Polyphenols Extraction from Different Grape Pomaces Using Natural Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Grape Pomace
2.3. DES Preparation
2.4. Solid-Liquid Extraction
2.5. Total Phenolic Content
2.6. HPLC/DAD/TOF
2.7. Statistics
3. Results
3.1. Pomace Dry Weight
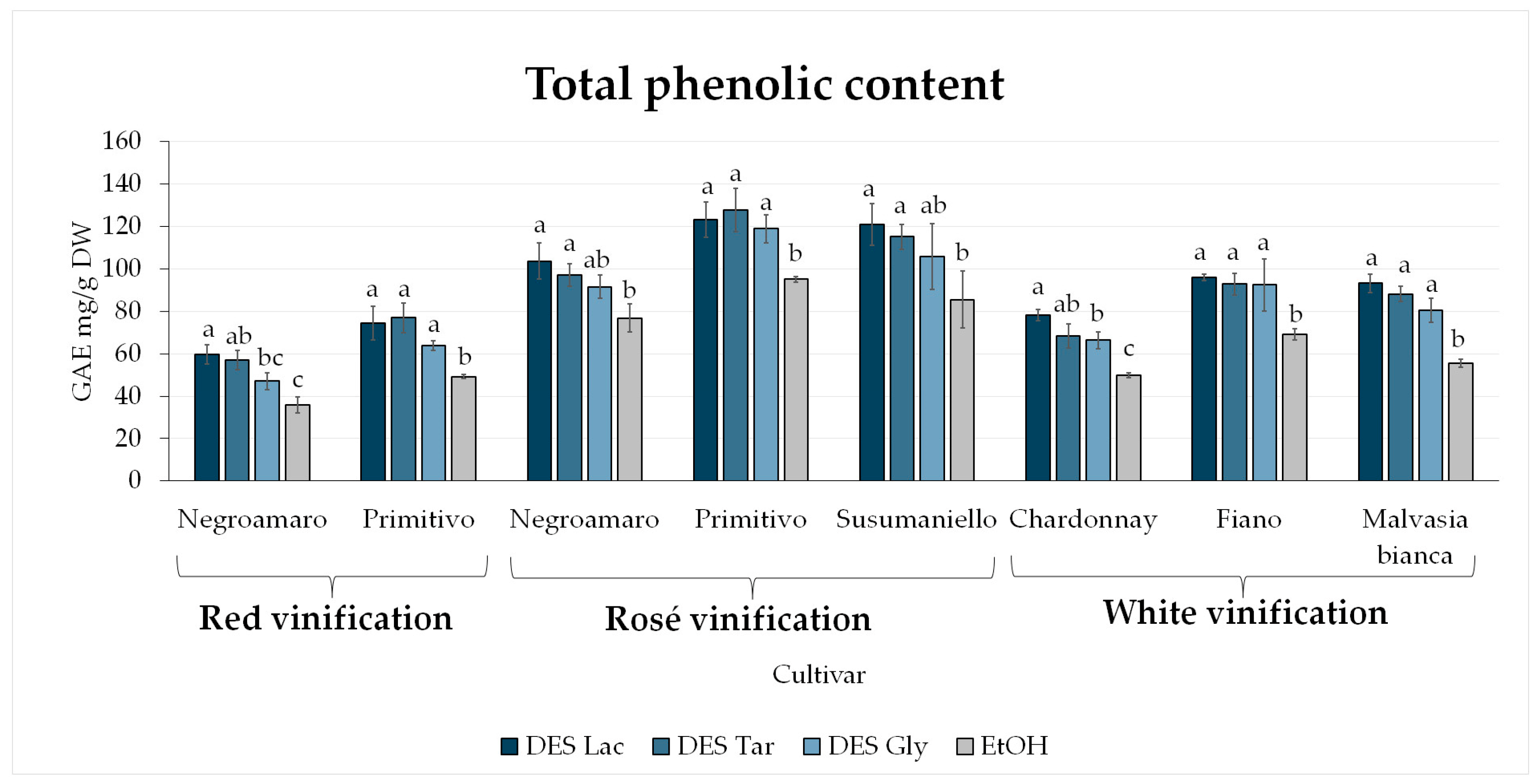
3.2. Total Phenolic Content
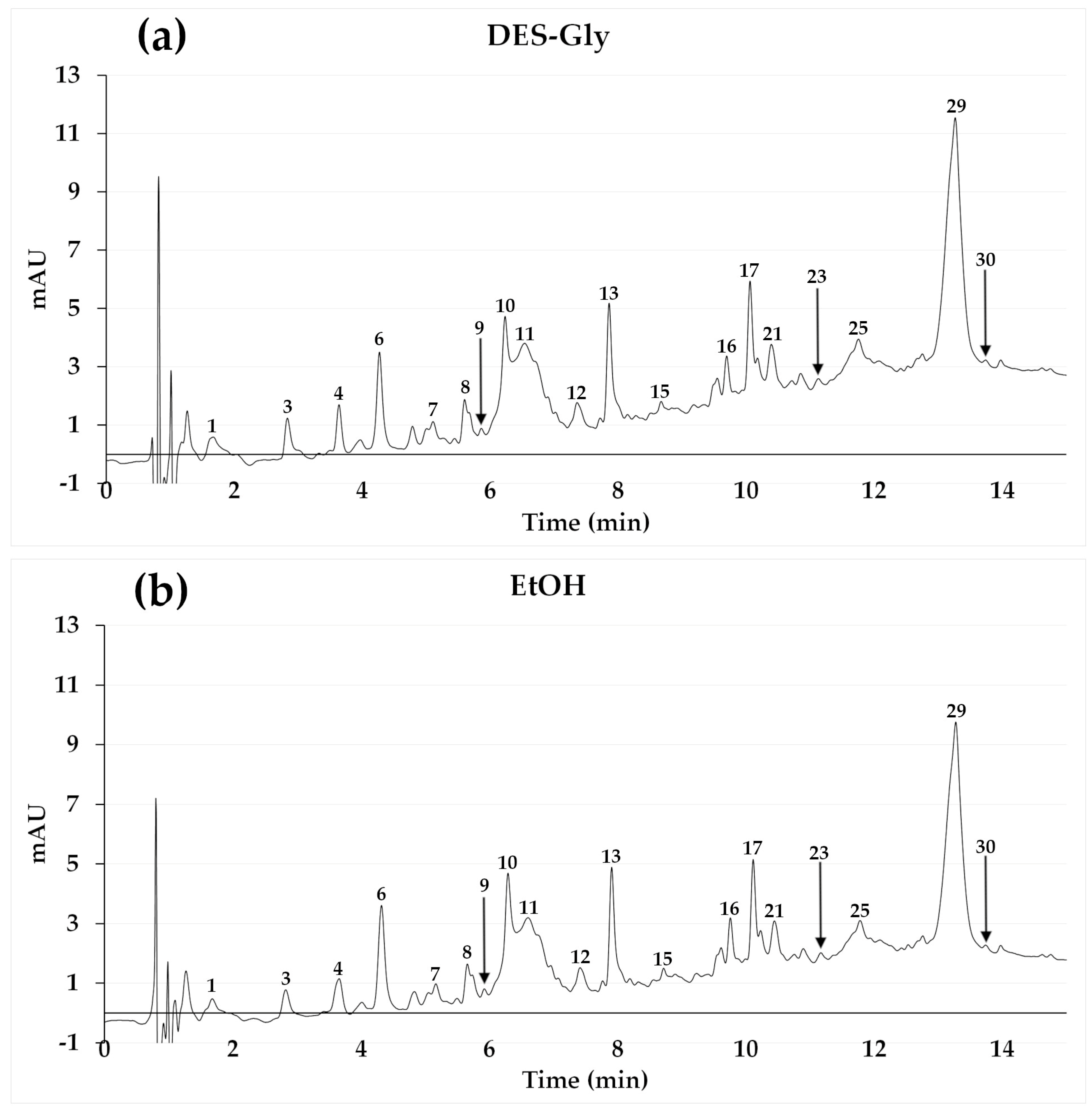
3.3. HPLC/DAD/TOF
3.3.1. Anthocyanins
3.3.2. Other Phenolic Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Q.; Neilson, A.P.; Stewart, A.C.; O’Keefe, S.F.; Kim, Y.-T.; McGuire, M.; Wilder, G.; Huang, H. Integrated Approach for the Valorization of Red Grape Pomace: Production of Oil, Polyphenols, and Acetone−Butanol−Ethanol. ACS Sustain. Chem. Eng. 2018, 6, 16279–16286. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. State of the World Vine and Wine Sector; OIV: Dijion, France, 2023. [Google Scholar]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for Recycling and Valorization of Grape Marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ahmedna, M. Functional Components of Grape Pomace: Their Composition, Biological Properties and Potential Applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, N.; Morassut, M.; Serra, M.C.; Cecchini, F. Determinazione dell’Impronta Carbonica dei Sottoprodotti della Vinificazione e loro Valenza Biologica. Ing. Dell’ambiente 2017, 4, 277–285. