citrus microcarpa). Part I: Volatiles, aromatic profiles and phenolic acids in the peel. Food Chem. 2012, 134, 686–695. [Google Scholar] [CrossRef]
- Miyazawa, N.; Fujita, A.; Kubota, K. Aroma character impact compounds in Kinokuni mandarin orange (Citrus kinokuni) compared with Satsuma mandarin orange (Citrus unshiu). Biosci. Biotechnol. Biochem. 2010, 74, 835–842. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, G.; Wu, H.; Liang, G.; Wang, H. Flavor deterioration of Mandarin juice during storage by MDGC-MS/O and GC-MS/PFPD. LWT Food Sci. Technol. 2022, 159, 113132. [Google Scholar] [CrossRef]
- Chisholm, M.G.; Jell, J.A.; Cass, D.M., Jr. Characterization of the major odorants found in the peel oil of Citrus reticulata Blanco cv. Clementine using gas chromatography–olfactometry. Flavour Fragr. J. 2003, 18, 275–281. [Google Scholar] [CrossRef]
- Dong, Y.; Shan, Y.; Li, P.; Jiang, L.; Liu, X. Nondestructive Characterization of Citrus Fruit by near-Infrared Diffuse Reflectance Spectroscopy (NIRDRS) with Principal Component Analysis (PCA) and Fisher Linear Discriminant Analysis (FLDA). Anal. Lett. 2022, 55, 2554–2563. [Google Scholar] [CrossRef]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of Citrus reticulata ‘Chachi’ by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef] [PubMed]
- Delahunty, C.M.; Eyres, G.; Dufour, J.-P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, A.; Chrysanthou, A.; Koutidou, M. Characterisation of volatile compounds of lupin protein isolate-enriched wheat flour bread. Food Res. Int. 2012, 48, 568–577. [Google Scholar] [CrossRef]
- Ledauphin, J.; Saint-Clair, J.F.; Lablanquie, O.; Guichard, H.; Founier, N.; Guichard, E.; Barillier, D. Identification of trace volatile compounds in freshly distilled Calvados and Cognac using preparative separations coupled with gas chromatography-mass spectrometry. J. Agric. Food Chem. 2004, 52, 5124–5134. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, A.; Collin, G.; Garneau, F.-X.; Gagnon, H.; Pichette, A. Aromas from Quebec. II. Composition of the Essential Oil of the Rhizomes and Roots of Asarum canadense L. J. Essent. Oil Res. 2010, 22, 164–169. [Google Scholar] [CrossRef]
- Choi, H.-S. Volatile constituents of satsuma mandarins growing in Korea. Flavour Fragr. J. 2004, 19, 406–412. [Google Scholar] [CrossRef]
- Mistry, B.S.; Reineccius, T.; Olson, L.K. Gas Chromatography–Olfactometry for the Determination of Key Odorants in Foods. In Techniques for Analyzing, 1st ed.; Marsili, R., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 265–292. [Google Scholar] [CrossRef]
- Tajima, K.; Tanaka, S.; Yamaguchi, T.; Fujita, M. Analysis of green and yellow yuzu peel oils (Citrus junos Tanaka). Novel aldehyde components with remarkably low odor thresholds. J. Agric. Food Chem. 1990, 38, 1544–1548. [Google Scholar] [CrossRef]





| No. | Compound | LRI I | CAS Number | Abundance (FID Peak Area) | Identification II | |||
|---|---|---|---|---|---|---|---|---|
| Iyokan | Ponkan | Shiranui | Unshiu Mikan | |||||
| 1 | Acetaldehyde 1,2 | 671 | 75-07-0 | 3,021,814 ± 113,450 b | 2,379,431 ± 66,713 c | 8,209,625 ± 229,458 a | - | LRI, MS, STD |
| 2 | Ethyl acetate 1,3,4,5 | 917 | 141-78-6 | 2,412,262 ± 223,644 a | Trace | Trace | 117,903 ± 10,373 b | LRI, MS, STD |
| 3 | Methyl butanoate 2 | 1001 | 623-42-7 | 494,504 ± 50,478 | Trace | Trace | - | LRI, MS, STD |
| 4 | α-Pinene 1,2,3,4,5,6 | 1038 | 80-56-8 | 72,767,898 ± 3,542,614 b | 84,955,972 ± 2,536,220 a | 37,673,143 ± 1,839,905 c | 7,405,861 ± 254,832 d | LRI, MS, STD |
| 5 | α-Thujene 1,3,4 | 1042 | 2867-05-2 | 33,907,447 ± 2,516,927 b | 48,208,167 ± 933,902 a | 14,676,075 ± 534,126 c | 364,519 ± 22,485 d | LRI, MS |
| 6 | Ethyl butanoate 1,2 | 1049 | 105-54-4 | 403,512 ± 41,054 | - | - | - | LRI, MS, STD |
| 7 | Fenchene 1,4 | 1077 | 471-84-1 | 251,486 ± 24,760 a | 218,369 ± 9024 ab | 235,149 ± 22,513 a | 190,388 ± 10,381 b | LRI, MS |
| 8 | Camphene 1,3,4,5 | 1082 | 79-92-5 | 1,118,843 ± 106,451 b | 1,311,309 ± 80,790 a | 253,555 ± 1996 c | 78,621 ± 6696 d | LRI, MS, STD |
| 9 | Hexanal 1,2,3,5 | 1099 | 66-25-1 | Trace | Trace | 90,117 ± 3010 | Trace | LRI, MS, STD |
| 10 | β-Pinene 1,3,4,5,6 | 1123 | 127-91-3 | 48,161,195 ± 3,394,260 a | 28,873,180 ± 869,334 b | 1,742,254 ± 75,939 c | 3,046,957 ± 164,865 c | LRI, MS, STD |
| 11 | Sabinene 1,3,4,5,6 | 1135 | 3387-41-5 | 4,186,389 ± 29,334 c | 52,753,832 ± 4,187,512 a | 19,442,711 ± 346,792 b | 487,989 ± 23,800 c | LRI, MS, STD |
| 12 | Myrcene 1,2,3,4,5,6 | 1175 | 123-35-3 | 181,746,657 ± 3,133,756 b | 223,400,905 ± 7,438,213 a | 185,541,609 ± 6,318,947 b | 35,207,496 ± 755,294 c | LRI, MS, STD |
| 13 | α-Phellandrene 1,2,3,4 | 1182 | 99-83-2 | 971,304 ± 135,772 c | 234,917 ± 28,355 d | 2,463,630 ± 62,843 a | 1,265,627 ± 40,262 b | LRI, MS, STD |
| 14 | α-Terpinene 1,2,3,4 | 1195 | 99-86-5 | 425,859 ± 20,341 b | 274,139 ± 23,765 b | 477,566 ± 2719 b | 5,378,465 ± 320,862 a | LRI, MS |
| 15 | Limonene 1,2,3,4,5,6 | 1229 | 138-86-3 | 7,794,558,186 ± 171,677,992 b | 9,003,005,342 ± 112,992,466 a | 8,066,891,387 ± 325,705,414 b | 1,135,791,679 ± 63,421,763 c | LRI, MS, STD |
| 16 | β-Phellandrene 1,3,4,5,6 | 1233 | 555-10-2 | 13,226,859 ± 1,327,534 c | 18,751,198 ± 426,585 a | 15,970,718 ± 524,327 b | 3,848,447 ± 10,554 d | LRI, MS |
| 17 | Ethyl hexanoate 1,2,3 | 1246 | 123-66-0 | 1,420,023 ± 161,014 | - | - | - | LRI, MS, STD |
| 18 | cis-β-Ocimene 1,3,4,5 | 1254 | 3338-55-4 | 1,501,939 ± 75,692 a | 1,208,799 ± 72,438 b | 674,214 ± 56,472 c | 214,089 ± 11,659 d | LRI, MS, STD |
| 19 | γ-Terpinene 1,2,3,4,5,6 | 1265 | 99-85-4 | 335,256,197 ± 27,081,641 b | 383,400,732 ± 8,802,941 a | 11,978,659 ± 292,078 d | 62,312,158 ± 604,708 c | LRI, MS, STD |
| 20 | trans-β-Ocimene 1,3,4,5 | 1267 | 3779-61-1 | 226,416,538 ± 13,752,207 a | 138,154,783 ± 17,504,308 b | 13,259,873 ± 1,075,416 c | 3,220,802 ± 250,457 c | LRI, MS, STD |
| 21 | p-Mentha-3,8-diene 1 | 1280 | 586-67-4 | 202,373 ± 8356 b | 341,690 ± 13,408 a | 167,391 ± 12,674 c | 84,575 ± 5882 d | LRI, MS |
| 22 | Hexyl acetate 1,3,4 | 1288 | 142-92-7 | 357,655 ± 25,769 a | - | - | 27,363 ± 523 b | LRI, MS, STD |
| 23 | p-Cymene 1,2,3,4,5,6 | 1294 | 99-87-6 | 34,255,941 ± 1,493,829 a | 33,859,784 ± 1,941,883 a | 2,807,393 ± 212,707 c | 14,139,510 ± 1,068,535 b | LRI, MS, STD |
