Research Progress in Tritium Processing Technologies: A Review
Abstract
:1. Introduction
2. Research Progress on Tritium Separation Technologies
2.1. Water Distillation
2.2. Thermal Diffusion
2.3. Membrane Technology
2.4. Adsorption Method
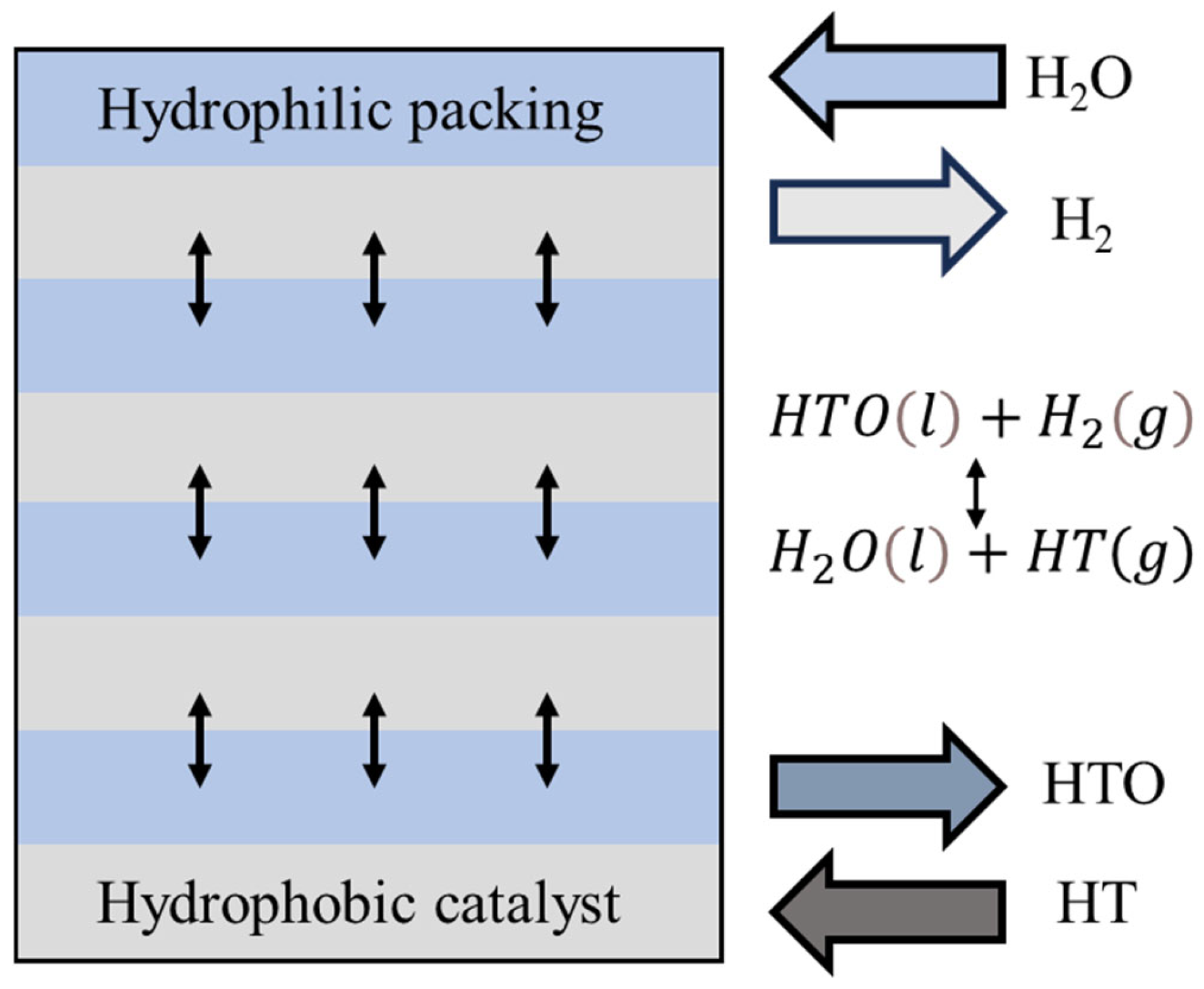
2.5. Catalytic Exchange
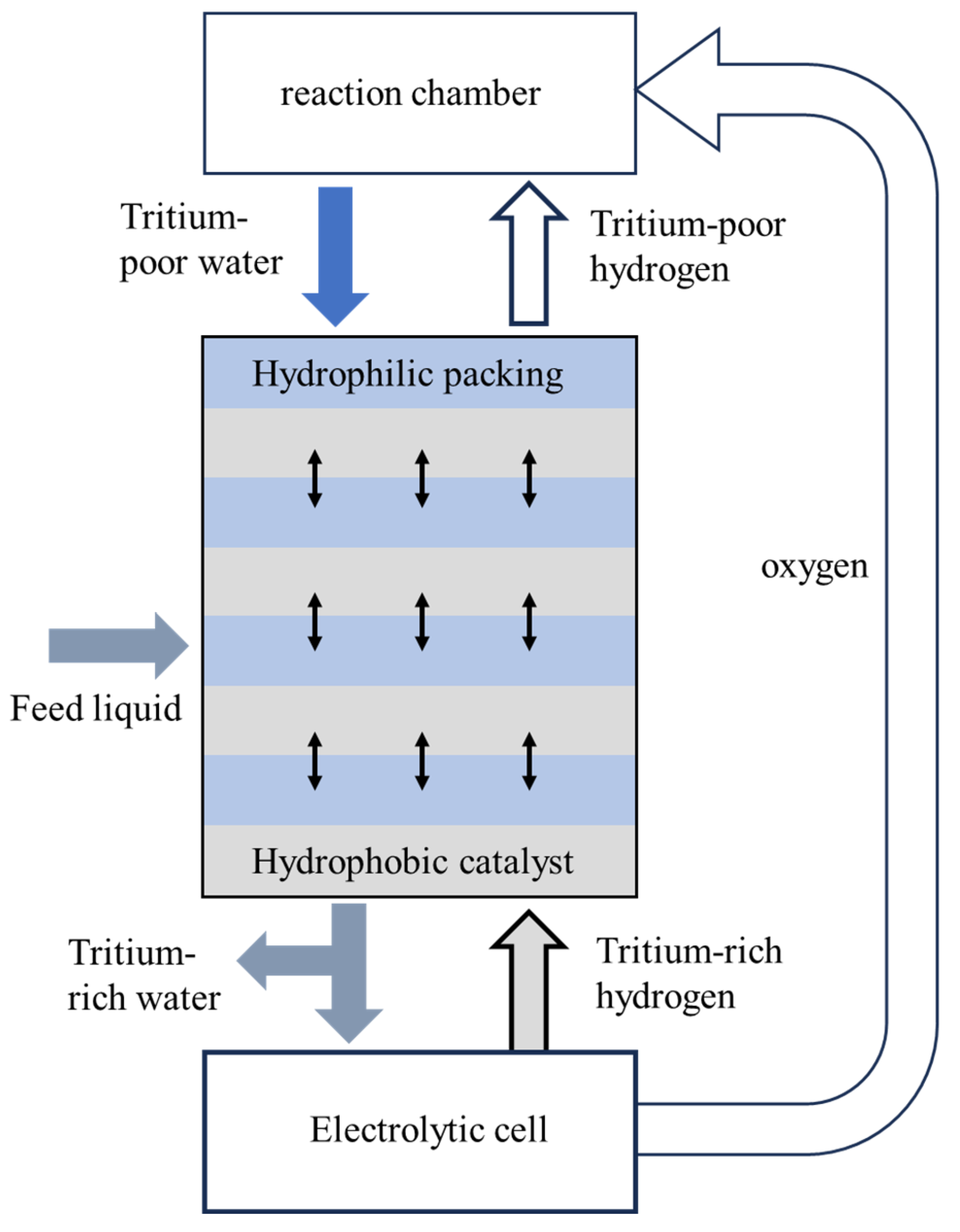
2.6. Electrolysis Process
3. Conclusions
- Significant Advancements in Tritium Separation: Recent developments in tritium separation technologies have been substantial, particularly with the integration and optimization of vapor phase catalytic exchange (VPCE), liquid phase catalytic exchange (LPCE), and combined electrolysis catalytic exchange (CECE) methods. By combining complementary techniques, the limitations of individual methods can be overcome, significantly improving the efficiency of tritium separation. For instance, the CECE process combines electrolysis and catalytic exchange, not only enhancing the separation factor of hydrogen isotopes but also reducing energy consumption in downstream processes, thereby offering a more efficient approach for handling high-concentration tritiated water.
- Advantages of solid polymer electrolyte (SPE) technology: SPE technology has demonstrated clear advantages in tritium separation, particularly in terms of reducing energy consumption and improving electrolysis efficiency. Compared to traditional alkaline electrolysis, SPE offers smaller equipment size, higher current density, longer lifespan, and simpler system design. This technology has become one of the leading solutions for processing tritiated water. SPE is especially effective in the treatment of high-concentration tritiated water and wastewater concentration, providing an innovative solution for enhancing tritium enrichment efficiency.
- Electrolyte operation with tritium generates safety concerns; the number of facilities for water detritiation is low, and characterization of packing materials is carried out at a relatively small scale.
