Determination of Metals in Walnut Oils by Means of an Optimized and Validated ICP-AES Method in Conventional and Organic Farming Type Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Instrumentation
2.3. Sample Collection and Preparation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Dissolution Procedure
3.2. Optimization of ICP-AES Conditions
3.3. Figures of Merit
3.4. Real Samples Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kalogiouri, N.P.; Manousi, N.; Rosenberg, E.; Zachariadis, G.A.; Samanidou, V.F. Advances in the Chromatographic Separation and Determination of Bioactive Compounds for Assessing the Nutrient Profile of Nuts. Curr. Anal. Chem. 2020, 16, 1–17. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Zachariadis, G.A. Determination of the Toxic and Nutrient Element Content of Almonds, Walnuts, Hazelnuts and Pistachios by ICP-AES. Separations 2021, 8, 28. [Google Scholar] [CrossRef]
- Savage, G.P.; Mcneil, D.L. Some nutritional advantages of walnuts. Acta Hortic. 2001, 544, 557–563. [Google Scholar] [CrossRef]
- Crews, C.; Hough, P.; Godward, J.; Brereton, P.; Lees, M.; Guiet, S.; Winkelmann, W. Study of the main constituents of some authentic walnut oils. J. Agric. Food Chem. 2005, 53, 4853–4860. [Google Scholar] [CrossRef] [PubMed]
- Fregapane, G.; Guisantes-Batan, E.; Ojeda-Amador, R.M.; Salvador, M.D. Development of functional edible oils enriched with pistachio and walnut phenolic extracts. Food Chem. 2020, 310, 125917. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Amador, R.M.; Salvador, M.D.; Gómez-Alonso, S.; Fregapane, G. Characterization of virgin walnut oils and their residual cakes produced from different varieties. Food Res. Int. 2018, 108, 396–404. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Fessi, H.; Elaissari, A. Plant oils: From chemical composition to encapsulated form use. Int. J. Pharm. 2021, 601, 120538. [Google Scholar] [CrossRef] [PubMed]
- Valero-Vello, M.; Peris-Mart, C.; Garc, J.; Zan, V.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D. Searching for the Antioxidant, Anti-Inflammatory, and Neuroprotective Potential of Natural Food and Nutritional. Foods 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, R.; Jin, Q.; Wang, X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and Juglans sigillata. Food Chem. 2019, 279, 279–287. [Google Scholar] [CrossRef]
- Zibaeenezhad, M.J.; Farhadi, P.; Attar, A.; Mosleh, A.; Amirmoezi, F.; Azimi, A. Effects of walnut oil on lipid profiles in hyperlipidemic type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Nutr. Diabetes 2017, 7, e259. [Google Scholar] [CrossRef] [PubMed]
- Batirel, S.; Yilmaz, A.M.; Sahin, A.; Perakakis, N.; Kartal Ozer, N.; Mantzoros, C.S. Antitumor and antimetastatic effects of walnut oil in esophageal adenocarcinoma cells. Clin. Nutr. 2018, 37, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F. HPLC Fingerprints for the Characterization of Walnuts and the Detection of Fraudulent Incidents. Foods 2021, 10, 2145. [Google Scholar]
- Kalogiouri, N.P.; Manousi, N.; Rosenberg, E.; Zachariadis, G.A.; Paraskevopoulou, A.; Samanidou, V. Exploring the volatile metabolome of conventional and organic walnut oils by solid-phase microextraction and analysis by GC-MS combined with chemometrics. Food Chem. 2021, 363, 130331. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska, A.; Zielińska, D.; Łepecka, A.; Trząskowska, M.; Neffe-Skocińska, K.; Kołożyn-Krajewska, D. Development of functional high-protein organic bars with the addition of whey protein concentrate and bioactive ingredients. Agriculture 2020, 10, 390. [Google Scholar] [CrossRef]
- European Commission, Regulation (EU) 2018/848 on organic production and labelling of organic product. Off. J. Eur. Union 2018, 2018, 1–92.