| 24 | Terpinolene 1,2,3,4,5,6 | 1304 | 586-62-9 | 50,351,353 ± 2,521,776 b | 63,825,352 ± 2,750,559 a | 25,708,236 ± 1,030,117 c | 1,1878,259 ± 930,233 d | LRI, MS, STD |
| 25 | Octanal 1,2,3,4,5,6 | 1306 | 124-13-0 | - | 282,649 ± 12,185 b | 391,863 ± 29,293 a | - | LRI, MS, STD |
| 26 | Isoterpinolene 1,4 | 1310 | 586-63-0 | 1,139,956 ± 71,812 b | 1,881,552 ± 179,005 a | 975,303 ± 80,256 b | 439,599 ± 48,054 c | LRI, MS |
| 27 | cis-2-Pentenol 1,6 | 1327 | 1576-95-0 | 59,984 ± 4403 | - | - | - | LRI, MS |
| 28 | cis-3-Hexenyl acetate 1,3,5 | 1328 | 3681-71-8 | 724,286 ± 31,438 a | - | - | 54,471 ± 2973 b | LRI, MS, STD |
| 29 | 6-Methyl-5-hepten-2-one 1,3,4 | 1351 | 110-93-0 | 20,061 ± 1708 b | - | 21,736 ± 2068 b | 33,168 ± 2662 a | LRI, MS, STD |
| 30 | Hexanol 1,2,3,5,6 | 1357 | 111-27-3 | 1,141,555 ± 71,011 a | 176,255 ± 9873 c | 629,718 ± 14,528 b | 245,510 ± 30,423 c | LRI, MS, STD |
| 31 | cis-Alloocimene 1,4,5 | 1379 | 673-84-7 | 1,674,019 ± 101,035 a | 846,605 ± 47,359 b | 498,268 ± 51,697 c | 232,381 ± 4693 d | LRI, MS, STD |
| 32 | cis-3-Hexenol 1,3,5,6 | 1392 | 928-96-1 | 1,560,263 ± 109,543 a | 807,807 ± 75,632 b | 935,481 ± 11,045 b | 67,410 ± 4843 c | LRI, MS, STD |
| 33 | Methyl octanoate 1,2 | 1395 | 111-11-5 | 165,456 ± 15,096 | - | - | - | LRI, MS, STD |
| 34 | trans-Alloocimene 1 | 1402 | 14947-20-7 | 893,954 ± 40,097 a | 531,250 ± 58,378 b | 360,579 ± 34,087 c | 207,150 ± 19,239 d | LRI, MS |
| 35 | trans-2-Hexenol 1,2,3,6 | 1410 | 928-95-0 | 125,465 ± 5396 | - | - | - | LRI, MS, STD |
| 36 | p-Mentha-1,3,8-triene 1,3,4 | 1413 | 18368-95-1 | 210,801 ± 20,445 a | 195,113 ± 7165 a | 213,968 ± 20,664 a | 66,809 ± 9454 b | LRI, MS |
| 37 | Hexyl butanoate 1,2,3 | 1421 | 2639-63-6 | 1,107,410 ± 103,495 | - | - | - | LRI, MS, STD |
| 38 | Ethyl octanoate 1,2,5 | 1438 | 106-32-1 | 395,035 ± 43,480 a | - | 24,480 ± 2627 b | - | LRI, MS, STD |
| 39 | p-Mentha-1,5,8-triene 2 | 1440 | 21195-59-5 | 120,366 ± 9076 a | 110,244 ± 6032 ab | 98,940 ± 8028 b | 48,492 ± 4867 c | LRI, MS |
| 40 | p-Cymenene 3 | 1457 | 1195-32-0 | 2,948,285 ± 207,196 a | 3,097,667 ± 204,365 a | 1,338,095 ± 130,297 b | 1,631,156 ± 111,077 b | LRI, MS |
| 41 | cis-Limonene oxide 1,3,4,6 | 1468 | 13837-75-7 | Trace | 118,353 ± 12,087 a | 95,713 ± 1556 b | Trace | LRI, MS |
| 42 | cis-3-Hexenyl butanoate 1,3 | 1471 | 16491-36-4 | 437,567 ± 41,062 | - | - | - | LRI, MS, STD |
| 43 | α-Cubebene 1,2,3,5 | 1473 | 17699-14-8 | 901,160 ± 54,638 a | 127,939 ± 5198 c | 266,232 ± 8816 d | 361,791 ± 29,736 b | LRI, MS |
| 44 | trans-Limonene oxide 3,4 | 1481 | 4959-35-7 | 132,628 ± 11,778 c | 1,524,017 ± 88,197 a | 1,343,757 ± 131,036 b | 54,539 ± 2665 c | LRI, MS |
| 45 | Octyl acetate 1,3,4,5 | 1483 | 112-14-1 | 1,041,047 ± 48,326 a | 435,829 ± 12,954 b | 205,589 ± 13,573 c | - | LRI, MS, STD |
| 46 | δ-Elemene 1,3,5,6 | 1487 | 20307-84-0 | 14,570,016 ± 778,325 a | 1,377,078 ± 95,264 b | 698,139 ± 36,489 b | 1,149,042 ± 28,839 b | LRI, MS |
| 47 | Citronellal 1,3,4 | 1493 | 106-23-0 | - | 183,173 ± 9278 a | 90,924 ± 6271 b | - | LRI, MS, STD |
| 48 | α-Ylangene 3,6 | 1503 | 14912-44-8 | 618,142 ± 28,949 a | 91,961 ± 6194 b | 78,245 ± 6377 b | 82,568 ± 5267 b | LRI, MS |
| 49 | α-Copaene 1,2,3,4,5,6 | 1513 | 3856-25-5 | 3,402,465 ± 500,759 a | - | 3,387,074 ± 254,593 b | 1,380,120 ± 151,456 b | LRI, MS |
| 50 | Decanal 1,2,3,4,5,6 | 1515 | 112-31-2 | 1,251,121 ± 65,784 b | 7,168,137 ± 497,569 a | 1,236,357 ± 121,466 b | Trace | LRI, MS, STD |
| 51 | Linalool 1,2,3,4,5,6 | 1552 | 78-70-6 | 19,839,139 ± 1,716,833 a | 7,061,014 ± 611,543 b | 2,156,911 ± 237,030 c | 122,394 ± 5602 d | LRI, MS, STD |
| 52 | cis-4-Decenal 3 | 1555 | 21662-09-9 | - | 66,948 ± 2022 | - | - | LRI, MS, STD |
| 53 | β-Cubebene 1,2,3 | 1557 | 13744-15-5 | 463,836 ± 22,840 a | - | 115,995 ± 10,154 b | 103,283 ± 1237 b | LRI, MS |
| 54 | Linalyl acetate 3,4 | 1566 | 115-95-7 | 48,017 ± 3747 | - | - | - | LRI, MS, STD |
| 55 | Octanol 1,2,3,4,5,6 | 1567 | 111-87-5 | 187,148 ± 10,299 c | 2,825,984 ± 268,182 a | 1,757,499 ± 186,038 b | 55,432 ± 5639 c | LRI, MS, STD |
| 56 | trans-α-Bergamotene 3,4 | 1576 | 13474-59-4 | 173,673 ± 5351 a | 130,315 ± 6438 b | - | - | LRI, MS |
| 57 | Nonyl acetate 4 | 1584 | 143-13-5 | 135,996 ± 8557 a | 51,267 ± 1102 b | 33,249 ± 3525 c | - | LRI, MS, STD |
| 58 | Methyl decanoate 1 | 1591 | 110-42-9 | - | - | 32,015 ± 2545 | - | LRI, MS |
| 59 | β-Copaene 1,2,3,4 | 1595 | 18252-44-3 | 770,008 ± 73,148 a | - | 140,828 ± 9270 b | 125,276 ± 3185 b | LRI, MS |
| 60 | Methylthymol 3,4 | 1602 | 1076-56-8 | - | 7,416,428 ± 577,654 | - | - | LRI, MS |
| 61 | β-Elemene 1,3,4,5,6 | 1611 | 515-13-9 | 4,396,452 ± 417,605 b | - | 876,753 ± 60,868 c | 4,979,190 ± 391,615 a | LRI, MS |
| 62 | Hexyl hexanoate 1 | 1615 | 6378-65-0 | 1,351,623 ± 100,303 | - | - | - | LRI, MS, STD |
| 63 | Undecanal 3,4,5,6 | 1617 | 112-44-7 | - | 566,099 ± 27,765 a | 488,674 ± 45,944 b | - | LRI, MS, STD |
| 64 | Terpinen-4-ol 1,2,3,4,6 | 1618 | 562-74-3 | - | 418,702 ± 19,007 a | - | 83,869 ± 6973 b | LRI, MS, STD |
| 65 | β-Caryophyllene 1,3,4,5 | 1624 | 87-44-5 | 3,932,499 ± 207,687 a | 353,044 ± 35,252 d | 764,800 ± 6263 c | 2,024,648 ± 183,248 b | LRI, MS, STD |
| 66 | β-Gurjunene | 1625 | 17334-55-3 | - | - | - | 192,441 ± 17,451 | LRI, MS |
| 67 | trans-Dihydrocarvone 2,3 | 1626 | 5948-04-9 | 532,126 ± 75,746 a | 169,144 ± 5807 b | 116,528 ± 15,675 bc | 69,907 ± 4068 c | LRI, MS |
| 68 | γ-Elemene 1,3,4,5,6 | 1656 | 29873-99-2 | 4,248,781 ± 146,454 a | 1,739,293 ± 95,084 b | - | 329,240 ± 25,738 c | LRI, MS |
| 69 | cis-3-Hexenyl hexanoate 1 | 1659 | 31501-11-8 | 565,004 ± 29,440 | - | - | - | LRI, MS, STD |
| 70 | trans-2-Decenal 1,3,5 | 1661 | 3913-81-3 | - | 1,248,632 ± 123,764 | - | - | LRI, MS, STD |
| 71 | Nonanol 1,3,4,5,6 | 1662 | 143-08-8 | 41,061 ± 2908 c | 332,089 ± 29,052 b | 659,399 ± 48,236 a | Trace | LRI, MS, STD |
| 72 | Citronellyl acetate 1,3,4,5,6 | 1671 | 150-84-5 | - | 705,591 ± 20,817 b | 1,353,091 ± 106,793 a | - | LRI, MS, STD |
| 73 | trans-β-Farnesene 1,3,4,6 | 1676 | 18794-84-8 | 4,342,285 ± 443,666 a | 2,665,085 ± 54,910 b | 347,988 ± 32,701 c | 134,819 ± 10,243 c | LRI, MS |
| 74 | Decyl acetate 3,4,5,6 | 1689 | 112-17-4 | - | 763,481 ± 72,735 a | 415,566 ± 15,990 b | - | LRI, MS, STD |
| 75 | γ-Muurolene 1,3,4,6 | 1693 | 30021-74-0 | 4,273,643 ± 306,833 a | 123,575 ± 8632 b | - | 235,926 ± 19,014 b | LRI, MS |