- Energy consumption challenges of electrolysis: While electrolysis methods offer high separation factors and the ability to concentrate tritium, their high energy consumption limits their economic viability when used alone for large-scale tritiated water processing. As a result, electrolysis is typically combined with other separation technologies, such as CECE, to fully utilize its separation efficiency while minimizing energy usage. Moving forward, further optimization of electrolysis energy efficiency will be crucial to achieving cost-effective, large-scale treatment of tritiated water.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, L.; Hu, H.; Sun, Z. Study on the behaviour of tritium in Purex process. J. Nucl. Radiochem. 1996, 18, 13–20. [Google Scholar]
- Zhang, X. 3H behaviour and control in reprocessing of spent fuel from LWR. Atom. Energy Sci. Technol. 1990, 24, 87–94. [Google Scholar]
- Hou, Y. Management of tritium in spent fuel reprocessing plant of power reactor. Ind. Sci. Trib. 2018, 17, 47–49. [Google Scholar]
- Uchiyama, G.; Fujine, S.; Maeda, M.; Sugikawa, S.; Tsujino, T. Behavior of tritium in the Purex process. Solvent Extr. Ion Exch. 1995, 13, 59–82. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H. The extraction mechanism of tritium in 30% TBP-kerosene-HNO3 system. J. Nucl. Radiochem. 1997, 19, 17–23. [Google Scholar]
- Hammond, G.S. A correlation of reaction rates. J. Am. Chem. Soc. 1955, 77, 334–338. [Google Scholar] [CrossRef]
- Shere, L.; Hill, A.K.; Mays, T.J.; Lawless, R.; Brown, R.; Perera, S.P. The next generation of low tritium hydrogen isotope separation technologies for future fusion power plants. Int. J. Hydrogen Energy 2024, 55, 319–338. [Google Scholar] [CrossRef]
- Kakiuchi, M. Distribution of isotopic water molecules, H2O, HDO, and D2O, in vapor and liquid phases in pure water and aqueous solution systems. Geochim. Cosmochim. Acta 2000, 64, 1485–1492. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanishi, T.; Okuno, K.; Yokogawa, N.; Tsuchiya, H.; Yoshida, H.; Kveton, O. Design study of feasible water detritiation systems for fusion reactor of ITER scale. J. Nucl. Sci. Technol. 1996, 33, 981–992. [Google Scholar] [CrossRef]
- Kveton, O.; Yoshida, H.; Koonce, J.; Haange, R.; Horikiri, H.; Sood, S.; Fong, C.; Kalyanam, K.; Busigin, A. Design of the water detritiation and isotope separation systems for ITER. Fusion Technol. 1995, 28, 636–640. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kato, Y.; Enokida, Y.; Yamamoto, I. Simultaneous solution of concentration profiles in vapor-liquid phases of wetted-wall distillation column for H2O-HTO isotope separation. J. Nucl. Sci. Technol. 1998, 35, 60–65. [Google Scholar] [CrossRef]
- Strigle, R.F., Jr.; Rukovena, F., Jr. Packed distillation column design. Chem. Eng. Progr. 1979, 75, 86–91. [Google Scholar]
- Yamanishi, T.; Kinoshita, M. Preliminary experimental study for cryogenic distillation column with small inner diameter, (I). J. Nucl. Sci. Technol. 1984, 21, 61–70. [Google Scholar] [CrossRef]
- Yamanishi, T.; Kinoshita, M. Preliminary experimental study for cryogenic distillation column with small inner diameter, (II). J. Nucl. Sci. Technol. 1984, 21, 853–861. [Google Scholar] [CrossRef]
- Magomedbekov, E.; Rastunova, I.; Kulov, N. Water distillation as a method for separation of hydrogen and oxygen isotopes: State of the art and prospects. Theor. Found. Chem. Eng. 2021, 55, 1–11. [Google Scholar] [CrossRef]
- Gligan, M.; Radoi, A.; Dronca, S.; Bidian, C.; Radu, D.; Axente, D. Experimental installation for oxygen isotope separation by nitrogen oxide distillation at low temperature; Instalatie experimentala pentru separarea izotopilor oxigenului prin distilarea oxidului de azot la temperaturi joase. Rev. Chim. 1997, 48, 335–339. [Google Scholar]
- Lazar, A.; Brad, S.; Vijulie, M.; Oubraham, A. Cryogenic distillation experimental stands for hydrogen isotopes separation. Fusion Eng. Des. 2019, 146, 1998–2001. [Google Scholar] [CrossRef]
- Bornea, A.; Zamfirache, M.; Stefanescu, I.; Preda, A.; Balteanu, O.; Stefan, I. Investigation related to hydrogen isotopes separation by cryogenic distillation. Fusion Sci. Technol. 2008, 54, 426–429. [Google Scholar] [CrossRef]
- Yamamoto, I.; Kaba, A.; Kanagawa, A. H2O-HTO isotope separation by distillation of water—A dynamics of HETP of SUS Dixon ring in a small packed column. Fusion Eng. Des. 1989, 10, 315–318. [Google Scholar] [CrossRef]
