Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry
Abstract
1. Introduction
2. Spirulina spp. Cultivation
- Suitable illumination: Both low and excessive light can limit microalgal growth. The PBR’s geometry and location determine the amount and distribution of light throughout the day and year, affecting the cultivation season length.
- Adequate carbon dioxide supply: Atmospheric CO2 levels are too low for optimal microalgae growth. Depending on the system, CO2 concentrations from 1 to 100% in the aeration gas are used.
- Efficient mixing: This prevents microalgae from settling and biofilm formation, ensuring uniform light distribution and promoting photosynthesis through short light/dark cycles.
- Appropriate construction material: It should prevent biofilm formation, be durable, resistant to solar radiation, and suitable for saline water.
- Effective oxygen release: Excess oxygen from photosynthesis can lower productivity by reducing photosynthetic activity. Managing oxygen levels is crucial.
- Suitable temperature: Overheating from sunlight can damage microalgae. In open systems, water evaporation helps control temperature, while closed systems require thermostatic regulation or surface spraying.
- Ease of cleaning and operation: The PBR should be easy to clean, sanitize, and operate effectively [36].
3. Nutritional Value of Spirulina (Macronutrients and Micronutrients)
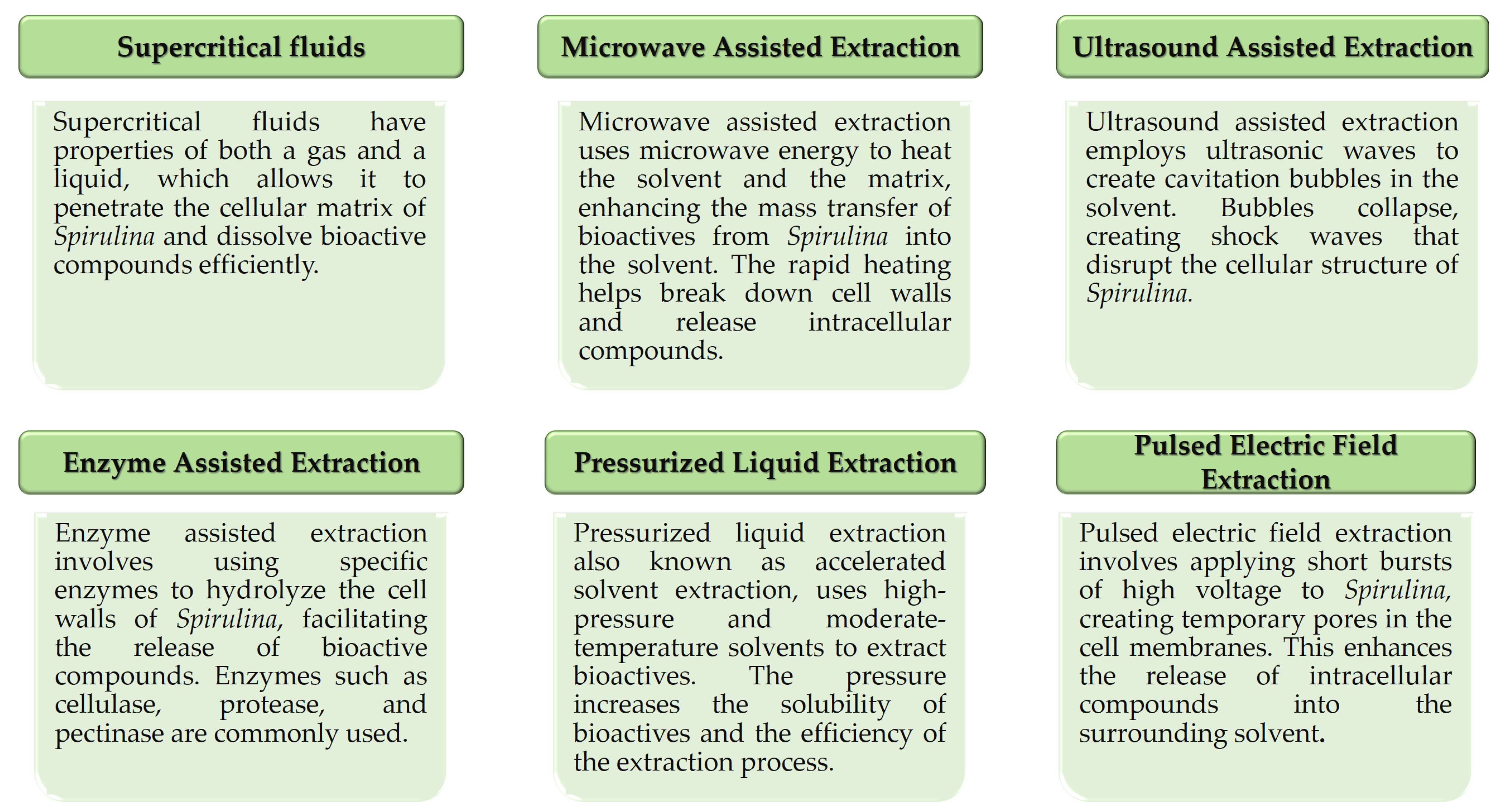
4. Bioactives’ Extraction from Spirulina
5. Spirulina spp. as Functional Food
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies Affecting the growth and cultivation of genus Spirulina: An investigative review on current trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Soma, K.; Kals, J.; Opiyo, M.A.; Ndambi, A.; García-Cubero, R.; Barbosa, M.J.; Rurangwa, E.; Vernooij, A. Toward sustainable food systems: Can Spirulina (Arthrospira platensis) become a sustainable source of protein to enhance the nutritional benefits of cultured Nile tilapia (Oreochromis niloticus)? Front. Sustain. Food Syst. 2024, 8, 1283150. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.d.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Istijanto; Arifin, Y.; Nurhayati. Examining costumer satisfaction and purchase intention toward a new product before its lauch: Cookies enricher with spirulina. Cogent. Bus. Manag. 2023, 10, 2257346. [Google Scholar] [CrossRef]
- Saraswathi, K.; Kavitha, C.N. Spirulina: Pharmacological Activities and Health Benefits. J. Young Pharm. 2023, 15, 441–447. [Google Scholar] [CrossRef]
- Seyidoglu, N.; Inan, S.; Aydin, C.; Seyidoglu, N.; Inan, S.; Aydin, C. A Prominent superfood: Spirulina platensis. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; Shiomi, N., Waisundara, V., Eds.; InTech: London, UK, 2017; pp. 1–28. [Google Scholar]
- Gutiérrez-Salmeán, G.; Fabila-Castillo, L.; Chamorro-Cevallos, G. Revisión Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. 2015, 32, 34–40. [Google Scholar]
- Mathur, M. Bioactive Molecules of Spirulina: A food supplement. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina microalgae and brain Health: A scoping review of experimental and clinical evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef]
- Wu, J.Y.; Tso, R.; Teo, H.S.; Haldar, S. The utility of algae as sources of high value nutritional ingredients, particularly for alternative/complementary proteins to improve human health. Front. Nutr. 2023, 10, 1277343. [Google Scholar] [CrossRef]
- Alcorta, A.; Porta, A.; Tárrega, A.; Alvarez, M.D.; Pilar Vaquero, M. Foods for plant-based diets: Challenges and innovations. Foods 2021, 10, 293. [Google Scholar] [CrossRef]
- Perez-Cueto, F.J.A.; Rini, L.; Faber, I.; Rasmussen, M.A.; Bechtold, K.B.; Schouteten, J.J.; De Steur, H. How barriers towards plant-based food consumption differ according to dietary lifestyle: Findings from a consumer survey in 10 EU countries. Int. J. Gastron. Food Sci. 2022, 29, 100587. [Google Scholar] [CrossRef]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A comprehensive review of the benefits of and the barriers to the switch to a plant-based diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Sanchez-Sabate, R.; Sabaté, J. Consumer attitudes towards environmental concerns of meat consumption: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 1220. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A review of recent strategies to improve the physical stability of phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- e Silva, A.d.S.; de Magalhães, W.T.; Moreira, L.M.; Rocha, M.V.P.; Bastos, A.K.P. Microwave-assisted extraction of polysaccharides from Arthrospira (Spirulina) platensis using the concept of green chemistry. Algal Res. 2018, 35, 178–184. [Google Scholar] [CrossRef]
- Tzachor, A.; Smidt-Jensen, A.; Ramel, A.; Geirsdóttir, M. Environmental impacts of large-scale Spirulina (Arthrospira platensis) production in Hellisheidi Geothermal Park Iceland: Life Cycle Assessment. Mar. Biotechnol. 2022, 24, 991. [Google Scholar]
- Sabrin, A.; Shukri, M.; Najihah, A.; Nor, M.; Faiz, M.; Amin, M.; Sukhairi, M.; Rasat, M.; Abas, M.A. Greening Spirulina Value Chain Towards Environmental Sustainability in Malaysia. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2023; p. 03005. [Google Scholar]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, functional, textural and sensory evaluation of Spirulina enriched green pasta: A potential dietary and health supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef]