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach that Is Increasingly Reaching its Maturity—A Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-Based Compounds from Grape Seeds: A Biorefinery Approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Oliveros, A.; Bautista-Hernández, I.; Castro-López, C.; Aguilar-Zárate, P.; Meza-Carranco, Z.; Rojas, R.; Michel, M.R.; Cristian Martínez-Ávila, G.G. Grape Pomace-Advances in Its Bioactivity, Health Benefits, and Food Applications. Foods 2024, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. Focus OIV 2023. Evolution of World Wine Production and Consumption by Colour; OIV: Dijion, France, 2023. [Google Scholar]
- Todd, R.; Baroutian, S. A Techno-Economic Comparison of Subcritical Water, Supercritical CO2 and Organic Solvent Extraction of Bioactives from Grape Marc. J. Clean. Prod. 2017, 158, 349–358. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Comm. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Database of Deep Eutectic Solvents and Their Physical Properties: A Review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers Based on Natural Deep Eutectic Mixtures to Enhance Anthocyanins Isolation from Grape Pomace by Pressurized Hot Water Extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Iannone, A.; Sapone, V.; Di Paola, L.; Cicci, A.; Bravi, M. Extraction of Anthocyanins from Grape (Vitis vinifera) Skins Employing Natural Deep Eutectic Solvents (NaDES). Chem. Eng. Trans. 2021, 87, 469–474. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojcic Redovnikovic, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Food and Feed Information Portal Food Additives. Available online: https://ec.europa.eu/food/food-feed-portal/screen/food-additives/search (accessed on 24 June 2024).
- Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.-I.; Mangelsdorf, I.; Mcardle, H.; Naska, A.; Neuhäuser-Berthold, M.; et al. Dietary Reference Values for Choline. EFSA J. 2016, 14, e04484. [Google Scholar] [CrossRef]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, M.; Tan, T.; Yan, A.; Guo, L.; Jiang, K.; Tan, C.; Wan, Y. Deep Eutectic Solvents Used as Extraction Solvent for the Determination of Flavonoids from Camellia oleifera Flowers by High-Performance Liquid Chromatography. Phytochem. Anal. 2018, 29, 639–648. [Google Scholar] [CrossRef]
- Frontini, A.; Negro, C.; Accogli, R.; Minonne, F.; Luvisi, A.; De Bellis, L. Valorization of a Local Italian Pear (Pyrus communis L. Cv. ‘Petrucina’). Foods 2024, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Negro, C.; Aprile, A.; Luvisi, A.; De Bellis, L.; Miceli, A. Antioxidant Activity and Polyphenols Characterization of Four Monovarietal Grape Pomaces from Salento (Apulia, Italy). Antioxidants 2021, 10, 1406. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 1 October 2020).
- Flamini, R.; De Rosso, M.; Bavaresco, L. Study of Grape Polyphenols by Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC/QTOF) and Suspect Screening Analysis. J. Anal. Methods Chem. 2015, 2015, 350259. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Ebrahimi, F.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Comparative Study on the Effect of Phenolics and Their Antioxidant Potential of Freeze-Dried Australian Beach-Cast Seaweed Species upon Different Extraction Methodologies. Pharmaceuticals 2023, 16, 773. [Google Scholar] [CrossRef] [PubMed]
- Candela, L.; Formato, M.; Crescente, G.; Piccolella, S.; Pacifico, S. Coumaroyl Flavonol Glycosides and More in Marketed Green Teas: An Intrinsic Value beyond Much-Lauded Catechins. Molecules 2020, 25, 1765. [Google Scholar] [CrossRef] [PubMed]
- Della Vedova, L.; Ferrario, G.; Gado, F.; Altomare, A.; Carini, M.; Morazzoni, P.; Aldini, G.; Baron, G. Liquid Chromatography–High-Resolution Mass Spectrometry (LC-HRMS) Profiling of Commercial Enocianina and Evaluation of Their Antioxidant and Anti-Inflammatory Activity. Antioxidants 2022, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Lazović, M.; Jović, M.D.; Petrović, M.; Dimkić, I.Z.; Gašić, U.M.; Milojković Opsenica, D.M.; Ristivojević, P.M.; Trifković, J. Potential Application of Green Extracts Rich in Phenolics for Innovative Functional Foods: Natural Deep Eutectic Solvents as Media for Isolation of Biocompounds from Berries. Food Funct. 2024, 15, 4122–4139. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, S.; Xu, Y.; Ettoumi, F.; Fang, J.; Yan, X.; Xie, Z.; Luo, Z.; Cheng, K. Valorization and Protection of Anthocyanins from Strawberries (Fragaria×ananassa Duch.) by Acidified Natural Deep Eutectic Solvent Based on Intermolecular Interaction. Food Chem. 2024, 447, 138971. [Google Scholar] [CrossRef]
- Jurić, T.; Uka, D.; Holló, B.B.; Jović, B.; Kordić, B.; Popović, B.M. Comprehensive Physicochemical Evaluation of Choline Chloride-Based Natural Deep Eutectic Solvents. J. Mol. Liq. 2021, 343, 116968. [Google Scholar] [CrossRef]