- De Lima, M.D.; Barbosa, R. Methods of authentication of food grown in organic and conventional systems using chemometrics and data mining algorithms: A review. Food Anal. Methods 2019, 12, 887–901. [Google Scholar] [CrossRef]
- Fiamegos, Y.; Papoci, S.; Dumitrascu, C.; Ghidotti, M.; Zdiniakova, T.; Ulberth, F.; de la Calle Guntiñas, M.B. Are the elemental fingerprints of organic and conventional food different? ED-XRF as screening technique. J. Food Compos. Anal. 2021, 99, 103854. [Google Scholar] [CrossRef]
- Lepri, F.G.; Chaves, E.S.; Vieira, M.A.; Ribeiro, A.S.; Curtius, A.J.; De Oliveira, L.C.C.; De Campos, R.C. Determination of trace elements in vegetable oils and biodiesel by atomic spectrometric techniques—A review. Appl. Spectrosc. Rev. 2011, 46, 175–206. [Google Scholar] [CrossRef]
- Momen, A.A.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Investigation of four digestion procedures for multi-element determination of toxic and nutrient elements in legumes by inductively coupled plasma-optical emission spectrometry. Anal. Chim. Acta 2006, 565, 81–88. [Google Scholar] [CrossRef]
- Laursen, K.H.; Schjoerring, J.K.; Kelly, S.D.; Husted, S. Authentication of organically grown plants—Advantages and limitations of atomic spectroscopy for multi-element and stable isotope analysis. TrAC Trends Anal. Chem. 2014, 59, 73–82. [Google Scholar] [CrossRef]
- Savio, M.; Ortiz, M.S.; Almeida, C.A.; Olsina, R.A.; Martinez, L.D.; Gil, R.A. Multielemental analysis in vegetable edible oils by inductively coupled plasma mass spectrometry after solubilisation with tetramethylammonium hydroxide. Food Chem. 2014, 159, 433–438. [Google Scholar] [CrossRef]
- Cindric, I.J.; Zeiner, M.; Steffan, I. Trace elemental characterization of edible oils by ICP-AES and GFAAS. Microchem. J. 2007, 85, 136–139. [Google Scholar] [CrossRef]
- Yin, L.L.; Qing, T.; Zhang, S.X.; Yin, K.X.; Qin, J.I.Q. Determination of Trace Elements in Edible Nuts in the Beijing Market by ICP-MS. Biomed. Environ. Sci. 2015, 28, 449–454. [Google Scholar]
- Bakircioglu, D.; Kurtulus, Y.B.; Yurtsever, S. Comparison of extraction induced by emulsion breaking, ultrasonic extraction and wet digestion procedures for determination of metals in edible oil samples in Turkey using ICP-OES. Food Chem. 2013, 138, 770–775. [Google Scholar] [CrossRef]