| 76 | α-Humulene 3,4,5,6 | 1699 | 6753-98-6 | 3,448,333 ± 286,530 a | 509,886 ± 30,877 c | 770,864 ± 47,609 c | 2,265,701 ± 232,056 b | LRI, MS |
| 77 | Neral 1,3,4,5 | 1704 | 106-26-3 | 232,615 ± 15,937 a | 70,784 ± 6092 b | 37,846 ± 1303 c | Trace | LRI, MS, STD |
| 78 | α-Terpineol 1,2,3,4,5,6 | 1710 | 98-55-5 | - | 1,510,068 ± 112,107 a | 893,486 ± 62,724 c | 972,896 ± 108,044 b | LRI, MS, STD |
| 79 | Dodecanal 3,4 | 1723 | 112-54-9 | 611,380 ± 15,376 b | 4,621,407 ± 273,589 a | 4,207,331 ± 331,690 a | - | LRI, MS, STD |
| 80 | β-Selinene 1,4,5,6 | 1725 | 17066-67-0 | 1,396,586 ± 167,426 b | 141,199 ± 16,442 c | 276,085 ± 24,848 c | 1,720,951 ± 131,977 a | LRI, MS |
| 81 | Germacrene D 1,3,4,5,6 | 1739 | 23986-74-5 | 2,604,458 ± 215,011 | - | - | - | LRI, MS, STD |
| 82 | Neryl acetate 1,3,4,6 | 1741 | 141-12-8 | 3,906,687 ± 322,516 a | 1,043,472 ± 120,990 b | 1,106,805 ± 63,115 b | - | LRI, MS, STD |
| 83 | δ-Selinene 1 | 1743 | 28624-23-9 | 2,923,547 ± 225,794 a | 472,730 ± 22,823 c | 1,110,507 ± 106,279 b | 999,774 ± 107,057 b | LRI, MS |
| 84 | Valencene 1,2,3,4 | 1744 | 4630-07-3 | 946,351 ± 73,168 c | - | 18,201,327 ± 1,406,097 a | 8,511,199 ± 755,215 b | LRI, MS, STD |
| 85 | α-Muurolene 1,3,4 | 1747 | 10208-80-7 | 2,139,599 ± 87,154 a | 156,714 ± 6062 b | - | - | LRI, MS |
| 86 | Geranial 1,3,4,5,6 | 1748 | 141-27-5 | 68,557 ± 5015 b | 239,032 ± 33,071 a | Trace | 2936 ± 147 c | LRI, MS, STD |
| 87 | α-Selinene 1,5 | 1753 | 473-13-2 | 1,601,701 ± 80,894 b | - | 1,222,031 ± 102,210 c | 2,854,491 ± 214,256 a | LRI, MS |
| 88 | α-Farnesene 1,3,4,5,6 | 1761 | 502-61-4 | 3,682,771 ± 215,845 b | 2,027,817 ± 105,590 c | 18,592,435 ± 1,527,910 a | 3,379,537 ± 78,648 bc | LRI, MS, STD |
| 89 | Geranyl acetate 1,3,4,5,6 | 1765 | 105-87-3 | 3,349,148 ± 188,570 | - | - | - | LRI, MS, STD |
| 90 | Decanol 3,4 | 1767 | 112-30-1 | 2,145,521 ± 196,674 c | 4,573,607 ± 334,157 b | 6,191,909 ± 580,822 a | Trace | LRI, MS, STD |
| 91 | Citronellol 1,2,3,4,5,6 | 1768 | 106-22-9 | 296,728 ± 24,666 c | 2,431,748 ± 247,414 a | 1,798,873 ± 182,943 b | Trace | LRI, MS, STD |
| 92 | δ-Cadinene 1,3,4,6 | 1780 | 483-76-1 | 7,045,004 ± 196,134 a | 835,474 ± 44,285 d | 1,542,340 ± 139,745 c | 1,940,735 ± 128,660 b | LRI, MS |
| 93 | γ-Cadinene 1,3,6 | 1784 | 39029-41-9 | 2,394,928 ± 186,049 a | 347,242 ± 33,169 b | - | 303,859 ± 26,715 b | LRI, MS |
| 94 | β-Sesquiphellandrene 3,4 | 1789 | 20307-83-9 | 257,824 ± 13,964 a | 202,586 ± 7225 b | - | - | LRI, MS |
| 95 | cis-4-Decenol | 1799 | 57074-37-0 | - | 56,380 ± 3687 b | 61,942 ± 3662 a | - | LRI, MS |
| 96 | Perillyl aldehyde 1,3,4,5,6 | 1818 | 2111-75-3 | Trace | 1,085,479 ± 100,983 a | 495,968 ± 34,962 b | - | LRI, MS, STD |
| 97 | α-Cadinene 3 | 1826 | 24406-05-1 | 1,467,487 ± 122,452 a | - | - | 145,415 ± 13,270 b | LRI, MS |
| 98 | trans-2-Decenol 5,6 | 1830 | 18409-18-2 | 238,075 ± 15,624 a | 145,406 ± 13,141 b | 117,321 ± 11,457 c | - | LRI, MS |
| 99 | trans-trans-2,4-Decadienal 1,3,5,6 | 1833 | 25152-84-5 | 290,521 ± 30,168 b | 542,977 ± 21,873 a | 108,927 ± 9438 c | - | LRI, MS, STD |
| 100 | trans-Carveol 1,3,4,6 | 1848 | 1197-07-5 | - | 54,800 ± 3675 b | 109,216 ± 9358 a | - | LRI, MS, STD |
| 101 | Calamenene 1,3 | 1850 | 483-77-2 | 795,574 ± 73,127 a | 101,296 ± 4162 c | 207,106 ± 11,690 d | 312,129 ± 24,238 b | LRI, MS |
| 102 | Undecanol 3,4 | 1852 | 112-42-5 | 84,785 ± 3126 a | 61,928 ± 2808 b | - | - | LRI, MS |
| 103 | cis-Carveol 1,3,4,6 | 1884 | 1197-06-4 | - | 250,251 ± 25,009 a | 157,451 ± 13,436 b | - | LRI, MS, STD |
| 104 | trans-2-Dodecenal 1,3,5 | 1885 | 20407-84-5 | 142,673 ± 14,121 a | 34,987 ± 3199 b | Trace | - | LRI, MS, STD |
| 105 | Benzyl alcohol 1,3,4,6 | 1898 | 100-51-6 | 77,059 ± 3097 a | 72,336 ± 6248 a | 71,040 ± 6005 a | - | LRI, MS, STD |
| 106 | trans-cis-2,6-Dodecadienal 5 | 1911 | 21662-13-5 | 93,919 ± 1358 b | 102,340 ± 1527 a | 31,910 ± 3351 c | - | LRI, MS, STD |
| 107 | Perillyl acetate 1,3,4,5,6 | 1925 | 15111-96-3 | 362,777 ± 14,110 a | 27,093 ± 1526 b | - | - | LRI, MS |
| 108 | Tetradecanal 3,4 | 1935 | 124-25-4 | - | 201,756 ± 20,358 | - | - | LRI, MS |
| 109 | α-Calacorene 4 | 1951 | 21391-99-1 | 460,545 ± 14,248 a | 58,725 ± 5455 c | 75,623 ± 3759 b | 92,329 ± 6458 b | LRI, MS |
| 110 | p-Menth-1-en-9-ol 3,6 | 1952 | 18479-68-0 | - | 71,104 ± 7850 a | 42,944 ± 3627 b | - | LRI, MS |
| 111 | Heptanoic acid 1 | 1964 | 111-14-8 | - | - | - | 26,743 ± 1102 | LRI, MS, STD |
| 112 | Dodecanol 3 | 1972 | 112-53-8 | - | 55,051 ± 5365 b | 147,603 ± 10,680 a | - | LRI, MS, STD |
| 113 | cis-Nerolidol 3 | 2010 | 142-50-7 | Trace | Trace | - | - | LRI, MS, STD |
| 114 | Perillyl alcohol 1,2,3,4,5,6 | 2012 | 536-59-4 | 291,604 ± 28,313 a | 185,643 ± 12,165 b | 112,364 ± 4774 c | 37,207 ± 3229 d | LRI, MS, STD |
| 115 | Methyleugenol 1 | 2029 | 93-15-2 | 288,659 ± 26,281 a | - | 44,808 ± 3267 b | - | LRI, MS, STD |
| 116 | trans-Nerolidol 3,5 | 2044 | 40716-66-3 | 132,973 ± 2854 a | 44,950 ± 4797 b | - | - | LRI, MS, STD |
| 117 | Octanoic acid 1,3,4,5,6 | 2064 | 124-07-2 | Trace | 38,430 ± 2295 b | 31,011 ± 3097 c | 55,451 ± 6204 a | LRI, MS, STD |
| 118 | Elemol 1,4,5,6 | 2098 | 639-99-6 | 129,980 ± 12,453 a | - | 48,784 ± 1458 b | - | LRI, MS |
| 119 | Methyl N-methylanthranilate 3,5 | 2107 | 85-91-6 | Trace | 24,816 ± 2151 | Trace | - | LRI, MS, STD |
| 120 | Globulol 3,4 | 2108 | 489-41-8 | 55,438 ± 5066 | - | - | - | LRI, MS |
| 121 | Nonanoic acid 1,3,4,5,6 | 2171 | 112-05-0 | 34,088 ± 2430 c | 13,818 ± 325 d | 38,801 ± 1404 b | 45,093 ± 3195 a | LRI, MS, STD |
| 122 | Thymol 3 | 2177 | 89-83-8 | 52,858 ± 1832 b | 1,168,809 ± 44,593 a | 20,512 ± 1747 b | 26,514 ± 2400 b | LRI, MS, STD |
| 123 | Eugenol 3 | 2194 | 97-53-0 | 411,866 ± 17,797 | - | Trace | - | LRI, MS, STD |
| 124 | Carvacrol 3,4 | 2231 | 499-75-2 | 37,632 ± 3220 b | 95,715 ± 8702 a | 14,500 ± 1335 c | 19,723 ± 1394 c | LRI, MS, STD |
| 125 | β-Sinensal 3 | 2255 | 60066-88-8 | 355,351 ± 4653 a | 243,727 ± 12,764 b | 19,655 ± 567 c | Trace | LRI, MS, STD |
| 126 | Isospathulenol 6 | 2272 | 88395-46-4 | 52,658 ± 2436 | - | - | - | LRI, MS |
| 127 | Decanoic acid 1,3,4,5,6 | 2277 | 334-48-5 | 28,821 ± 1238 | Trace | Trace | Trace | LRI, MS, STD |
| 128 | p-Menth-8-ene-1,2-diol 3,5 | 2288 | 1946-00-5 | 279,513 ± 26,598 b | 563,909 ± 48,359 a | 536,560 ± 24,663 a | 317,897 ± 19,309 b | LRI, MS |