- Fukada, S. Tritium isotope separation by water distillation column packed with silica-gel beads. J. Nucl. Sci. Technol. 2004, 41, 619–623. [Google Scholar] [CrossRef]
- Fukada, S. Tritium isotope separation using adsorption-distillation column. Fusion Sci. Technol. 2005, 48, 140–143. [Google Scholar] [CrossRef]
- Fukada, S. Transient behavior of enrichment of tritium water in adsorption-distillation column. J. Nucl. Sci. Technol. 2006, 43, 423–426. [Google Scholar] [CrossRef]
- Miho, Y.; Fukada, S.; Motomura, T.; Mizutani, J.; Hirano, S.; Arimoto, M.; Takeuchi, T. Tritium water distillation assisted with adsorption and isotopic exchange. Fusion Sci. Technol. 2017, 71, 326–332. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Bhanja, K.; Mohan, S. Simulation studies of the characteristics of a cryogenic distillation column for hydrogen isotope separation. Int. J. Hydrogen Energy 2016, 41, 5003–5018. [Google Scholar] [CrossRef]
- Park, D.; Urm, J.J.; Lee, J.U.; Chang, M.H.; Lee, J.M. Dynamic optimization of cryogenic distillation operation for hydrogen isotope separation in fusion power plant. Int. J. Hydrogen Energy 2021, 46, 24135–24148. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Li, J.; Ran, G.; Wang, H.; Xia, X.; Hou, J.; Lin, Q.; Xiao, C. Dynamic behavior and control strategy of cryogenic distillation column for hydrogen isotope separation in CFETR. Fusion Eng. Des. 2020, 160, 112018. [Google Scholar] [CrossRef]
- Cristescu, I.; Cristescu, I.R.; Dörr, L.; Glugla, M.; Hellriegel, G.; Michling, R.; Murdoch, D.; Schäfer, P.; Welte, S.; Wurster, W. Commissioning of water detritiation and cryogenic distillation systems at TLK in view of ITER design. Fusion Eng. Des. 2007, 82, 2126–2132. [Google Scholar] [CrossRef]
- Cristescu, I.; Cristescu, I.; Dörr, L.; Glugla, M.; Murdoch, D. Integrated tests of water detritiation and cryogenic distillation in view of iter design. Fusion Sci. Technol. 2007, 52, 667–671. [Google Scholar] [CrossRef]
- Cristescu, I. Enhanced configuration of a water detritiation system; impact on ITER Isotope Separation System based cryogenic distillation. Fusion Eng. Des. 2016, 109, 1404–1407. [Google Scholar] [CrossRef]
- Ana, G.; Vladulescu, F.; Ana, R.; Pasca, G.; Niculescu, A. Thermal analysis of a cryogenic distillation column for hydrogen isotopes separation. Fusion Eng. Des. 2019, 146, 1868–1871. [Google Scholar] [CrossRef]
- Xia, X. Influencing factors of hydrogen isotopes separation by cryogenic distillation. Nucl. Tech. 2006, 29, 221–224. [Google Scholar]
- Niculescu, A.; Constantin, T.; Ana, G.; Draghia, M. Dynamic simulation of a multicomponent distillation column for DT separation. Fusion Eng. Des. 2017, 124, 752–756. [Google Scholar] [CrossRef]
- Urm, J.J.; Park, D.; Lee, J.U.; Chang, M.H.; Lee, J.M. Design study of a cryogenic distillation column for hydrogen isotope separation system. Fusion Eng. Des. 2021, 172, 112736. [Google Scholar] [CrossRef]
- Urm, J.J.; Park, D.; Choi, J.H.; Lee, J.U.; Chang, M.H.; Lee, J.M. Dynamic optimization study for cryogenic distillation in hydrogen isotope separation system. IFAC-Pap. 2022, 55, 168–173. [Google Scholar] [CrossRef]
- Xue, X.; Chu, X.; Zhang, M.; Wei, F.; Liang, C.; Liang, J.; Li, J.; Cheng, W.; Deng, K.; Liu, W. High hydrogen isotope separation efficiency: Graphene or catalyst? ACS Appl. Mater. Interfaces 2022, 14, 32360–32368. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.H.; Zhao, Q.K.; Chen, C.A. Review of water hydrogen isotope separation technology. J. Nucl. Radiochem. 2024, 46, 85–99. [Google Scholar] [CrossRef]
- Wu, Z.H.; Hu, S.L. Tritium removal from tritiated heavy water. J. Isot. 2021, 34, 89–95. [Google Scholar] [CrossRef]
- Kobayashi, N.; Hirano, R.; Enokida, Y.; Yamamoto, I. H2-HT Separation using “cryogenic-wall” Thermal diffusion column with heated-tube. Fusion Sci. Technol. 2003, 44, 415–419. [Google Scholar] [CrossRef]
- Arita, T.; Yamanishi, T.; Iwai, Y.; Nishi, M.; Yamamoto, I. Experimental study for parameters affecting separation factor of cryogenic wall thermal diffusion column. Fusion Sci. Technol. 2002, 41, 1116–1120. [Google Scholar] [CrossRef]
- Shimizu, M.; Takeshita, K. Numerical study on extraction of tritium generated in HMR by way of system composed of EXEL-process and thermal diffusion column cascade. Nukleonika 2002, 47, 89–93. [Google Scholar]
