A Two-Dimensional Thiotitanate Ion Exchanger with High Cs+ Removal Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Rb-TiS2
2.2.2. Characterization Techniques
2.2.3. Batch Adsorption Experiments
3. Results
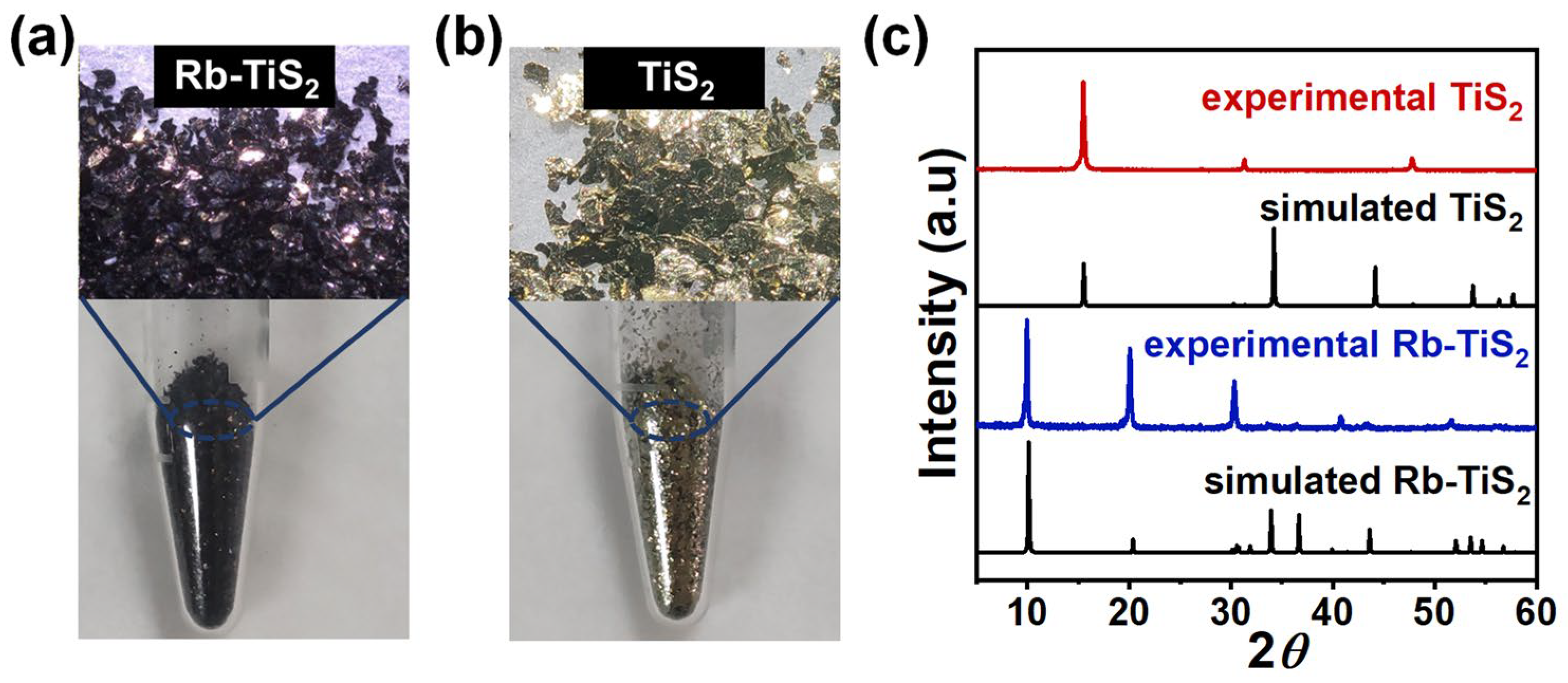
3.1. Synthetic Optimization
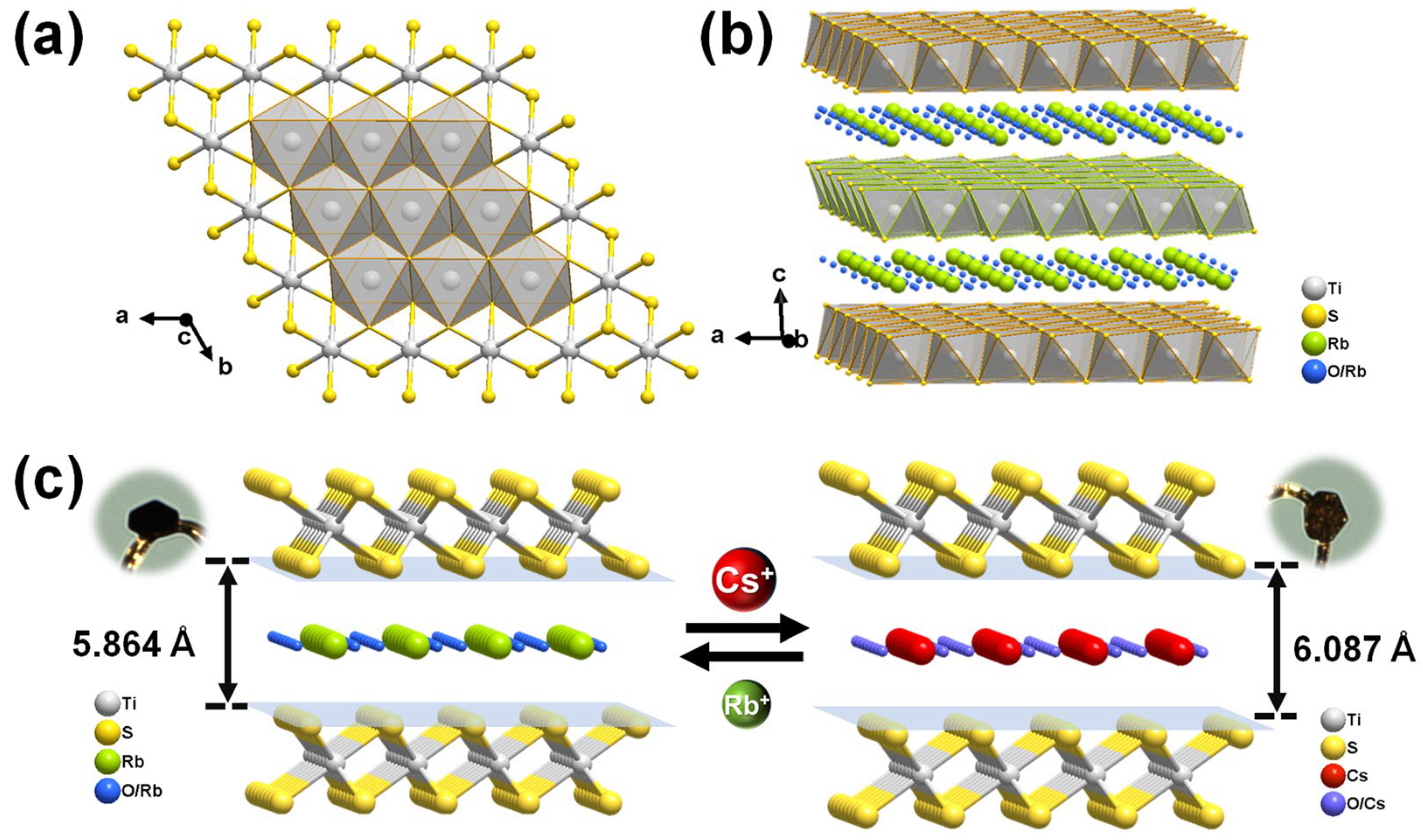
3.2. Structural Description and Removal Mechanism
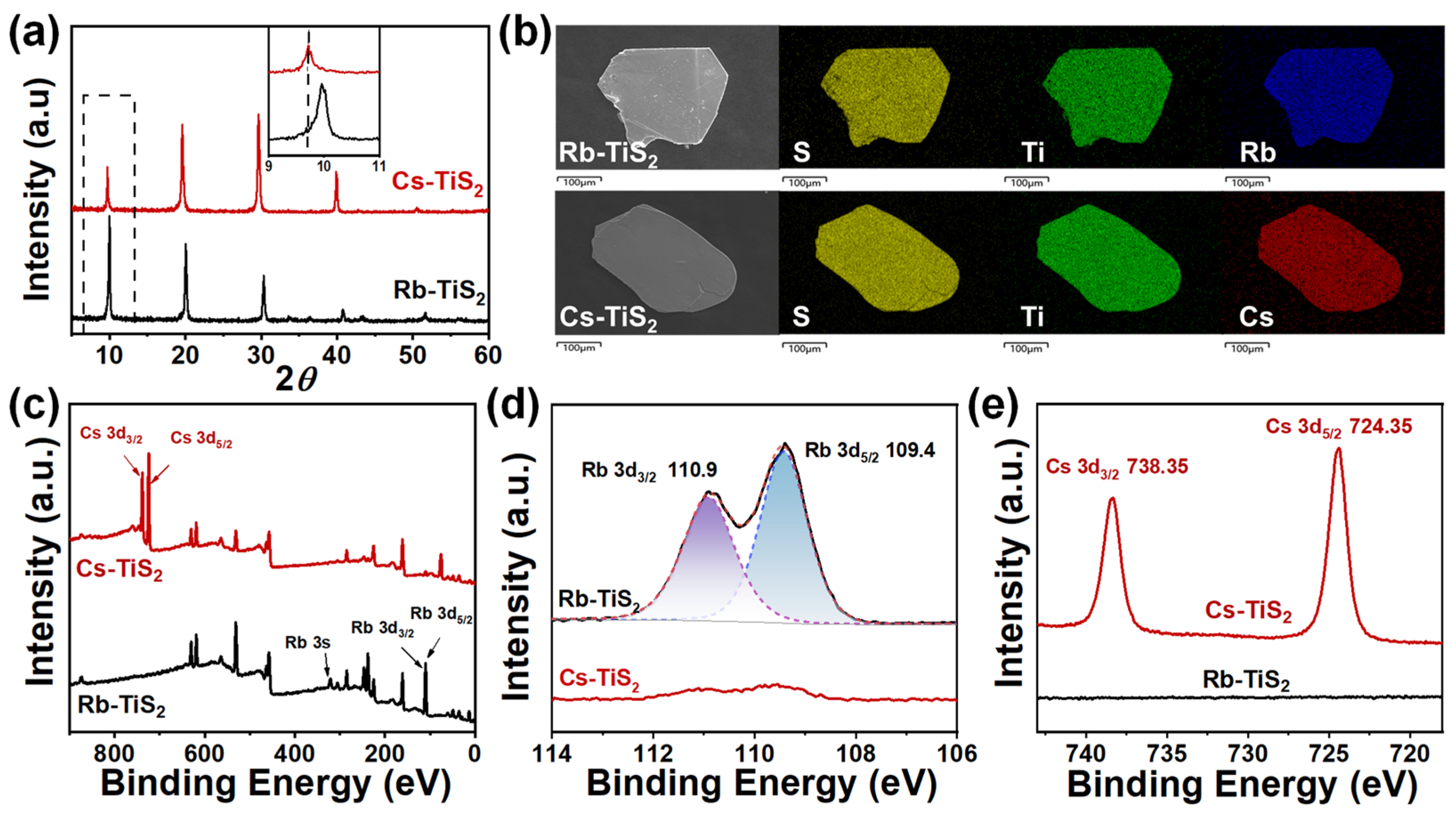
3.3. Characterization of Rb-TiS2 and Cs-TiS2
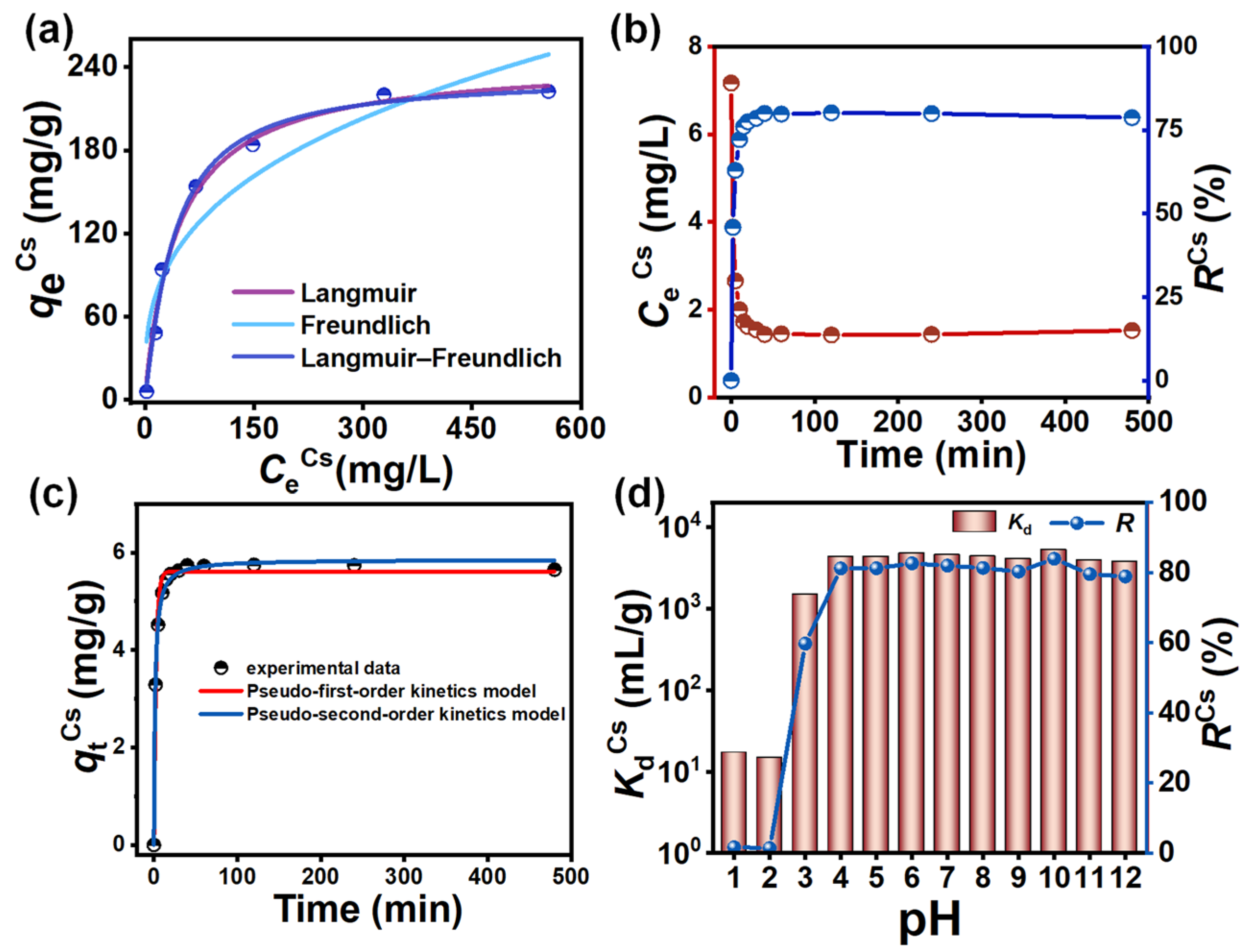
3.4. Adsorption Isotherm Study
3.5. Adsorption Kinetics Studies
3.6. Effect of pH on the Cs+ Adsorption
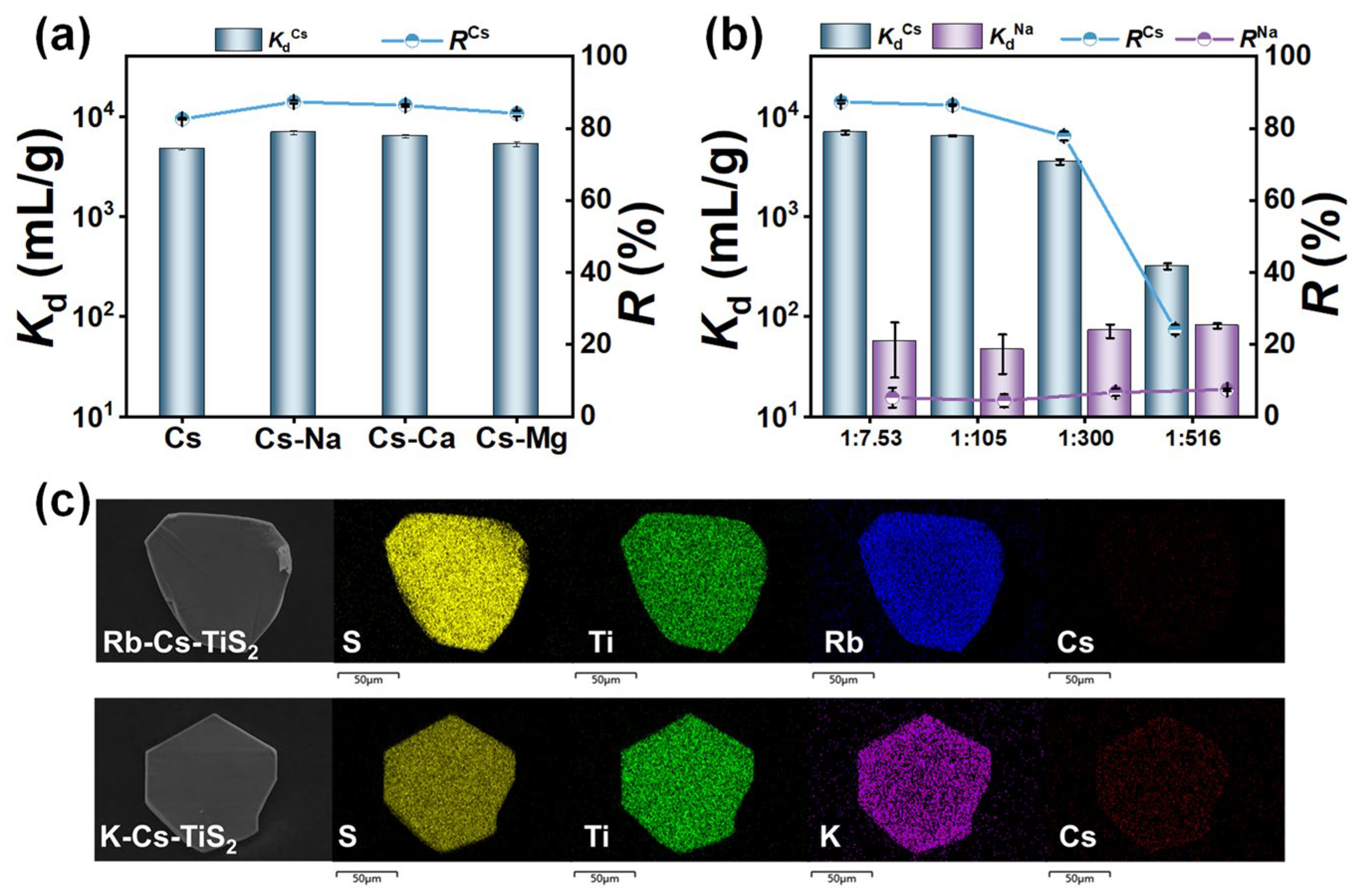
3.7. Competitive Adsorption Studies
3.8. Adsorption and Desorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bruno, J.; Ewing, R.C. Spent nuclear fuel. Elements 2006, 2, 343–349. [Google Scholar] [CrossRef]
- Burns, P.C.; Ewing, R.C.; Navrotsky, A. Nuclear Fuel in a Reactor Accident. Science 2012, 335, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.H.; Yang, Y.S.; Song, K.W. Radioactivity release from the Fukushima accident and its consequences: A review. Prog. Nucl. Energy 2014, 74, 61–70. [Google Scholar] [CrossRef]
- Matsuda, K.; Yamamoto, S.; Miyamoto, K. Comparison of Cs-137 uptake, depuration and continuous uptake, originating from feed, in five salmonid fish species. J. Environ. Radioact. 2020, 222, 106350. [Google Scholar] [CrossRef]
- Morimoto, M.; Kobayashi, J.; Kino, Y. Radiation dose and gene expression analysis of wild boar 10 years after the Fukushima Daiichi Nuclear Plant accident. Sci. Rep. 2022, 12, 18653. [Google Scholar] [CrossRef]
- Nash, K.L.; Braley, J.C. Chemistry of Radioactive Materials in the Nuclear Fuel Cycle; Nash, K.L.L., Gregg, J., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 3–22. [Google Scholar]