- Xu, M.; Ran, L.; Chen, N.; Fan, X.; Ren, D.; Yi, L. Polarity-Dependent Extraction of Flavonoids from Citrus Peel Waste Using a Tailor-Made Deep Eutectic Solvent. Food Chem. 2019, 297, 124970. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Probst, Y.; Price, W.E.; Kelso, C. Relative Comparisons of Extraction Methods and Solvent Composition for Australian Blueberry Anthocyanins. J. Food Comp. Anal. 2022, 105, 104232. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. 2017, 56, 9782–9785. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G. Valorization of Artichoke Outer Petals by Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents (NADES) for the Recovery of Phenolic Compounds. J. Sci. Food Agric. 2024, 104, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Mu, L.; Yan, G.L.; Liang, N.N.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Lovino, R.; Baiano, A.; Pati, S.; Faccia, M.; Gambacorta, G. Phenolic composition of red grapes grown in southern Italy. Ital. J. Food Sci. 2006, 2, 177–186. [Google Scholar]
- Nicoletti, I.; Bello, C.; De Rossi, A.; Corradini, D. Identification and Quantification of Phenolic Compounds in Grapes by HPLC-PDA-ESI-MS on a Semimicro Separation Scale. J. Agric. Food Chem. 2008, 56, 8801–8808. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, Y.; Liu, D.; Li, Z.; Zhang, X.X.; Li, J.M.; Huang, J.H.; Wang, J.; Pan, Q.H. Evolution of Phenolic Compounds and Sensory in Bottled Red Wines and Their Co-Development. Food Chem. 2015, 172, 565–574. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Dangles, O.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Physico-Chemical and Chromatic Characterization of Malvidin 3-Glucoside-Vinylcatechol and Malvidin 3-Glucoside-Vinylguaiacol Wine Pigments. J. Agric. Food Chem. 2010, 58, 9744–9752. [Google Scholar] [CrossRef]
- Zhao, Q.; Duan, C.Q.; Wang, J. Anthocyanins Profile of Grape Berries of Vitis amurensis, Its Hybrids and Their Wines. Int. J. Mol. Sci. 2010, 11, 2212–2218. [Google Scholar] [CrossRef]
- Dipalmo, T.; Crupi, P.; Pati, S.; Clodoveo, M.L.; Di Luccia, A. Studying the Evolution of Anthocyanin-Derived Pigments in a Typical Red Wine of Southern Italy to Assess Its Resistance to Aging. LWT 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Le Scanff, M.; Marchal, A. New Insights about Astilbin, a Sweet Polyphenol: Effect of Grape Variety and Stems Addition during Winemaking. Oeno One 2024, 58, 7872. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.; Chauhan, S.; Nair, A.; Sharma, P. Astilbin: A Promising Unexplored Compound with Multidimensional Medicinal and Health Benefits. Pharmacol. Res. 2020, 158, 104894. [Google Scholar] [CrossRef]
- Mei, J.; Chen, X.; Wang, P.; Wu, Y.; Yi, Y.; Ying, G. Production of Taxifolin from Astilbin by Fungal Biotransformation. Catalysts 2022, 12, 1037. [Google Scholar] [CrossRef]
- Landrault, N.; Larronde, F.; Delaunay, J.C.; Castagnino, C.; Vercauteren, J.; Merillon, J.M.; Gasc, F.; Cros, G.; Teissedre, P.L. Levels of Stilbene Oligomers and Astilbin in French Varietal Wines and in Grapes during Noble Rot Development. J. Agric. Food Chem. 2002, 50, 2046–2052. [Google Scholar] [CrossRef]
- Bavaresco, L.; Lucini, L.; Busconi, M.; Flamini, R.; de Rosso, M. Wine Resveratrol: From the Ground Up. Nutrients 2016, 8, 222. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Fanizzi, F.P.; Maffia, M. A Comparative Study of Phenols in Apulian Italian Wines. Foods 2017, 6, 24. [Google Scholar] [CrossRef]