| 129 | α-Sinensal 3 | 2360 | 17909-77-2 | 195,811 ± 7979 b | 293,104 ± 17,654 a | Trace | Trace | LRI, MS, STD |
| 130 | Indole 1,6 | 2488 | 120-72-9 | 65,046 ± 3222 | Trace | Trace | Trace | LRI, MS, STD |
| 131 | Nootkatone 1,2,3,4 | 2580 | 4674-50-4 | - | - | 111,415 ± 8811 | - | LRI, MS, STD |
| Total peak area | 8,932,836,018 ± 176,904,541 b | 10,155,518,812 ± 121,237,952 a | 8,488,970,363 ± 326,245,173 c | 1,323,601,924 ± 65,326,925 d | ||||
| No. | Compound | LRI I | CAS Number | Iyokan | Ponkan | Shiranui | Unshiu Mikan | Identification II |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene 1,2,3,4,5,6 | 1038 | 80-56-8 | 1546.03 ± 42.22 b | 1027.75 ± 19.21 c | 2847.14 ± 43.34 a | 352.98 ± 8.61 d | LRI, MS, STD |
| 2 | α-Thujene 1,3,4 | 1042 | 2867-05-2 | 2562.59 ± 27.55 b | 897.25 ± 34.33 c | 2721.30 ± 9.81 a | 135.32 ± 3.74 d | LRI, MS |
| 3 | Camphene 1,3,4,6 | 1082 | 79-92-5 | 17.27 ± 0.39 a | 10.25 ± 0.58 b | 3.26 ± 0.29 d | 4.62 ± 0.12 c | LRI, MS, STD |
| 4 | Hexanal 1,2,3,6 | 1099 | 66-25-1 | 21.36 ± 0.63 a | 11.69 ± 0.37 c | 18.77 ± 1.76 b | 19.04 ± 0.92 b | LRI, MS, STD |
| 5 | β-Pinene 1,3,4,5,6 | 1123 | 127-91-3 | 1666.40 ± 4.81 a | 738.89 ± 30.38 b | 238.43 ± 4.22 c | 191.32 ± 7.29 d | LRI, MS, STD |
| 6 | Sabinene 1,3,4,5,6 | 1135 | 3387-41-5 | 1354.84 ± 12.66 c | 2416.02 ± 46.50 b | 12,553.31 ± 258.90 a | 77.80 ± 2.45 d | LRI, MS, STD |
| 7 | 1-Penten-3-ol 1,5 | 1165 | 616-25-1 | 16.59 ± 0.43 b | - | - | 20.11 ± 0.57 a | LRI, MS |
| 8 | Myrcene 1,2,3,4,5,6 | 1175 | 123-35-3 | 8660.65 ± 69.73 b | 4064.46 ± 45.72 c | 13,831.64 ± 102.60 a | 926.11 ± 39.92 d | LRI, MS, STD |
| 9 | α-Phellandrene 1,2,3,4 | 1182 | 99-83-2 | 15.88 ± 0.32 a | 3.88 ± 0.25 b | Trace | 15.88 ± 0.64 a | LRI, MS, STD |
| 10 | α-Terpinene 1,2,3,4 | 1195 | 99-86-5 | Trace | Trace | Trace | 72.49 ± 1.37 | LRI, MS |
| 11 | Limonene 1,2,3,4,5,6 | 1229 | 138-86-3 | 421,726.10 ± 2687.60 b | 194,306.44 ± 3782.63 c | 972,976.48 ± 8890.99 a | 44,510.21 ± 2182.40 d | LRI, MS, STD |
| 12 | β-Phellandrene 1,3,4,5,6 | 1233 | 555-10-2 | 524.16 ± 1.01 b | 355.07 ± 6.98 c | 883.25 ± 65.27 a | 182.37 ± 7.03 d | LRI, MS |
| 13 | trans-2-Hexenal 1,2,3 | 1239 | 6728-26-3 | - | - | - | 7.75 ± 0.32 | LRI, MS, STD |
| 14 | cis-β-Ocimene 1,3,4,6 | 1254 | 3338-55-4 | 89.24 ± 3.34 a | 13.33 ± 0.86 b | Trace | Trace | LRI, MS, STD |
| 15 | Pentanol 1,3 | 1259 | 71-41-0 | - | - | - | 11.17 ± 0.31 | LRI, MS, STD |
| 16 | γ-Terpinene 1,2,3,4,5,6 | 1265 | 99-85-4 | 31,682.10 ± 197.90 a | 11,476.35 ± 326.57 b | 223.70 ± 2.26 d | 2789.72 ± 83.74 c | LRI, MS, STD |
| 17 | trans-β-Ocimene 1,3,4,6 | 1267 | 3779-61-1 | Trace | 14.03 ± 0.70 b | 5368.88 ± 54.14 a | Trace | LRI, MS, STD |
| 18 | Hexyl acetate 1,3,4 | 1288 | 142-92-7 | 13.26 ± 0.34 | - | - | - | LRI, MS, STD |
| 19 | p-Cymene 1,2,3,4,5,6 | 1294 | 99-87-6 | 1219.77 ± 7.69 a | 358.64 ± 23.87 b | 32.93 ± 1.52 d | 132.26 ± 5.62 c | LRI, MS, STD |
| 20 | Terpinolene 1,2,3,4,5,6 | 1304 | 586-62-9 | 1046.39 ± 120.46 a | 1053.84 ± 22.78 a | 311.17 ± 8.69 b | Trace | LRI, MS, STD |
| 21 | Octanal 1,2,3,4,5,6 | 1306 | 124-13-0 | 29.08 ± 2.05 c | 415.92 ± 45.24 a | 350.37 ± 20.03 b | 53.78 ± 1.83 c | LRI, MS, STD |
| 22 | cis-2-Pentenol 1,5 | 1327 | 1576-95-0 | - | - | - | 19.73 ± 0.95 | LRI, MS |
| 23 | cis-3-Hexenyl acetate 1,3,6 | 1328 | 3681-71-8 | 13.18 ± 0.30 | - | - | - | LRI, MS, STD |
| 24 | Prenol 1,3,5 | 1328 | 556-82-1 | 43.75 ± 1.79 a | 14.16 ± 0.91 c | 19.95 ± 2.09 b | 22.81 ± 1.05 b | LRI, MS, STD |
| 25 | 6-Methyl-5-hepten-2-one 1,3,4 | 1351 | 110-93-0 | 8.04 ± 0.42 a | 2.67 ± 0.17 c | - | 3.26 ± 0.05 b | LRI, MS, STD |
| 26 | Hexanol 1,2,3,5,6 | 1357 | 111-27-3 | 192.86 ± 1.30 a | 26.77 ± 0.99 c | 96.13 ± 2.04 d | 69.85 ± 1.13 b | LRI, MS, STD |
| 27 | cis-Alloocimene 1,4,6 | 1379 | 673-84-7 | 23.17 ± 0.94 a | 7.15 ± 0.43 b | - | - | LRI, MS, STD |
| 28 | cis-3-Hexenol 1,3,5,6 | 1392 | 928-96-1 | 172.16 ± 3.78 b | 33.25 ± 0.73 c | 344.19 ± 2.67 a | 175.24 ± 2.04 b | LRI, MS, STD |
| 29 | Methyl octanoate 1,2 | 1395 | 111-11-5 | 2.44 ± 0.04 | - | - | - | LRI, MS, STD |
| 30 | Nonanal 1,2,3,4,5,6 | 1407 | 124-19-6 | 46.67 ± 3.46 b | 38.19 ± 3.76 b | 259.41 ± 18.56 a | 11.69 ± 0.13 c | LRI, MS, STD |
| 31 | p-Mentha-1,3,8-triene 1,3,4 | 1413 | 18368-95-1 | 18.02 ± 0.67 b | 20.45 ± 1.56 b | 23.88 ± 2.75 a | 11.74 ± 0.49 c | LRI, MS |
| 32 | Hexyl butanoate 1,2,3 | 1421 | 2639-63-6 | 19.49 ± 0.57 | - | - | - | LRI, MS, STD |
| 33 | p-Mentha-1,5,8-triene 2 | 1440 | 21195-59-5 | 14.04 ± 0.22 a | 13.95 ± 0.40 a | 7.11 ± 0.28 c | 11.86 ± 0.96 b | LRI, MS |
| 34 | p-Cymenene 3 | 1457 | 1195-32-0 | - | 22.13 ± 1.47 a | - | 21.33 ± 0.91 a | LRI, MS |
| 35 | Heptanol 1,2,3,5 | 1460 | 111-70-6 | - | - | 33.46 ± 2.78 a | 15.43 ± 0.51 b | LRI, MS, STD |
| 36 | cis-Linalool oxide 1,3 | 1464 | 5989-33-3 | Trace | 91.27 ± 9.03 | Trace | Trace | LRI, MS, STD |
| 37 | Acetic acid 1,3,4,5,6 | 1465 | 64-19-7 | - | - | 52.73 ± 4.84 b | 67.73 ± 2.85 a | LRI, MS, STD |
| 38 | cis-Limonene oxide 1,3,4,5 | 1468 | 13837-75-7 | 30.54 ± 0.50 b | 26.14 ± 1.44 c | 160.10 ± 1.23 a | 11.18 ± 0.54 d | LRI, MS |
| 39 | cis-3-Hexenyl butanoate 1,3 | 1471 | 16491-36-4 | 6.86 ± 0.37 | - | - | - | LRI, MS, STD |
| 40 | trans-Sabinene hydrate 3,4 | 1476 | 17699-16-0 | 176.89 ± 2.25 c | 693.19 ± 35.08 a | 429.44 ± 2.85 b | 199.96 ± 8.79 c | LRI, MS |
| 41 | trans-Limonene oxide 3,4 | 1481 | 4959-35-7 | 39.86 ± 1.87 d | 89.40 ± 2.13 b | 272.71 ± 2.32 a | 47.57 ± 1.56 c | LRI, MS |
| 42 | trans-Linalool oxide 1,3 | 1482 | 34995-77-2 | Trace | Trace | Trace | 44.67 ± 3.79 | LRI, MS, STD |
| 43 | Octyl acetate 1,3,4,6 | 1483 | 112-14-1 | - | 21.48 ± 1.93 b | 43.52 ± 2.07 a | - | LRI, MS, STD |
| 44 | δ-Elemene 1,3,5,6 | 1487 | 20307-84-0 | 3610.47 ± 323.05 a | 176.09 ± 3.21 b | 104.63 ± 3.73 b | 262.67 ± 9.59 b | LRI, MS |
| 45 | Citronellal 1,3,4 | 1493 | 106-23-0 | 8.23 ± 0.49 c | 85.57 ± 3.09 b | 1049.29 ± 8.39 a | - | LRI, MS, STD |
| 46 | 2-Ethylhexanol 3 | 1500 | 104-76-7 | - | Trace | - | 12.60 ± 0.77 | LRI, MS, STD |
| 47 | α-Ylangene 3,5 | 1503 | 14912-44-8 | 21.67 ± 0.27 | - | - | - | LRI, MS |
| 48 | α-Copaene 1,2,3,4,5,6 | 1513 | 3856-25-5 | 322.48 ± 12.79 b | 26.50 ± 2.04 c | 2898.51 ± 132.15 a | 103.83 ± 1.33 c | LRI, MS |
| 49 | Decanal 1,2,3,4,5,6 | 1515 | 112-31-2 | 335.47 ± 8.28 c | 583.00 ± 44.89 b | 1419.40 ± 128.08 a | 4.59 ± 0.26 d | LRI, MS, STD |