- Jalili, S.; Aryan, S.; Mousavinezhad, S.A.; Moeini, H.; Dehnad, D. Optimizing Spirulina platensis, Chlorella vulgaris microalgae and curcumin application in functional cheese production and investigating its physicochemical properties and sensory evaluation by RSM. J. Food Meas. Charact. 2023, 18, 1144–1157. [Google Scholar] [CrossRef]
- Herrera, M.; Viera, I.; Roca, M. Study of the authentic composition of the novel green foods: Food colorants and coloring foods. Food Res. Int. 2023, 170, 112974. [Google Scholar] [CrossRef]
- Shkolnikov Lozober, H.; Okun, Z.; Parvari, G.; Shpigelman, A. The Effect of Storage and Pasteurization (Thermal and High-Pressure) Conditions on the Stability of Phycocyanobilin and Phycobiliproteins. Antioxidants 2023, 12, 568. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2016, 8, 1462. [Google Scholar]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966. [Google Scholar] [PubMed]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Cruz, J.D.; Vasconcelos, V. Legal Aspects of microalgae in the European food sector. Foods 2023, 13, 124. [Google Scholar] [CrossRef]
- Zrimec, M.B.; Sforza, E.; Pattaro, L.; Carecci, D.; Ficara, E.; Idà, A.; Ferrer-Ledo, N.; Canziani, S.; Mangini, S.; Lazar, B.; et al. Advances in Spirulina cultivation: Techniques, challenges, and applications. In New Insights Into Cyanobacteria—Fundamentals, Culture Techniques and Biotechnological Uses of Microalgae and Cyanobacteria; Sevaro, I.A.A., Martinez-Burgos, W., Ordonez, J., Eds.; InTech: London, UK, 2024; pp. 1–27. [Google Scholar]
- Pisanello, D.; Caruso, G. EU Regulation on Novel Foods. In Novel Foods in the European Union; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–29. [Google Scholar]
- Siddhnath; Surasani, V.K.R.; Singh, A.; Singh, S.M.; Hauzoukim; Murthy, L.N.; Baraiya, K.G. Bioactive compounds from micro-algae and its application in foods: A review. Discov. Food 2024, 4, 27. [Google Scholar] [CrossRef]
- Sánchez, A.S.; Nogueira, I.B.R.; Kalid, R.A. Uses of the reject brine from inland desalination for fish farming, Spirulina cultivation, and irrigation of forage shrub and crops. Desalination 2015, 364, 96–107. [Google Scholar] [CrossRef]
- Branyikova, I.; Lucakova, S. Technical and physiological aspects of microalgae cultivation and productivity—Spirulina as a promising and feasible choice. Org. Agric. 2021, 11, 269–276. [Google Scholar] [CrossRef]
- Khoo, K.S.; Chew, K.W.; Yew, G.Y.; Leong, W.H.; Chai, Y.H.; Show, P.L.; Chen, W.H. Recent advances in downstream processing of microalgae lipid recovery for biofuel production. Bioresour. Technol. 2020, 304, 122996. [Google Scholar] [CrossRef]
- Tambiev, A.K.; Vasilieva, S.G.; Lukyanov, A.A. Manifestation of salt tolerance of Spirulina platensis and Spirulina maxima cyanobacteria of the genus Arthrospira (Spirulina). Mosc. Univ. Biol. Sci. Bull. 2011, 66, 133–137. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Singh, R.N.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Hossain, M.S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A review on Spirulina: Alternative media for cultivation and nutritive value as an aquafeed. Rev. Aquac. 2020, 12, 2371–2395. [Google Scholar] [CrossRef]
- Pandey, J.; Tiwari, A.; Mishra, R. Evaluation of Biomass Production of Spirulina maxima on Different Reported Media. J. Algal Biomass Utln 2010, 1, 70–81. [Google Scholar]
- Costa, L.R.; Tonoli, G.H.D.; Milagres, F.R.; Hein, P.R.G. Artificial neural network and partial least square regressions for rapid estimation of cellulose pulp dryness based on near infrared spectroscopic data. Carbohydr. Polym. 2019, 224, 115186. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, H.; Wu, X.; Wang, C.; Zhou, T.; Liu, Y.; Ruan, R.; Zheng, H. Continuous cultivation of Arthrospira platensis for phycocyanin production in large-scale outdoor raceway ponds using microfiltered culture medium. Bioresour. Technol. 2019, 287, 121420. [Google Scholar] [CrossRef]
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.K.; Munawaroh, H.S.H.; Kumar, P.S.; Huy, N.D.; Show, P.L. Perspective of Spirulina culture with wastewater into a sustainable circular bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef]
- Arora Soni, R.; Sudhakar, K.; Rana, R. Influence of temperature and light intensity on the growth performance of Spirulina platensis. Int. J. Emerg. Technol. 2019, 10, 19–22. [Google Scholar]
- Kumari, A.; Pathak, A.K.; Guria, C. Cost-effective cultivation of Spirulina platensis Using NPK Fertilizer. Agric. Res. 2015, 4, 261–271. [Google Scholar] [CrossRef]
- Villaró-Cos, S.; Guzmán Sánchez, J.L.; Acién, G.; Lafarga, T. Research trends and current requirements and challenges in the industrial production of Spirulina as a food source. Trends Food Sci. Technol. 2024, 143, 104280. [Google Scholar] [CrossRef]
- Bumandalai, O.; Bayliss, K.L.; Moheimani, N.R. Innovative processes for combating contaminants in fresh Spirulina. Algal Res. 2024, 78, 103397. [Google Scholar] [CrossRef]
- Girotto, F.; Biasi, V.O.; Mirto, A.; Piazza, L. Challenges for greening Spirulina value chain in terms of freshwater input—A case study. Chem. Eng. Trans. 2021, 87, 355–360. [Google Scholar]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and economic aspects of Spirulina-based biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- The European Market Potential for Chlorella and Spirulina for Health Products|CBI. Available online: https://www.cbi.eu/market-information/natural-ingredients-health-products/chlorella-and-spirulina/market-potential (accessed on 29 July 2024).