- Ashraf, M.A.; Akib, S.; Maah, M.J.; Yusoff, I.; Balkhair, K.S. Cesium-137: Radio-Chemistry, Fate, and Transport, Remediation, and Future Concerns. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1740–1793. [Google Scholar] [CrossRef]
- Mitrovic, B.M.; Vitorovic, G.; Vicentijevic, M.; Vitorovic, D.; Pantelic, G.; Lazarevic-Macanovic, M. Comparative study of 137Cs distribution in broilers and pheasants and possibilities for protection. Radiat. Environ. Biophys. 2012, 51, 79–84. [Google Scholar] [CrossRef]
- Cardis, E.; Howe, G.; Ron, E.; Bebeshko, V.; Bogdanova, T.; Bouville, A.; Carr, Z.; Chumak, V.; Davis, S.; Demidchik, Y.; et al. Cancer consequences of the Chernobyl accident: 20 years on. J. Radiol. Prot. 2006, 26, 127–140. [Google Scholar] [CrossRef]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 9405. [Google Scholar] [CrossRef]
- Haque, M.; Dayem, S.B.; Tasnim, N.T.; Islam, M.R.; Shakil, M.S. Biological impact of Chernobyl radiation: A review of recent progress. Int. J. Radiat. Biol. 2024, 100, 1405–1415. [Google Scholar] [CrossRef]
- Clemons, J.; Blumenberg, A. The Goiania incident, the semiotics of danger, and the next 10,000 years. Clin. Toxicol. 2023, 61, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Tosri, C.; Chusreeaeom, K.; Limtiyayotin, M.; Sukin, N.; Jompuk, P. Comparative effect of high energy electron beam and 137Cs gamma ray on survival, growth and chlorophyll content in curcuma hybrid ‘laddawan’ and determine proper dose for mutations breeding. Emir. J. Food Agric. 2019, 31, 321–327. [Google Scholar] [CrossRef]
- Fentiman, I.S.; Deshmane, V.; Tong, D.; Winter, J.; Mayles, H.; Chaudary, M.A. Caesium137 implant as sole radiation therapy for operable breast cancer:: A phase II trial. Radiother. Oncol. 2004, 71, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Artun, O. A study of nuclear structure for 244Am, 238Pu, 210Po, 147Pm, 137Cs, 90Sr and 63Ni nuclei used in nuclear battery. Mod. Phys. Lett. A 2017, 32, 750117. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuang, S.T. Removal of cesium ions from aqueous solutions using various separation technologies. Rev. Environ. Sci. Bio-Technol. 2019, 18, 231–269. [Google Scholar] [CrossRef]
