Techniques and Methods for Fatty Acid Analysis in Lipidomics: Exploring Pinus cembroides Kernels as a Sustainable Food Resource
Abstract
:1. Introduction
2. The Biogeographic and Physiographic Context of P. cembroides
3. Chemical and Fatty Acids Composition of Pine Nuts and P. cembroides Kernels
4. Oils Extraction and Fatty Acids Analyses in Pine Nuts and P. cembroides spp. Kernel
4.1. Fatty Acids Extraction in Pine Nuts and P. Cembroides spp. Kernel
4.2. Processing, Separation and Detection Techniques of Fatty Acids in Pine Nuts and P. cembroides Kernels
4.2.1. Derivatization
4.2.2. TLC
4.2.3. LC and GC
4.2.4. Fatty Acids Detections: FID, NMR and MS
4.3. Post-Acquisition Data Processing, Bioinformatics and Computational Tools to Analyse Lipid Pathways of Fatty Acids in Pine Nuts and P. cembroides Kernels
5. Conclusions
6. Future Directions
- Integration of different techniques and methods: the integration of High-Resolution Techniques and Non-Destructive techniques, as advanced tools like UHPLC, HPLC, or MDGC are employed for improved separation and resolution; non-invasive techniques, such as NMR spectroscopy, to assess fatty acid content without damaging seeds; and GC-FID to improve the resolution of different fatty acid isomers [76,105].
- Multi-omics integration and environmental impact studies: combining lipidomics with other omics studies to understand broader metabolic pathways affecting fatty acid synthesis [62]. Investigating how environmental factors influence fatty acid profiles across Pinus species. This could be achieved through open-access platforms that analyze the territories on a regional scale [106].
- Bioinformatics and Machine Learning: Using computational tools to analyze complex datasets and predict fatty acid compositions [26].
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Resare, S.K.; Gordon, L.J.; Lindborg, R.; Piipponen, J.; Van Rysselberge, P.; Rouet-Leduc, J.; Röös, E. An exploration of biodiversity limits to grazing ruminant milk and meat production. Nat. Sustain. 2024, 7, 1160–1170. [Google Scholar] [CrossRef]
- Fu, J.; Li, P.; Lin, Y.; Du, H.; Liu, H.; Zhu, W.; Ren, H.J. Fight for carbon neutrality with state-of-the-art negative carbon emission technologies. Eco-Environ. Health 2022, 1, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Munialo, S.; Siddique, K.H.; Barker, N.P.; Onyango, C.M.; Amissah, J.N.; Wamalwa, L.N.; Sibanda, L.M. Reorienting research investments toward under-researched crops for sustainable food systems. Food Energy Secur. 2024, 13, 538. [Google Scholar] [CrossRef]
- Balta, I.; Stef, L.; Pet, I.; Iancu, T.; Stef, D.; Corcionivoschi, N. Essential fatty acids as biomedicines in cardiac health. Biomedicines 2021, 9, 1466. [Google Scholar] [CrossRef]
- Motti, R. Wild Edible Plants: A Challenge for Future Diet and Health. Plants 2022, 11, 344. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus species as prospective reserves of bioactive compounds with potential use in functional food—Current state of knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Briand, C.H. One hundred years of piñon nuts, a largely forgotten wild food crop from the American Southwest (1850–1950). Trees For. People 2024, 18, 100705. [Google Scholar] [CrossRef]
- Casas, A.; Blancas, J.; Lira, R. Ethnobotany of Mexico Interactions of People and Plants in Mesoamerica, 1st ed.; Springer: New York, NY, USA, 2016; pp. 1–560. [Google Scholar]
- Montes, J.R.; Peláez, P.; Willyard, A.; Moreno-Letelier, A.; Piñero, D.; Gernandt, D.S. Phylogenetics of Pinus Subsection Cembroides Engelm. (Pinaceae) Inferred from Low-Copy Nuclear Gene Sequences. Syst. Bot. 2019, 44, 501–518. [Google Scholar] [CrossRef]
- Madrid-Aispuro, R.E.; Prieto-Ruiz, J.A.; Aldrete, A.; Hernández-Díaz, J.C.; Wehenkel, C.; Chávez-Simental, J.A.; Mexal, J.G. Alternative substrates and fertilization doses in the production of Pinus cembroides Zucc. in nursery. Forests 2020, 11, 71. [Google Scholar] [CrossRef]
- Padilla-Martínez, J.R.; Paul, C.; Husmann, K.; Corral-Rivas, J.J.; Von Gadow, K. Grouping tree species to estimate basal area increment in temperate multispecies forests in Durango, Mexico. For. Ecosyst. 2024, 11, 100158. [Google Scholar] [CrossRef]