- Shah, M.A.R.; Zhu, F.; Cui, Y.; Hu, X.; Chen, H.; Kayani, S.I.; Huo, S. Mechanistic insights into the nutritional and therapeutic potential of Spirulina (Arthrospira) spp.: Challenges and opportunities. Trends Food Sci. Technol. 2024, 151, 104648. [Google Scholar] [CrossRef]
- Citi, V.; Torre, S.; Flori, L.; Usai, L.; Aktay, N.; Dunford, N.T.; Lutzu, G.A.; Nieri, P. Nutraceutical Features of the Phycobiliprotein C-Phycocyanin: Evidence from Arthrospira platensis (Spirulina). Nutrients 2024, 16, 1752. [Google Scholar] [CrossRef] [PubMed]
- Farkha, T.K.J. Fatty Acids, Minerals composition, and antimicrobial activity of the blue-green microalga (Spirulina platensis) isolated from Qilyasan stream, Sulaimani, Kurdistan, Iraq. Egypt J. Aquat. Biol. Fish. 2023, 27, 187–201. [Google Scholar] [CrossRef]
- Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the benefits of phycocyanin: From Spirulina cultivation to its widespread applications. Pharmaceuticals 2023, 16, 592. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, B.; Gupta, S.K.; Cho, W.M.; Kim, J.K.; Lee, S.R.; Jeong, K.H.; Lee, D.J.; Choi, H.C. Microalgae potential and multiple roles—Current progress and future prospects—An overview. Sustainability 2016, 8, 1215. [Google Scholar] [CrossRef]
- Park, W.S.; Kim, H.J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.S.; Kang, C.M.; Ferruzzi, M.G.; Ahn, M.J. Two classes of pigments, carotenoids and c-phycocyanin, in Spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Liestianty, D.; Rodianawati, I.; Arfah, R.A.; Assa, A.; Patimah; Sundari; Muliadi. Nutritional analysis of Spirulina sp. to promote as superfood candidate. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 13th Joint Conference on Chemistry (13th JCC), Semarang, Indonesia, 7–8 September 2018; IOP Publishing: Bristol, UK, 2019; Volume 509, p. 012031. [Google Scholar]
- Rahim, A.; Çakir, C.; Ozturk, M.; Şahin, B.; Soulaimani, A.; Sibaoueih, M.; Nasser, B.; Eddoha, R.; Essamadi, A.; El Amiri, B. Chemical characterization and nutritional value of Spirulina platensis cultivated in natural conditions of Chichaoua region (Morocco). S. Afr. J. Bot. 2021, 141, 235–242. [Google Scholar] [CrossRef]
- Hernández-Lepe, M.A.; López-Díaz, J.A.; Juárez-Oropeza, M.A.; Hernández-Torres, R.P.; Wall-Medrano, A.; Ramos-Jiménez, A. Effect of Arthrospira (Spirulina) maxima supplementation and a systematic physical exercise program on the body composition and cardiorespiratory fitness of overweight or obese subjects: A double-blind, randomized, and crossover controlled trial. Mar. Drugs 2018, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, R.; Bragança, L.; da Silva, M.V.; Oliveira, R.S. Challenges and Solutions for sustainable food systems: The potential of home hydroponics. Sustainability 2024, 16, 817. [Google Scholar] [CrossRef]
- Michael, A.; Kyewalyanga, M.S.; Mtolera, M.S.; Lugomela, C.V. Antioxidants activity of the cyanobacterium, Arthrospira (Spirulina) fusiformis cultivated in a low-cost medium. Afr. J. Food Sci. 2018, 12, 188–195. [Google Scholar]
- Yin, C.; Daoust, K.; Young, A.; Tebbs, E.J.; Harper, D.M. Tackling community undernutrition at Lake Bogoria, Kenya: The potential of Spirulina (Arthrospira fusiformis) as a food supplement. Afr. J. Food. 2017, 17, 11603–11615. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Liu, R.; Qin, S.; Li, W. Phycocyanin: Anti-inflammatory effect and mechanism. Biomed. Pharmacother. 2022, 153, 113362. [Google Scholar] [CrossRef]
- Nova, M.; Citterio, S.; Martegani, E.; Colombo, S. Unraveling the anti-aging properties of phycocyanin from the cyanobacterium Spirulina (Arthrospira platensis). Int. J. Mol. Sci. 2024, 25, 4215. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Morales, I.S.; Trujillo-Valle, L.; Márquez-Rocha, F.J.; Hernández, J.F.L. Tocopherols, phycocyanin and superoxide dismutase from microalgae: As potential food antioxidants. Appl. Food Biotechnol. 2018, 5, 19–27. [Google Scholar]
- Choopani, A.; Poorsoltan, M.; Fazilati, M.; Latifi, A.M.; Salavati, H. Spirulina: A source of gamma-linoleic acid and its applications. J. Appl. Biotechnol. Rep. 2016, 3, 483–488. [Google Scholar]
- Jie, X.; Xuan, C.J.; Xi, W.T.; Fan, L.; Jie, X.; Xuan, C.J.; Xi, W.T.; Fan, L. Gamma-linolenic Acid from a Blue-Green alga Spirulina platensis Might Alleviate PM2.5 Induced Damages in A549 Cells. Biomed. Environ. Sci. 2023, 36, 299–303. [Google Scholar]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulina platensis. Food Chem. 2008, 109, 580–586. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Spirulina lipids alleviate oxidative stress and inflammation in mice fed a high-fat and high-sucrose diet. Mar. Drugs 2020, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Wu, W.; Xu, Y.; Li, X.; Qiu, Q.; Chen, H. Effects on Spirulina supplementation on immune cells’ parameters of elite college athletes. Nutrients 2022, 14, 4346. [Google Scholar] [CrossRef] [PubMed]
- Hirahashi, T.; Matsumoto, M.; Hazeki, K.; Saeki, Y.; Ui, M.; Seya, T. Activation of the human innate immune system by Spirulina: Augmentation of interferon production and NK cytotoxicity by oral administration of hot water extract of Spirulina platensis. Int. Immunopharmacol. 2002, 2, 423–434. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Liu, Y.; Yang, Y.; Shi, F.; Cheong, K.L.; Teng, B. Immunostimulatory effects of polysaccharides from Spirulina platensis in vivo and vitro and their activation mechanism on RAW246.7 macrophages. Mar. Drugs 2020, 18, 538. [Google Scholar] [CrossRef]
- Cai, B.; Zhao, X.; Luo, L.; Wan, P.; Chen, H.; Pan, J. Structural characterization, and in vitro immunostimulatory and antitumor activity of an acid polysaccharide from Spirulina platensis. Int. J. Biol. Macromol. 2022, 196, 46–53. [Google Scholar] [CrossRef]
- Akao, Y.; Ebihara, T.; Masuda, H.; Saeki, Y.; Akazawa, T.; Hazeki, K.; Hazeki, O.; Matsumoto, M.; Seya, T. Enhancement of antitumor natural killer cell activation by orally administered Spirulina extract in mice. Cancer Sci. 2009, 100, 1494–1501. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yang, S.; Lu, Z.; Li, G.; Liu, J.; Zhou, B.; Wu, D.; Wang, L. Isolation, Purification, Characterization, and Immunomodulatory Activity Analysis of α-Glucans from Spirulina platensis. ACS Omega 2021, 6, 21384–21394. [Google Scholar] [CrossRef]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally occurring immune modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef] [PubMed]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid.-Based Complement. Altern. Med. 2011, 2011, 531053. [Google Scholar] [CrossRef]
- Janda-Milczarek, K.; Szymczykowska, K.; Jakubczyk, K.; Kupnicka, P.; Skonieczna-Żydecka, K.; Pilarczyk, B.; Tomza-Marciniak, A.; Ligenza, A.; Stachowska, E.; Dalewski, B. Spirulina supplements as a source of mineral nutrients in the daily diet. Appl. Sci. 2023, 13, 1011. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.H. Spirulina platensis, a super food? J. Cell. Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Aouir, A.; Amiali, M.; Bitam, A.; Benchabane, A.; Raghavan, V.G. Comparison of the biochemical composition of different Arthrospira platensis strains from Algeria, Chad and the USA. J. Food Meas. Charact. 2017, 11, 913–923. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and technological advancements in the possible food applications of Spirulina and their health benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial effects of Spirulina consumption on brain health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef]
- Stunda-Zujeva, A.; Berele, M.; Lece, A.; Šķesters, A. Comparison of antioxidant activity in various spirulina containing products and factors affecting it. Sci. Rep. 2023, 13, 4529. [Google Scholar] [CrossRef]
- Stunda-Zujeva, A.; Berele, M. Algae as a Functional Food: A Case Study on Spirulina. In Value-Added Products from Algae: Phycochemical Production and Applications; Springer International Publishing: Cham, Switzerland, 2024; pp. 563–594. [Google Scholar]
- Alshuniaber, M.A.; Krishnamoorthy, R.; AlQhtani, W.H. Antimicrobial activity of polyphenolic compounds from Spirulina against food-borne bacterial pathogens. Saudi J. Biol. Sci. 2021, 28, 459. [Google Scholar] [CrossRef]