- Tošić, S.B.; Mitić, S.S.; Velimirović, D.S.; Stojanović, G.S.; Pavlović, A.N.; Pecev-Marinković, E.T. Elemental composition of edible nuts: Fast optimization and validation procedure of an ICP-OES method. J. Sci. Food Agric. 2015, 95, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Ennoukh, F.E.; Bchitou, R.; Mohammed, F.; Guillaume, D.; Harhar, H.; Bouhaouss, A. Study of the effects of extraction methods on Argan oil quality through its metal content. Ind. Crops Prod. 2017, 109, 182–184. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Giakisikli, G.; Zachariadis, G.A. The HyperSep SCX micro-cartridge for on-line flow injection inductively coupled plasma atomic emission spectrometric determination of trace elements in biological and environmental samples. Anal. Methods 2011, 3, 2108–2114. [Google Scholar] [CrossRef]
- IUPAC. Compendium in Chemical Terminology, Version 2014; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Zhu, F.; Fan, W.; Wang, X.; Qu, L.; Yao, S. Health risk assessment of eight heavy metals in nine varieties of edible vegetable oils consumed in China. Food Chem. Toxicol. 2011, 49, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.F.; Papai, R.; Luz, M.S.; Gaubeur, I. Analytical extraction procedure combined with atomic and mass spectrometry for the determination of tin in edible oil samples, and the potential application to other chemical elements. J. Food Compos. Anal. 2021, 96, 103759. [Google Scholar] [CrossRef]
- Carneiro, A.F.; Carneiro, C.N.; de N. Pires, L.; Teixeira, L.S.G.; Azcarate, S.M.; de S. Dias, F. D-optimal mixture design for the optimization of extraction induced by emulsion breaking for multielemental determination in edible vegetable oils by microwave-induced plasma optical emission spectrometry. Talanta 2020, 219, 121218. [Google Scholar]
- Juranović Cindrić, I.; Zeiner, M.; Hlebec, D. Mineral composition of elements in walnuts and walnut oils. Int. J. Environ. Res. Public Health 2018, 15, 2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanidou, V.F.; Christodoulou, E.A.; Papadoyannis, I.N. Determination of fluoroquinolones in edible animal tissue samples by high performance liquid chromatography after solid phase extraction. J. Sep. Sci. 2005, 28, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Nițu, S.; Pădure, M.; Tămaș, A.; Rusnac, L.M. Comparative analysis of walnuts and peanuts oils. Stud. Univ. Babes-Bolyai Chem. 2019, 64, 229–237. [Google Scholar] [CrossRef]