- Herrera-Soto, G.; González-Cásares, M.; Pompa-García, M.; Camarero, J.J.; Solís-Moreno, R. Growth of Pinus cembroides Zucc. in response to hydroclimatic variability in four sites forming the species latitudinal and longitudinal distribution limits. Forests 2018, 9, 440. [Google Scholar] [CrossRef]
- Valero-Galván, J.; Reyna-González, M.; Chico-Romero, P.A.; Martínez-Ruiz, N.D.R.; Núñez-Gastélum, J.A.; Monroy-Sosa, A.; Gonzalez Fernandez, R. Seed characteristics and nutritional composition of pine nut from five populations of P. cembroides from the States of Hidalgo and Chihuahua, Mexico. Molecules 2019, 24, 2057. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Cruz, J.C.; Ramírez, M.A. Additive equations for estimating aboveground biomass of Pinus cembroides Zucc. Madera Bosques 2020, 26, 1–13. [Google Scholar] [CrossRef]
- Carlón-Allende, T.; Mendoza, M.E.; Villanueva Díaz, J.; Li, Y. Climatic response of Pinus cembroides Zucc. radial growth in Sierra del Cubo, Guanajuato, Mexico. Trees Struct. Funct. 2018, 32, 1387–1399. [Google Scholar] [CrossRef]
- Constante-García, V.; Villanueva-Díaz, J.; Cerano-Paredes, J.; Cornejo-Oviedo, E.H.; Valencia Manzo, S. Dendrocronología de Pinus cembroides Zucc. y reconstrucción de precipitación estacional para el sureste de coahuila. Rev. Cien. For. Mex 2009, 34, 17–39. [Google Scholar]
- González-Ávalos, J.; García-Moya, E.; Vargas-Hernández, J.J.; Trinidad-Santos, A.; Romero-Manzanares, A.; Cetina-Alcalá, V.M. Evaluation of production and cones and seeds analysis of Pinus cembroides Zucc. Rev. Chapingo Ser. Cienc. For. Y Del Ambiente 2006, 12, 133–138. [Google Scholar]
- Teneva, O.; Petkova, Z.; Toshev, N.; Solakov, N.; Loginovska, K.; Platov, Y. Effect of roasting on the chemical and lipid composition of pine nuts in two regions in Russia. Heliyon 2024, 10, e34576. [Google Scholar] [CrossRef]
- Moscetti, R.; Berhe, D.H.; Agrimi, M.; Haff, R.P.; Liang, P.; Ferri, S.; Massantini, R. Pine nut species recognition using NIR spectroscopy and image analysis. J. Food Eng. 2021, 292, 110357. [Google Scholar] [CrossRef]
- Awan, H.U.M.; Pettenella, D. Pine nuts: A review of recent sanitary conditions and market development. Forests 2017, 8, 367. [Google Scholar] [CrossRef]
- Takala, R.; Ramji, D.P.; Choy, E. The Beneficial Effects of Pine Nuts and Its Major Fatty Acid, Pinolenic Acid, on Inflammation and Metabolic Perturbations in Inflammatory Disorders. Int. J. Mol. Sci. 2023, 24, 1171. [Google Scholar] [CrossRef]
- Incegul, Y.; Aksu, M.; Kiralan, S.S.; Kiralan, M.; Ozkan, G. Cold pressed pine (Pinus koraiensis) nut oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications, 1st ed.; Ramadan, M.F., Ed.; Elsevier: Zagazig, Egypt, 2020; Volume 1, pp. 525–536. [Google Scholar]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Factories 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Calder, P.C. A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid and their potential health benefits. Prog. Lipid Res. 2021, 82, 101097. [Google Scholar] [CrossRef] [PubMed]
- Kehelpannala, C.; Rupasinghe, T.; Hennessy, T.; Bradley, D.; Ebert, B.; Roessner, U. The state of the art in plant lipidomics. R. Soc. Chem. 2021, 17, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Chappel, J.R.; Kirkwood-Donelson, K.I.; Reif, D.M.; Baker, E.S. From big data to big insights: Statistical and bioinformatic approaches for exploring the lipidome. Anal. Bioanal. Chem. 2024, 416, 2189–2202. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, H.; Lv, X.; Chen, H.; Wei, F. Mass Spectrometry Based on Chemical Derivatization Has Brought Novel Discoveries to Lipidomics: A Comprehensive Review. Crit. Rev. Anal. Chem. 2025, 55, 21–52. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G.S. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef]
- Özel, H.B.; Şevik, H.; Onat, S.M.; Yiğit, N. The Effect of Geographic Location and Seed Storage Time on the Content of Fatty Acids in Stone Pine (Pinus pinea L.) Seeds. Bioresources 2022, 17, 5038–5048. [Google Scholar] [CrossRef]