- Dasankoppa, F.S.; Sholapur, H.N.; Byahatti, A.; Abbas, Z.; Komal, S.K.; Subrata, K. Application of Response Surface optimization methodology in designing ordispersible tablets of antdiabetic drug. J. Young Pharm. 2020, 12, 173–177. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Li, X.; Lai, X.; Yang, M.; Liu, F.; Luan, F.; Meng, X. Polysaccharides from Spirulina platensis: Extraction methods, structural features and bioactivities diversity. Int. J. Biol. Macromol. 2023, 231, 123211. [Google Scholar] [CrossRef]
- Kuhnholz, J.; Glockow, T.; Siebecke, V.; Le, A.T.; Tran, L.D.; Noke, A. Comparison of different methods for extraction of phycocyanin from the cyanobacterium Arthrospira maxima (Spirulina). J. Appl. Phycol. 2024, 36, 1725–1735. [Google Scholar] [CrossRef]
- Neag, E.; Stupar, Z.; Varaticeanu, C.; Senila, M.; Roman, C. Optimization of lipid extraction from Spirulina spp. by ultrasound application and mechanical stirring using the Taguchi method of experimental design. Molecules 2022, 27, 6794. [Google Scholar] [CrossRef]
- Fabre, J.-F.; Niangoran, N.U.F.; Gaignard, C.; Buso, D.; Mouloungui, Z.; Valentin, R. Extraction, purification and stability of C-phycocyanin from Arthrospira platensis. Eur. Food Res. Technol. 2022, 248, 1583–1599. [Google Scholar] [CrossRef]
- Nikolova, K.; Petkova, N.; Mihaylova, D.; Gentscheva, G.; Gavrailov, G.; Pehlivanov, I.; Andonova, V. Extraction of phycocyanin and chlorophyll from Spirulina by “Green methods”. Separations 2024, 11, 57. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Huang, Y.; Yuan, Y.; Ma, Q.; Du, M.; Cai, T.; Cai, Y. Extraction of polysaccharide from Spirulina and evaluation of its activities. Evid. Based Complement. Altern. Med. 2018, 2018, 3425615. [Google Scholar] [CrossRef] [PubMed]
- Nahid, N.; Azam, A.; Ejhieh, N.; Reza, A. Intensified Phycobiliprotein Extraction from Spirulina platensis by Freezing and Ultrasound Methods. Iran. J. Chem. Chem. Eng. 2023, 42, 601–617. [Google Scholar]
- Verdasco-Martín, C.M.; Díaz-Lozano, A.; Otero, C. Advantageous enzyme selective extraction process of essential Spirulina oil. Catal. Today 2020, 346, 121–131. [Google Scholar] [CrossRef]
- Silva, N.C.; Graton, I.S.; Duarte, C.R.; Barrozo, M.A.S. Effects of infrared and microwave radiation on the bioactive compounds of microalga Spirulina platensis after continuous and intermittent drying. Molecules 2023, 28, 5963. [Google Scholar] [CrossRef]
- Colla, L.M.; Bertol, C.D.; Ferreira, D.J.; Bavaresco, J.; Costa, J.A.V.; Bertolin, T.E. Thermal and photo-stability of the antioxidant potential of Spirulina platensis powder. Braz. J. Biol. 2017, 77, 332–339. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Functional and Bioactive Properties of protein extracts generated from Spirulina platensis and Isochrysis galbana T-Iso. Appl. Sci. 2021, 11, 3964. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Pallarés, N.; Dawidowicz, K.; Ruiz, M.J.; Barba, F.J. Enhancing nutrient recovery and bioactive compound extraction from Spirulina through supercritical fluid extraction: Implications for SH-SY5Y Cell Viability. Foods 2023, 12, 2509. [Google Scholar] [CrossRef]
- Katari, J.K.; Uz Zama Khan, M.R.; Trivedi, V.; Das, D. Extraction, purification, characterization and bioactivity evaluation of high purity C-phycocyanin from Spirulina sp. NCIM 5143. Process. Biochem. 2023, 130, 322–333. [Google Scholar] [CrossRef]
- Lauceri, R.; Cavone, C.; Chini Zittelli, G.; Kamburska, L.; Musazzi, S.; Torzillo, G. High purity grade phycocyanin recovery by decupling cell lysis from the pigment extraction: An innovative approach. Food Bioprocess Technol. 2023, 16, 111–121. [Google Scholar] [CrossRef]
- Khandual, S.; Sanchez, E.O.L.; Andrews, H.E.; de la Rosa, J.D.P. Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: Efficient extraction process and stability evaluation of phycocyanin. BMC Chem. 2021, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, S.; Kyriakopoulou, K.; Tzovenis, I.; Krokida, M. Environmental impact of phycocyanin recovery from Spirulina platensis cyanobacterium. Innov. Food Sci. Emerg. Technol. 2017, 44, 217–223. [Google Scholar] [CrossRef]
- Muhammad, M.; Mohammad, S.; Ketut, S.I.; Rahmatang. Environmental-friendly extraction methods to produce bioactive compounds in seaweed. Res. J. Chem. Environ. 2023, 27, 114–121. [Google Scholar]
- Cardoso, L.G.; Lombardi, A.T.; de Jesus Silva, J.S.; Lemos, P.V.F.; Costa, J.A.V.; de Souza, C.O.; Druzian, J.I.; Chinalia, F.A. Scaling-up production of Spirulina sp. LEB18 grown in aquaculture wastewater. Aquaculture 2021, 544, 737045. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K.S.M.S. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of phycocyanin—A natural blue colorant from dried Spirulina biomass: Influence of processing parameters and extraction techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99, 1042–1047. [Google Scholar] [CrossRef]
- Lee, C.W.; Bae, G.Y.; Bae, S.H.; Suh, H.J.; Jo, K. Increased thermal stability of phycocyanin extracted from Spirulina platensis by cysteine addition during enzyme extraction. Food Sci. Technol. 2021, 42, e15021. [Google Scholar] [CrossRef]
- Benelhadj, S.; Douiri, S.; Ghouilli, A.; Hassen, R.B.; Keshk, S.M.A.S.; El-kott, A.; Attia, H.; Ghorbel, D. Extraction of Arthrospira platensis (Spirulina) proteins via Osborne sequential procedure: Structural and functional characterizations. J. Food Compos. Anal. 2023, 115, 104984. [Google Scholar] [CrossRef]
- Costa, E.; Ribeiro, M.; Filipe-Ribeiro, L.; Cosme, F.; Nunes, F.M. Protein extraction from Arthrospira platensis for use in food processing. Med. Sci. Forum 2024, 23, 8. [Google Scholar] [CrossRef]
- Silva, A.S.; Ghisi, E. Evaluation of capabilities of different global sensitivity analysis techniques for building energy simulation: Experiment on design variables. Ambient. Construído 2021, 21, 89–111. [Google Scholar] [CrossRef]
- Sela, K.; Budhijanto, W.; Budiman, A. Protein Extraction from Spirulina platensis by Using Ultrasound Assisted Extraction: Effect of Solvent Types and Extraction Time. Key Eng. Mater. 2021, 872, 33–37. [Google Scholar] [CrossRef]
- Chi, H.; Nguyen Doan Duy, L.; The Duy, P.; Thi Thanh Hoa, L.; Thi Xuan Quynh, N.; Dang Ninh, N. Protein Extraction from Spirulina platensis with The Cellulase Enzyme Assistance. J. Tech. Educ. Sci. 2022, 17, 25–32. [Google Scholar]
- Purdi, T.S.; Setiowati, A.D.; Ningrum, A. Ultrasound-assisted extraction of Spirulina platensis protein: Physicochemical characteristic and techno-functional properties. J. Food Meas. Charact. 2023, 17, 5474–5486. [Google Scholar] [CrossRef]
- Rajakumar, M.S.; Muthukumar, K. Influence of pre-soaking conditions on ultrasonic extraction of Spirulina platensis proteins and its recovery using aqueous biphasic system. Sep. Sci. Technol. 2018, 53, 2034–2043. [Google Scholar] [CrossRef]
- Parimi, N.S.; Singh, M.; Kastner, J.R.; Das, K.C.; Forsberg, L.S.; Azadi, P. Optimization of protein extraction from Spirulina platensis to generate a potential co-product and a biofuel feedstock with reduced nitrogen content. Front. Energy Res. 2015, 3, 136957. [Google Scholar] [CrossRef]
- Sánchez-Zurano, A.; Morillas-España, A.; González-López, C.V.; Lafarga, T. Optimisation of protein recovery from Arthrospira platensis by ultrasound-assisted isoelectric solubilisation/precipitation. Processes 2020, 8, 1586. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef]
- Thi Dong Phuong, N.; Van Anh, L.T.; Thi Dieu Huong, L.; Thi My Yen, P. Extraction of polysaccharide from Spirulina platensis—Advantage of freeze-thaw method. Vietnam J. Biotechnol. 2021, 18, 693–699. [Google Scholar] [CrossRef]
- Al-Badwy, A.H.; Khalil, A.M.; Bashal, A.H.; Kebeish, R. Polysaccharides from Spirulina platensis (PSP): Promising biostimulants for the green synthesis of silver nanoparticles and their potential application in the treatment of cancer tumors. Microb. Cell Factories 2023, 22, 247. [Google Scholar] [CrossRef] [PubMed]
- Kalsum, L.; Dewi, E.; Margarety, E.; Ningsih, A.S. Lipid extraction from microalgae Spirulina platensis for raw materials of Biodiesel. J. Phys. Conf. Ser. 2019, 1167, 012051. [Google Scholar] [CrossRef]
- Pinto, L.F.R.; Ferreira, G.F.; Beatriz, F.P.; Cabral, F.A.; Maciel Filho, R. Lipid and phycocyanin extractions from Spirulina and economic assessment. J. Supercrit. Fluids 2022, 184, 105567. [Google Scholar] [CrossRef]