- Glugla, M.; Cristescu, I.R.; Cristescu, I.; Demange, D. Hydrogen isotope separation by permeation through palladium membranes. J. Nucl. Mater. 2006, 355, 47–53. [Google Scholar] [CrossRef]
- Luo, D.; Shen, C.; Meng, D. Hydrogen isotope separation factors on palladium alloy membranes. Fusion Sci. Technol. 2002, 41, 1142–1145. [Google Scholar] [CrossRef]
- Bulubasa, G.; Niculescu, A.; Craciun, M.; Bucur, C.; Ana, G.; Bornea, A. Investigations on hydrogen isotope separation factor employing palladium-based solid metallic membranes. Fusion Sci. Technol. 2024, 1–5. [Google Scholar] [CrossRef]
- Bulubasa, G.; Niculescu, A.; Ana, G.; Bucur, C.; Ștefan, I.; Crăciun, M.; Bornea, A. Investigations on 3he: Hydrogen isotope separation employing palladium/silver membranes. Fusion Sci. Technol. 2024, 80, 411–415. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, G.; Chen, M.; Wang, X. Tritium aging effects on hydrogen permeation through Pd8.5Y0.19Ru alloy membrane. Fusion Eng. Des. 2011, 86, 2220–2222. [Google Scholar] [CrossRef]
- Sevigny, G.J.; Motkuri, R.K.; Gotthold, D.W.; Fifield, L.S.; Frost, A.P.; Bratton, W. Separation of Tritiated Water Using Graphene Oxide Membrane; Pacific Northwest National Lab.: Richland, WA, USA, 2015. [Google Scholar]
- García-Arroyo, E.; Campos-Martínez, J.; Bartolomei, M.; Pirani, F.; Hernández, M.I. Molecular hydrogen isotope separation by a graphdiyne membrane: A quantum-mechanical study. Phys. Chem. Chem. Phys. 2022, 24, 15840–15850. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guan, D.; Chen, M.; Zhang, X.; Yang, H.; Xu, C. Graphene oxide composited with different size of organic molecules for hydrogen isotopic water separation in membrane distillation. J. Environ. Chem. Eng. 2023, 11, 111389. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Y.; Cao, L.; Li, S.; Liu, X.; Fan, R. Enhanced separation performance of graphene oxide membrane through modification with Graphitic carbon Nitride. Water 2024, 16, 967. [Google Scholar] [CrossRef]
- Lozada-Hidalgo, M.; Hu, S.; Marshall, O.; Mishchenko, A.; Grigorenko, A.N.; Dryfe, R.A.W.; Radha, B.; Grigorieva, I.V.; Geim, A.K. Sieving hydrogen isotopes through two-dimensional crystals. Science 2016, 351, 68–70. [Google Scholar] [CrossRef]
- Lozada-Hidalgo, M.; Zhang, S.; Hu, S.; Esfandiar, A.; Grigorieva, I.V.; Geim, A.K. Scalable and efficient separation of hydrogen isotopes using graphene-based electrochemical pumping. Nat. Commun. 2017, 8, 15215. [Google Scholar] [CrossRef] [PubMed]
- Poltavsky, I.; Zheng, L.; Mortazavi, M.; Tkatchenko, A. Quantum Tunneling of Thermal Protons Through Pristine Graphene. J. Chem. Phys. 2018, 148, 204707. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yang, H.; Shao, Z. Radiation resistance of graphene in tritiated water. Fusion Eng. Des. 2024, 200, 114213. [Google Scholar] [CrossRef]
- Sun, D.; Chen, M.; Zhang, X.; Xu, C.; Zhang, G. Separation of hydrogen isotopic water by multi-walled carbon nanotube (MWCNT) membrane and graphene oxide (GO)-MWCNT composite membranes. Sep. Purif. Technol. 2024, 344, 127275. [Google Scholar] [CrossRef]
- Sun, D.; Li, R.; Wen, M.; Zhang, X.; Chen, M.; Yang, H.; Guan, D.; Xu, C.; Zhang, G. Hydrogen isotopic water separation in membrane distillation through BN, MoS2 and their heterostructure membranes. Sep. Purif. Technol. 2023, 314, 123634. [Google Scholar] [CrossRef]
- Koyanaka, H.; Fukutani, S.; Miyatake, H. Tritium separation from heavy water using a membrane containing deuterated manganese dioxide. J. Radioanal. Nucl. Chem. 2019, 322, 1889–1895. [Google Scholar] [CrossRef]
- Koyanaka, H.; Fukutani, S. Tritium separation from parts-per-trillion-level water by a membrane with protonated manganese dioxide. J. Radioanal. Nucl. Chem. 2018, 318, 175–182. [Google Scholar] [CrossRef]
- So, S.H.; Oh, H. A mini-review of the current progress and future challenges of zeolites for hydrogen isotopes separation through a quantum effect. Int. J. Hydrogen Energy 2024, 50, 539–560. [Google Scholar] [CrossRef]
- Lim, D.W.; Ha, J.; Oruganti, Y.; Moon, H.R. Hydrogen separation and purification with MOF-based materials. Mater. Chem. Front. 2021, 5, 4022–4041. [Google Scholar] [CrossRef]
- Sircar, A.; Devi, V.G.; Yadav, D.; Mishra, J.S.; Gangradey, R.; Gayathry, J.; Tomar, R.; Dhorajiya, P.B.; Dave, P. Study and characterization of potential adsorbent materials for the design of the hydrogen isotopes extraction and analysis system. Fusion Eng. Des. 2021, 166, 112308. [Google Scholar] [CrossRef]