- Nergiz, C.; Dönmez, I. Chemical composition and nutritive value of Pinus pinea L. seeds. Food Chem. 2004, 86, 365–368. [Google Scholar] [CrossRef]
- Martínez-Sánchez, J.N.; Cuéllar-Rodríguez, L.G.; Yerena Yamallel, J.I.; Cavazos, M.T.; Gárate-Escamilla, H.A. Comparison of climatic databases in modeling the potential distribution of Pinus cembroides Zucc. Rev. Mex. Cienc. For. 2023, 14, 135–158. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress-a review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Loewe-Muñoz, V.; Del Río, R.; Delard, C.; Balzarini, M. Effect of fertilization on Pinus pinea cone to seed and kernel yields. For. Ecol. Manag. 2023, 545, 121249. [Google Scholar] [CrossRef]
- Hernández Moreno, M.M.; Islas Gutiérrez, J.; Guerra de la Cruz, V. Margins of commercialization of the pinion (Pinus cembroides subesp. orizabensis) in Tlaxcala, , Mexico. Rev. Mex. De Cienc. Agric. 2011, 2, 265–279. [Google Scholar]
- Burney, O.; Aldrete, A.; Alvarez-Reyes, R.; Prieto-Ruíz, J.A.; Sánchez-Velazquez, J.R.; Mexal, J.G. México—Addressing challenges to reforestation. J. For. 2015, 113, 404–413. [Google Scholar] [CrossRef]
- Sheridan, R.A.; Fulé, P.Z.; Lee, M.E.; Nielsen, E.A. Identifying Social-ecological Linkages to Develop a Community Fire Plan in Mexico. Conserv. Soc. 2015, 13, 395–406. [Google Scholar] [CrossRef]
- Solís-Mendoza, L.E.; Sánchez-Nupan, L.O.; Castro-Torres, R.B.; De la Mora de la Mora, G.; Kozak, R.; Peterson St Laurent, G.; Galicia, L. Scaling up in community forest enterprises: The case of central Mexico. Socio-Ecol. Prac. Res. 2024, 6, 347–366. [Google Scholar] [CrossRef]
- Galicia-Sarmiento, L.; Solís-Mendoza, L.E.; Sánchez-Nupan, L.O.; Castro-Torres, R.B.; Kozak, R.; St-Laurent, G.P. Limitations and opportunities for scaling up in four forest community enterprises of central México. Econ. Soc. Y Territ. 2023, 23, 89–130. [Google Scholar]
- García-Zubia, L.C.; Hernández-Velasco, J.; Hernández-Díaz, J.C.; Simental-Rodríguez, S.L.; López-Sánchez, C.A.; Quiñones-Pérez, C.Z.; Wehenkel, C. Spatial genetic structure in Pinus cembroides Zucc. At population and landscape levels in central and northern Mexico. PeerJ 2019, 7, e8002. [Google Scholar] [CrossRef]
- Alva-Rodríguez, S.; López-Upton, J.; Vargas-Hernández, J.J.; Ruiz-Posadas, L.D.M. Biomass and growth of Pinus cembroides Zucc. and Pinus orizabensis D. K. Bailey & Hawksworth in response to water deficit. Rev. Chapingo Ser. Cienc. For. Y Del Ambiente 2019, 26, 71–83. [Google Scholar]
- Avalas, J.G.; Moya, E.G.; Alcalá, V.M.C.; Hernández, J.J.V.; Santos, A.T.; Manzanares, A.R. Variación Morfológica e índice de calidaden plantas de Pinus cembroides var. cembroides Zucc. Rev. Mex. Cienc. For. 2005, 30, 29–44. [Google Scholar]
- Méndez, A.; Rivera-Valentín, E.G.; Schulze-Makuch, D.; Filiberto, J.; Ramírez, R.M.; Wood, T.E.; Haqq-Misra, J. Habitability Models for Astrobiology. Astrobiology 2021, 21, 1017–1027. [Google Scholar] [CrossRef]
- Cockell, C.S.; Simons, M.; Castillo-Rogez, J.; Higgins, P.M.; Kaltenegger, L.; Keane, J.T.; Vance, S.D. Sustained and comparative habitability beyond Earth. Nat. Astron. 2024, 8, 30–38. [Google Scholar] [CrossRef]
- Encina-Domínguez, J.A.; Estrada-Castillón, E.; Mellado, M.; González-Montelongo, C.; Arévalo, J.R. Livestock Grazing Impact on Species Composition and Richness Understory of the Pinus cembroides Zucc. Forest in Northeastern Mexico. Forests 2022, 13, 1113. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Saleem, M. An Ecoregion-Based Approach to Protecting Half the Terrestrial Realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]
- CONABIO. Potal de Información Geospacial (CONABIO 2024). Sistema Nacional de Información Sobre Biodiversidad (SNIB). Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 9 November 2024).
- Hartsell, J.A.; Copeland, S.M.; Munson, S.M.; Butterfield, B.J.; Bradford, J.B. Gaps and hotspots in the state of knowledge of pinyon-juniper communities. For. Ecol. Manag. 2020, 455, 117628. [Google Scholar] [CrossRef]
- Dinerstein, E. RESOLVE Ecoregions. 2017. Available online: https://ecoregions.appspot.com/ (accessed on 9 November 2024).