- Tapia, P.E.; Silva, A.M.; Delerue-Matos, C.; Moreira, M.; Rodrigues, F.; Torres Carro, R.; Santi, M.D.; Ortega, M.G.; Blázquez, M.A.; Arena, M.E.; et al. Exploring the Phytochemical Composition and the Bioactive Properties of Malbec and Torrontés Wine Pomaces from the Calchaquíes Valleys (Argentina) for Their Sustainable Exploitation. Foods 2024, 13, 1795. [Google Scholar] [CrossRef]

| Solvent | HBA | HBD | Molar Ratio | Final Water Content (v/v) | pH |
|---|---|---|---|---|---|
| DES-Lac | Choline chloride | Lactic acid | 1:1 | 50% | 1.47 |
| DES-Tar | Choline chloride | Tartaric acid | 1:1 | 50% | 0.56 |
| DES-Gly | Choline chloride | Glycerol | 1:1 | 50% | 2.76 |
| Vinification Method | Sample | Dry Weight (%) |
|---|---|---|
| Red | Negroamaro | 49.4 ± 0.4 |
| Primitivo | 49.5 ± 0.8 | |
| Rosé | Negroamaro | 39.7 ± 1.4 |
| Primitivo | 39.6 ± 1.8 | |
| Susumaniello | 42.6 ± 1.7 | |
| White | Chardonnay | 35.2 ± 1.3 |
| Fiano | 38.6 ± 1.1 | |
| Malvasia bianca | 29.1 ± 2.1 |
| ID | RT | Name | Formula [M-H]− | MW exp [M-H]− | MW calc [M-H]− | Δ ppm | Ref. |
|---|---|---|---|---|---|---|---|
| A | 11.313 | Delphinidin 3-O-glucoside | C21H21O12 | 465.1034 | 465.1028 | −1.34 | [25,27] |
| B | 13.020 | Cyanidin 3-O-glucoside | C21H21O11 | 449.1081 | 449.1078 | −0.66 | [25,27] |
| C | 14.393 | Petunidin 3-O-glucoside | C22H23O12 | 479.1184 | 479.1187 | −0.57 | [25,27] |
| D | 16.093 | Peonidin 3-O-glucoside | C22H23O11 | 463.1248 | 463.1235 | −2.8 | [25,27] |
| E | 17.140 | Malvidin 3-O-glucoside 1 | C23H25O12 | 493.1359 | 493.1341 | −3.84 | [25,27] |
| F | 20.673 | Petunidin 3-(6’acetyl)glucoside | C24H25O13 | 521.1314 | 521.129 | −4.58 | [25,27] |
| G | 22.140 | Peonidin 3-(6’acetyl)-glucoside | C24H25O12 | 505.1365 | 505.1341 | −4.83 | [25,27] |
| H | 22.973 | Malvidin 3-(6′acetyl)-glucoside | C25H27O13 | 535.1461 | 535.1446 | −2.84 | [25,27] |
| I | 23.805 | Malvidin 3-(6′caffeoil)-glucoside | C32H31O15 | 655.1669 | 655.1657 | −1.79 | [25,27] |
| J | 24.186 | Petunidin 3-(6′coumaroyl)-glucoside | C31H29O14 | 625.1558 | 625.1552 | −0.93 | [25,27] |
| K | 24.747 | Malvidin 3-(6′coumaroyl)-glucoside | C32H31O14 | 639.1725 | 639.1708 | −2.6 | [25,27] |
| L | 25.579 | Malvidin 3-O-glucoside 4 vinylphenol | C31H29O13 | 609.1619 | 609.1603 | −2.6 | [25] |
| M | 25.845 | Malvidin 3-O-glucoside 4 vinylguaiacol | C32H31O14 | 639.1733 | 639.1708 | −3.8 | [25] |
| Red Vinification | Rosé Vinification | ||||
|---|---|---|---|---|---|
| Negroamaro | Primitivo | Negroamaro | Primitivo | Susumaniello | |
| Delphinidin 3-O-glucoside 1 (µg/g DW) | |||||
| DES-Lac | 237 ± 55 a | 84 ± 9 a | 2169 ± 266 a | 365 ± 18 a | 2572 ± 105 a |
| DES-Tar | 214 ± 12 a | 106 ± 20 a | 2033 ± 167 a | 365 ± 9 a | 2487 ± 158 a |
| DES-Gly | 38 ± 6 b | 15 ± 3 b | 867 ± 288 b | 151 ± 26 c | 1581 ± 367 b |
| EtOH | 191 ± 34 a | 89 ± 7 a | 1721 ± 154 a | 225 ± 42 b | 1815 ± 200 b |
| Cyanidin 3-O-glucoside 1 (µg/g DW) | |||||
| DES-Lac | 120 ± 9 a | 59 ± 4 a | 2218 ± 358 a | 240 ± 8 a | 1914 ± 175 ab |
| DES-Tar | 110 ± 11 ab | 61 ± 9 a | 2283 ± 170 a | 241 ± 6 a | 2047 ± 156 ab |
| DES-Gly | 88 ± 11 b | 47 ± 4 a | 2014 ± 183 a | 207 ± 14 a | 1689 ± 88 b |
| EtOH | 123 ± 5 a | 55 ± 7 a | 2130 ± 306 a | 168 ± 22 b | 1695 ± 108 ab |
| Petunidin 3-O-glucoside 1 (µg/g DW) | |||||
| DES-Lac | 479 ± 43 ab | 208 ± 9 b | 4315 ± 561 ab | 994 ± 57 b | 4076 ± 189 b |
| DES-Tar | 554 ± 28 a | 323 ± 41 a | 5548 ± 474 a | 1411 ± 65 a | 5431 ± 369 a |
| DES-Gly | 329 ± 45 b | 174 ± 12 b | 4180 ± 659 b | 1094 ± 124 b | 4165 ± 390 b |
| EtOH | 573 ± 15 a | 300 ± 43 a | 5115 ± 642 ab | 985 ± 143 b | 4377 ± 342 b |
