Simple Green Purification of Spilanthol from Natural Deep Eutectic Solvent and Ethanolic Acmella oleracea (L.) R.K. Jansen Extracts Using Solid-Phase Extraction
Abstract
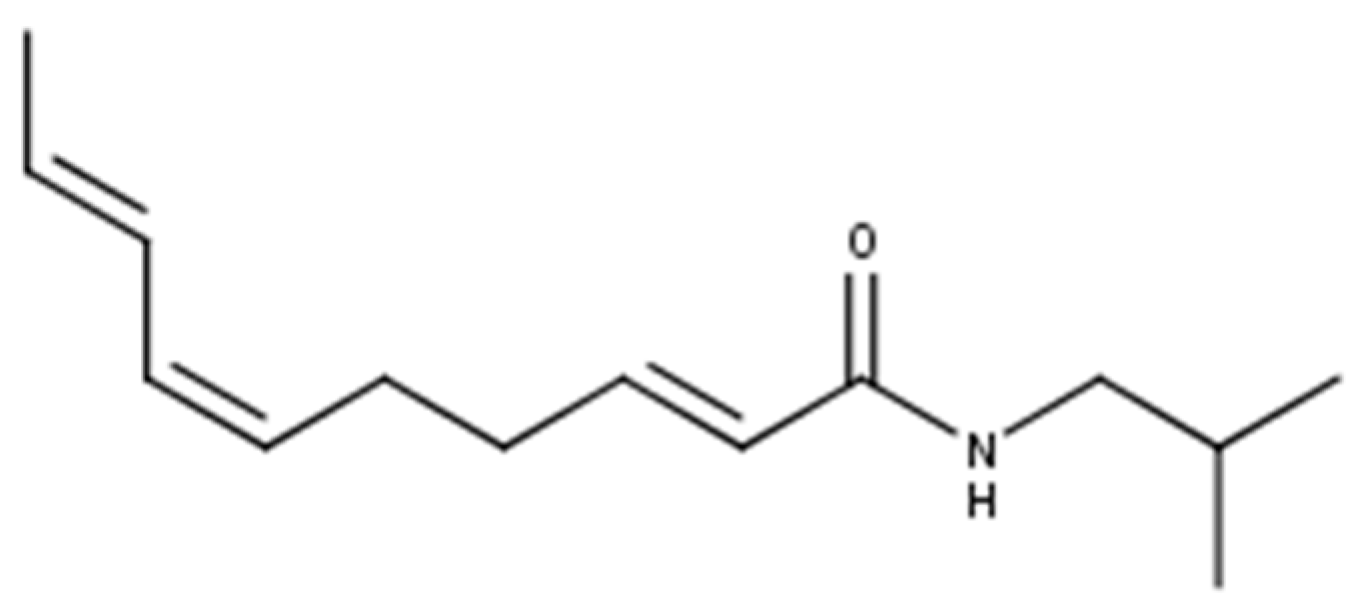
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Solvents
2.3. Preparation and Synthesis of NADES
2.4. Extraction Methods
2.4.1. Extractions with NADES
2.4.2. Extraction with Ethanol
2.5. Solid-Phase Extraction (SPE) Methods
2.5.1. SPE for the Recovery of Spilanthol from NADES Extracts
2.5.2. SPE for the Recovery of Spilanthol from Ethanolic Extracts
2.6. HPLC-DAD Analysis for Relative Quantification of Spilanthol in SPE Fractions
2.7. NMR Analysis for Purity Determination and Quantification of Isolated Spilanthol
2.8. Statistical Analysis
3. Results and Discussion
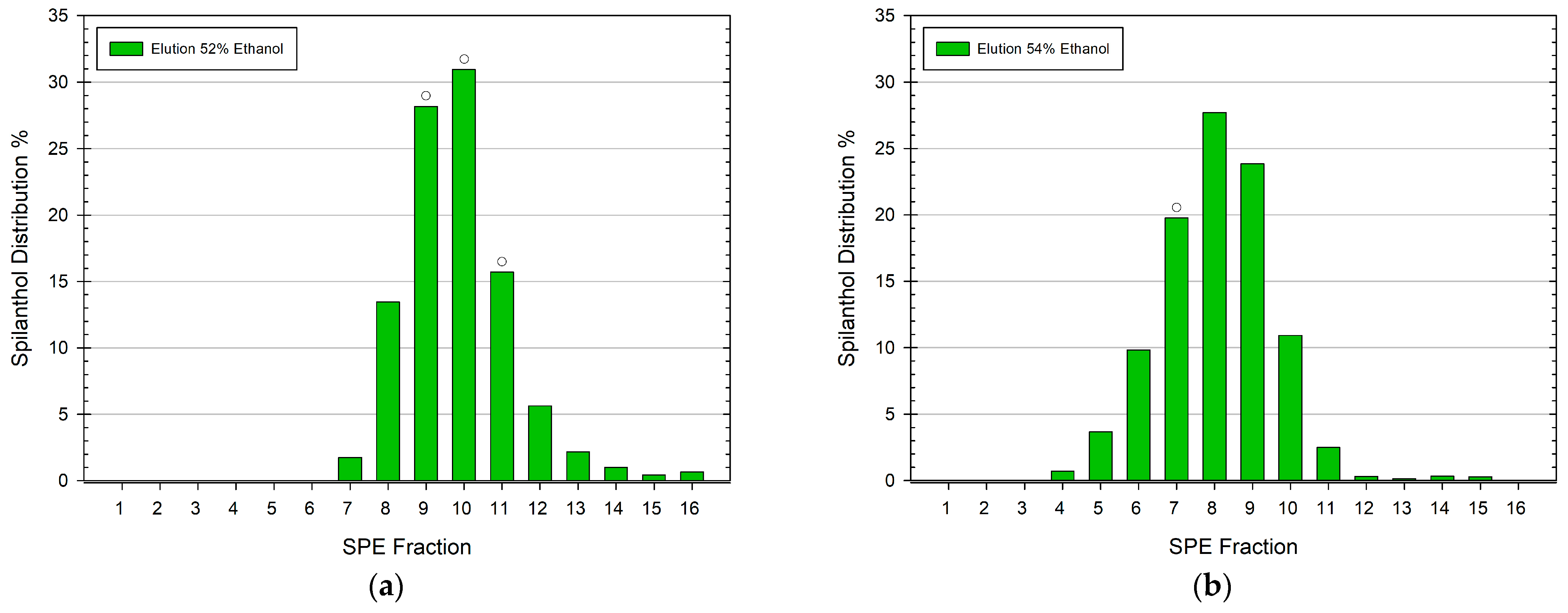
3.1. General Method Development for the SPE Elution of Spilanthol with Ethanol
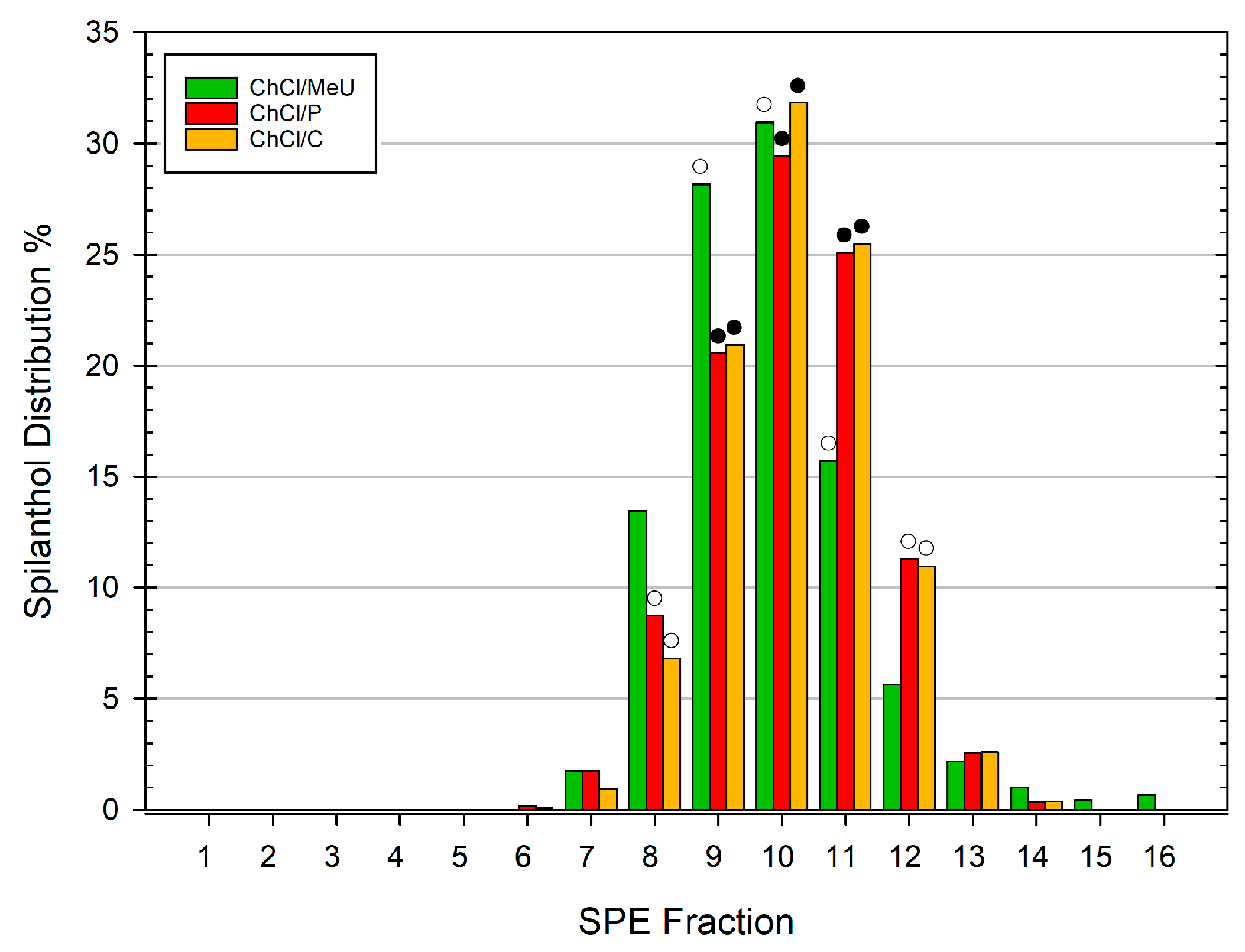
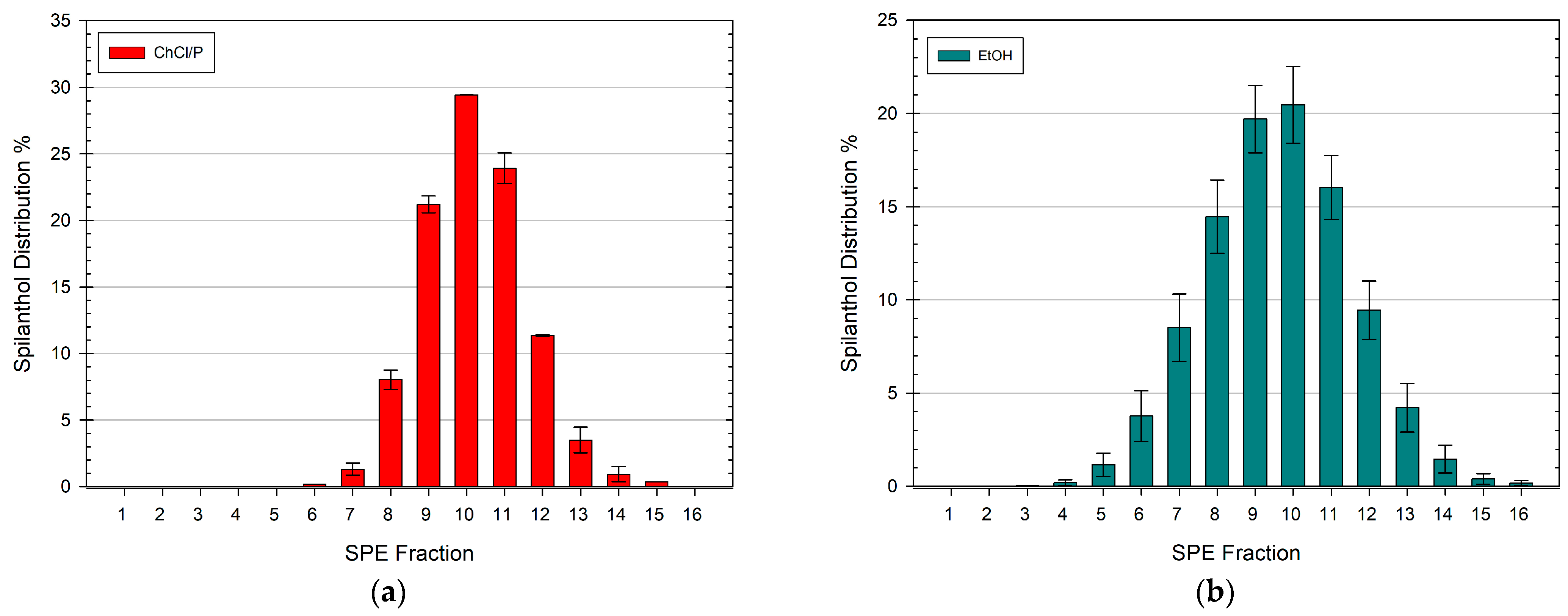
3.2. Spilanthol Distribution in SPE Fractions of Different NADES Extracts
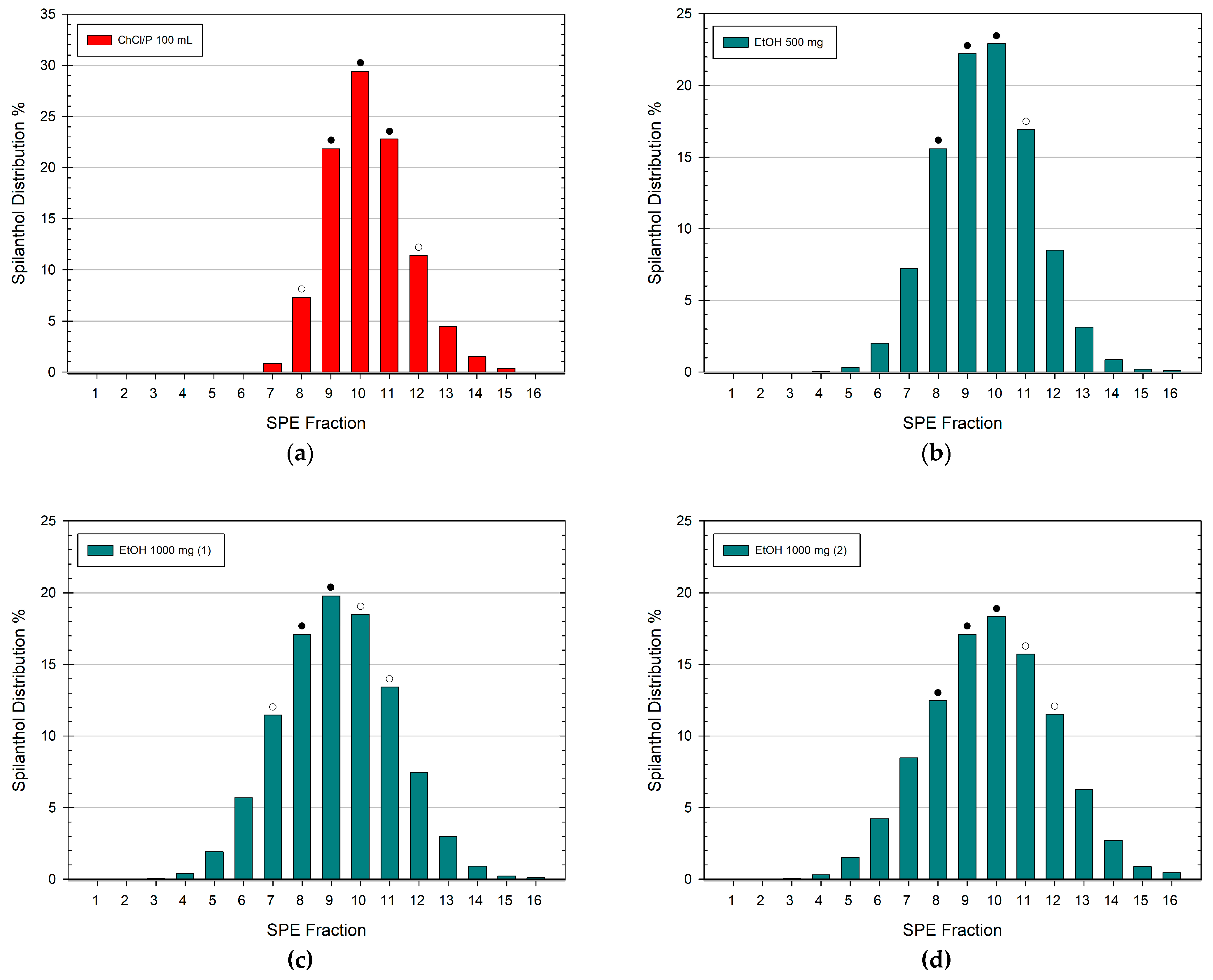
3.3. Spilanthol Distribution in SPE Fractions of Ethanolic Extracts
3.4. Scale-Up and Isolation of Spilanthol from NADES and Ethanolic Extracts
3.5. Purity and Quantification of Isolated Spilanthol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bae, S.S.; Ehrmann, B.M.; Ettefagh, K.A.; Cech, N.B. A validated liquid chromatography-electrospray ionization-mass spectrometry method for quantification of spilanthol in Spilanthes acmella (L.) Murr. Phytochem. Anal. 2010, 21, 438–443. [Google Scholar] [CrossRef]