- Kadri, N.; Khettal, B.; Aid, Y.; Kherfellah, S.; Sobhi, W.; Barragan-Montero, V. Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria, their lipid profiles and volatile contents. Food Chem. 2015, 188, 184–192. [Google Scholar] [CrossRef]
- Kobler, H.; Monakhova, Y.B.; Kuballa, T.; Tschiersch, C.; Vancutsem, J.; Thielert, G.; Lachenmeier, D.W. Nuclear magnetic resonance spectroscopy and chemometrics to identify pine nuts that cause taste disturbance. J. Agric. Food Chem. 2011, 59, 6877–6881. [Google Scholar] [CrossRef]
- Treviño, M.G.M.; Ruíz, N.L.T.; Treviño, A.P.E.; Do-Vale, D.O.G.; Moreno, F.R. Development and Analysis of a Product Made from Pink Pine Nuts in the South of Nuevo León. Int. J. Food Eng. 2021, 7, 29–34. [Google Scholar] [CrossRef]
- Gómez-García, E.; Martínez-Chamorro, E.; García-Méijome, A.; Rozados-Lorenzo, M.J. Modelling resin production distributions for Pinus pinaster Ait. stands in NW Spain. Ind. Crops Prod. 2022, 176, 114316. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Sun, Y.; Chen, J.; Liu, Q.; Dong, S. Genetic diversity and conservation of Siberian apricot (Prunus sibirica L.) based on microsatellite markers. Sci. Rep. 2023, 13, 11245. [Google Scholar] [CrossRef]
- López-Mata, L. Proteins, amino acids and fatty acids composition of nuts from the Mexican endemic rarity, Pinus maximartinezii, and its conservation implications. Interciencia 2001, 26, 606–610. [Google Scholar]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Wickramasinghe. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Bhargava, S.; De la Puente-Secades, S.; Schurgers, L.; Jankowski, J. Lipids and lipoproteins in cardiovascular diseases: A classification. Trends Endocrinol. Metab. 2022, 33, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Szabó, A. Review of Eukaryote Cellular Membrane Lipid Composition, with Special Attention to the Fatty Acids. Int. J. Mol. Sci. 2023, 24, 15693. [Google Scholar] [CrossRef]
- Matsushita, Y.; Nakagawa, H.; Koike, K. Lipid metabolism in oncology: Why it matters, how to research, and how to treat. Cancers 2021, 13, 474. [Google Scholar] [CrossRef]
- Park, J.; Choi, J.; Kim, D.D.; Lee, S.; Lee, B.; Lee, Y.; Oh, Y.K. Bioactive lipids and their derivatives in biomedical applications. Korean Soc. Appl. Pharmacol. 2021, 29, 465. [Google Scholar] [CrossRef]
- Aldana, J.; Romero-Otero, A.; Cala, M.P. Exploring the lipidome: Current lipid extraction techniques for mass spectrometry analysis. Metabolites 2020, 10, 231. [Google Scholar] [CrossRef]
- Chiu, H.H.; Kuo, C.H. Gas chromatography-mass spectrometry-based analytical strategies for fatty acid analysis in biological samples. J. Food Drug Anal. 2020, 28, 60–73. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, J.L. Mass-spectrometry-based lipidomics. J. Sep. Sci. 2018, 41, 351–372. [Google Scholar] [CrossRef]
- Castro-Garibay, S.L.; Cruz-Arvizu, O.; Monroy-González, I.; Abarca-Cervantes, A.D.; Cruz-Larios, I.J.; Arguello-Hernández, M. Pinus cembroides, and P. orizabensis grafts, a viable option for pink pine nut production. New For. 2024, 55, 1787–1799. [Google Scholar] [CrossRef]
- Lahlou, A.; Lyashenko, S.; Chileh-Chelh, T.; Belarbi, E.H.; Torres-García, I.; Álvarez-Corral, M.; Guil-Guerrero, J.L. Fatty acid profiling in the genus Pinus in relation to its chemotaxonomy and nutritional or pharmaceutical properties. Phytochemistry 2023, 206, 113517. [Google Scholar] [CrossRef]