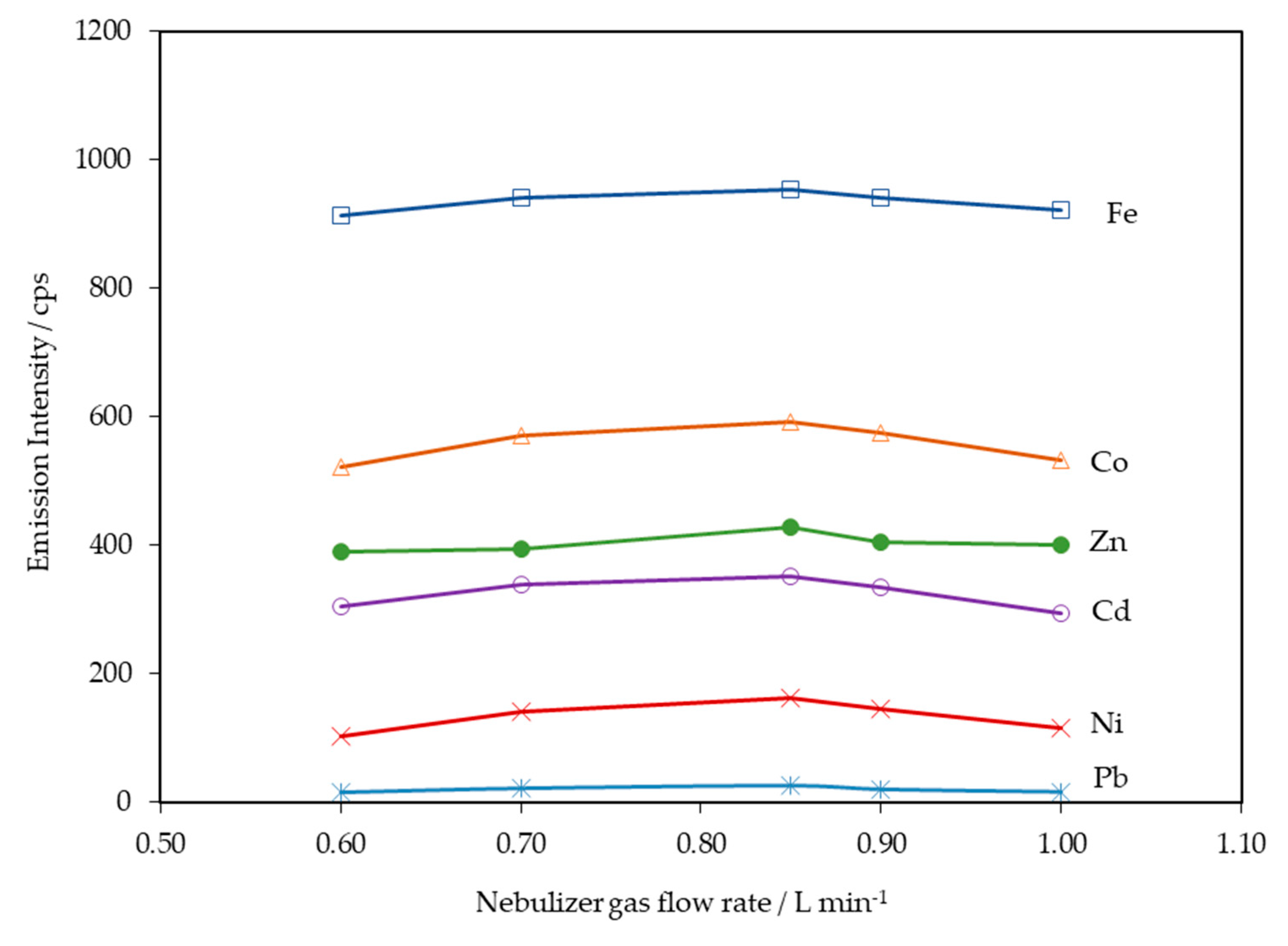


| RF generator | 40 MHz, free-running |
| RF incident power | 1350 W (optimized) |
| Auxiliary Argon gas flow rate | 0.6 L min−1 |
| Plasma Argon gas flow rate | 15.0 L min−1 |
| Nebulizer Argon gas flow rate | 0.85 L min−1 (optimized) |
| Nebulizer uptake flow rate | 1.50 mL min−1 (optimized) |
| Element | Spectral Line (nm) | Slope ± Standard Deviation (cps mg−1 L) | r2 | LOD 1 (μg g−1) | LOQ 2 (μg g−1) | Upper Limit of Linear Range (μg g−1) |
|---|---|---|---|---|---|---|
| Ag | 328.068 | 20,800 ± 171 | 0.9997 | 0.58 | 1.94 | 2500 |
| Al | 237.313 | 352 ± 0.8 | 0.9999 | 0.49 | 1.64 | 2500 |
| B | 249.772 | 3645 ± 50.6 | 0.9992 | 0.48 | 1.59 | 2500 |
| Ba | 233.527 | 1125 ± 7.1 | 0.9998 | 0.66 | 2.22 | 2500 |
| Ca | 396.847 | 1,715,367 ± 6567 | 0.9999 | 0.65 | 2.18 | 2500 |
| Cd | 226.502 | 756 ± 4.4 | 0.9999 | 1.53 | 5.09 | 2500 |
| Co | 238.892 | 1177 ± 1.4 | 0.9998 | 0.76 | 2.53 | 2500 |
| Cr | 357.869 | 43,901 ± 170 | 0.9998 | 0.29 | 0.95 | 2500 |
| Cu | 324.752 | 65,948 ± 440 | 0.9998 | 0.45 | 1.49 | 2500 |
| Fe | 238.204 | 1962 ± 12.4 | 0.9998 | 1.20 | 4.01 | 2500 |
| Mg | 280.271 | 50,276 ± 540 | 0.9995 | 0.14 | 0.47 | 2500 |
| Mn | 257.610 | 23,317 ± 242 | 0.9996 | 0.09 | 0.28 | 2500 |
| Ni | 232.003 | 315 ± 0.6 | 0.9999 | 1.65 | 5.49 | 2500 |
| Pb | 217.000 | 47.1 ± 0.6 | 0.9993 | 2.43 | 8.11 | 2500 |
| Zn | 213.857 | 813 ± 4.3 | 0.9999 | 0.72 | 2.40 | 2500 |
| Element | Added (μg g−1) | Conventional Walnut Oil | Organic Walnut Oil | ||||
|---|---|---|---|---|---|---|---|
| Found (μg g−1) | RR% | RSD% | Found (μg g−1) | RR% | RSD% | ||
| Ag | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 90.0 ± 4.4 | 90.0 | 4.9 | 93.1 ± 2.6 | 93.1 | 2.8 | |
| 500 | 447 ± 11.6 | 89.4 | 2.6 | 482 ± 26.2 | 96.5 | 5.4 | |
| 2500 | 2263 ± 11.3 | 90.5 | 0.5 | 2263 ± 9.1 | 90.5 | 0.4 | |
| Al | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 110 ± 5.1 | 110.2 | 4.6 | 111 ± 1.2 | 110.8 | 1.1 | |
| 500 | 487 ± 8.0 | 97.4 | 1.6 | 510 ± 13.0 | 102.0 | 5.9 | |
| 2500 | 2331 ± 23.0 | 93.3 | 1.0 | 2215 ± 2.3 | 88.6 | 0.1 | |
| Β | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 90.8 ± 7.6 | 90.8 | 8.4 | 92.1 ± 0.9 | 92.1 | 1.0 | |