- Boonen, J.; Baert, B.; Burvenich, C.; Blondeel, P.; De Saeger, S.; De Spiegeleer, B. LC-MS profiling of N-alkylamides in Spilanthes acmella extract and the transmucosal behaviour of its main bio-active spilanthol. J. Pharm. Biomed. Anal. 2010, 53, 243–249. [Google Scholar] [CrossRef]
- Savic, S.; Petrovic, S.; Savic, S.; Cekic, N. Identification and photostability of N-alkylamides from Acmella oleracea extract. J. Pharm. Biomed. Anal. 2021, 195, 113819. [Google Scholar] [CrossRef]
- Bellumori, M.; Zonfrillo, B.; Maggini, V.; Bogani, P.; Gallo, E.; Firenzuoli, F.; Mulinacci, N.; Innocenti, M. Acmella oleracea (L.) R.K. Jansen: Alkylamides and phenolic compounds in aerial parts and roots of in vitro seedlings. J. Pharm. Biomed. Anal. 2022, 220, 114991. [Google Scholar] [CrossRef]
- Rios, M.Y.; Aguilar-Guadarrama, A.B.; Del Gutiérrez, M.C. Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae). J. Ethnopharmacol. 2007, 110, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; Rios, M.Y.; Aguilar-Guadarrama, A.B. Antinociceptive effect of Heliopsis longipes extract and affinin in mice. Planta Medica 2010, 76, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Nomura, E.C.O.; Rodrigues, M.R.A.; da Silva, C.F.; Hamm, L.A.; Nascimento, A.M.; de Souza, L.M.; Cipriani, T.R.; Baggio, C.H.; Werner, M.F.d.P. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J. Ethnopharmacol. 2013, 150, 583–589. [Google Scholar] [CrossRef]