- Sagrero-Nieves, L. Fatty Acid Composition of (Pinus cembroides) Oil from Phenotypes Mexican Pine Nut Three Seed Coat. J. Sci. Food Agric. 1992, 59, 413–414. [Google Scholar] [CrossRef]
- Wolff, R.L.; Marpeau, A.M. Δ5-olefinic acids in the edible seeds of nut pines (Pinus cembroides edulis) from the United States. J. Am. Oil Chem. Soc. 1997, 74, 613–614. [Google Scholar] [CrossRef]
- Wolff, R.L.; Deluc, L.G.; Marpeau, A.M.; Comps, B. Chemotaxonomic differentiation of conifer families and genera based on the seed oil fatty acid compositions: Multivariate analyses. Trees 1997, 12, 57. [Google Scholar] [CrossRef]
- Wolff, R.L.; Deluc, L.G.; Marpeau, A.M.; Comps, B. Pinus gerardiana Wallichex. D. Don.—A review. Phytomedicine Plus 2021, 1, 100024. [Google Scholar]
- Wolff, R.L.; Bayard, C.C. Fatty acid composition of some pine seed oils. J. Am. Oil Chem. Soc. 1995, 72, 1043–1046. [Google Scholar] [CrossRef]
- Bagci, E.Y.Ü.P.; Karaagacli, Y.A.L.Ç.I.N. Fatty Acid and Tocochromanol Patterns of Turkish Pines. Acta Bbiologica Cracovensia Ser. Bot. 2004, 46, 95–100. [Google Scholar]
- Xu, T.; Hu, C.; Xuan, Q.; Xu, G. Recent advances in analytical strategies for mass spectrometry-based lipidomics. Anal. Chim. Acta 2020, 1137, 156–169. [Google Scholar] [CrossRef]
- Ferreira, I.J.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M. Green emerging extraction technologies to obtain high-quality vegetable oils from nuts: A review. Innov. Food Sci. Emerg. Technol. 2022, 76, 102931. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, Q.; Gu, H.; Yang, L. An approach for extraction of kernel oil from Pinus pumila using homogenate-circulating ultrasound in combination with an aqueous enzymatic process and evaluation of its antioxidant activity. J. Chromatogr. A 2016, 1471, 68–79. [Google Scholar] [CrossRef]
- Wolff, R.L.; Pédrono, F.; Pasquier, E.; Marpeau, A.M. General Characteristics of Pinus spp. Seed Fatty Acid Compositions, and Importance of ∆5-Olefinic Acids in the Taxonomy and Phylogeny of the Genus. Lipids 2000, 35, 1–22. [Google Scholar] [CrossRef]
- Tabassum, R.; Ripatti, S. Integrating lipidomics and genomics: Emerging tools to understand cardiovascular diseases. Cell. Mol. Life Sci. 2021, 78, 2565–2584. [Google Scholar] [CrossRef] [PubMed]
- Camunas-Alberca, S.M.; Moran-Garrido, M.; Sáiz, J.; Gil-de-la-Fuente, A.; Barbas, C.; Gradillas, A. Integrating the potential of ion mobility spectrometry-mass spectrometry in the separation and structural characterisation of lipid isomers. Front. Mol. Biosci. 2023, 10, 1112521. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, J.; Nath, L.R.; Gowda, S.G.; Gowda, D.; Hui, S.P. Analysis and functions of bioactive lipids in food. Discov. Food 2024, 4, 107. [Google Scholar] [CrossRef]
- Mason, M.E.; Waller, G.R. Dimethoxypropane Induced Transesterification of Fats and Oils in Preparation of Methyl Esters for Gas Chromatographic Analysis. Anal. Chem. 1964, 36, 583–586. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Xia, F.; Wan, J. Chemical derivatization strategy for mass spectrometry-based lipidomics. Mass Spectrom. Rev. 2023, 42, 432–452. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar-Yadav, R.; Kumar-Shrivastava, N.; Kumar, R.; Kumar, D.; Singh, J.; Yadav, S.; Nazam-Ansari, M.; Saeedan, A.S.; Kaithwas, G. Optimization of novel method for isolation of high purity food grade α-linolenic acid from Linum usitatissimum seeds. LWT 2023, 189, 115466. [Google Scholar] [CrossRef]