| 50 | Camphor 3,4,6 | 1532 | 76-22-2 | - | 6.35 ± 0.49 | - | - | LRI, MS, STD |
| 51 | Linalool 1,2,3,4,5,6 | 1552 | 78-70-6 | 14,830.16 ± 89.11 a | 10,414.26 ± 251.76 b | 4571.26 ± 31.33 c | 3138.83 ± 151.14 d | LRI, MS, STD |
| 52 | cis-4-Decenal 3 | 1555 | 21662-09-9 | Trace | Trace | 16.78 ± 1.77 | - | LRI, MS, STD |
| 53 | β-Cubebene 1,2,3 | 1557 | 13744-15-5 | 88.60 ± 5.80 a | - | 76.34 ± 9.02 b | 50.90 ± 0.71 c | LRI, MS |
| 54 | Linalyl acetate 3,4 | 1566 | 115-95-7 | 3.56 ± 0.21 b | 163.55 ± 4.65 a | - | Trace | LRI, MS, STD |
| 55 | Octanol 1,2,3,4,5,6 | 1567 | 111-87-5 | 134.70 ± 1.12 c | 637.51 ± 20.88 b | 764.67 ± 17.89 a | 126.74 ± 1.53 c | LRI, MS, STD |
| 56 | Isopulegol | 1568 | 89-79-2 | - | 233.34 ± 15.78 a | 8.86 ± 0.88 b | - | LRI, MS, STD |
| 57 | trans-α-Bergamotene 3,4 | 1576 | 13474-59-4 | 23.94 ± 0.80 a | 7.09 ± 0.12 b | - | - | LRI, MS |
| 58 | Nonyl acetate 4 | 1584 | 143-13-5 | 27.21 ± 0.26 a | - | - | 1.80 ± 0.18 b | LRI, MS, STD |
| 59 | β-Copaene 1,2,3,4 | 1595 | 18252-44-3 | 260.38 ± 3.28 a | 21.37 ± 0.17 b | 15.81 ± 0.45 c | 23.75 ± 1.03 b | LRI, MS |
| 60 | Methylthymol 3,4 | 1602 | 1076-56-8 | 86.03 ± 2.76 b | 245.82 ± 16.28 a | 17.46 ± 0.55 c | Trace | LRI, MS |
| 61 | β-Elemene 1,3,4,5,6 | 1611 | 515-13-9 | 1081.83 ± 6.25 a | 47.80 ± 2.62 d | 249.55 ± 1.62 c | 826.68 ± 8.86 b | LRI, MS |
| 62 | Undecanal 3,4,5,6 | 1617 | 112-44-7 | - | 25.52 ± 1.08 b | 232.09 ± 2.47 a | - | LRI, MS, STD |
| 63 | Terpinen-4-ol 1,2,3,4,5 | 1618 | 562-74-3 | 506.49 ± 11.96 a | 361.16 ± 10.69 b | 93.98 ± 2.38 d | 141.41 ± 2.38 c | LRI, MS, STD |
| 64 | β-Caryophyllene 1,3,4,6 | 1624 | 87-44-5 | 319.12 ± 17.49 a | 16.81 ± 1.46 d | 144.07 ± 0.83 b | 98.54 ± 1.39 c | LRI, MS, STD |
| 65 | Butanoic acid 1,3 | 1626 | 107-92-6 | 170.96 ± 3.10 | Trace | - | - | LRI, MS, STD |
| 66 | trans-p-Mentha-2,8-dien-1-ol 3,4,5 | 1640 | 7212-40-0 | 42.56 ± 2.56 c | 69.65 ± 4.11 b | 229.51 ± 2.49 a | 31.79 ± 0.99 d | LRI, MS |
| 67 | γ-Elemene 1,3,4,5,6 | 1656 | 29873-99-2 | 1088.56 ± 6.51 a | 171.83 ± 1.46 b | 65.52 ± 1.18 c | 59.06 ± 0.76 c | LRI, MS |
| 68 | trans-2-Decenal 1,3,6 | 1661 | 3913-81-3 | 49.56 ± 3.24 b | 27.40 ± 0.50 c | 150.85 ± 4.72 a | - | LRI, MS, STD |
| 69 | Nonanol 1,3,4,5,6 | 1662 | 143-08-8 | 48.60 ± 3.24 b | 30.49 ± 1.73 c | 148.49 ± 1.87 a | 17.84 ± 0.99 d | LRI, MS, STD |
| 70 | Alloaromadendrene 3,4 | 1671 | 25246-27-9 | - | - | 21.18 ± 0.42 a | 7.89 ± 0.31 b | LRI, MS |
| 71 | Citronellyl acetate 1,3,4,5,6 | 1671 | 150-84-5 | 50.12 ± 1.70 b | 31.31 ± 2.63 c | 503.32 ± 26.13 a | 6.00 ± 0.22 c | LRI, MS, STD |
| 72 | 2-Methylbutanoic acid 3 | 1675 | 116-53-0 | 13.22 ± 0.18 a | 6.58 ± 0.21 b | Trace | 1.53 ± 0.08 c | LRI, MS, STD |
| 73 | trans-β-Farnesene 1,3,4,5 | 1676 | 18794-84-8 | 647.95 ± 8.61 a | 210.45 ± 6.81 b | - | 2.09 ± 0.03 c | LRI, MS |
| 74 | cis-p-Mentha-2,8-dien-1-ol 3,5 | 1686 | 3886-78-0 | 29.24 ± 0.94 c | 43.98 ± 1.26 b | 219.54 ± 2.62 a | 6.31 ± 0.10 d | LRI, MS |
| 75 | Decyl acetate 3,4,5,6 | 1689 | 112-17-4 | 271.03 ± 2.20 a | 24.02 ± 0.62 c | 47.72 ± 1.43 b | 4.48 ± 0.07 d | LRI, MS, STD |
| 76 | γ-Muurolene 1,3,4,5 | 1693 | 30021-74-0 | - | - | 7.04 ± 0.58 a | 7.56 ± 0.34 a | LRI, MS |
| 77 | α-Humulene 3,4,5,6 | 1699 | 6753-98-6 | 233.95 ± 4.58 a | 15.04 ± 1.06 c | 88.20 ± 1.44 b | 91.18 ± 2.86 b | LRI, MS |
| 78 | Neral 1,3,4,6 | 1704 | 106-26-3 | - | 119.40 ± 3.18 a | 113.92 ± 1.66 b | 46.30 ± 2.14 c | LRI, MS, STD |
| 79 | α-Terpineol 1,2,3,4,5,6 | 1710 | 98-55-5 | 1418.51 ± 8.53 c | 3096.50 ± 118.53 a | 1161.46 ± 6.29 d | 1735.62 ± 78.57 b | LRI, MS, STD |
| 80 | trans-trans-2,4-Nonadienal 1,3 | 1718 | 5910-87-2 | 58.59 ± 0.54 b | 69.32 ± 4.05 b | 422.66 ± 13.79 a | 3.57 ± 0.04 c | LRI, MS |
| 81 | Dodecanal 3,4 | 1723 | 112-54-9 | 117.17 ± 1.08 b | 138.65 ± 8.09 b | 845.33 ± 27.57 a | 10.72 ± 0.11 c | LRI, MS, STD |
| 82 | Germacrene D 1,3,4,5,6 | 1739 | 23986-74-5 | 2981.07 ± 22.58 a | 130.87 ± 4.58 c | 91.65 ± 12.29 d | 256.30 ± 4.09 b | LRI, MS, STD |
| 83 | Neryl acetate 1,3,4,5 | 1741 | 141-12-8 | 585.07 ± 15.97 a | 46.35 ± 3.96 c | 519.50 ± 10.06 b | - | LRI, MS, STD |
| 84 | Valencene 1,2,3,4 | 1744 | 4630-07-3 | Trace | Trace | 3189.66 ± 94.74 a | 433.32 ± 20.27 b | LRI, MS, STD |
| 85 | cis-Carvyl acetate 1,4 | 1746 | 1205-42-1 | Trace | - | - | Trace | LRI, MS, STD |
| 86 | α-Muurolene 1,3,4 | 1747 | 10208-80-7 | 34.42 ± 2.49 a | 3.75 ± 0.12 b | - | - | LRI, MS |
| 87 | Geranial 1,3,4,5,6 | 1748 | 141-27-5 | 24.27 ± 0.75 b | 37.33 ± 2.08 a | 7.47 ± 0.73 c | 0.46 ± 0.01 d | LRI, MS, STD |
| 88 | trans-Carvyl acetate 5 | 1750 | 1134-95-8 | 118.93 ± 3.69 a | - | - | 9.92 ± 0.51 b | LRI, MS, STD |
| 89 | α-Selinene 1,6 | 1753 | 473-13-2 | - | - | 301.59 ± 15.00 a | 19.59 ± 0.74 b | LRI, MS |
| 90 | α-Farnesene 1,3,4,5,6 | 1761 | 502-61-4 | 869.01 ± 19.08 b | 220.12 ± 12.40 c | 7344.18 ± 81.10 a | 854.32 ± 15.50 b | LRI, MS, STD |
| 91 | Bicyclogermacrene 3,4,6 | 1763 | 24703-35-3 | 207.10 ± 21.72 a | 22.81 ± 1.04 b | - | - | LRI, MS |
| 92 | Carvone 1,3,4,5,6 | 1764 | 99-49-0 | 13.96 ± 0.29 b | 10.76 ± 0.59 b | 287.92 ± 14.47 a | 5.04 ± 0.03 b | LRI, MS, STD |
| 93 | Geranyl acetate 1,3,4,5,6 | 1765 | 105-87-3 | 639.92 ± 17.29 a | - | - | 8.49 ± 0.13 b | LRI, MS, STD |
| 94 | Decanol 3,4 | 1767 | 112-30-1 | 237.25 ± 4.88 b | 236.61 ± 13.02 b | 765.29 ± 18.08 a | 75.53 ± 0.51 c | LRI, MS, STD |
| 95 | Citronellol 1,2,3,4,5,6 | 1768 | 106-22-9 | 42.72 ± 2.35 c | 356.28 ± 5.81 b | 1825.12 ± 12.90 a | Trace | LRI, MS, STD |
| 96 | δ-Cadinene 1,3,4,5 | 1780 | 483-76-1 | 364.00 ± 4.92 a | 21.87 ± 1.71 d | 185.70 ± 15.41 b | 95.85 ± 2.95 c | LRI, MS |
| 97 | trans-cis-2,4-Decadienal 1,5,6 | 1784 | 25152-83-4 | 21.02 ± 0.75 a | 8.10 ± 0.60 b | 7.59 ± 0.64 b | - | LRI, MS |
| 98 | β-Sesquiphellandrene 3,4 | 1789 | 20307-83-9 | 100.14 ± 2.57 a | 28.71 ± 1.52 b | - | - | LRI, MS |
| 99 | Nerol 1,3,4,5 | 1807 | 106-25-2 | 127.81 ± 3.15 a | 77.23 ± 3.80 c | 106.79 ± 5.99 b | 52.84 ± 1.88 d | LRI, MS, STD |
| 100 | Perillyl aldehyde 1,3,4,5,6 | 1818 | 2111-75-3 | Trace | 132.64 ± 1.59 b | 245.25 ± 24.98 a | 47.38 ± 0.22 c | LRI, MS, STD |
| 101 | Hexyl octanoate 3 | 1820 | 1117-55-1 | 241.35 ± 5.11 | - | - | - | LRI, MS |
| 102 | trans-2-Decenol 5,6 | 1830 | 18409-18-2 | 43.72 ± 0.72 b | 20.23 ± 1.45 c | 68.05 ± 5.17 a | - | LRI, MS |