- Fang, D.; Wang, Y.; Liu, H.; Zhang, H.; Ye, X.; Li, Q.; Li, J.; Wu, Z. Efficient extraction of Rb+ and Cs+ by a precipitation flotation process with ammonium phosphowolframate as precipitant. Colloid Surf. A-Physicochem. Eng. Asp. 2021, 608, 125581. [Google Scholar] [CrossRef]
- Wang, J.L.; Zhuang, S.T. Cesium separation from radioactive waste by extraction and adsorption based on crown ethers and calixarenes. Nucl. Eng. Technol. 2020, 52, 328–336. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553. [Google Scholar] [CrossRef]
- Tang, J.H.; Sun, H.Y.; Ma, W.; Feng, M.L.; Huang, X.Y. Recent Progress in Developing Crystalline Ion Exchange Materials for the Removal of Radioactive Ions. Chin. J. Struct. Chem. 2020, 39, 2157–2171. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Metal sulfide ion exchangers: Superior sorbents for the capture of toxic and nuclear waste-related metal ions. Chem. Sci. 2016, 7, 4804–4824. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Feng, M.; Huang, X. Metal chalcogenides as ion-exchange materials for the efficient removal of key radionuclides: A review. Fundam. Res. 2024, in press. [CrossRef]
- Zhao, Q.; Wang, S.; Wu, Y.C.; Wang, Y.X.; Ma, S.S.; Shih, K.M. Layered metal sulfides with MaSbc− framework (M = Sb, In, Sn) as ion exchangers for the removal of Cs(I) and Sr(II) from radioactive effluents: A review. Front. Chem. 2023, 11, 1292979. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.J.; Kanatzidis, M.G. Highly Efficient and Rapid Cs+ Uptake by the Layered Metal Sulfide K2xMnxSn3−xS6 (KMS-1). J. Am. Chem. Soc. 2009, 131, 6599–6607. [Google Scholar] [CrossRef] [PubMed]