- Khouja, M.; Páscoa, R.M.N.J.; Melo, D.; Costa, A.S.G.; Nunes, M.A.; Khaldi, A.; Messaoud, C.; Oliveira, M.B.P.P.; Alves, R.C. Lipid Profile Quantification and Species Discrimination of Pine Seeds through NIR Spectroscopy: A Feasibility Study. Foods 2022, 11, 3939. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Yalım, N.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Karrar, E. The effect of different roasting processes on the total phenol, flavonoid, polyphenol, fatty acid composition and mineral contents of pine nut (Pinus pinea L.) seeds. J. Food Meas. Charact. 2025, 19, 238–251. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Mourtzinos, I.; Rosenberg, E.; Zachariadis, G.A. A Rapid GC-FID Method for the Determination of Fatty Acids in Walnut Oils and Their Use as Markers in Authenticity Studies. Food Anal. Methods 2022, 15, 761–771. [Google Scholar] [CrossRef]
- Koch, E.; Wiebel, M.; Hopmann, C.; Kampschulte, N.; Schebb, N.H. Rapid quantification of fatty acids in plant oils and biological samples by LC-MS. Anal. Bioanal. Chem. 2021, 413, 5439–5451. [Google Scholar] [CrossRef] [PubMed]
- Abhyankar, N.; Szalai, V. Challenges and Advances in the Application of Dynamic Nuclear Polarization to Liquid-State NMR Spectroscopy. J. Phys. Chem. B 2021, 125, 5171–5190. [Google Scholar] [CrossRef] [PubMed]
- Skakovskii, E.D.; Tychinskaya, L.Y.; Gaidukevich, O.A.; Klyuev, A.Y.; Kulakova, A.N.; Petlitskaya, N.M.; Rykove, S.V. NMR analysis of oils from pine nuts (Pinus sibirica) and seeds of common pine (Pinus silvestris L.). J. Appl. Spectrosc. 2007, 74, 584–588. [Google Scholar] [CrossRef]
- Terskikh, V.V.; Feurtado, J.A.; Borchardt, S.; Giblin, M.; Abrams, S.R.; Kermode, A.R. In vivo 13C NMR metabolite profiling: Potential for understanding and assessing conifer seed quality. J. Exp. Bot. 2005, 56, 2253–2265. [Google Scholar] [CrossRef]
- Hoffmann, N.; Mayer, G.; Has, C.; Kopczynski, D.; Machot, F.A.; Schwudke, D.; Ahrends, R.; Marcus, K.; Eisenacher, M.; Turewicz, M. A Current Encyclopedia of Bioinformatics Tools, Data Formats and Resources for Mass Spectrometry Lipidomics. Metabolites 2022, 12, 584. [Google Scholar] [CrossRef]
- Gerhardtova, I.; Jankech, T.; Majerova, P.; Piestansky, J.; Olesova, D.; Kovac, A.; Jampilek, J. Recent Analytical Methodologies in Lipid Analysis. Int. J. Mol. Sci. 2024, 25, 2249. [Google Scholar] [CrossRef]
- Chen, Y.; Li, E.M.; Xu, L.Y. Guide to Metabolomics Analysis: A Bioinformatics Workflow. Metabolites 2022, 2, 357. [Google Scholar] [CrossRef]
- Klukowski, P.; Riek, R.; Güntert, P. NMRtist: An online platform for automated biomolecular NMR spectra analysis. Bioinformatics 2023, 39, 2249. [Google Scholar] [CrossRef]
- Akyol, S.; Ugur, Z.; Yilmaz, A.; Ustun, I.; Kumar-Gorti, S.K.; Oh, K.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Maddens, M.E.; et al. Lipid Profiling of Alzheimer’s Disease Brain Highlights Enrichment in Glycerol (phospho)lipid, and Sphingolipid Metabolism. Cells 2021, 10, 2591. [Google Scholar] [CrossRef]
- Ahluwalia, K.; Ebright, B.; Chow, K.; Dave, P.; Mead, A.; Poblete, R.; Louie, S.G.; Asante, I. Lipidomics in Understanding Pathophysiology and Pharmacologic Effects in Inflammatory Diseases: Considerations for Drug Development. Metabolites 2022, 12, 333. [Google Scholar] [CrossRef]
- Rina, S.; Ying, H.; Shan, Y.; Du, W.; Liu, Y.; Li, R.; Deng, D. Application of Machine Learning to Tree Species Classification Using Active and Passive Remote Sensing: A Case Study of the Duraer Forestry Zone. Remote Sens. 2023, 15, 2596. [Google Scholar] [CrossRef]