- Rizwanul Fattah, I.M.; Noraini, M.Y.; Mofijur, M.; Silitonga, A.S.; Badruddin, I.A.; Yunus Khan, T.M.; Ong, H.C.; Mahlia, T.M.I. Lipid Extraction Maximization and Enzymatic Synthesis of Biodiesel from Microalgae. Appl. Sci. 2020, 10, 6103. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Adetya, N.P. Response surface optimization of lipid and protein extractions from Spirulina platensis using ultrasound assisted osmotic shock method. Food Sci. Biotechnol. 2018, 27, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Nikitine, C.; Zaier, O.; Lépine, O.; Tanzi, C.D.; Vian, A.; Chemat, F.; Badens, E. Oil extraction from enriched Spirulina platensis microalgae usingă supercritical carbon dioxide. J. Supercrit. Fluids 2017, 119, 289–296. [Google Scholar] [CrossRef]
- Alotaiby, S.; Zhao, X.; Boesch, C.; Sergeeva, N.N. Sustainable approach towards isolation of photosynthetic pigments from Spirulina and the assessment of their prooxidant and antioxidant properties. Food Chem. 2024, 436, 137653. [Google Scholar] [CrossRef]
- Martins, R.; Mouro, C.; Pontes, R.; Nunes, J.; Gouveia, I. Natural Deep Eutectic Solvent Extraction of Bioactive Pigments from Spirulina platensis and Electrospinning Ability Assessment. Polymers 2023, 15, 1574. [Google Scholar] [CrossRef] [PubMed]
- Marzorati, S.; Schievano, A.; Idà, A.; Verotta, L. Carotenoids, chlorophylls and phycocyanin from Spirulina: Supercritical CO2 and water extraction methods for added value products cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Bougatef, H.; Hadrich, F.; Gazbar, M.; Sila, A.; Chamkha, M.; Bougatef, A. Development of a novel method for the extraction of phycocyanin pigment from Spirulina platensis and assessment of its antioxidant, antimicrobial, and anticancer activities. Biomass Convers. Biorefinery 2024, 1–13. [Google Scholar] [CrossRef]
- Julianti, E.; Susanti; Singgih, M.; Neti Mulyani, L. Optimization of extraction method and characterization of phycocyanin pigment from spirulina platensis. J. Math. Fundam. Sci. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Ahmad, A.M.R.; Intikhab, A.; Zafar, S.; Farooq, U.; Shah, H.B.U.; Akram, S.; Abid, J.; Parveen, Z.; Iqbal, S. Spirulina, an FDA-Approved Functional Food: Worth the Hype? Cell. Mol. Biol. 2023, 69, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Andaregie, A.; Sasaki, S.; Shimura, H.; Chikasada, M.; Sato, S.; Addisu, S.; Astatkie, T.; Takagi, I. Promoting Spirulina-enriched bread for primary school children in Ethiopia: Assessing parental willingness to purchase through information nudging. Appl. Food Res. 2024, 4, 100403. [Google Scholar] [CrossRef]
- Fanzo, J.; Rudie, C.; Sigman, I.; Grinspoon, S.; Benton, T.G.; Brown, M.E.; Covic, N.; Fitch, K.; Golden, C.D.; Grace, D.; et al. Sustainable food systems and nutrition in the 21st century: A report from the 22nd annual Harvard Nutrition Obesity Symposium. Am. J. Clin. Nutr. 2022, 115, 18. [Google Scholar] [CrossRef]
- Çakmakçı, R.; Salık, M.A.; Çakmakçı, S. Assessment and principles of environmentally sustainable food and agriculture systems. Agriculture 2023, 13, 1073. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Afraz, M.T.; Yılmaz, B.B.; Adil, M.; Arshad, N.; Goksen, G.; Ali, M.; Zeng, X.A. Recent progress in natural seaweed pigments: Green extraction, health-promoting activities, techno-functional properties and role in intelligent food packaging. J. Agric. Food Res. 2024, 15, 100991. [Google Scholar] [CrossRef]
- Bürck, M.; Fratelli, C.; de Amarante, M.C.A.; Braga, A.R.C. Unveiling the potential of Spirulina biomass—A Glimpse into the future circular economy using green and blue ingredients. Biomass 2024, 4, 704–719. [Google Scholar] [CrossRef]
- Asghari, A.; Fazilati, M.; Latifi, A.M.; Salavati, H.; Choopani, A. A Review on antioxidant properties of Spirulina. J. Appl. Biotechnol. Rep. Rev. Artic. J. Appl. Biotechnol. Rep. 2016, 3, 345–351. [Google Scholar]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for skin care: A bright blue future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Li, T.T.; Tong, A.J.; Liu, Y.Y.; Huang, Z.R.; Wan, X.Z.; Pan, Y.Y.; Jia, R.B.; Liu, B.; Chen, X.H.; Zhao, C. Polyunsaturated fatty acids from microalgae Spirulina platensis modulates lipid metabolism disorders and gut microbiota in high-fat diet rats. Food Chem. Toxicol. 2019, 131, 110558. [Google Scholar] [CrossRef]
- Chei, S.; Oh, H.J.; Song, J.H.; Seo, Y.J.; Lee, K.; Kim, K.J.; Lee, B.Y. Spirulina maxima extract prevents activation of the NLRP3 inflammasome by inhibiting ERK signaling. Sci. Rep. 2020, 10, 2075. [Google Scholar] [CrossRef] [PubMed]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan Bhatt, A.; Kumar Nishad, D.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis- In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]
- Prete, V.; Abate, A.C.; Di Pietro, P.; De Lucia, M.; Vecchione, C.; Carrizzo, A. Beneficial effects of Spirulina supplementation in the management of cardiovascular diseases. Nutrients 2024, 16, 642. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.J. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Chaouachi, M.; Vincent, S.; Groussard, C. A Review of the health-promoting properties of Spirulina with a focus on athletes’ performance and recovery. J. Diet. Suppl. 2024, 21, 210–241. [Google Scholar] [CrossRef] [PubMed]
- Zen, C.K.; Tiepo, C.B.V.; da Silva, R.V.; Reinehr, C.O.; Gutkoski, L.C.; Oro, T.; Colla, L.M. Development of functional pasta with microencapsulated Spirulina: Technological and sensorial effects. J. Sci. Food Agric. 2020, 100, 2018–2026. [Google Scholar] [CrossRef]
- Hernández-López, I.; Alamprese, C.; Cappa, C.; Prieto-Santiago, V.; Abadias, M.; Aguiló-Aguayo, I. Effect of Spirulina in Bread Formulated with Wheat Flours of Different Alveograph Strength. Foods 2023, 12, 3724. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Effect of spirulina addition on the physicochemical and structural properties of extruded snacks. Food Sci. Technol. 2017, 37, 16–23. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernández, F.G.; Castellari, M.; Villaró, S.; Bobo, G.; Aguiló-Aguayo, I. Effect of microalgae incorporation on the physicochemical, nutritional, and sensorial properties of an innovative broccoli soup. LWT 2019, 111, 167–174. [Google Scholar] [CrossRef]
- Batista de Oliveira, T.T.; Miranda dos Reis, I.; Bastos de Souza, M.; da Silva Bispo, E.; Fonseca Maciel, L.; Druzian, J.I.; Lordelo Guimarães Tavares, P.P.; de Oliveira Cerqueira, A.; dos Santos Boa Morte, E.; Abreu Glória, M.B.; et al. Microencapsulation of Spirulina sp. LEB-18 and its incorporation in chocolate milk: Properties and functional potential. LWT 2021, 148, 111674. [Google Scholar] [CrossRef]
- Zarzycki, P.; Wirkijowska, A.; Blicharz-Kania, A.; Marzec, A.; Kramarczuk, P.; Kowalska, H.; Kowalska, J. Effect of type of flour and microalgae (Chlorella vulgaris) on the rheological, microstructural, textural, and sensory properties of vegan muffins. Appl. Sci. 2023, 13, 7632. [Google Scholar] [CrossRef]
- Niccolai, A.; Venturi, M.; Galli, V.; Pini, N.; Rodolfi, L.; Biondi, N.; D’Ottavio, M.; Batista, A.P.; Raymundo, A.; Granchi, L.; et al. Development of new microalgae-based sourdough “crostini”: Functional effects of Arthrospira platensis (Spirulina) addition. Sci. Rep. 2019, 9, 19433. [Google Scholar] [CrossRef]
- Oliveira, B.C.C.; Machado, M.; Machado, S.; Costa, A.S.G.; Bessada, S.; Alves, R.C.; Oliveira, M.B.P.P. Algae incorporation and nutritional improvement: The case of a whole-wheat pasta. Foods 2023, 12, 3039. [Google Scholar] [CrossRef]
- Arslan, R.; Aksay, S. Investigation of sensorial and physicochemical properties of yoghurt colored with phycocyanin of Spirulina platensis. J. Food Process. Preserv. 2022, 46, e15941. [Google Scholar] [CrossRef]
- Nourmohammadi, N.; Soleimanian-Zad, S.; Shekarchizadeh, H. Effect of Spirulina (Arthrospira platensis) microencapsulated in alginate and whey protein concentrate addition on physicochemical and organoleptic properties of functional stirred yogurt. J. Sci. Food Agric. 2020, 100, 5260–5268. [Google Scholar] [CrossRef]
- Golmakani, M.T.; Soleimanian-Zad, S.; Alavi, N.; Nazari, E.; Eskandari, M.H. Effect of Spirulina (Arthrospira platensis) powder on probiotic bacteriologically acidified feta-type cheese. J. Appl. Phycol. 2019, 31, 1085–1094. [Google Scholar] [CrossRef]