- Mertz, J.L.; Fard, Z.H.; Malliakas, C.D.; Manos, M.J.; Kanatzidis, M.G. Selective Removal of Cs+, Sr2+, and Ni2+ by K2xMgxSn3−xS6 (x = 0.5–1) (KMS-2) Relevant to Nuclear Waste Remediation. Chem. Mater. 2013, 25, 2116–2127. [Google Scholar] [CrossRef]
- Jiang, Z.Z.; Liu, G.L.; Ma, C.; Guo, Y.F.; Duo, J.; Li, M.L.; Deng, T.L. Cesium removal from wastewater: High-efficient and reusable adsorbent K1.93Ti0.22Sn3S6.43. Chemosphere 2022, 305, 135406. [Google Scholar] [CrossRef]
- Sarma, D.; Malliakas, C.D.; Subrahmanyam, K.S.; Islama, S.M.; Kanatzidis, M.G. K2xSn4−xS8−x (x = 0.65–1): A new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO22+ ions. Chem. Sci. 2016, 7, 1121–1132. [Google Scholar] [CrossRef]
- Yu, J.M.; Luo, D.; Ma, Z.J.; Zheng, B.; Cheng, F.F.; Xiong, W.W. Effective Enrichment of Low-Concentration Rare-Earth Ions by Three-Dimensional Thiostannate K2Sn2S5. ACS Appl. Mater. Interfaces 2021, 13, 55188–55197. [Google Scholar] [CrossRef]
- Qi, X.H.; Du, K.Z.; Feng, M.L.; Li, J.R.; Du, C.F.; Zhang, B.; Huang, X.Y. A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property. J. Mater. Chem. A 2015, 3, 5665–5673. [Google Scholar] [CrossRef]
- Feng, M.L.; Sarma, D.; Gao, Y.J.; Qi, X.H.; Li, W.A.; Huang, X.Y.; Kanatzidis, M.G. Efficient Removal of [UO2]2+, Cs+, and Sr2+ Ions by Radiation-Resistant Gallium Thioantimonates. J. Am. Chem. Soc. 2018, 140, 11133–11140. [Google Scholar] [CrossRef]
- Tang, J.H.; Jin, J.C.; Li, W.A.; Zeng, X.; Ma, W.; Li, J.L.; Lv, T.T.; Peng, Y.C.; Feng, M.L.; Huang, X.Y. Highly selective cesium(I) capture under acidic conditions by a layered sulfide. Nat. Commun. 2022, 13, 658. [Google Scholar] [CrossRef]
- Wu, M.; Sul, W.P.; Jasutkar, N.; Huang, X.Y.; Li, J. An open-framework bimetallic chalcogenide structure K3Rb3Zn4Sn3Se13 built on a unique Zn49Sn3Se1612− cluster: Synthesis, crystal structure, ion exchange and optical properties. Mater. Res. Bull. 2005, 40, 21–27. [Google Scholar] [CrossRef]