- Iwai, Y.; Uzawa, M.; Yamanishi, T. Effect of cation on HTO/H2O separation and dehydration characteristics of Y-type zeolite adsorbent. Fusion Sci. Technol. 2008, 54, 462–465. [Google Scholar] [CrossRef]
- Iwai, Y.; Oka, N.; Yamanishi, T. Influence of framework silica-to-alumina ratio on the water adsorption and desorption characteristics of MHI-CaX/CaY zeolite. J. Phys. Chem. Solids 2009, 70, 881–888. [Google Scholar] [CrossRef]
- Borisevich, O.; Antunes, R.; Demange, D. Comparison of MFI-ZSM5 and NaA zeolite-type tubular membranes for the separation of water vapour from helium for tritium processes in future fusion reactors. Fusion Eng. Des. 2017, 125, 134–138. [Google Scholar] [CrossRef]
- Tulenko, J.; Kim, Y.M.; Baney, R.; Powers, K.; Koopman, B. Screening experiments for removal of low-level Tritiated water (HTO). Trans. Am. Nucl. Soc. 2003, 89, 253–256. [Google Scholar] [CrossRef]
- Taguchi, A.; Akai, R.; Saito, M.; Torikai, Y.; Matsuyama, M.; Ogura, M.; Uchida, S. Tritium removal from tritiated water using mesoporous silica. Fusion Sci. Technol. 2011, 60, 1395–1398. [Google Scholar] [CrossRef]
- Vergari, L.; Scarlat, R.O. The impact of neutron irradiation, graphite oxidation and fluorination on tritium uptake into and desorption from graphite in molten salt environments. Fusion Eng. Des. 2021, 168, 112627. [Google Scholar] [CrossRef]
- Yeon, J.W.; Yang, J. Effects of temperature and hydrogen peroxide on the selective adsorption of HTO on activated carbon in tritiated water. J. Radioanal. Nucl. Chem. 2022, 331, 4569–4576. [Google Scholar] [CrossRef]
- Edao, Y.; Iwai, Y.; Mori, H.; Itoi, N.; Goto, T.; Kumada, N. Selective adsorption properties of layered titanate for tritiated water. Fusion Eng. Des. 2024, 202, 114426. [Google Scholar] [CrossRef]
- Park, S.C.; Son, S.K.; Ahn, M.Y.; Ying, A.; Cho, S.; Park, Y.H.; Lee, Y. Hydrogen adsorption performance for large-scale cryogenic molecular sieve bed. Fusion Eng. Des. 2019, 146, 1863–1867. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Chen, C.; Yang, M.; Gong, Y.; Hou, J.; Xiao, C.; Cong, H.; Huang, H.; Wang, H.; et al. Superhydrophilic metal-organic frameworks film modified surface for tritium removal from tritiated heavy water. Microporous Mesoporous Mater. 2023, 348, 112387. [Google Scholar] [CrossRef]
- Iwai, Y.; Yamanishi, T.; Nishi, M.; Suzuki, Y.; Kurita, K.; Shimazaki, M. Application of pressure swing adsorption to water detritiation process. J. Nucl. Sci. Technol. 2005, 42, 566–572. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sakuragawa, C.; Mu, T.; Tachibana, K.; Ishihara, M.; Tomita, M.; Sugimoto, H. Removal of tritiated water molecules by isotope exchange reaction between H2O vapor and tritium water. Heliyon 2024, 10, e33956. [Google Scholar] [CrossRef] [PubMed]
- Vijulie, M.; Lazar, A.; Brad, S.; Balteanu, O.; Stefan, I.; Bucur, C.; Moraru, C.; Sofilca, N. Method of determination and optimization of the control parameters for an LPCE process. Fusion Eng. Des. 2019, 146, 1725–1728. [Google Scholar] [CrossRef]
- Lu, Z.; Fu, X.; Li, J.; Hou, J.; Ran, G.; Xiao, C.; Wang, X. Superhydrophobic Pt@SBA-15 catalyst for tritium separation in liquid phase catalytic exchange. Int. J. Hydrogen Energy 2023, 48, 1979–1987. [Google Scholar] [CrossRef]
- Ionita, G.; Bornea, A.; Braet, J.; Popescu, I.; Stefanescu, I.; Bidica, N.; Varlam, C.; Postolache, C.; Matei, L. Endurance test for SCK-CEN catalytic mixed packing, proposed for water detritiation system at JET. Fusion Sci. Technol. 2005, 48, 112–115. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Gou, K.; Yang, H.; Ren, X.; Peng, B. Effects of residual double bonds on the catalytic activity and stability of Pt/SDB hydrophobic catalysts. RSC Adv. 2015, 5, 45420–45425. [Google Scholar] [CrossRef]
- Fan, Y.; Chao, W.; Liu, C.; Lin, S. Synthesis and properties of fluorosilane treated nano-Al2O3 hybrid modified styrene-divinylbenzene copolymers. Colloids Surf. A 2024, 700, 134835. [Google Scholar] [CrossRef]
- Ionita, G.; Bucur, C.; Spiridon, I.; Stefanescu, I. An assessment on hydrogen isotopes separation by liquid phase catalytic exchange process. J. Radioanal. Nucl. Chem. 2015, 305, 117–126. [Google Scholar] [CrossRef]