- Almeida, L.M.R.; da Silva Cruz, L.F.; Machado, B.A.S.; Nunes, I.L.; Costa, J.A.V.; de Souza Ferreira, E.; Lemos, P.V.F.; Druzian, J.I.; de Souza, C.O. Effect of the addition of Spirulina sp. biomass on the development and characterization of functional food. Algal Res. 2021, 58, 102387. [Google Scholar] [CrossRef]
- Saharan, V.; Jood, S. Effect of storage on Spirulina platensis powder supplemented breads. J. Food Sci. Technol. 2021, 58, 978. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez De Marco, E.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of spirulina biomass on the technological and nutritional quality of bread wheat pasta. LWT Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- Montevecchi, G.; Santunione, G.; Licciardello, F.; Köker, Ö.; Masino, F.; Antonelli, A. Enrichment of wheat flour with Spirulina. Evaluation of thermal damage to essential amino acids during bread preparation. Food Res. Int. 2022, 157, 111357. [Google Scholar] [CrossRef]
- Üstün-Aytekin, Ö.; Çoban, I.; Aktaş, B. Nutritional value, sensory properties, and antioxidant activity of a traditional kefir produced with Arthrospira platensis. J. Food Process. Preserv. 2022, 46, e16380. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A.; Majewska, B. Effect of Spirulina (Spirulina platensis) addition on textural and quality properties of cookies. Ital. J. Food Sci. 2018, 30, 2018–2020. [Google Scholar]
- Erem, F. Investigation of the effects of corn flour, Spirulina powder, and buffalo yogurt on the quality characteristics of gluten-free muffins. Food Sci. Technol. Int. 2024. [Google Scholar] [CrossRef]
- State of Technology Review-Algae Bioenergy An IEA Bioenergy Inter-Task Strategic Project. 2017. Available online: https://www.ieabioenergy.com/wp-content/uploads/2017/01/IEA-Bioenergy-Algae-report-update-20170114.pdf (accessed on 15 June 2024).
- Spirulina Market by Product Type (Powder, Tablets, Capsules, Flakes, Phycocyanin), Distribution Channel (Business Channel, Consumer Channel), Application (Nutraceuticals, Food & Beverages, Animal Feed, Cosmetics, Agriculture)—Global Forecast to 2030. Available online: https://www.giiresearch.com/report/meti1266319-spirulina-market-by-product-type-powder-tablets.html (accessed on 29 July 2024).

| Challenges | Solutions | |
|---|---|---|
| Environmental Control | Temperature:Spirulina requires temperatures between 35 °C and 37 °C [42]. Light: Needs optimal light conditions for photosynthesis [42]. pH Levels: Requires a highly alkaline environment (pH 8.5–10.5) [1]. Water Quality: High-quality water is essential. | Temperature Control: Use of greenhouses or temperature-regulated systems can help maintain optimal temperatures. Light Management: Implementing artificial lighting or shading systems can control light intensity. pH Regulation: Regular monitoring and adjustment of pH using buffers can maintain the required alkalinity. Water Treatment: Utilizing filtration and water treatment systems to ensure water quality and prevent contamination. |
| Contamination | Competing Algae and Microorganisms: Invasion by other microorganisms. Predators: Protozoa and zooplankton feeding on Spirulina. | Sterile Techniques: Implementing sterile techniques during inoculation and handling. Closed Systems: Using closed photobioreactors reduces the risk of contamination. Biocontrol Agents: Employing beneficial microorganisms or biocides to control unwanted species. |
| Nutrient Supply Challenges | Nutrient Requirements: Consistent supply of nutrients. Cost of Nutrients: High costs of nutrients like nitrogen, phosphorus, and trace elements [43]. | Nutrient Recycling: Implementing nutrient recycling systems can reduce costs. Alternative Sources: Using cheaper or more sustainable nutrient sources, such as agricultural byproducts. Optimized Formulations: Developing optimized nutrient formulations to minimize waste. |
| Production System Challenges | Open vs. Closed Systems: Balancing contamination risk and cost [44,45]. Scalability: Maintaining efficiency and consistency at larger scales. | Hybrid Systems: Combining the advantages of open and closed systems. Modular Designs: Using modular photobioreactors for easier scalability. Automation: Implementing automated monitoring and control systems to maintain optimal conditions. |
| Harvesting and Processing Challenges | Efficient Harvesting: Need for efficient and cost-effective harvesting methods [46]. Post-Harvest Processing: Energy-intensive drying and processing methods [46]. | Innovative Harvesting Techniques: Using methods like flocculation, electroflotation, or membrane filtration. Energy-Efficient Drying: Implementing solar drying or low-energy dehydration techniques. Integrated Processing: Developing integrated processing systems that combine harvesting and drying to reduce energy use. |
| Economic and Market Factors Challenges | Production Costs: High costs of cultivation and processing [47]. Market Demand: Fluctuations in demand and prices [48]. Regulatory Issues: Compliance with food safety and quality regulations. | Cost Reduction: Streamlining operations and using cost-effective technologies. Market Diversification: Exploring new markets and applications for Spirulina. Regulatory Compliance: Staying updated with regulations and implementing quality control systems to ensure compliance. |
| Spirulina Component | Mechanism of Action |
|---|---|
| Phycocyanin | Antioxidant Activity: Phycocyanin scavenges reactive oxygen species (ROS) and reactive nitrogen species (RNS), reducing oxidative stress at the cellular level. It protects cells from damage by neutralizing free radicals, which can otherwise lead to chronic inflammation and various diseases, including cancer [62,63,64,65]. |
| Anti-Inflammatory Effects: Phycocyanin inhibits the enzyme cyclooxygenase-2 (COX-2), which is involved in the synthesis of pro-inflammatory prostaglandins. It also downregulates the expression of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). This dual action helps in reducing inflammation and providing relief from inflammatory conditions such as arthritis [62,63,64,65]. | |
| Gamma-Linolenic Acid | Anti-Inflammatory Pathway: Once ingested, GLA is converted in the body to dihomo-γ-linolenic acid (DGLA), which competes with arachidonic acid for the same enzymatic pathways. This competition results in the production of anti-inflammatory prostaglandins (PGE1) instead of pro-inflammatory ones (PGE2). This shift helps to reduce inflammation, making GLA effective in managing conditions such as rheumatoid arthritis and eczema [66,67,68,69,70]. |
| Skin Health: GLA’s role in maintaining skin barrier integrity and reducing transepidermal water loss makes it beneficial in treating skin conditions like atopic dermatitis [66,67,68,69,70]. | |
| Polysaccharides | Immune Modulation: These polysaccharides enhance the activity of macrophages, natural killer (NK) cells, and T-lymphocytes. They stimulate the production of cytokines such as interferon-gamma (IFN-γ) and interleukin-1 (IL-1), which boost the immune response against infections and tumors [71,72,73,74,75,76]. |
| Antiviral Activity: The sulfated polysaccharides in Spirulina can inhibit the replication of viruses by mimicking heparan sulfate, a molecule that viruses typically bind to for entry into host cells. By blocking this interaction, these polysaccharides prevent viruses like HIV and herpes simplex virus from attaching to and entering human cells, thereby reducing infection rates [71,72,73,74,75,76]. | |
| Vitamins and Minerals | B-Complex Vitamins: These are essential for energy metabolism, particularly in converting carbohydrates, fats, and proteins into energy. They also play a key role in maintaining nerve function and red blood cell formation [5,77,78,79,80,81,82,83]. |
| Vitamin E: As a potent antioxidant, vitamin E protects cells from oxidative damage by neutralizing free radicals. This vitamin is particularly important for skin health and immune function [5,77,78,79,80,81,82,83]. | |
| Iron: The bioavailable iron in Spirulina helps in the formation of hemoglobin and myoglobin, which are crucial for oxygen transport in the blood and muscle tissues. This makes Spirulina an excellent supplement for preventing or treating iron-deficiency anemia [5,77,78,79,80,81,82,83]. | |