- Dallazen, J.L.; da Luz, B.B.; Maria-Ferreira, D.; Nascimento, A.M.; Cipriani, T.R.; de Souza, L.M.; Geppetti, P.; de Paula Werner, M.F. Local effects of natural alkylamides from Acmella oleracea and synthetic isobutylalkyl amide on neuropathic and postoperative pain models in mice. Fitoterapia 2022, 160, 105224. [Google Scholar] [CrossRef] [PubMed]
- Yien, R.M.K.; Gomes, A.C.C.; Fiorot, R.G.; Miranda, A.L.P.; Neves, G.A.; da Silva Andrade, B.; Costa, F.N.; Tributino, J.L.M.; Simas, N.K. Alkylamides from Acmella oleracea: Antinociceptive effect and molecular docking with cannabinoid and TRPV1 receptors. Nat. Prod. Res. 2023, 37, 3136–3144. [Google Scholar] [CrossRef]
- de Alcantara, B.N.; Kobayashi, Y.T.; Barroso, K.F.; da Silva, I.D.R.; de Almeida, M.B.; Barbosa, W.L.R. Pharmacognostic analyses and evaluation of the in vitro antimicrobial activity of Acmella oleracea (L.) RK Jansen (Jambu) floral extract and fractions. J. Med. Plants Res. 2015, 9, 91–96. [Google Scholar] [CrossRef]
- Peretti, P.; Rodrigues, E.T.; de Souza Junior, B.M.; Bezerra, R.M.; Fernández, E.G.; de Sousa, F.F.O.; Pinheiro, M.T. Spilanthol content of Acmella oleracea subtypes and their bactericide and antibiofilm activities against Streptococcus mutans. S. Afr. J. Bot. 2021, 143, 17–24. [Google Scholar] [CrossRef]
- dos Santos, M.B.V.; Gontijo, D.C.; Nascimento, M.F.A.D.; de Paula, R.C.; Bellei, J.C.B.; Raimundo, F.O.; Scopel, K.K.G.; de Oliveira, A.B.; Mourão, R.H.V. In Vitro and in Vivo Antimalarial Activity, Cytotoxicity and Phytochemical HRMS2 Profile of Plants from the Western Pará State, Brazilian Amazonia. Chem. Biodivers. 