- Stefan, I.; Balteanu, O.; Stefan, L.; Bucur, C.; Vijulie, M.; Moraru, C.; Sofilca, N. Computer based architecture to control water detritiation process. Fusion Eng. Des. 2019, 146, 2613–2617. [Google Scholar] [CrossRef]
- Sakharovski, Y.; Tkachenko, V. A new way for improving efficiency of CECE-process based hydrogen isotope separation plant. J. Radioanal. Nucl. Chem. 2015, 304, 357–360. [Google Scholar] [CrossRef]
- Li, P.; Guo, L.; Xiong, R.; Luo, J.; Wen, M.; Yao, Y.; Zhang, Z.; Song, J.; Shi, Y.; Tang, T. Separation process study of liquid phase catalytic exchange reaction based on the Pt/C/PTFE catalysts. Chin. J. Chem. Eng. 2019, 27, 1837–1845. [Google Scholar] [CrossRef]
- Sohn, S.H.; Lee, K.J. Deactivation of hydrophobic Pt/SDBC catalyst in the WTRF LPCE column for tritium separation. J. Nucl. Sci. Technol. 2006, 43, 874–883. [Google Scholar] [CrossRef]
- Miller, J.; Graham, W.; Celovsky, S.; Tremblay, J.; Everatt, A. Design and operational experience with a pilot-scale CECE detritiation process. Fusion Sci. Technol. 2002, 41, 1077–1081. [Google Scholar] [CrossRef]
- Alekseev, I.A.; Bondarenko, S.D.; Fedorchenko, O.A.; Vasyanina, T.V.; Konoplev, K.A.; Arkhipov, E.A.; Voronina, T.V.; Grushko, A.I.; Tchijov, A.S.; Uborsky, V.V. Heavy water detritiation by combined electrolysis catalytic exchange at the experimental industrial plant. Fusion Eng. Des. 2003, 69, 33–37. [Google Scholar] [CrossRef]
- Wu, D.; Yin, Y.; Ruan, H.; Huang, D.; Hu, S. Study on the deep purification of tritiated light water based on combined electrolysis and catalytic exchange. Contemp. Chem. Ind. 2021, 50, 1144–1147. [Google Scholar] [CrossRef]
- Heinze, S.; Giroux, P.; Ducret, D.; Colson, J.C.; Vuillemin, B. Bipolar electrolysis for tritium recovery from weakly active tritiated water. Fusion Eng. Des. 2001, 58–59, 429–432. [Google Scholar] [CrossRef]
- Heinze, S.; Bussiere, P.; Pelletier, T. French experience in tritiated water management. Fusion Eng. Des. 2003, 69, 67–70. [Google Scholar] [CrossRef]
- Heinze, S.; Ducret, D.; Verdin, J.P.; Pelletier, T. Isotopic enrichment of tritiated water by a bipolar electrolysis process. Fusion Sci. Technol. 2002, 41, 1160–1164. [Google Scholar] [CrossRef]
- Jurkin, D.; Müllen, G.; Aign, J. Development and performance of a multibipolar HTO electrolysis system. Fusion Sci. Technol. 2011, 60, 1403–1406. [Google Scholar] [CrossRef]
- Isobe, K.; Yamanishi, T. Development of high efficiency electrode for highly tritiated water processing. Fusion Sci. Technol. 2011, 60, 1387–1390. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, W.; Ding, W.; Li, T. Preparation of two-dimensional material composite proton exchange membrane and its performance in protium-tritium separation. Mod. Chem. Ind. 2024, 44, 177–182. [Google Scholar] [CrossRef]
- Zabolockis, R.; Sondars, M.; Vaivars, G.; Reinholds, I.; Gostilo, V.; Malgin, V.; Kizilov, A.; Lescinskis, A.; Felsharuk, A.; Avotina, L.; et al. Graphene-based electrochemical system for tritium enrichment. Nucl. Fusion 2024, 64, 026022. [Google Scholar] [CrossRef]
- Whitehorne, T.; Muirhead, C.; Thomson, S.; Li, H.; Carson, R.; Boniface, H.; Suppiah, S. Study of Electrolyzer materials at high tritium concentrations. Fusion Sci. Technol. 2021, 77, 26–32. [Google Scholar] [CrossRef]
- Thomson, S.; Carson, R.; Ratnayake, A.; Muirhead, C.; Li, H.; Castillo, I.; Boniface, H.; Suppiah, S.; Robinson, J. Characterization of commercial proton exchange membrane materials after exposure to beta and gamma radiation. Fusion Sci. Technol. 2015, 67, 443–446. [Google Scholar] [CrossRef]
- Iwai, Y.; Sato, K.; Hiroki, A.; Tamada, M.; Hayashi, T.; Yamanishi, T. Recent R&D results on polymeric materials for a SPE-type high-level tritiated water electrolyzer system. Fusion Eng. Des. 2010, 85, 1421–1425. [Google Scholar] [CrossRef]
- Pushkarov, O.V.; Zubko, A.V.; Sevruk, I.M.; Dolin, V.V. Membrane properties of montmorillonite, saponite and clinoptilolite during electroosmotic fractionation of hydrogen isotopes. Mineral. J. 2020, 42, 23–32. [Google Scholar] [CrossRef]
- Ogata, Y.; Sakuma, Y.; Ohtani, N.; Kotaka, M. Tritium separation from heavy water by electrolysis with solid polymer electrolyte. J. Radioanal. Nucl. Chem. 2003, 255, 539–541. [Google Scholar] [CrossRef]
- Wei, F.; Xue, X.; Chu, X.; Zeng, Y.; Liu, W. Theoretical analysis of two-dimensional crystals on separation of hydrogen isotopes in solid polymer electrolyte (SPE) electrolysis. Mater. Today Commun. 2021, 29, 102736. [Google Scholar] [CrossRef]