- Ferrari, R.; Lachs, L.; Pygas, D.R.; Humanes, A.; Sommer, B.; Figueira, W.F.; Edwards, A.J.; Bythell, J.C.; Guest, J.R. Photogrammetry as a tool to improve ecosystem restoration. Trends Ecol. Evol. 2021, 36, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Lausch, A.; Erasmi, S.; King, D.; Magdon, P.; Heurich, M. Understanding Forest Health with Remote Sensing -Part I—A Review of Spectral Traits, Processes and Remote-Sensing Characteristics. Remote Sens. 2016, 8, 1029. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Luo, Y.; Cao, J.; Xie, R.; Li, S. Integrating satellite-derived climatic and vegetation indices to predict smallholder maize yield using deep learning. Agric. For. Meteorol. 2021, 311, 108666. [Google Scholar] [CrossRef]
- Bian, L.; Zhang, H.; Ge, Y.; Čepl, J.; Stejskal, J.; EL-Kassaby, Y.A. Closing the gap between phenotyping and genotyping: Review of advanced, image-based phenotyping technologies in forestry. Ann. For. Sci. 2022, 79, 22. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R. Remote sensing of temperate and boreal forest phenology: A review of progress, challenges and opportunities in the intercomparison of in-situ and satellite phenological metrics. For.Ecol. Manag. 2021, 480, 118663. [Google Scholar] [CrossRef]
- Castruita-Esparza, L.U.; Correa-Díaz, A.; Villanueva-Díaz, J.; Cervantes-Martínez, R.; Ortiz-Reyes, A.D. Impacto de Ips confusus Wood & Bright, 1992 en el incremento radial de Pinus cembroides Zucc. Rev. Mex. Cienc. For. 2024, 15, 4–28. [Google Scholar] [CrossRef]
- Escobar-Flores, J.G.; Lopez-Sanchez, C.A.; Sandoval, S.; Marquez-Linares, M.A.; Wehenkel, C. Predicting Pinus monophylla forest cover in the Baja California Desert by remote sensing. PeerJ 2018, 6, 4603. [Google Scholar] [CrossRef]
- Fukushima, A.; Kusano, M.; Redestig, H.; Arita, M.; Saito, K. Integrated omics approaches in plant systems biology. Curr. Opin. Chem. Biol. 2009, 13, 532–538. [Google Scholar] [CrossRef]
- Maldonado-Alconada, A.M.; Castillejo, M.Á.; Rey, M.-D.; Labella-Ortega, M.; Tienda-Parrilla, M.; Hernández-Lao, T.; Honrubia-Gómez, I.; Ramírez-García, J.; Guerrero-Sanchez, V.M.; López-Hidalgo, C.; et al. Multiomics Molecular Research into the Recalcitrant and Orphan Quercus ilex Tree Species: Why, What for, and How. Int. J. Mol. Sci. 2022, 23, 9980. [Google Scholar] [CrossRef]
- Meikle, T.G.; Huynh, K.; Giles, C.; Meikle, P.J. Clinical lipidomics: Realizing the potential of lipid profiling. J. Lipid Res. 2021, 62, 100127. [Google Scholar] [CrossRef]
- Yang, L.; Driscol, J.; Sarigai, S.; Wu, Q.; Chen, H.; Lippitt, C.D. Google Earth Engine and Artificial Intelligence (AI): A Comprehensive Review. Remote Sens. 2022, 14, 3256. [Google Scholar] [CrossRef]

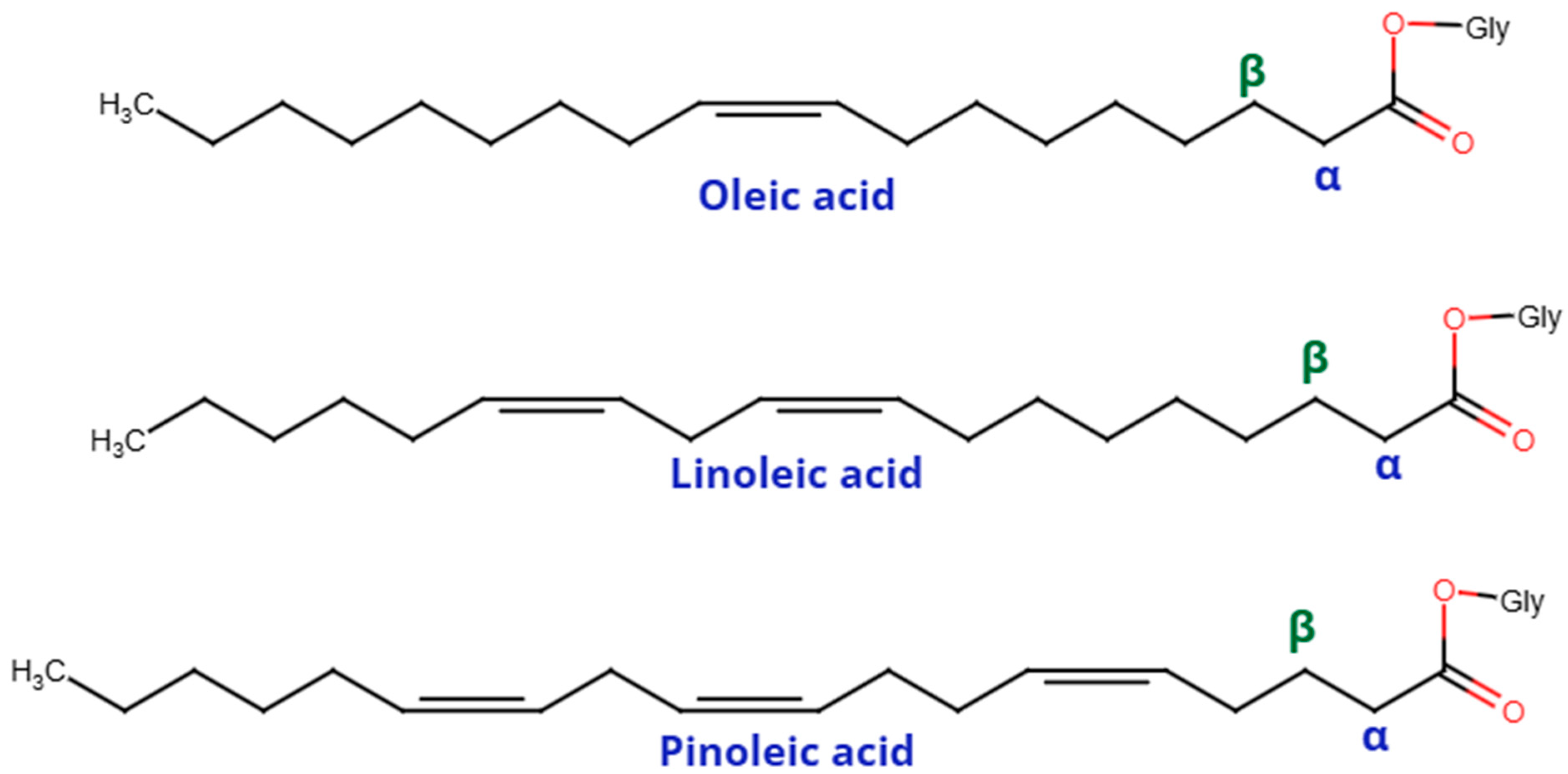
| Species | Location | Moisture (%) | Protein (%) | Fat (%) | Carbohydrates (%) | Ash (%) | Ref. |
|---|---|---|---|---|---|---|---|
| P. pinea | Mediterranean Europe and Near East | 5 | 32–34 | 45–48 | 7–14 | 5 | [30] |
| P. halepensis | Mediterranean Basin | 8 | 27 | 37 | 6 | 7 | [49] |
| P. pinaster | Western Mediterranean countries | 8 | 16 | 24 | 2 | 5 | [49,52] |
| P. canariensis | Canary Islands | 9 | 17 | 23.9 | 4 | 5 | [49] |
| P. gerardiana | Himalayas of India | - | 14 | 51 | 23 | - | [20] |
| P. edulis * | Southwestern US and Northern Mexico | - | 14 | 62–71 | 18 | - | [20] |
| P. sibrica | China, Russia and Mongolia | - | 19 | 51–75 | 12 | - | [20,53] |
| P. monophyla * | Southwestern US and Northern Mexico | - | 10 | 23 | 54 | - | [20] |