| Calcium and Magnesium: These minerals are vital for bone health, muscle function, and nerve transmission. They also play a role in cardiovascular health by regulating blood pressure and heart rhythm [5,77,78,79,80,81,82,83]. | |
| Selenium Antioxidant Defense: Selenium’s role in the formation of selenoproteins enhances the body’s antioxidant defense system, reducing the risk of chronic diseases linked to oxidative stress, including cancer and cardiovascular diseases [5,77,78,79,80,81,82,83]. Selenium Thyroid Function: Selenium is crucial for the synthesis of thyroid hormones, which regulate metabolism, growth, and development. Adequate selenium intake can help prevent thyroid-related disorders [5,77,78,79,80,81,82,83]. | |
| Phenolic Compounds | Antioxidant Action: Phenolic compounds scavenge free radicals and chelate metal ions, reducing oxidative stress. This action protects cells from DNA damage, which can lead to mutations and cancer development [50,80,81,83,84,85,86]. |
| Anti-Cancer Potential: Some phenolic compounds in Spirulina can induce apoptosis (programmed cell death) in cancer cells and inhibit their proliferation by interfering with cell cycle progression. This makes Spirulina a potential adjunct in cancer therapy [50,80,81,83,84,85,86]. |
| Challenges | Possible Solutions |
|---|---|
| Complex Matrix: Spirulina has a complex matrix with various components like proteins, polysaccharides, lipids, and pigments, which can interfere with the extraction of specific bioactive compounds [89,90,91]. | Use selective extraction methods such as supercritical fluid extraction or enzyme-assisted extraction to target specific bioactive compounds while minimizing interference from other components [92,93,94,95]. |
| Thermal Sensitivity: Many bioactive compounds in Spirulina, such as phycocyanin and vitamins, are sensitive to heat, which can lead to their degradation during extraction [96,97,98]. | Employ non-thermal extraction techniques like cold press extraction, ultrasonic-assisted extraction, or supercritical CO2; extraction to preserve heat-sensitive bioactives. |
| Solubility Issues: Some bioactive compounds have poor solubility in conventional solvents, making their extraction challenging [3,99,100]. | Utilize a combination of solvents or surfactants, or explore solvent-free techniques like supercritical fluid extraction to enhance the solubility and extraction efficiency of these compounds. |
| Yield and Purity: Achieving high yield and purity of specific bioactive compounds can be difficult due to the presence of multiple compounds with similar properties [101,102,103]. | Optimize extraction conditions (e.g., solvent type, pH, temperature, and time) and implement purification techniques such as chromatography or membrane filtration to improve yield and purity. |
| Environmental and Health Concerns: The use of organic solvents in extraction processes can pose environmental and health risks [18,104,105]. | Adopt green extraction methods such as supercritical fluid extraction, pressurized liquid extraction, or ionic liquid-based extraction, which are more environmentally friendly and safer. |
| Scalability: Scaling up laboratory-scale extraction processes to industrial levels can be challenging due to differences in efficiency and cost [105,106]. | Conduct pilot-scale studies to optimize and validate extraction processes before full-scale production. Utilize scalable and cost-effective technologies. |
| Extractable Component | Extraction Method and Extraction Solvent | Extraction Yield | Reference |
|---|---|---|---|
| Proteins | Osborne sequential extraction procedure. | Extracted three fractions of albumins (51.5%), globulins 2.4%), and prolamins (46.1%). | [114] |
| Agitation, bead milling, ultrasound, and protein isolation via precipitation using ethanol. | The most successful protein extraction method involved bead milling for 24 h, the addition of 1 M NaCl, and pH adjustment to 7, followed by precipitation with 75% ethanol, as shown in Figure 2. This method yielded a protein content of 58.19% ± 6.23, with an extraction yield of 23.66%. | [115] | |
| Ultrasound-assisted process followed by isoelectric precipitation. | Extraction yield of 40.0 ± 1.91 wt%, and a protein yield of 43.6 ± 0.93 wt%. | [116] | |
| Ultrasound-assisted extraction using methanol–ethanol mixtures as solvent. | Protein recovery from dry Spirulina was 42.55 ± 0.43%, obtained by using 20 mL of the mixture of methanol and ethanol at 50 min of extraction time. | [117] | |
| Cellulase enzyme supported the recovery process. | The highest protein recovery efficiency (40.13 ± 2.87%) was 50 UI/g dry algae for enzyme activity, 1:20 for the ratio of dry algae/solvent, and 7.0 for pH value with a process temperature of 50 °C for 90 min. | [118] | |
| Ultrasonic-assisted extraction. | UAE working at 80% amplitude for 30 min under a duty cycle of 60% could significantly improve the protein yield to 76.83% compared with conventional extraction without ultrasound (32.48%). | [119] | |
| Ultrasonic aqueous extraction at acidic, alkali, and neutral conditions was studied. S. platensis was soaked in the solvent for 12 h prior to extraction. | Neutral conditions showed a higher protein release of 18.6 mg/mL at optimum conditions (solid–liquid ratio 1:20, 80% ultrasound amplitude, 100% ultrasound duty cycle, and 4 °C). | [120] | |
| High-pressure homogenization and subsequent protein isolation by solubilization at alkaline pH followed by precipitation at acidic pH. | The optimized process conditions were found to be pH 11.38, solubilization time of 35 min, and biomass concentration of 3.6% (w/w) solids for the solubilization step, and pH 4.01 and precipitation time of 60 min for the precipitation step. At the optimized conditions, a protein yield of 60.7% (w/w) was obtained. | [121] | |
| Solubilization and precipitation. | A sonication pre-treatment (400 W, 24 kHz, 2 min) resulted in the solubilization of 96.2% of total proteins. The optimized precipitation conditions, which were pH 3.89 over 45 min, resulted in a protein recovery of 75.2%. | [122] | |
| Polysaccharides | Maceration with a hot alkali solution and further fractionated by DEAE-52 cellulose and Sephadex G-100 chromatography into two purified fractions | [75] | |
| Hot-water extraction, alkali extraction, ultrasonic-assisted extraction, and freeze/thaw extraction | The alkali extraction method was determined as the optimal method, with the optimized extraction process consisting of a solid–liquid ratio of 1:50, a pH value of 10.25, a temperature of 89.24 °C, and a time of 9.99 h. The final extract contained 71.65% of polysaccharide and 8.54% of protein. | [93] | |
| Solid-liquid aqueous extraction | A polysaccharide yield of around 8.3% dry weight was obtained under the following optimized conditions: solid-to-liquid ratio of 1:45, temperature of 90 °C, and time of 120 min. | [123] | |
| Hot water, ultrasonic- assisted, lye and freeze/thaw extraction | The efficiency of four extraction methods was 8.35%, 65%, 76.9%, and 85.1% for hot water, lye, and ultrasound-assisted and freeze/thaw extraction methods, respectively. | [124] | |
| Hot water extraction | Total carbohydrate contents record 325 ± 9.5 mg/g in the soluble polysaccharide fraction. | [125] | |
| Microwave-assisted process, using water as a solvent | To achieve the best extraction conditions, the biomass was treated for 20 min to eliminate pigments and lipids. Then, the biomass was extracted for 1 min at a power of 434 W, with a biomass/solvent ratio of 1:30 w/v and no additional contact. This resulted in a carbohydrate content of 127 ± 5 mg/g of biomass. | [17] | |
| Lipids | Ultrasound-assisted extraction and mechanical stirring | The lipid content of the extract at optimal conditions was 8.7%. The predominant fatty acids were palmitic acid (44.5%), linoleic acid (14.9%), and gamma-linolenic acid (13.4%). | [90] |
| Maceration, osmotic, and Soxhlet extraction methods | Lipids obtained from maceration, osmotic, and Soxhlet extraction methods were 5.5%, 0.6%, and 9%. | [126] | |
| Extractions using green solvents: supercritical CO2 (scCO2), high-pressure (HP) ethanol, and scCO2 with ethanol as a co-solvent (60 °C, 400 bar); compared to conventional lab-scale extractions: Soxhlet, Bligh and Dyer (B&D), and ultrasound-assisted extraction | Total extract yield using B&D showed the highest value (11.6%), followed by HP ethanol (11.4%); nevertheless, scCO2 resulted in better selectivity for fatty acid content (0.57 g/g lipids). | [127] | |