| Peonidin 3-O-glucoside 1 (µg/g DW) | |||||
| DES-Lac | 371 ± 63 a | 495 ± 37 a | 4351 ± 558 a | 3425 ± 210 ab | 11892 ± 650 ab |
| DES-Tar | 347 ± 24 a | 573 ± 83 a | 4334 ± 187 a | 3658 ± 184 a | 12249 ± 808 a |
| DES-Gly | 325 ± 41 a | 502 ± 53 a | 4083 ± 143 a | 3305 ± 173 ab | 10648 ± 333 b |
| EtOH | 429 ± 29 a | 552 ± 89 a | 4234 ± 642 a | 2800 ± 356 b | 10763 ± 547 ab |
| Malvidin-3-O-glucoside (mg/g DW) | |||||
| DES-Lac | 3.5 ± 0.1 a | 4.5 ± 0.9 a | 21.5 ± 2.1 a | 20.9 ± 0.9 a | 29.4 ± 1.3 a |
| DES-Tar | 3.2 ± 0.2 a | 4.3 ± 0.5 a | 20.4 ± 1.4 a | 21.9 ± 0.9 a | 28.9 ± 1.6 ab |
| DES-Gly | 3.0 ± 0.4 a | 4.1 ± 0.3 a | 18.9 ± 1.2 a | 20.1 ± 0.8 ab | 26.3 ± 0.8 b |
| EtOH | 3.3 ± 0.7 a | 4.2 ± 0.7 a | 19.9 ± 2.5 a | 18.7 ± 2.0 b | 26.6 ± 1.3 b |
| Malvidin 3-(6′caffeoil)-glucoside 1 (µg/g DW) | |||||
| DES-Lac | 230 ± 24 b | 458 ± 68 ab | 1415 ± 187 ab | 2530 ± 143 ab | 7475 ± 239 ab |
| DES-Tar | 195 ± 27 b | 388 ± 61 b | 1124 ± 81 b | 2384 ± 364 b | 6684 ± 716 b |
| DES-Gly | 259 ± 33 ab | 513 ± 42 ab | 1639 ± 71 a | 3068 ± 135 a | 8211 ± 255 a |
| EtOH | 331 ± 55 a | 585 ± 103 a | 1713 ± 228 a | 2567 ± 316 ab | 8315 ± 510 a |
| Malvidin 3-(6′acetyl)-glucoside 1 (µg/g DW) | |||||
| DES-Lac | 281 ± 17 ab | 1041 ± 153 a | 1054 ± 125 a | 2678 ± 60 a | 2186 ± 71 ab |
| DES-Tar | 242 ± 31 b | 924 ± 97 a | 1046 ± 75 a | 2798 ± 125 a | 2210 ± 151 a |
| DES-Gly | 275 ± 25 ab | 911 ± 97 a | 962 ± 37 a | 2513 ± 155 a | 1873 ± 84 b |
| EtOH | 336 ± 54 a | 1102 ± 156 a | 940 ± 77 a | 2140 ± 186 b | 1915 ± 153 ab |
| Malvidin 3-O-glucoside 4 vinylphenol 1 (µg/g DW) 1 | |||||
| DES-Lac | 254 ± 44 a | 217 ± 25 a | 910 ± 134 a | 2480 ± 118 a | 1082 ± 42 a |
| DES-Tar | 98 ± 3 b | 201 ± 22 a | 844 ± 37 a | 2609 ± 107 a | 1086 ± 18 a |
| DES-Gly | 95 ± 2 b | 197 ± 3 a | 831 ± 48 a | 2527 ± 175 a | 995 ± 43 b |
| EtOH | 108 ± 3 b | 221 ± 6 a | 860 ± 6 a | 2003 ± 213 b | 917 ± 35 c |
| Malvidin 3-O-glucoside 4 vinylguaiacol 1 (mg/g DW) | |||||
| DES-Lac | 3.3 ± 0.2 a | 7.9 ± 0.7 a | 7.5 ± 0.7 a | 17.9 ± 0.5 a | 6.8 ± 0.2 a |
| DES-Tar | 3.0 ± 0.3 a | 7.3 ± 0.6 a | 7.4 ± 0.3 a | 18.7 ± 0.8 a | 6.8 ± 0.4 a |
| DES-Gly | 2.8 ± 0.4 a | 7.2 ± 0.7 a | 7.1 ± 0.3 a | 17.1 ± 1.0 a | 5.8 ± 0.2 b |
| EtOH | 3.6 ± 0.6 a | 8.2 ± 1.3 a | 7.0 ± 0.6 a | 14.7 ± 1.3 b | 5.9 ± 0.5 ab |
| ID | RT | Name | Formula [M-H]− | MW exp [M-H]− | MW calc [M-H]− | Δ ppm | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 1.714 | Gallic acid Hexoside | C13H15O10 | 331.0659 | 331.0671 | 3.6 | [25] |
| 2 | 2.827 | Caffeic acid | C9H7O4 | 179.0348 | 179.035 | 1.27 | [25] |
| 3 | 2.847 | Caffeic acid glucuronide | C15H15O10 | 355.0661 | 355.0671 | 2.68 | [28] |
| 4 | 3.680 | (Epi)Catechin-(4,8″)-(Epi)Catechin | C30H25O12 | 577.1342 | 577.1351 | 1.58 | [25] |
| 5 | 4.027 | (Epi)Catechin-(4,8″)-(Epi)Catechin | C30H25O12 | 577.1349 | 577.1351 | 0.48 | [25] |
| 6 | 4.327 | Catechin 1 | C15H13O6 | 289.0718 | 289.0718 | −0.14 | [25] |
| 7 | 5.167 | Coumaroyl Hexoside Is. 1 | C15H17O8 | 325.0937 | 325.0929 | −2.4 | [25] |
| 8 | 5.663 | (Epi)Catechin-(4,8″)-(Epi)Catechin | C30H25O12 | 577.1343 | 577.1351 | 1.54 | [25] |
| 9 | 5.927 | Coumaroyl Hexoside Is. 2 | C15H17O8 | 325.0925 | 325.0929 | 1.24 | [25] |
| 10 | 6.296 | Epicatechin | C15H13O6 | 289.0714 | 289.0718 | 1.28 | [25] |
| 11 | 6.603 | Tetrahydroxy-Dimethoxy-Flavanone-Hexoside | C23H25O13 | 509.1293 | 509.1301 | 1.54 | [25] |