| 103 | trans-trans-2,4-Decadienal 1,3,5,6 | 1833 | 25152-84-5 | 41.99 ± 3.76 b | 28.21 ± 1.10 c | 83.79 ± 1.02 a | 0.96 ± 0.04 d | LRI, MS, STD |
| 104 | trans-Carveol 1,3,4,5 | 1848 | 1197-07-5 | Trace | 101.32 ± 1.49 b | 256.05 ± 4.79 a | 81.54 ± 3.18 c | LRI, MS, STD |
| 105 | Undecanol 3,4 | 1852 | 112-42-5 | 10.68 ± 0.85 b | - | 26.66 ± 1.37 a | - | LRI, MS |
| 106 | Hexanoic acid 1,3,4,5 | 1855 | 142-62-1 | 1292.19 ± 11.72 a | Trace | 5.26 ± 0.42 b | 4.79 ± 0.07 b | LRI, MS, STD |
| 107 | Geraniol 1,2,3,4,5 | 1858 | 106-24-1 | 88.38 ± 5.03 a | 36.98 ± 0.90 b | 23.06 ± 1.87 c | 10.09 ± 0.20 d | LRI, MS, STD |
| 108 | Germacrene B 3,4,5,6 | 1863 | 15423-57-1 | 106.39 ± 3.04 | - | - | - | LRI, MS |
| 109 | p-Cymen-8-ol 3,4 | 1867 | 1197-01-9 | - | 26.07 ± 0.82 a | 14.24 ± 1.21 b | 10.87 ± 0.34 c | LRI, MS |
| 110 | Geranyl acetone 1,5 | 1871 | 3796-70-1 | 21.53 ± 1.81 a | 2.44 ± 0.10 b | 3.94 ± 0.18 b | - | LRI, MS, STD |
| 111 | Isopiperitenone 3,5,6 | 1877 | 529-01-1 | 4.72 ± 0.18 c | 136.22 ± 2.10 a | 124.74 ± 1.09 b | 2.66 ± 0.09 c | LRI, MS |
| 112 | cis-Carveol 1,3,4,5 | 1884 | 1197-06-4 | 28.87 ± 1.89 b | 32.73 ± 0.78 b | 127.55 ± 8.54 a | 10.65 ± 0.26 c | LRI, MS, STD |
| 113 | trans-2-Dodecenal 1,3,6 | 1885 | 20407-84-5 | 17.22 ± 0.88 a | 12.32 ± 0.36 b | - | - | LRI, MS, STD |
| 114 | Lauryl acetate 5 | 1893 | 112-66-3 | 19.39 ± 0.54 a | 2.35 ± 0.06 c | 3.03 ± 0.29 b | 1.59 ± 0.02 d | LRI, MS |
| 115 | Benzyl alcohol 1,3,4,5 | 1898 | 100-51-6 | 12.94 ± 0.29 a | 4.35 ± 0.14 b | 12.54 ± 0.94 a | 5.13 ± 0.75 b | LRI, MS, STD |
| 116 | trans-cis-2,6-Dodecadienal 6 | 1911 | 21662-13-5 | 19.21 ± 0.12 b | 5.28 ± 0.44 c | 30.54 ± 0.07 a | - | LRI, MS, STD |
| 117 | Perillyl acetate 1,3,4,5,6 | 1925 | 15111-96-3 | 105.69 ± 0.85 a | - | 6.53 ± 0.50 b | 5.89 ± 0.32 b | LRI, MS |
| 118 | Tetradecanal 3,4 | 1935 | 124-25-4 | 13.14 ± 0.32 c | 33.67 ± 0.55 b | 38.95 ± 1.17 a | 1.41 ± 0.08 d | LRI, MS |
| 119 | p-Menth-1-en-9-ol 3,5 | 1952 | 18479-68-0 | 27.65 ± 1.29 c | 35.15 ± 0.74 b | 53.80 ± 4.26 a | 19.47 ± 0.67 d | LRI, MS |
| 120 | Heptanoic acid 1 | 1960 | 111-14-8 | 13.48 ± 0.60 a | 1.96 ± 0.18 d | 6.19 ± 0.06 b | 4.72 ± 0.15 c | LRI, MS, STD |
| 121 | Cubebol 3 | 1964 | 23445-02-5 | 37.79 ± 0.40 b | - | 52.90 ± 0.53 a | 8.62 ± 0.14 c | LRI, MS |
| 122 | β-Ionone 1,2,3 | 1965 | 14901-07-6 | 9.29 ± 0.11 a | 2.46 ± 0.14 b | 1.64 ± 0.01 c | 0.91 ± 0.03 d | LRI, MS, STD |
| 123 | Dodecanol 3 | 1972 | 112-53-8 | 46.43 ± 0.57 b | 41.85 ± 2.33 c | 63.29 ± 0.47 a | 11.83 ± 0.40 d | LRI, MS, STD |
| 124 | Caryophyllene oxide 1,4 | 2000 | 1139-30-6 | - | Trace | 8.52 ± 0.68 | Trace | LRI, MS, STD |
| 125 | trans-trans-2,4-Decadienol 5 | 2005 | 18409-21-7 | 81.89 ± 3.48 b | 16.29 ± 0.76 c | 124.09 ± 5.34 a | 3.02 ± 0.22 d | LRI, MS |
| 126 | cis-Nerolidol 3 | 2010 | 142-50-7 | 9.57 ± 0.07 a | 3.22 ± 0.06 c | 6.90 ± 0.06 b | Trace | LRI, MS, STD |
| 127 | Perillyl alcohol 1,2,3,4,5,6 | 2012 | 536-59-4 | 239.36 ± 1.75 a | 115.90 ± 2.03 c | 172.59 ± 1.43 b | 113.32 ± 3.94 c | LRI, MS, STD |
| 128 | Methyleugenol 1 | 2029 | 93-15-2 | 42.23 ± 2.02 | - | - | - | LRI, MS, STD |
| 129 | trans-Nerolidol 3,6 | 2044 | 40716-66-3 | 233.52 ± 1.06 a | 25.13 ± 1.04 c | 130.59 ± 8.90 b | Trace | LRI, MS, STD |
| 130 | Octanoic acid 1,3,4,5,6 | 2064 | 124-07-2 | 839.13 ± 5.04 a | 125.11 ± 4.79 c | 180.19 ± 4.60 b | 34.44 ± 1.62 d | LRI, MS, STD |
| 131 | Germacrene D-4-ol 3,4,5 | 2076 | 198,991-79-6 | 74.18 ± 5.06 b | 10.44 ± 0.92 c | 89.44 ± 1.48 a | 14.71 ± 0.21 c | LRI, MS |
| 132 | Elemol 1,4,5,6 | 2098 | 639-99-6 | 373.36 ± 29.69 a | 34.47 ± 2.25 c | 167.76 ± 2.48 b | 42.68 ± 1.29 c | LRI, MS |
| 133 | Methyl N-methylanthranilate 3,6 | 2107 | 85-91-6 | 8.27 ± 0.60 a | 0.56 ± 0.02 b | 8.10 ± 0.25 a | - | LRI, MS, STD |
| 134 | Globulol 3,4 | 2108 | 489-41-8 | 14.32 ± 0.36 a | - | 13.29 ± 0.89 b | 8.06 ± 0.17 c | LRI, MS |
| 135 | Cumin alcohol 3 | 2117 | 536-60-7 | 9.61 ± 0.68 a | 4.36 ± 0.35 b | Trace | 2.34 ± 0.22 c | LRI, MS |
| 136 | Hexadecanal 5 | 2148 | 629-80-1 | 89.14 ± 1.10 a | 68.61 ± 1.97 b | 33.67 ± 0.82 c | 8.36 ± 0.18 d | LRI, MS |
| 137 | Nonanoic acid 1,3,4,5,6 | 2171 | 112-05-0 | 19.75 ± 1.03 b | 22.01 ± 2.16 b | 69.78 ± 1.75 a | 15.75 ± 0.61 c | LRI, MS, STD |
| 138 | Thymol 3 | 2177 | 89-83-8 | 13.83 ± 1.11 b | 260.88 ± 2.35 a | 7.48 ± 0.27 c | 8.05 ± 0.16 c | LRI, MS, STD |
| 139 | Eugenol 3 | 2194 | 97-53-0 | 70.78 ± 2.75 a | 0.94 ± 0.01 b | Trace | - | LRI, MS, STD |
| 140 | Viridiflorol 3,4 | 2220 | 552-02-3 | - | - | Trace | 1.32 ± 0.02 | LRI, MS |
| 141 | Methyl palmitate 1,3,5 | 2226 | 112-39-0 | 10.09 ± 0.20 b | 43.66 ± 0.88 a | - | 6.56 ± 0.05 c | LRI, MS |
| 142 | Carvacrol 3,4 | 2231 | 499-75-2 | 19.63 ± 0.14 c | 52.40 ± 1.05 a | 21.20 ± 0.42 b | Trace | LRI, MS, STD |
| 143 | 4-Vinylguaiacol 1,4 | 2236 | 7786-61-0 | 438.96 ± 4.15 b | 2041.59 ± 9.36 a | 383.24 ± 1.96 c | 200.05 ± 5.91 d | LRI, MS, STD |
| 144 | Citronellic acid | 2254 | 502-47-6 | 14.12 ± 0.14 c | 36.58 ± 0.85 b | 91.26 ± 1.87 a | 10.86 ± 0.46 d | LRI, MS |
| 145 | β-Sinensal 3 | 2255 | 60066-88-8 | 559.29 ± 5.18 a | 203.25 ± 16.71 b | Trace | Trace | LRI, MS, STD |
| 146 | Isospathulenol 5 | 2272 | 88395-46-4 | 25.12 ± 1.90 | - | Trace | - | LRI, MS |
| 147 | 2,3-Dihydrofarnesol | 2275 | 51411-24-6 | 21.38 ± 1.54 b | Trace | 76.48 ± 6.84 a | 9.17 ± 0.58 c | LRI, MS |
| 148 | Decanoic acid 1,3,4,5,6 | 2277 | 334-48-5 | 74.83 ± 5.39 c | 108.21 ± 0.86 b | 635.75 ± 5.00 a | 17.43 ± 0.65 d | LRI, MS, STD |
| 149 | p-Menth-8-ene-1,2-diol 3,6 | 2288 | 1946-00-5 | 64.54 ± 3.59 a | 16.48 ± 1.51 c | 40.69 ± 1.12 b | 8.71 ± 0.32 d | LRI, MS |
| 150 | trans-trans-Farnesol 1 | 2289 | 502-67-0 | 30.59 ± 0.86 a | 1.55 ± 0.12 d | 21.47 ± 0.64 b | 8.07 ± 0.27 c | LRI, MS |
| 151 | trans-8-Hydroxylinalool 3 | 2311 | 75991-61-6 | 1770.52 ± 86.59 a | 238.71 ± 6.94 c | 500.81 ± 13.63 b | 421.04 ± 19.21 b | LRI, MS |
| 152 | cis-trans-Farnesol 3 | 2333 | 3790-71-4 | 295.09 ± 14.43 a | 32.70 ± 0.95 d | 51.63 ± 1.82 c | 70.17 ± 3.20 b | LRI, MS, STD |
| 153 | α-Sinensal 3 | 2360 | 17909-77-2 | 825.85 ± 7.70 a | 520.53 ± 7.31 b | 21.51 ± 0.90 c | 0.70 ± 0.02 d | LRI, MS, STD |
| 154 | trans-trans-Farnesol 5 | 2380 | 106-28-5 | 481.36 ± 18.88 a | 32.53 ± 0.46 c | 402.77 ± 19.07 b | 1.59 ± 0.10 d | LRI, MS, STD |
| 155 | Isoeugenol 3,5 | 2385 | 97-54-1 | 99.50 ± 3.54 a | 10.32 ± 0.37 b | 7.53 ± 0.51 b | 9.38 ± 0.10 b | LRI, MS, STD |