- Manos, M.J.; Iyer, R.G.; Quarez, E.; Liao, J.H.; Kanatzidis, M.G. {Sn[Zn4Sn4S17]}6−: A robust open framework based on metal-linked penta-supertetrahedral [Zn4Sn4S17]10− clusters with ion-exchange properties. Angew. Chem.-Int. Edit. 2005, 44, 3552–3555. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Q.; Jia, M.C.; Wang, X.W.; Du, Z.H.; Men, J.F.; Ding, H. Preparation of potassium niobium sulfide and its selective adsorption properties for Sr2+ and Co2+. J. Radioanal. Nucl. Chem. 2019, 322, 377–387. [Google Scholar] [CrossRef]
- Zhang, M.D.; Gu, P.; Yan, S.; Dong, L.H.; Zhang, G.H. Na/Zn/Sn/S (NaZTS): Quaternary metal sulfide nanosheets for efficient adsorption of radioactive strontium ions. Chem. Eng. J. 2020, 379, 122227. [Google Scholar] [CrossRef]
- Wei, C.; Liu, J.; Zhao, Y.; Jia, S.; Gao, Y.; Saha, R.A.; Torchio, R.; Zhang, T.; Yang, L.; Sun, H.; et al. Efficient capture of Cs+ and Sr2+ by layered thioniobates and thiotantalate and insight into the structure-property relationship. Sci. China-Chem 2025. [Google Scholar] [CrossRef]
- Sunshine, S.A.; Kang, D.; Ibers, J.A. A New Low-Temperature Route to Metal Polychalcogenides: Solid-State Synthesis of K4Ti3S14, A Novel One-Dimensional Compound. J. Am. Chem. Soc. 1987, 109, 6202–6204. [Google Scholar] [CrossRef]
- Klepp, K.O. K2TiS3, A New Thiotitanate(lV)with Pentacoordinate Titanium. Z. Naturforsch. (B) 1992, 47, 201–204. [Google Scholar] [CrossRef]
- Itoh, Y.; Suematsu, H. Stability of Two-Dimensional Intercalant Structure in KxTiS2. Mater. Sci. Forum 1992, 91–93, 375–380. [Google Scholar] [CrossRef]
- Bichon, J.; Danot, M.; Rouxel, J. Systematique Structurale Pour Les Series D’intercalaires MxTiS2 (M = Li, Na, K, Rb, Cs). C. R. Des Seances De L’academie Des Sci. Ser. C Sci. Chim. 1973, 276, 1283–1286. [Google Scholar]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation-Effective of Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics; World Scientific: Singapore, 1998. [Google Scholar]