| 500 | 475 ± 19.2 | 94.9 | 4.2 | 442 ± 22.9 | 88.3 | 5.2 | |
| 2500 | 2231 ± 88.9 | 89.3 | 4.0 | 2234 ± 75.9 | 89.3 | 3.4 | |
| Ba | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 96.8 ± 7.2 | 96.8 | 7.4 | 98.8 ± 1.6 | 98.8 | 1.6 | |
| 500 | 407 ± 1.0 | 81.4 | 0.2 | 401 ± 19.6 | 80.2 | 4.9 | |
| 2500 | 2253 ± 37.4 | 90.1 | 1.7 | 2266 ± 18.8 | 90.6 | 0.8 | |
| Ca | 0 | 19.8 ± 0.3 | - | 1.5 | 61.7 ± 2.9 | - | 4.6 |
| 100 | 116 ± 10.4 | 96.6 | 8.9 | 167 ± 0.7 | 105.2 | 0.4 | |
| 500 | 541 ± 7.1 | 104.3 | 1.3 | 568 ± 11.6 | 101.2 | 2.0 | |
| 2500 | 2150 ± 34.5 | 85.0 | 1.6 | 2390 ± 91.3 | 93.1 | 3.8 | |
| Cd | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 97.6 ± 4.4 | 97.6 | 4.5 | 99.5 ± 2.6 | 99.5 | 2.6 | |
| 500 | 453 ± 2.7 | 90.5 | 0.6 | 467 ± 23.8 | 93.4 | 5.1 | |
| 2500 | 2277 ± 10.2 | 91.1 | 0.5 | 2280 ± 4.8 | 91.2 | 0.2 | |
| Co | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 105 ± 7.6 | 104.6 | 7.3 | 97.5 ± 2.6 | 97.5 | 2.1 | |
| 500 | 434 ± 14.8 | 86.8 | 3.4 | 459 ± 22.4 | 91.7 | 4.9 | |
| 2500 | 2310 ± 12.9 | 92.4 | 0.6 | 2312 ± 3.0 | 92.5 | 0.1 | |
| Cr | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 90.1 ± 4.3 | 90.1 | 4.8 | 101 ± 1.3 | 100.6 | 1.3 | |
| 500 | 475 ± 7.3 | 95.1 | 1.5 | 494 ± 31.5 | 98.9 | 6.4 | |
| 2500 | 2387 ± 12.9 | 95.5 | 0.5 | 2403 ± 22.3 | 96.1 | 0.9 | |
| Cu | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 91.0 ± 4.9 | 91.0 | 5.4 | 101 ± 2.0 | 101.1 | 2.0 | |
| 500 | 520 ± 8.8 | 104.1 | 1.7 | 494 ± 33.4 | 98.8 | 6.8 | |
| 2500 | 2358 ± 92.8 | 94.3 | 3.9 | 2617 ± 20.9 | 104.7 | 0.8 | |
| Fe | 13.8 ± 1.2 | - | 8.7 | 21.8 ± 1.0 | - | 4.6 | |
| 100 | 114 ± 6.6 | 100.3 | 5.8 | 119 ± 0.9 | 97.5 | 0.8 | |
| 500 | 507 ± 8.8 | 101.3 | 1.7 | 541 ± 33.4 | 103.8 | 6.2 | |
| 2500 | 2427 ± 20.1 | 96.5 | 0.8 | 2441 ± 19.3 | 96.8 | 0.8 | |
| Mg | 1.5 ± 0.1 | - | 4.5 | 11.7 ± 1.1 | - | 9.6 | |
| 100 | 100 ± 6.9 | 98.9 | 6.9 | 110 ± 1.6 | 98.1 | 1.5 | |
| 500 | 424 ± 2.1 | 94.0 | 0.5 | 439 ± 23.1 | 85.5 | 5.3 | |
| 2500 | 2329 ± 7.2 | 93.1 | 0.3 | 2331 ± 11.4 | 92.8 | 0.5 | |
| Mn | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 104 ± 6.3 | 104.9 | 6.0 | 102 ± 1.2 | 101.8 | 1.2 | |
| 500 | 432 ± 1.8 | 86.4 | 0.4 | 435 ± 27.3 | 87.0 | 6.3 | |
| 2500 | 2385 ± 21.5 | 95.4 | 0.9 | 2384 ± 50.1 | 95.4 | 2.1 | |