| 156 | Isoelemicin | 2416 | 5273-85-8 | 1.83 ± 0.17 b | 1.85 ± 0.10 b | 1.86 ± 0.07 b | 2.48 ± 0.06 a | LRI, MS |
| 157 | Indole 1,5 | 2488 | 120-72-9 | 174.90 ± 0.68 a | 17.79 ± 0.87 b | - | Trace | LRI, MS, STD |
| 158 | Lauric acid 1,5 | 2489 | 143-07-7 | 175.75 ± 2.00 c | 329.07 ± 16.07 a | 241.77 ± 2.18 b | 28.93 ± 0.47 d | LRI, MS, STD |
| 159 | Nootkatone 1,2,3,4 | 2580 | 4674-50-4 | - | - | 671.70 ± 16.03 a | 34.55 ± 0.87 b | LRI, MS, STD |
| 160 | Vanillin 3,5 | 2591 | 121-33-5 | 43.56 ± 3.62 a | 16.29 ± 0.94 c | 36.53 ± 0.52 b | 11.42 ± 0.87 d | LRI, MS, STD |
| 161 | Perillic acid | 2594 | 7694-45-3 | 324.83 ± 9.08 c | 446.45 ± 41.12 b | 617.22 ± 27.09 a | 68.24 ± 5.91 d | LRI, MS |
| 162 | 3-Oxo-α-ionol | 2673 | 34318-21-3 | 31.83 ± 1.49 a | 23.51 ± 0.46 b | 7.80 ± 0.75 d | 10.31 ± 0.40 c | LRI, MS |
| 163 | Myristic acid 1,3,5,6 | 2708 | 544-63-8 | 132.07 ± 3.05 a | 52.98 ± 4.25 c | 118.09 ± 2.31 b | 35.50 ± 0.93 d | LRI, MS, STD |
| 164 | Palmitic acid 1,3,4,5,6 | 2921 | 57-10-3 | 1169.99 ± 6.73 a | 318.77 ± 29.03 c | 552.40 ± 14.57 b | 540.39 ± 14.60 b | LRI, MS, STD |
| Total concentration | 516,373.36 ± 3164.30 b | 242,460.52 ± 3667.19 c | 1,051,185.35 ± 8702.54 a | 60,849.01 ± 2289.68 d |
| No. | Compound | LRI a | Ref. LRI b | Odour Quality c | Flavour Dilution Factor d | |||
|---|---|---|---|---|---|---|---|---|
| Iyokan | Ponkan | Shiranui | Unshiu Mikan | |||||
| 1 | α-Pinene | 1045 | 1028 I | piney, green, fresh | 125 | 125 | 625 | 5 |
| 2 | α-Thujene | 1062 | 1028 I | woody, green, fresh | 5 | 125 | 625 | 5 |
| 3 | Camphene | 1088 | 1071 I | woody, herbal, terpenic | - | - | 625 | 5 |
| 4 | Unknown | 1095 | - | creamy, sweet, cooked | 1 | - | - | - |
| 5 | Hexanal | 1105 | 1083 I | fresh, green, fatty | 25 | 25 | 1 | 1 |
| 6 | β-Pinene | 1121 | 1112 I | woody, pine, green | 25 | 25 | 5 | 125 |
| 7 | Myrcene | 1180 | 1161 I | peppery, terpenic | 1 | 625 | 3125 | 125 |
| 8 | Unknown | 1189 | - | floral, aldehydic, waxy | 1 | - | 1 | 625 |
| 9 | Limonene | 1218 | 1200 I | citrusy, fresh, sweet | 125 | 125 | 3125 | 625 |
| 10 | β-Phellandrene | 1230 | 1211 I | minty, terpenic | 1 | 625 | 125 | - |
| 11 | cis-β-Ocimene | 1249 | 1235 I | herbal, floral | 1 | - | - | - |
| 12 | γ-Terpinene | 1259 | 1246 I | oily, woody, citrusy | 25 | 125 | - | 1 |
| 13 | trans-β-Ocimene | 1266 | 1250 I | citrusy, green, woody | - | - | 125 | - |
| 14 | Unknown | 1279 | - | indole, animalic, phenolic | 1 | - | - | - |
| 15 | p-Cymene | 1284 | 1272 I | woody, fresh, citrusy | - | 1 | - | 1 |
| 16 | Terpinolene | 1296 | 1283 I | fresh, sweet, fruity | - | 1 | - | 1 |
| 17 | Octanal | 1303 | 1289 I | green, waxy, citrusy | 1 | 5 | 125 | 1 |
| 18 | Unknown | 1316 | - | fatty, metallic | - | 1 | - | - |
| 19 | Unknown | 1332 | - | sweet, floral | 1 | - | - | - |
| 20 | Prenol | 1337 | 1320 I | fruity, green, floral | 25 | - | - | 25 |
| 21 | Unknown | 1343 | - | juicy, sweet, terpenic | - | 625 | - | - |
| 22 | Unknown | 1352 | - | floral | - | 5 | - | - |
| 23 | Unknown | 1355 | - | buttery, creamy | 1 | - | - | - |
| 24 | Hexanol | 1369 | 1355 I | fruity, sweet, green | 25 | 5 | 5 | 125 |
| 25 | cis-3-Hexenol | 1394 | 1382 I | fresh, green, herbal | 25 | 1 | 25 | 5 |
| 26 | Unknown | 1390 | - | sulphury, tropical | - | 25 | - | - |
| 27 | Nonanal | 1404 | 1391 I | fresh, floral, citrusy | 5 | 5 | 125 | 625 |
| 28 | Unknown | 1412 | - | albedo, floral, green | 1 | - | - | - |
| 29 | cis-Linalool oxide | 1452 | 1444 I | floral, woody, sweet | - | 5 | - | - |
| 30 | cis-Limonene oxide | 1458 | 1452 I | fresh, citrusy | 5 | 5 | 25 | 5 |
| 31 | trans-Limonene oxide | 1465 | 1462 I | fresh, citrusy | - | 5 | 125 | 1 |
| 32 | Unknown | 1470 | - | cooked, fermented, earthy | 5 | - | 125 | 5 |
| 33 | Acetic acid | 1478 | 1449 I | sharp, pungent, sour | 1 | 5 | - | - |
| 34 | Citronellal | 1490 | 1478 I | sweet, herbal, waxy | - | 25 | 5 | - |
| 35 | α-Copaene | 1499 | 1492 I | woody, spicy | - | 1 | 5 | - |
| 36 | Decanal | 1508 | 1498 I | floral, waxy, citrusy | 1 | 125 | 625 | 125 |
| 37 | 2-Ethylhexanol | 1527 | 1491 I | citrusy, fresh, sweet | - | 5 | - | 1 |
| 38 | β-Cubebene | 1542 | 1545 I | citrusy, fruity, radish | 25 | - | 5 | 5 |
| 39 | cis-4-Decenal | 1550 | 1544 I | citrusy, aldehydic, cardamom | 25 | - | 125 | - |
| 40 | Linalool | 1567 | 1547 I | citrusy, floral, woody | 125 | 125 | 25 | 3125 |
| 41 | Octanol | 1572 | 1557 I | green, citrusy, waxy | 5 | 5 | 125 | - |
| 42 | Unknown | 1575 | - | terpenic | - | 5 | - | - |
| 43 | β-Elemene | 1595 | 1591 I | sweet, herbal, fresh | - | 5 | 125 | - |
| 44 | Methylthymol | 1603 | 1590 I | woody, smoky, burnt | - | 5 | - | 5 |
| 45 | Terpinen-4-ol | 1614 | 1602 I | woody, peppery, sweet | - | 5 | 1 | 125 |
| 46 | Unknown | 1625 | - | sulphury, grapefruit, woody | - | - | 1 | - |
| 47 | Undecanal | 1628 | 1604 I | waxy, soapy, floral | - | - | 125 | - |
| 48 | Unknown | 1636 | - | woody, earthy | 1 | - | - | - |
| 49 | Unknown | 1650 | - | floral | - | 25 | - | - |
| 50 | trans-2-Decenal | 1657 | 1644 I | waxy, fatty, cilantro | - | 5 | 5 | - |
| 51 | Butanoic acid | 1660 | 1625 I | sharp, acetic, cheese | 5 | 5 | - | - |
| 52 | Nonanol | 1669 | 1660 I | fatty, floral, citrusy | - | - | 1 | 5 |
| 53 | trans-β-Farnesene | 1680 | 1664 I | woody, citrusy, sweet | - | 5 | - | - |
| 54 | Unknown | 1695 | - | juicy, sweet, vanilla | - | 5 | - | 5 |
| 55 | 2-Methylbutanoic acid | 1702 | 1662 I | acidic, fruity, cheesy | 5 | - | - | - |
| 56 | α-Terpineol | 1713 | 1697 I | citrusy, woody, floral | 5 | 3125 | 5 | 125 |
| 57 | trans-trans-2,4-Nonadienal | 1714 | 1700 I | fatty, green, floral | 1 | - | 5 | 1 |
| 58 | Germacrene D | 1720 | 1710 I | woody, spicy | 5 | - | 5 | 5 |
| 59 | Dodecanal | 1728 | 1711 I | waxy, citrusy, floral | 5 | 25 | 5 | - |
| 60 | Valencene | 1730 | 1730 I | sweet, fresh, oily | - | 1 | - | - |
| 61 | Neryl acetate | 1736 | 1724 I | floral, soapy, citrusy | 5 | - | - | 5 |
| 62 | cis-Carvyl acetate | 1746 | 1731 I | green, herbaceous | 1 | - | - | 1 |
| 63 | Carvone | 1751 | 1740 I | minty, spicy, caraway | 1 | 1 | 5 | - |
| 64 | α-Farnesene | 1763 | 1746 I | citrusy, floral, green | 5 | 25 | 5 | 1 |
| 65 | Unknown | 1767 | - | green, spicy, mango | - | 125 | - | - |
| 66 | Geranyl acetate | 1772 | 1752 I | floral, green | 1 | - | - | - |