2024, 21, e202301082. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Buitimea-Cantúa, N.E.; Rocha-Pizaña, M.d.R.; Rosas-Burgos, E.C.; Hernández-Morales, A.; Molina-Torres, J. Antifungal and anti-aflatoxigenic activity of Heliopsis longipes roots and affinin/spilanthol against Aspergillus parasiticus by downregulating the expression of alfD and aflR genes of the aflatoxins biosynthetic pathway. J. Environ. Sci. Health B 2020, 55, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Bakondi, E.; Singh, S.B.; Hajnády, Z.; Nagy-Pénzes, M.; Regdon, Z.; Kovács, K.; Hegedűs, C.; Madácsy, T.; Maléth, J.; Hegyi, P.; et al. Spilanthol Inhibits Inflammatory Transcription Factors and iNOS Expression in Macrophages and Exerts Anti-inflammatory Effects in Dermatitis and Pancreatitis. Int. J. Mol. Sci. 2019, 20, 4308. [Google Scholar] [CrossRef]
- de Freitas-Blanco, V.S.; Monteiro, K.M.; de Oliveira, P.R.; de Oliveira, E.C.S.; de Oliveira Braga, L.E.; de Carvalho, J.E.; Rodrigues, R.A.F. Spilanthol, the Principal Alkylamide from Acmella oleracea, Attenuates 5-Fluorouracil-Induced Intestinal Mucositis in Mice. Planta Medica 2019, 85, 203–209. [Google Scholar] [CrossRef]
- Savic, S.M.; Cekic, N.D.; Savic, S.R.; Ilic, T.M.; Savic, S.D. ‘All-natural’ anti-wrinkle emulsion serum with Acmella oleracea extract: A design of experiments (DoE) formulation approach, rheology and in vivo skin performance/efficacy evaluation. Int. J. Cosmet. Sci. 2021, 43, 530–546. [Google Scholar] [CrossRef]