| Ni | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 102 ± 6.4 | 101.9 | 6.5 | 95.3 ± 5.3 | 95.3 | 5.6 | |
| 500 | 453 ± 1.2 | 90.7 | 0.3 | 459 ± 16.7 | 91.8 | 3.6 | |
| 2500 | 2372 ± 32.8 | 94.9 | 1.4 | 2369 ± 5.0 | 94.8 | 0.2 | |
| Pb | 0 | <LOD | - | - | <LOD | - | - |
| 100 | 105 ± 8.5 | 105.3 | 8.1 | 86.4 ± 8.5 | 86.4 | 3.3 | |
| 500 | 488 ± 20.4 | 97.5 | 4.2 | 446 ± 30.8 | 89.2 | 6.9 | |
| 2500 | 2314 ± 104.1 | 92.6 | 4.5 | 2276 ± 53.5 | 91.0 | 2.4 | |
| Zn | 0 | 8.1 ± 0.5 | - | 6.4 | 15.6 ± 0.8 | - | |
| 100 | 105 ± 10.3 | 97.0 | 9.8 | 111 ± 1.2 | 95.2 | 1.1 | |
| 500 | 498 ± 2.8 | 97.9 | 0.6 | 526 ± 13.0 | 102.0 | 2.5 | |
| 2500 | 2303 ± 10.4 | 91.8 | 0.5 | 2320 ± 13.2 | 92.2 | 0.6 | |
| BCR 278-R | Certified Value (μg g−1) | Found 1 | texp. | Recovery (%) |
|---|---|---|---|---|
| Cd | 0.348 ± 0.007 | 0.331 ± 0.016 | 1.840 | 95.1 |
| Cr2 | 0.78 ± 0.06 2 | 0.74 ± 0.04 | 1.732 | 94.9 |
| Cu | 9.45 ± 0.13 | 9.31 ± 0.6 | 0.404 | 98.5 |
| Mn | 7.69 ± 0.23 | 7.56 ± 0.28 | 0.804 | 98.3 |
| Pb | 2.00 ± 0.04 | 1.89 ± 3.5 | 1.270 | 94.5 |
| Zn | 83.1 ± 1.7 | 80.0 ± 1.7 | 1.534 | 96.3 |
| Sample | Ag | Al | B | Ba | Ca | Cd | Co | Cr | Cu | Fe | Mg | Mn | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CWO1 | nd 1 | nd | nd | nd | 19.7 ± 0.3 | nd | nd | nd | nd | 13.8 ± 1.2 | 1.5 ± 0.07 | nd | nd | nd | 8.1 ± 0.5 |
| CWO2 | nd | nd | nd | nd | 11.23 ± 0.3 | nd | nd | nd | nd | 5.5 ± 0.3 | 1.9 ± 0.08 | nd | nd | nd | 8.9 ± 0.8 |
| CWO3 | nd | nd | nd | nd | 11.2 ± 1.1 | nd | nd | nd | nd | 6.7 ± 0.4 | 1.6 ± 0.13 | nd | nd | nd | 11.8 ± 1.1 |
| CWO4 | nd | nd | nd | nd | 5.8 ± 0.4 | nd | nd | nd | nd | nd | 1.8 ± 0.09 | nd | nd | nd | 9.3 ± 0.2 |
| CWO5 | nd | nd | nd | nd | 45.3 ± 3.5 | nd | nd | nd | nd | 4.01 ± 0.3 | 2.7 ± 0.22 | nd | nd | nd | 10.4 ± 0.9 |
| CWO6 | nd | nd | nd | nd | 6.5 ± 0.6 | nd | nd | nd | nd | nd | 1.2 ± 0.11 | nd | nd | nd | 10.0 ± 0.6 |
| CWO7 | nd | nd | nd | nd | 6.1 ± 0.5 | nd | nd | nd | nd | nd | 1.1 ± 0.10 | nd | nd | nd | 9.4 ± 0.8 |
| CWO8 | nd | nd | nd | nd | 5.1 ± 0.4 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 14.7 ± 1.3 |
| CWO9 | nd | nd | nd | nd | 10.0 ± 0.9 | nd | nd | nd | nd | nd | 0.75 ± 0.06 | nd | nd | nd | 11.4 ± 1.1 |
| CWO10 | nd | nd | nd | nd | 33.5 ± 2.5 | nd | nd | nd | nd | nd | 0.45 ± 0.03 | nd | nd | nd | 14.5 ± 1.2 |