| P. koraiensis | Asia | 3 | 15 | 64 | 12 | 2 | [18,20] |
| P. sabiniana | California (United States) | - | 28 | 56 | 9 | - | [20] |
| P. cembra | Swiss | 17–18 | 50–59 | 17 | - | [54] | |
| P. cembroides | Central and North America | 15 | 16–19 | 48–58 | 19–32 | 3 | [13] |
| P. maximartinezii * | Central and North America | 5 | 31 | 42 | 2 | 4 | [54] |
| Species | Solvents (Extraction)/Detection and Separation | Fatty Acids (%) | Reference |
|---|---|---|---|
| * P. maximartinnezii | AOAC method/GC-FID | Linoleic (52), oleic (31), palmitic (9) | [54] |
| * P. cembroides (phenotype brown) | Hexane/GC-FID | Linoleic (45), oleic (37), palmitic (7) | [65] |
| * P. cembroides (phenotype fawn) | Hexane/GC-FID | Linoleic (43), oleic (42), palmitic (6) | [65] |
| * P. cembroides (phenotype black) | Hexane/GC-FID | Linoleic (33), oleic (47), palmitic (8) | [65] |
| * P. cembroides edulis | CH-Cl3-MeOH/GC-MS/LC-MS | Oleic (47), linoleic (41) | [66] |
| P. cembra | CH-Cl3-MeOH/GC-FID | Linoleic (45), oleic (23), pinoleic (19) | [67] |
| P. gerardiana | - | Palmitic (11), oleic (52), linoleic (43) | [68] |
| P. sibirica | n-hexane/GC-FID | Oleic (24), linoleic (43), pinoleic (16) | [18] |
| P. koraiensis | n-hexane/GC-FID | Oleic (33), linoleic (41), pinoleic (18) | [18] |
| CH-Cl3-MeOH/GC-FID | Oleic (24), linoleic (48), pinoleic (15) | [69] | |
| P. pinea | AOAC method/GC-FID | Palmitic (6), oleic (39), linoleic (48) | [30] |
| Hydro-distilled/GC-MS | Oleic (35), linoleic (53), palmitic (7) | [49] | |
| P. halepensis | Hydro-distilled/GC-MS | Oleic (25), linoleic (59), palmitic (5) | [49] |
| P. pinaster | Hydro-distilled/GC-MS | Palmitic (30), oleic (18), linoleic (52 | [49] |
| P. canariensis | Hydro-distilled/GC-MS | Linoleic (65), oleic (17), arachirid (6) | [49] |
| P. sylvestris | Heptane/GC-FID | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Herrera, L.R.; Contreras-Medina, L.M.; Feregrino-Pérez, A.A.; Cedillo, C.; Soto-Zarazúa, G.M.; Ramos-López, M.A.; Tejeda, S.; Amador-Enríquez, E.; Montoya-Morado, E. Techniques and Methods for Fatty Acid Analysis in Lipidomics: Exploring Pinus cembroides Kernels as a Sustainable Food Resource. Separations 2025, 12, 41. https://doi.org/10.3390/separations12020041
León-Herrera LR, Contreras-Medina LM, Feregrino-Pérez AA, Cedillo C, Soto-Zarazúa GM, Ramos-López MA, Tejeda S, Amador-Enríquez E, Montoya-Morado E. Techniques and Methods for Fatty Acid Analysis in Lipidomics: Exploring Pinus cembroides Kernels as a Sustainable Food Resource. Separations. 2025; 12(2):41. https://doi.org/10.3390/separations12020041
Chicago/Turabian StyleLeón-Herrera, Luis Ricardo, Luis Miguel Contreras-Medina, Ana Angélica Feregrino-Pérez, Christopher Cedillo, Genaro Martín Soto-Zarazúa, Miguel Angel Ramos-López, Samuel Tejeda, Eduardo Amador-Enríquez, and Enrique Montoya-Morado. 2025. "Techniques and Methods for Fatty Acid Analysis in Lipidomics: Exploring Pinus cembroides Kernels as a Sustainable Food Resource" Separations 12, no. 2: 41. https://doi.org/10.3390/separations12020041
APA StyleLeón-Herrera, L. R., Contreras-Medina, L. M., Feregrino-Pérez, A. A., Cedillo, C., Soto-Zarazúa, G. M., Ramos-López, M. A., Tejeda, S., Amador-Enríquez, E., & Montoya-Morado, E. (2025). Techniques and Methods for Fatty Acid Analysis in Lipidomics: Exploring Pinus cembroides Kernels as a Sustainable Food Resource. Separations, 12(2), 41. https://doi.org/10.3390/separations12020041