| 12 | 7.430 | 3-O-Galloyl (Epi)Catechin-(4,8″)-(Epi)Catechin | C37H29O16 | 729.1455 | 729.1461 | 0.8 | [25] |
| 13 | 7.916 | 3-O-Galloyl (Epi)Catechin-(4,8″)-(Epi)Catechin | C37H29O16 | 729.1464 | 729.1461 | −0.35 | [25] |
| 14 | 8.368 | Myricetin 3 Hexoxide | C21H19O13 | 479.0842 | 479.0831 | −2.35 | [25,27] |
| 15 | 8.736 | Tetrahydroxy-Dimethoxy-Flavanone-Hexoside | C23H25O13 | 509.1294 | 509.1301 | 1.38 | [25] |
| 16 | 9.776 | Epicatechin gallate | C22H17O10 | 441.0829 | 441.0827 | −0.43 | [28,29] |
| 17 | 10.116 | Quercetin 3-O-Hexuronide | C21H17O13 | 477.0671 | 477.0675 | 0.74 | [25,27] |
| 18 | 10.144 | Quercetin | C15H9O7 | 301.0347 | 301.0354 | 2.1 | [27] |
| 19 | 10.243 | Quercetin 3-β-D-glucoside 1 | C14H23O17 | 463.0914 | 463.0941 | 5.69 | [25,27] |
| 20 | 10.450 | Piceatannol | C14H11O4 | 243.0671 | 243.0663 | −3.39 | [27] |
| 21 | 10.463 | Dihydroquercetin 3-O-Rhamnoside (Astilbin) | C21H21O11 | 449.1103 | 449.1089 | −2.98 | [25,27] |
| 22 | 10.552 | Larycitrin 3-O-Hexoside | C22H21O13 | 493.0988 | 493.0988 | −0.08 | [25,27] |
| 23 | 11.194 | Quercetin 3-O-Rhamnoside | C21H19O11 | 447.0941 | 447.0933 | −1.92 | [25] |
| 24 | 11.643 | Kaempferol 7-O-Hexuronide | C21H17O12 | 461.0704 | 461.0725 | 4.59 | [25,27] |
| 25 | 11.803 | Kaempferol 3-O-glucoside 1 | C21H19O11 | 447.0932 | 447.0933 | 0.29 | [25,27] |
| 26 | 12.450 | Syringetin 3-O-Hexoside | C23H23O13 | 507.1149 | 507.1144 | −0.88 | [25,27] |
| 27 | 12.788 | Piceid | C20H20O8 | 389.1261 | 389.1242 | −4.9 | [27] |
| 28 | 12.987 | Viniferin | C28H21O6 | 453.1348 | 453.1344 | −0.86 | [27] |
| 29 | 13.290 | Malvidin 3-(6′caffeoil)-glucoside | C32H31O15 | 655.1660 | 655.1668 | 1.28 | [30] |
| 30 | 13.481 | Resveratrol 1 | C14H11O3 | 227.0717 | 227.0714 | −1.63 | [25,27] |
| 31 | 16.184 | Kaempferol | C15H9O6 | 285.0417 | 285.0405 | −4.26 | [25] |
| Red Vinification | Rosé Vinification | White Vinification | ||||||
|---|---|---|---|---|---|---|---|---|
| Negra. | Primi. | Negra. | Primi. | Susum. | Chard. | Fiano | MalBia | |
| Catechin (mg/g DW) | ||||||||
| DES-Lac | 0.9 ± 0.1 b | 1.6 ± 0.1 b | 3.9 ± 0.4 bc | 4.9 ± 0.5 bc | 2.1 ± 0.3 b | 2.6 ± 0.5 c | 2.1 ± 0.4 bc | 3.7 ± 0.4 b |
| DES-Tar | 0.8 ± 0.1 b | 1.5 ± 0.1 b | 2.4 ± 0.2 c | 3.2 ± 0.1 c | 1.4 ± 0.2 b | 2.1 ± 0.2 c | 1.6 ± 0.2 c | 1.9 ± 0.2 c |
| DES-Gly | 0.7 ± 0.1 b | 1.9 ± 0.3 b | 5.1 ± 0.3 b | 6.6 ± 0.7 b | 2.2 ± 0.2 b | 3.5 ± 0.1 b | 2.5 ± 0.3 b | 4.4 ± 0.5 b |
| EtOH | 3.0 ± 0.5 a | 5.2 ± 0.9 a | 8.8 ± 1.7 a | 9.1 ± 1.3 a | 4.1 ± 0.7 a | 6.1 ± 0.3 a | 5.0 ± 0.5 a | 8.7 ± 0.9 a |
| Epicatechin 1 (mg/g DW) | ||||||||
| DES-Lac | 0.4 ± 0.1 c | 0.6 ± 0.1 c | 0.9 ± 0.1 b | 1.3 ± 0.2 b | 0.8 ± 0.1 b | 1.4 ± 0.1 d | 1.3 ± 0.3 c | 1.2 ± 0.1 c |
| DES-Tar | 0.9 ± 0.1 b | 1.2 ± 0.1bc | 1.4 ± 0.1 b | 1.9 ± 0.2 b | 1.2 ± 0.1 b | 1.9 ± 0.1 c | 1.6 ± 0.1 c | 1.1 ± 0.3 c |
| DES-Gly | 0.8 ± 0.1 b | 1.9 ± 0.2 b | 3.7 ± 0.3 a | 4.7 ± 0.3 a | 2.3 ± 0.2 a | 4.8 ± 0.2 b | 5.7 ± 0.2 b | 4.0 ± 0.3 b |
| EtOH | 2.1 ± 0.2 a | 3.3 ± 0.6 a | 4.7 ± 0.8 a | 5.1 ± 0.7 a | 2.7 ± 0.4 a | 5.7 ± 0.4 a | 6.5 ± 0.5 a | 5.7 ± 0.6 a |
| Quercetin 3-O-hexuronide 2 (µg/g DW) | ||||||||
| DES-Lac | 115 ± 9 ab | 281 ± 25 a | 453 ± 20 a | 1684 ± 205 ab | 1264 ±155 a | 392 ± 52 a | 884 ± 169 a | 620 ± 19 a |
| DES-Tar | 99 ± 7 b | 264 ± 15 a | 476 ± 33 a | 1696 ± 131 ab | 1194 ± 76 a | 374 ± 41 a | 766 ± 90 a | 523 ± 57 a |