- de Freitas Gomes Lima, T.M.; da Silva, L.M.R.; de Sousa, P.H.M.; Magalhaes, F.E.; Ricardo, N.M.P.S.; Vieira, I.G.P.; de Sousa Sabino, L.B.; de Figueiredo, R.W. Bioactive jambu extract (Acmella ciliata) as source of spilanthol for the development of a functional vegetable gelatin. Food Biosci. 2024, 61, 104706. [Google Scholar] [CrossRef]
- Castro-Ruiz, J.E.; Rojas-Molina, A.; Luna-Vázquez, F.J.; Rivero-Cruz, F.; García-Gasca, T.; Ibarra-Alvarado, C. Affinin (Spilanthol), Isolated from Heliopsis longipes, Induces Vasodilation via Activation of Gasotransmitters and Prostacyclin Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Grymel, M.; Mazurkiewicz, R.; Bajkacz, S.; Bilik, J.; Kowalczyk, S. Extraction, Purification, Quantification, and Stability of Bioactive Spilanthol from Acmella oleracea. Planta Medica 2023, 89, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Santos, P.; Seabra, I.; Júnior, R.; Braga, M.; de Sousa, H. Spilanthol from Spilanthes acmella flowers, leaves and stems obtained by selective supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 61, 62–70. [Google Scholar] [CrossRef]
- de Freitas Blanco, V.S.; Michalak, B.; Zelioli, Í.A.M.; de Oliveira, A.D.S.S.; Rodrigues, M.V.N.; Ferreira, A.G.; Garcia, V.L.; Cabral, F.A.; Kiss, A.K.; Rodrigues, R.A.F. Isolation of spilanthol from Acmella oleracea based on Green Chemistry and evaluation of its in vitro anti-inflammatory activity. J. Supercrit. Fluids 2018, 140, 372–379. [Google Scholar] [CrossRef]