- Amesh, P.; Suneesh, A.S.; Venkatesan, K.A.; Maheswari, R.U.; Vijayalakshmi, S. Preparation and ion exchange studies of cesium and strontium on sodium iron titanate. Sep. Purif. Technol. 2020, 238, 116393. [Google Scholar] [CrossRef]
- Asgari, P.; Mousavi, S.H.; Aghayan, H.; Ghasemi, H.; Yousefi, T. Nd-BTC metal-organic framework (MOF); synthesis, characterization and investigation on its adsorption behavior toward cesium and strontium ions. Microchem. J. 2019, 150, 104188. [Google Scholar] [CrossRef]
- Bayülken, S.; Basçetin, E.; Güçlü, K.; Apak, R. Investigation and Modeling of Cesium(I) Adsorption by Turkish Clays: Bentonite, Zeolite, Sepiolite, and Kaolinite. Environ. Prog. Sustain. Energy 2011, 30, 70–80. [Google Scholar] [CrossRef]
- Park, Y.; Lee, Y.C.; Shin, W.S.; Choi, S.J. Removal of cobalt, strontium and cesium from radioactive laundry wastewater by ammonium molybdophosphate-polyacrylonitrile (AMP-PAN). Chem. Eng. J. 2010, 162, 685–695. [Google Scholar] [CrossRef]
- Gupta, K.; Yuan, B.L.; Chen, C.; Varnakavi, N.; Fu, M.L. K2xMnxSn3−xS6 (x = 0.5–0.95) (KMS-1) immobilized on the reduced graphene oxide as KMS-1/r-GO aerogel to effectively remove Cs+ and Sr2+ from aqueous solution. Chem. Eng. J. 2019, 369, 803–812. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Cheng, L.; Zhao, Y.M.; Li, M.Y.; Wang, Z.Z.; Wang, J.; Wang, C.; Wang, K.Y. Structural investigation of the efficient capture of Cs+ and Sr2+ by a microporous Cd-Sn-Se ion exchanger constructed from mono-lacunary supertetrahedral clusterst. Inorg. Chem. Front. 2022, 9, 2880–2894. [Google Scholar] [CrossRef]