| DES-Gly | 94 ± 15 b | 286 ± 28 a | 522 ± 16 a | 1805 ± 140 a | 1198 ± 25 a | 396 ± 8 a | 941 ± 45 a | 620 ± 31 a |
| EtOH | 141 ± 26 a | 335 ± 53 a | 561 ± 99 a | 1282 ± 210 b | 1223 ± 140 a | 445 ± 13 a | 992 ± 29 a | 668 ± 97 a |
| Quercetin-3-β-D-glucoside (µg/g DW) | ||||||||
| DES-Lac | 33 ± 7 b | 43 ± 6 b | 463 ± 26 a | 438 ± 75 a | 565 ± 84 a | 539 ± 42 b | 736 ± 99 bc | 477 ± 21 ab |
| DES-Tar | 26 ± 5 b | 38 ± 5 b | 413 ± 32 a | 440 ± 30 a | 521 ± 39 a | 519 ± 45 b | 624 ± 95 c | 398 ± 33 b |
| DES-Gly | 34 ± 4 ab | 42 ± 6 b | 476 ± 25 a | 469 ± 41 a | 530 ± 15 a | 478 ± 14 b | 815 ± 18 b | 495 ± 28 ab |
| EtOH | 49 ± 9 a | 59 ± 10 a | 526 ± 95 a | 433 ± 78 a | 586 ± 45 a | 687 ± 29 a | 993 ± 58 a | 557 ± 74 a |
| Astilbin 2 (µg/g DW) | ||||||||
| DES-Lac | 18 ± 5 ab | 64 ± 10 b | 27 ± 7 b | 91 ± 8 a | 21 ± 2 b | 248 ± 21 bc | 56 ± 7 bc | 19 ± 3 ab |
| DES-Tar | 12 ± 1 c | 54 ± 2 b | 25 ± 1 ab | 91 ± 4 a | 22 ± 2 b | 222 ± 23 c | 48 ± 7 c | 15 ± 2 b |
| DES-Gly | 15 ± 2 bc | 64 ± 11 ab | 31 ± 2 ab | 106 ± 8 a | 28 ± 2 a | 293 ± 16 ab | 70 ± 3 ab | 20 ± 1 ab |
| EtOH | 23 ± 3 a | 85 ± 13 a | 35 ± 7 a | 101 ± 7 a | 28 ± 2 a | 323 ± 18 a | 84 ± 11 a | 22 ± 3 a |
| Kaempferol-3-O-glucoside (µg/g DW) | ||||||||
| DES-Lac | 4 ± 1 b | 8 ± 1 b | 37 ± 4 b | 72 ± 10 a | 71 ± 10ab | 212 ± 25 b | 82 ± 15 b | 66 ± 3 c |
| DES-Tar | 4 ± 1 b | 9 ± 1 b | 41 ± 5 b | 81 ± 8 a | 75 ± 8 ab | 251 ± 30 b | 81 ± 15 b | 69 ± 6 bc |
| DES-Gly | 4 ± 1 b | 9 ± 1 b | 45 ± 2 ab | 90 ± 8 a | 81 ± 2 ab | 220 ± 9 b | 110 ± 6 a | 87 ± 6 b |
| EtOH | 6 ± 1 a | 13 ± 1 a | 52 ± 6 a | 89 ± 14 a | 93 ± 9 a | 363 ± 11 a | 132 ± 3 a | 108 ± 12 a |
| Malvidin 3-(6′caffeoil)-glucoside 3 (µg/g DW) | ||||||||
| DES-Lac | 210 ± 22 b | 371 ± 61 ab | 1231 ± 168 ab | 1897 ± 129 ab | 5905 ± 215 ab | <LoD | <LoD | <LoD |
| DES-Tar | 175 ± 25 b | 341 ± 55 b | 933 ± 73 b | 2074 ± 327 b | 5147 ± 644 b | <LoD | <LoD | <LoD |
| DES-Gly | 207 ± 30 ab | 436 ± 37 ab | 1328 ± 64 a | 2731 ± 121 a | 7554 ± 229 a | <LoD | <LoD | <LoD |
| EtOH | 291 ± 49 a | 462 ± 53 a | 1525 ± 205 a | 1951 ± 285 ab | 6985 ± 459 a | <LoD | <LoD | <LoD |
| Resveratrol (µg/g DW) | ||||||||
| DES-Lac | 19 ± 3 b | 25 ± 1 b | 49 ± 1 ab | 22 ± 5 a | 45 ± 4 ab | <LoQ | 21 ± 5 a | <LoD |
| DES-Tar | 9 ± 1 b | 14 ± 2 b | 25 ± 1 b | 10 ± 2 c | 33 ± 2 c | <LoQ | 12 ± 2 b | <LoQ |
| DES-Gly | 22 ± 3 b | 25 ± 1 b | 42 ± 3 ab | 13 ± 2 bc | 40 ± 7 bc | <LoQ | 12 ± 1 b | <LoQ |
| EtOH | 53 ± 9 a | 49 ± 8 a | 54 ± 10 a | 20 ± 2 ab | 52 ± 7 a | <LoD | 12 ± 3 b | <LoD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frontini, A.; Luvisi, A.; Negro, C.; Apollonio, M.; Accogli, R.; De Pascali, M.; De Bellis, L. Polyphenols Extraction from Different Grape Pomaces Using Natural Deep Eutectic Solvents. Separations 2024, 11, 241. https://doi.org/10.3390/separations11080241
Frontini A, Luvisi A, Negro C, Apollonio M, Accogli R, De Pascali M, De Bellis L. Polyphenols Extraction from Different Grape Pomaces Using Natural Deep Eutectic Solvents. Separations. 2024; 11(8):241. https://doi.org/10.3390/separations11080241
Chicago/Turabian StyleFrontini, Alessandro, Andrea Luvisi, Carmine Negro, Massimiliano Apollonio, Rita Accogli, Mariarosaria De Pascali, and Luigi De Bellis. 2024. "Polyphenols Extraction from Different Grape Pomaces Using Natural Deep Eutectic Solvents" Separations 11, no. 8: 241. https://doi.org/10.3390/separations11080241