- Barbosa, A.F.; Pereira, C.D.S.S.; Mendes, M.F.; Junior, R.N.D.C.; De Carvalho, M.G.; Maia, J.G.S.; Sabaa-Srur, A.U.O. Spilanthol Content in the Extract Obtained by Supercritical CO2 at Different Storage Times of Acmella oleracea L. J. Food Process Eng. 2017, 40, e12441. [Google Scholar] [CrossRef]
- Spinozzi, E.; Pavela, R.; Bonacucina, G.; Perinelli, D.R.; Cespi, M.; Petrelli, R.; Cappellacci, L.; Fiorini, D.; Scortichini, S.; Garzoli, S.; et al. Spilanthol-rich essential oil obtained by microwave-assisted extraction from Acmella oleracea (L.) R.K. Jansen and its nanoemulsion: Insecticidal, cytotoxic and anti-inflammatory activities. Ind. Crops Prod. 2021, 172, 114027. [Google Scholar] [CrossRef]
- Alperth, F.; Feistritzer, T.; Huber, M.; Kunert, O.; Bucar, F. Natural Deep Eutectic Solvents for the Extraction of Spilanthol from Acmella oleracea (L.) R.K.Jansen. Molecules 2024, 29, 612. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Cannavacciuolo, C.; Pagliari, S.; Frigerio, J.; Giustra, C.M.; Labra, M.; Campone, L. Natural Deep Eutectic Solvents (NADESs) Combined with Sustainable Extraction Techniques: A Review of the Green Chemistry Approach in Food Analysis. Foods 2022, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- González-Laredo, R.F.; Sayago-Monreal, V.I.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Landeros-Macías, L.F.; Rosales-Castro, M. Natural deep eutectic solvents (NaDES) as an emerging technology for the valorisation of natural products and agro-food residues: A review. Int. J. Food Sci. Technol. 2023, 58, 6660–6673. [Google Scholar] [CrossRef]