- Datta, S.J.; Moon, W.K.; Choi, D.Y.; Hwang, I.C.; Yoon, K.B. A Novel Vanadosilicate with Hexadeca-Coordinated Cs+ Ions as a Highly Effective Cs+ Remover. Angew. Chem.-Int. Edit. 2014, 53, 7203–7208. [Google Scholar] [CrossRef]
- Sun, H.Y.; Chen, Z.H.; Hu, B.; Tang, J.H.; Yang, L.; Guo, Y.L.; Yao, Y.X.; Feng, M.L.; Huang, X.Y. Boosting selective Cs+ uptake through the modulation of stacking modes in layered niobate-based perovskites. Nat. Commun. 2024, 15, 8681. [Google Scholar] [CrossRef]
- Chen, Z.H.; Jia, S.Q.; Sun, H.Y.; Tang, J.H.; Guo, Y.L.; Yao, Y.X.; Pan, T.Y.; Feng, M.L.; Huang, X.Y. All-in-one treatment: Capture and immobilization of 137 Cs by ultra-stable inorganic solid acid materials HMMoO6•nH2O (M = Ta, Nb). Water Res. 2024, 255, 121459. [Google Scholar] [CrossRef]
- Yan, J.; Wu, J.T.; Li, B.H.; Zhang, Y.D.; Yang, Y.; Li, J.; Zhang, B. Economically friendly and facilely synthesized layered metal sulfide for efficient removal of aqueous cesium. J. Solid State Chem. 2024, 337, 124834. [Google Scholar] [CrossRef]
- Wang, K.Y.; Li, M.Y.; Cheng, L.; Hao, X.; Wang, C. Tailoring supertetrahedral cadmium/tin selenide clusters into a robust framework for efficient elimination of Cs+, Co2+, and Ni2+ ions. Inorg. Chem. Front. 2024, 11, 3229–3244. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Li, J.; Yang, Y.; Wang, Y.N.; Zhang, Y.D.; Liu, X.Z. Rapid and Selective Uptake of Radioactive Cesium from Water by a Microporous Zeolitic-like Sulfide. Inorg. Chem. 2023, 62, 12843–12850. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.F.; Ni, S.; Xing, H.F.; Meng, Q.Y.; Bian, Y.Y.; Xu, Z.H.; Rong, M.; Liu, H.Z.; Yang, L.R. Cation-Intercalated Lamellar MoS2 Adsorbent Enables Highly Selective Capture of Cesium. ACS Appl. Mater. Interfaces 2023, 15, 49095–49106. [Google Scholar] [CrossRef]