| OWO1 | nd | nd | nd | nd | 61.7 ± 2.8 | nd | nd | nd | nd | 21.7 ± 1.0 | 11.7 ± 1.1 | nd | nd | nd | 15.6 ± 0.8 |
| OWO2 | nd | nd | nd | nd | 59.5 ± 7.3 | nd | nd | nd | nd | 4.6 ± 0.6 | 10.7 ± 0.91 | nd | nd | nd | 10.8 ± 1.0 |
| OWO3 | nd | nd | nd | nd | 25.5 ± 2.1 | nd | nd | nd | nd | 12.4 ± 1.6 | 5.5 ± 0.4 | nd | nd | nd | 6.4 ± 0.6 |
| OWO4 | nd | nd | nd | nd | 67.4 ± 3.3 | nd | nd | nd | nd | 33.8 ± 2.3 | 8.5 ± 0.6 | nd | nd | nd | 11.2 ± 1.0 |
| OWO5 | nd | nd | nd | nd | 52.3 ± 4.5 | nd | nd | nd | nd | 45.6 ± 4.1 | 8.2 ± 0.2 | nd | nd | nd | 9.8 ± 0.8 |
| OWO6 | nd | nd | nd | nd | 47.4 ± 6.3 | nd | nd | nd | nd | 41.1 ± 3.9 | 7.4 ± 0.5 | nd | nd | nd | 10.5 ± 1.0 |
| OWO7 | nd | nd | nd | nd | 1.6 ± 0.1 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 10.4 ± 1.0 |
| OWO8 | nd | nd | nd | nd | 20.3 ± 1.9 | nd | nd | nd | nd | nd | 1.23 ± 0.1 | nd | nd | nd | 7.9 ± 0.6 |
| OWO9 | nd | nd | nd | nd | 19.5 ± 1.2 | nd | nd | nd | nd | nd | 0.91 ± 0.08 | nd | nd | nd | 11.8 ± 0.8 |
| OWO10 | nd | nd | nd | nd | 17.3 ± 1.3 | nd | nd | nd | nd | nd | 0.73 ± 0.04 | nd | nd | nd | 11.3 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Kalogiouri, N.P.; Anthemidis, A.; Zachariadis, G.A. Determination of Metals in Walnut Oils by Means of an Optimized and Validated ICP-AES Method in Conventional and Organic Farming Type Samples. Separations 2021, 8, 169. https://doi.org/10.3390/separations8100169
Manousi N, Kalogiouri NP, Anthemidis A, Zachariadis GA. Determination of Metals in Walnut Oils by Means of an Optimized and Validated ICP-AES Method in Conventional and Organic Farming Type Samples. Separations. 2021; 8(10):169. https://doi.org/10.3390/separations8100169
Chicago/Turabian StyleManousi, Natalia, Natasa P. Kalogiouri, Aristidis Anthemidis, and George A. Zachariadis. 2021. "Determination of Metals in Walnut Oils by Means of an Optimized and Validated ICP-AES Method in Conventional and Organic Farming Type Samples" Separations 8, no. 10: 169. https://doi.org/10.3390/separations8100169
APA StyleManousi, N., Kalogiouri, N. P., Anthemidis, A., & Zachariadis, G. A. (2021). Determination of Metals in Walnut Oils by Means of an Optimized and Validated ICP-AES Method in Conventional and Organic Farming Type Samples. Separations, 8(10), 169. https://doi.org/10.3390/separations8100169