- Wawoczny, A.; Gillner, D. The Most Potent Natural Pharmaceuticals, Cosmetics, and Food Ingredients Isolated from Plants with Deep Eutectic Solvents. J. Agric. Food Chem. 2023, 71, 10877–10900. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Figueroa-Espinoza, M.C.; Durand, E. Application of Deep Eutectic Solvents (DES) for Phenolic Compounds Extraction: Overview, Challenges, and Opportunities. J. Agric. Food Chem. 2017, 65, 3591–3601. [Google Scholar] [CrossRef]



| Extract Source, SPE | Spilanthol 1 [mg] | Initial Sample 2 [mg] | Spilanthol Purity [%] | Final Sample 3 [mg] | Spilanthol Purity [%] |
|---|---|---|---|---|---|
| ChCl/P, Fraction 9 | 3.62 | 6.44 | 56.21 | 4.27 | 84.78 |
| ChCl/P, Fraction 10 | 4.73 | 5.99 | 78.96 | 5.24 | 90.27 |
| ChCl/P, Fraction 11 | 3.86 | 4.61 | 83.73 | 4.10 | 94.15 |
| Total | 12.21 | 17.04 | 71.65 | 13.61 | 89.71 |
| 500 mg Ethanolic Extract, Fraction 8 | 2.40 | 3.21 | 74.77 | 2.94 | 81.63 |
| 500 mg Ethanolic Extract, Fraction 9 | 3.11 | 3.78 | 82.28 | 3.52 | 88.35 |
| 500 mg Ethanolic Extract, Fraction 10 | 3.11 | 3.94 | 78.93 | 3.42 | 90.94 |
| Total | 8.62 | 10.93 | 78.87 | 9.88 | 87.25 |
| 1000 mg Ethanolic Extract (1), Fraction 8 | 4.97 | 6.37 | 78.02 | 5.73 | 86.74 |
| 1000 mg Ethanolic Extract (1), Fraction 9 | 5.52 | 6.59 | 83.76 | 6.29 | 87.76 |
| 1000 mg Ethanolic Extract (1), Fraction 10 | 4.97 | 6.30 | 78.89 | 5.57 | 89.23 |
| Total | 15.46 | 19.26 | 80.27 | 17.59 | 87.89 |