- Wen, X.; Yang, H.; Wu, B.; Yang, H. The development and application of solid polymer electrolysis enrichment device of tritium in water. Nucl. Electron. Detect. Technol. 2003, 23, 583–586. [Google Scholar]
- Xue, X.; Zhang, M.; Wei, F.; Liang, C.; Liang, J.; Li, J.; Cheng, W.; Deng, K.; Liu, W. Gold as an efficient hydrogen isotope separation catalyst in proton exchange membrane water electrolysis. Int. J. Hydrogen Energy 2022, 47, 26842–26849. [Google Scholar] [CrossRef]
- Zhao, Q.; Pang, M.; Tang, C.; Xiang, X.; Wang, X.; Chen, J.; Chen, C. Molybdenum disulfide nanosheets rich in edge sites for efficient hydrogen isotope separation by water electrolysis. Electrochim. Acta 2023, 464, 142780. [Google Scholar] [CrossRef]
- Ando, S.; Komatsuzaki, T.; Okada, M.; Kataoka, N. Effects of additives and electrolytic treatment to remove tritium from contaminated water. Heliyon 2023, 9, e17031. [Google Scholar] [CrossRef] [PubMed]
- Varlam, C.; Vagner, I.; Făurescu, I.; Bornea, A.; Făurescu, D.; Bogdan, D. Tritium behavior in water and gas produced by a fully tritium-compatible Electrolyzer. Fusion Sci. Technol. 2024, 80, 391–398. [Google Scholar] [CrossRef]
- Zeng, N.; Hu, C.; Lv, C.; Liu, A.; Hu, L.; An, Y.; Li, P.; Chen, M.; Zhang, X.; Wen, M.; et al. Large-current density and high-durability proton exchange membrane water electrolysis for practical hydrogen isotope separation. Sep. Purif. Technol. 2023, 310, 123148. [Google Scholar] [CrossRef]
- Kalyanam, K.M.; Sood, S.K. A Comparison of Process Characteristics for the Recovery of Tritium from Heavy Water and Light Water Systems. Fusion Technol. 1988, 14, 524–528. [Google Scholar] [CrossRef]
- Souers, P.C. Cryogenic Hydrogen Data Pertinent to Magnetic Fusion Energy; Lawrence Livermore Laboratory, University of California: Livermore, CA, USA, 1979. [Google Scholar]
- Roy, L.P. Influence of temperature on the electrolytic separation factor of hydrogen isotopes. Can. J. Chem. 1962, 40, 1452–1460. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Zakrzewska-Trznadel, G.; Miljević, N.R.; Van Hook, W.A. Membrane distillation employed for separation of water isotopic compounds. Sep. Sci. Technol. 1995, 30, 1653–1667. [Google Scholar] [CrossRef]
- Michling, R.; Bekris, N.; Cristescu, I.; Lohr, N.; Plusczyk, C.; Welte, S.; Wendel, J. Water detritiation processing of JET purified waste water using the TRENTA facility at Tritium Laboratory Karlsruhe. Fusion Eng. Des. 2013, 88, 2361–2365. [Google Scholar] [CrossRef]
- Hook, V.; Alexander, W. Vapor pressures of the isotopic waters and ices. J. Phys. Chem. 1968, 72, 1234–1244. [Google Scholar] [CrossRef]

| Technology | Tritium Separation Factor | Operating Temperature (K) | Industrial Feasibility | Technical Challenge |
|---|---|---|---|---|
| VPCE | 0.47 [105] | 473 [105] | Industrialized | Have been replaced |
| LPCE | 0.14 [105] | 298 [105] | Industrialized | Catalyst optimization, process equipment optimization |
| CECE | 150–250 [106] | Industrialized | Catalyst optimization, process equipment optimization | |
| Water distillation | 1.056 [107] | 333 [107] | Industrialized | |
| Cryogenic distillation | 1.82 [108] | 21–24 [108] | Industrialized | The technology is complex and the security risk is high |
| Electrolysis process | 10 [109] | 353 [109] | Mainly used in laboratories | Low processing efficiency |
| Membrane permeation | H/D: 1.05–1.09 [110] | Have matured | High membrane cost |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Sun, Y.; Chen, Q.; Li, T.; Liu, F.; Yan, T.; Zheng, W. Research Progress in Tritium Processing Technologies: A Review. Separations 2025, 12, 33. https://doi.org/10.3390/separations12020033
Zhao Z, Sun Y, Chen Q, Li T, Liu F, Yan T, Zheng W. Research Progress in Tritium Processing Technologies: A Review. Separations. 2025; 12(2):33. https://doi.org/10.3390/separations12020033
Chicago/Turabian StyleZhao, Ziqian, Yandong Sun, Qi Chen, Tianchi Li, Fang Liu, Taihong Yan, and Weifang Zheng. 2025. "Research Progress in Tritium Processing Technologies: A Review" Separations 12, no. 2: 33. https://doi.org/10.3390/separations12020033
APA StyleZhao, Z., Sun, Y., Chen, Q., Li, T., Liu, F., Yan, T., & Zheng, W. (2025). Research Progress in Tritium Processing Technologies: A Review. Separations, 12(2), 33. https://doi.org/10.3390/separations12020033