| Solvent extraction, Soxhlet extraction, and ultrasonic-assisted extraction | The highest percentage oil yield was obtained by ultrasonication (6.6% for Spirulina sp.) followed by the Soxhlet and solvent extraction processes. | [128] | |
| Ultrasound-assisted osmotic shock method | Ultrasound irradiation increased lipid yields to 6.65% in the presence of 11.9% NaCl, a solvent/biomass ratio of 12:1 v/w, and a 22 min extraction time. | [129] | |
| Supercritical carbon dioxide (CO2) extraction | Extract analyses showed that oil extracts contained chlorophylls a and b, and beta-carotene. | [130] | |
| Pigments | Pulsed electric fields pre-treatment combined with the binary mixture EtOH/H2O, 50:50, v/v, for 60–120 min | Recovery of 55–60%, 85–90%, and 60–70% was obtained for chlorophylls, carotenoids, and total phenolic compounds | [100] |
| Ultrasonic- and microwave-assisted extraction | When phycocyanin was extracted using ultrasound rather than microwave, the yield and purity index of the product were higher. The maximum yield of 14.88 mg/g and a purity index of 1.60 was attained after 1 h at 40 °C and 40 kHz ultrasonic wave frequency. | [92] | |
| Ultrasonication, centrifugation, freezing/thawing cycle, and water extraction | This approach succeeded in producing three main types of photosynthetic pigments: carotenoids, chlorophylls, and phycocyanin from the same batch of Spirulina. | [131] | |
| Ultrasound-assisted extraction using deep eutectic solvents | Optimal extraction parameters were temperature: 60 °C; extraction cycle time: 20 min; and a solvent-to-biomass ratio of 70 mL/mg. Under optimal conditions, the experimental value for total pigment yield was 165.19 ± 1.01 mg/g dry matter. | [132] | |
| Supercritical CO2 extraction | The total carotenoid, chlorophyll a, and chlorophyll b contents in the extracts were equal to 3.5 ± 0.2 mg/g, 5.7 ± 0.2 mg/g, and 3.4 ± 0.3 mg/g, respectively (by dry Spirulina weight). | [133] | |
| Four-step process including cell lysis through freezing/thawing, maceration, centrifugation, and tangential microfiltration | A phycocyanin purity index of 1.32 was achieved. | [134] | |
| Freezing/thawing extraction with 0.01 M phosphate buffer pH 7 | A phycocyanin purity index of 1.729 was achieved. | [135] |
| Food Product | Amount of Added Spirulina | Enriched Food Product Properties | Reference |
|---|---|---|---|
| Pasta | From 2 to 15% of Spirulina sp. CCC 540 powder | Green color pasta with nutritional and functional fortification resulting in an increase in its protein, total phenols, flavonoids, iron, and calcium content by up to 77.47%, 76.62%, 162.88%, 296.99%, and 57.27%, respectively. | [20] |
| Pasta | Pasta formulations included free Spirulina or microencapsulated Spirulina | The microencapsulation protected the antioxidant potential of Spirulina in 37.8% of the conditions of pasta cooking. | [150] |
| Bread | 1.5 and 2.5% of Arthrospira sp. to flour weight | Spirulina addition significantly affected bread weight and volume. | [151] |
| Extruded snacks | From 0.4 to 2.6% of Spirulina sp. LEB 18 | Higher concentrations of microalga produced snacks with higher protein content, total color difference (ΔE), and compact structure. | [152] |
| Broccoli soup | 0.5 to 2.0% (w/v) of Spirulina sp., Chlorella sp., or Tetraselmis sp. | Incorporation of freeze-dried microalgae biomass into the broccoli soup resulted in lower L* values, increased content of polyphenols, and higher antioxidant capacity. | [153] |
| Functional cheese | Spirulina platensis and Chlorella vulgaris microalgae | Cheese enriched with Spirulina platensis (3%), Chlorella vulgaris (1.467%), and curcumin (1%) had a higher antioxidant activity and iron content than the control cheese. | [21] |
| Chocolate milk | Spray-dried microencapsulated Spirulina sp. LEB-18 was used in concentrations of 5 and 8.75% | The incorporation of different microencapsulated microalgae in chocolate milk presented an increase in protein content and reduced total lipids. | [154] |
| Vegan muffins | Chlorella vulgaris in concentrations of 0, 0.5, 1.0, and 1.5% (g/100 g flour) | Incorporating a mixture of spelt and wheat flour along with a 1.5% addition of microalgae made the dough more viscous, leading to a fine, porous microstructure and a crumbly texture in the muffins. | [155] |
| Sourdough “crostini” | Three concentrations of Arthrospira platensis F&M-C256 were tested: 2, 6, and 10% (w/w) | A. platensis F&M-C256 “crostini” showed higher protein content compared to the control. | [156] |
| Whole-wheat pasta | 2% of Himanthalia elongata and 1.5% of Spirulina | A significant increase in fat (70.4%), protein (29.7%), ash (26.5%), and total amino acid contents in the raw algae-enriched pasta. The antioxidant activity was also higher. | [157] |
| Yogurt | Spirulina platensis in three different concentrations (0.5, 1, 1.5%, and a control without Spirulina) | The protein content of colored yogurt increased proportionally with added Spirulina powder. An increase in Spirulina content caused it to decrease in L*, a*, and b* values. | [158] |
| Yogurt | Spirulina (Arthrospira platensis) microencapsulated in alginate | During the storage, microencapsulated Spirulina yogurt had a higher pH value and showed the lowest syneresis and a constant increase in viscosity. | [159] |
| Feta cheese | 0.5 or 1% of Spirulina (Arthrospira platensis) powder | After 60 days of storage, there were significantly higher viable counts of Lactobacillus casei in Spirulina samples in comparison with the control. | [160] |
| Functional sauce | 0 (control), 2, 3, and 4% Spirulina sp. biomass (wt%) | Significantly increased protein, fiber, ash, monounsaturated fatty acid, and mineral contents and storage stability. | [161] |
| Bread | 2, 4, 6, and 8% of Spirulina platensis powder | 6% Spirulina-supplemented breads provided higher contents of total phenolic content and antioxidant activity. | [162] |
| Pasta | 5, 10, and 20 g/100 g, of Spirulina platensis biomass | The incorporation of Spirulina resulted in an increase in protein content; however, protein digestibility was reduced as microalgae content increased. | [163] |
| Bread | 1 and 2% of Spirulina platensis powder | The concentration of proteins significantly increased in the samples enriched with 1% Spirulina (3.17%) and 2% Spirulina (5.12%), while at the same time, the gluten content decreased by 5.62% and 7.41%, respectively. | [164] |
| Kefir | Arthrospira platensis at 0.05, 0.1, 0.5, 1, and 2% (w/v) | The addition of 1% A. platensis increased amino acid contents, palmitic and oleic acid content, and the antioxidant activities (FRAP and DPPH) of kefir. | [165] |
| Cookies | 1, 2, and 3% of Spirulina platensis powder | Hardness increased with the Spirulina content. Microalgae had a negative impact on the overall sensory quality. | [166] |
| Gluten-free muffins | 1, 2, and 3% of Spirulina platensis powder | Increasing the Spirulina platensis powder content made the muffins firmer and caused a decrease in the L*, a*, b*, and browning index values of the muffins. | [167] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marjanović, B.; Benković, M.; Jurina, T.; Sokač Cvetnić, T.; Valinger, D.; Gajdoš Kljusurić, J.; Jurinjak Tušek, A. Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry. Separations 2024, 11, 257. https://doi.org/10.3390/separations11090257
Marjanović B, Benković M, Jurina T, Sokač Cvetnić T, Valinger D, Gajdoš Kljusurić J, Jurinjak Tušek A. Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry. Separations. 2024; 11(9):257. https://doi.org/10.3390/separations11090257
Chicago/Turabian StyleMarjanović, Blaženko, Maja Benković, Tamara Jurina, Tea Sokač Cvetnić, Davor Valinger, Jasenka Gajdoš Kljusurić, and Ana Jurinjak Tušek. 2024. "Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry" Separations 11, no. 9: 257. https://doi.org/10.3390/separations11090257
APA StyleMarjanović, B., Benković, M., Jurina, T., Sokač Cvetnić, T., Valinger, D., Gajdoš Kljusurić, J., & Jurinjak Tušek, A. (2024). Bioactive Compounds from Spirulina spp.—Nutritional Value, Extraction, and Application in Food Industry. Separations, 11(9), 257. https://doi.org/10.3390/separations11090257