| 1000 mg Ethanolic Extract (2), Fraction 8 | 3.11 | 4.07 | 76.41 | 3.57 | 87.11 |
| 1000 mg Ethanolic Extract (2), Fraction 9 | 3.37 | 4.64 | 72.63 | 3.62 | 93.09 |
| 1000 mg Ethanolic Extract (2), Fraction 10 | 3.82 | 4.62 | 82.68 | 4.08 | 93.63 |
| Total | 10.30 | 13.33 | 77.27 | 11.27 | 91.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alperth, F.; Erhart, S.; Kunert, O.; Bucar, F. Simple Green Purification of Spilanthol from Natural Deep Eutectic Solvent and Ethanolic Acmella oleracea (L.) R.K. Jansen Extracts Using Solid-Phase Extraction. Separations 2024, 11, 251. https://doi.org/10.3390/separations11080251
Alperth F, Erhart S, Kunert O, Bucar F. Simple Green Purification of Spilanthol from Natural Deep Eutectic Solvent and Ethanolic Acmella oleracea (L.) R.K. Jansen Extracts Using Solid-Phase Extraction. Separations. 2024; 11(8):251. https://doi.org/10.3390/separations11080251
Chicago/Turabian StyleAlperth, Fabian, Sebastian Erhart, Olaf Kunert, and Franz Bucar. 2024. "Simple Green Purification of Spilanthol from Natural Deep Eutectic Solvent and Ethanolic Acmella oleracea (L.) R.K. Jansen Extracts Using Solid-Phase Extraction" Separations 11, no. 8: 251. https://doi.org/10.3390/separations11080251
APA StyleAlperth, F., Erhart, S., Kunert, O., & Bucar, F. (2024). Simple Green Purification of Spilanthol from Natural Deep Eutectic Solvent and Ethanolic Acmella oleracea (L.) R.K. Jansen Extracts Using Solid-Phase Extraction. Separations, 11(8), 251. https://doi.org/10.3390/separations11080251