- Sun, H.Y.; Hu, B.; Lv, T.T.; Guo, Y.L.; Yao, Y.X.; Yang, L.; Feng, M.L.; Huang, X.Y. Efficient Co-Adsorption and Highly Selective Separation of Cs+ and Sr2+ with a K+-Activated Niobium Germanate by the pH Control. Small 2023, 19, 2208212. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, F.Q.; Hao, X.; Li, M.Y.; Cheng, L.; Wang, C.; Wang, K.Y. Open-framework hybrid zinc/tin selenide as an ultrafast adsorbent for Cs+, Ba2+, Co2+, and Ni2+. J. Hazard. Mater. 2023, 458, 132038. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Cheng, L.; Wang, K.Y.; Hao, X.; Wang, J.; Zhu, J.Y.; Sun, M.; Wang, C. pH-Controlled Switch over Coadsorption and Separation for Mixed Cs+ and Sr2+ by an Acid-Resistant Potassium Thioantimonate. Adv. Funct. Mater. 2022, 32, 2112717. [Google Scholar] [CrossRef]
- Li, W.A.; Li, J.R.; Zhang, B.; Sun, H.Y.; Jin, J.C.; Huang, X.Y.; Feng, M.L. Layered Thiostannates with Distinct Arrangements of Mixed Cations for the Selective Capture of Cs+, Sr2+, and Eu3+ Ions. ACS Appl. Mater. Interfaces 2021, 13, 10191–10201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.; Jia, S.; Zhao, Y.; Liu, J.; Sun, H.; Feng, M.; Huang, X. A Two-Dimensional Thiotitanate Ion Exchanger with High Cs+ Removal Performance. Separations 2025, 12, 104. https://doi.org/10.3390/separations12050104
Wei C, Jia S, Zhao Y, Liu J, Sun H, Feng M, Huang X. A Two-Dimensional Thiotitanate Ion Exchanger with High Cs+ Removal Performance. Separations. 2025; 12(5):104. https://doi.org/10.3390/separations12050104
Chicago/Turabian StyleWei, Chang, Shaoqing Jia, Yingying Zhao, Jiating Liu, Haiyan Sun, Meiling Feng, and Xiaoying Huang. 2025. "A Two-Dimensional Thiotitanate Ion Exchanger with High Cs+ Removal Performance" Separations 12, no. 5: 104. https://doi.org/10.3390/separations12050104
APA StyleWei, C., Jia, S., Zhao, Y., Liu, J., Sun, H., Feng, M., & Huang, X. (2025). A Two-Dimensional Thiotitanate Ion Exchanger with High Cs+ Removal Performance. Separations, 12(5), 104. https://doi.org/10.3390/separations12050104