| 67 | Decanol | 1781 | 1760 I | fatty, waxy, citrusy | - | - | 5 | 1 |
| 68 | Citronellol | 1787 | 1765 I | floral, waxy, citrusy | 1 | 125 | 25 | 1 |
| 69 | Perillyl aldehyde | 1790 | 1793 I | fresh, green, cirtusy | - | 625 | 25 | - |
| 70 | Nerol | 1815 | 1797 I | sweet, floral, citrusy | - | - | - | 5 |
| 71 | trans-trans-2,4-Decadienal | 1825 | 1811 I | aldehydic, citrusy | 1 | - | 1 | 1 |
| 72 | Unknown | 1838 | - | nutty, beany | - | - | 5 | - |
| 73 | trans-Carveol | 1846 | 1845 I | caraway, green, floral | - | 625 | 5 | - |
| 74 | Geraniol | 1862 | 1847 I | floral, waxy, citrusy | 25 | 5 | 1 | - |
| 75 | trans-2-Dodecenal | 1867 | 1867 I | metallic, mandarin, waxy | - | 625 | 1 | - |
| 76 | Hexanoic acid | 1877 | 1846 I | fatty, fruity | 125 | 5 | 25 | - |
| 77 | cis-Carveol | 1882 | 1861 I | caraway, green, herbal | 125 | 5 | 25 | 125 |
| 78 | Benzyl alcohol | 1895 | 1870 I | floral, phenolic | - | 1 | 1 | 5 |
| 79 | Lauryl acetate | 1898 | 1892 I | sweet, fresh, waxy | - | 1 | 1 | 5 |
| 80 | trans-cis-2,6-Dodecadienal | 1906 | 1894 I | waxy, green, mandarin | - | 5 | 3125 | - |
| 81 | Perillyl acetate | 1918 | 1902 I | spicy, phenolic, fruity | 5 | - | - | 5 |
| 82 | p-Menth-1-en-9-ol | 1940 | 1933 I | fruity, herbal | 1 | 5 | - | 5 |
| 83 | Unknown | 1970 | - | pith, sweet, floral | 1 | - | - | - |
| 84 | Dodecanol | 1979 | 1966 I | earthy, soapy, waxy | 5 | 25 | 5 | - |
| 85 | Unknown | 1992 | - | fresh, juicy, floral | 5 | - | 1 | 625 |
| 86 | trans-trans-2,4-Decadienol | 2002 | 1994 II | fatty, waxy, fruity | - | 1 | 5 | 5 |
| 87 | Perillyl alcohol | 2044 | 2016 I | green, spicy, floral | 5 | 3125 | 25 | 5 |
| 88 | trans-Nerolidol | 2053 | 2042 I | sweet, floral | 25 | 25 | - | 5 |
| 89 | Germacrene D-4-ol | 2055 | 2069 I | citrusy, sweet | - | 5 | 1 | - |
| 90 | Unknown | 2067 | - | floral, citrusy, albedo | 25 | - | 25 | - |
| 91 | Unknown | 2078 | - | sulphury, spicy, grapefruit | 25 | 25 | 5 | - |
| 92 | Methyl N-methylanthranilate | 2091 | 2077 I | sweet, musty, phenolic | 1 | 5 | 1 | - |
| 93 | Octanoic acid | 2093 | 2060 I | fatty, waxy, cheesy | - | 25 | 25 | - |
| 94 | Elemol | 2096 | 2080 I | woody, spicy, floral | 5 | 5 | - | 5 |
| 95 | Unknown | 2104 | - | sweet, juicy, floral | 1 | - | 125 | - |
| 96 | Cumin alcohol | 2116 | 2113 I | cumin, spicy, leathery | - | 25 | - | 1 |
| 97 | Unknown | 2126 | - | green, juicy, sweet | 5 | 5 | 25 | 5 |
| 98 | Hexadecanal | 2158 | 2135 I | woody | 25 | - | - | - |
| 99 | Unknown | 2160 | - | sweet, mandarin, juicy | - | 5 | - | 5 |
| 100 | Unknown | 2191 | - | spicy, peely | - | 25 | - | - |
| 101 | Thymol | 2213 | 2189 I | herbal, spicy, phenolic | 5 | 25 | - | - |
| 102 | 4-Vinylguaiacol | 2221 | 2188 I | spicy, clove, smoky | - | 5 | - | 5 |
| 103 | Carvacrol | 2242 | 2236 I | spicy, woody, smoky | 5 | 5 | 125 | - |
| 104 | β-Sinensal | 2244 | 2238 I | fresh, citrusy, waxy | 5 | 625 | - | - |
| 105 | Unknown | 2252 | - | meaty, sulphury | 1 | - | - | - |
| 106 | Isospathulenol | 2267 | 2227 I | woody | 125 | - | 5 | - |
| 107 | Unknown | 2276 | - | vanilla, spicy, phenolic | - | 25 | - | - |
| 108 | 2,3-Dihydrofarnesol | 2283 | 2262 III | floral, fruity | 625 | 5 | 125 | 25 |
| 109 | Decanoic acid | 2294 | 2276 I | sour, fatty, citrusy | - | 25 | 1 | - |
| 110 | Unknown | 2307 | - | sweet, phenolic, spicy | - | 3125 | - | - |
| 111 | trans-8-Hydroxylinalool | 2334 | 2284 I | citrusy, lemon, alcoholic | 1 | 5 | 1 | 25 |
| 112 | α-Sinensal | 2345 | 2304 I | citrusy, powdery, sour | 1 | 125 | - | 25 |
| 113 | Isoeugenol | 2358 | 2318 I | spicy, woody, floral | - | 625 | 3125 | 1 |
| 114 | trans-trans-Farnesol | 2374 | 2356 I | woody, floral, green | - | - | 3125 | - |
| 115 | Isoelemicin | 2387 | 2389 IV | spicy, floral | 5 | 1 | - | - |
| 116 | Indole | 2460 | 2445 I | animalic, floral | 1 | 1 | - | - |
| 117 | Unknown | 2472 | - | green, floral, peely | 1 | - | 5 | - |
| 118 | Unknown | 2489 | - | sweet, woody, powdery | 1 | 125 | 5 | - |
| 119 | Unknown | 2529 | - | peely, earthy, herbal | - | 1 | - | - |
| 120 | Nootkatone | 2548 | 2530 I | grapefruit, peely, floral | - | - | 5 | 5 |
| 121 | Unknown | 2551 | - | woody, earthy, green | 5 | 1 | - | - |
| 122 | Vanillin | 2581 | 2568 I | sweet, vanilla, phenolic | 25 | - | 25 | 125 |
| 123 | Unknown | 2606 | - | sweet, coumaric | - | 25 | - | - |
| 124 | Unknown | 2612 | - | green, sour | - | 25 | - | - |
| 125 | Perillic acid | 2649 | 2640 I | floral, sweet | 1 | 125 | - | 625 |
| 126 | 3-Oxo-α-ionol | 2667 | 2639 I | spicy | 1 | - | - | - |
| 127 | Unknown | 2722 | - | spicy, woody, clove | - | 25 | - | 1 |
| 128 | Unknown | 2737 | - | phenolic, spicy, vanilla | - | 125 | 5 | 5 |
| 129 | Unknown | 2824 | - | woody, spicy, phenolic | 625 | - | 5 | 5 |
| 130 | Unknown | 2901 | - | spicy, clove, phenolic | - | 625 | 5 | 5 |
| 131 | Unknown | 2922 | - | green, woody, sweet | 25 | 3125 | - | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Goh, R.M.V.; Huang, Y.; Ee, K.-H.; Pua, A.; Tan, D.; Zhang, S.; Jublot, L.; Liu, S.Q.; Yu, B. Characterisation of the Volatile Compounds and Key Odourants in Japanese Mandarins by Gas Chromatography–Mass Spectrometry and Gas Chromatography–Olfactometry. Separations 2024, 11, 237. https://doi.org/10.3390/separations11080237
Li L, Goh RMV, Huang Y, Ee K-H, Pua A, Tan D, Zhang S, Jublot L, Liu SQ, Yu B. Characterisation of the Volatile Compounds and Key Odourants in Japanese Mandarins by Gas Chromatography–Mass Spectrometry and Gas Chromatography–Olfactometry. Separations. 2024; 11(8):237. https://doi.org/10.3390/separations11080237
Chicago/Turabian StyleLi, Lingyi, Rui Min Vivian Goh, Yunle Huang, Kim-Huey Ee, Aileen Pua, Daphne Tan, Shanbo Zhang, Lionel Jublot, Shao Quan Liu, and Bin Yu. 2024. "Characterisation of the Volatile Compounds and Key Odourants in Japanese Mandarins by Gas Chromatography–Mass Spectrometry and Gas Chromatography–Olfactometry" Separations 11, no. 8: 237. https://doi.org/10.3390/separations11080237
APA StyleLi, L., Goh, R. M. V., Huang, Y., Ee, K.-H., Pua, A., Tan, D., Zhang, S., Jublot, L., Liu, S. Q., & Yu, B. (2024). Characterisation of the Volatile Compounds and Key Odourants in Japanese Mandarins by Gas Chromatography–Mass Spectrometry and Gas Chromatography–Olfactometry. Separations, 11(8), 237. https://doi.org/10.3390/separations11080237





