Abstract
The presence of antibiotic residue in eggs is a current issue due to the increasingly important phenomenon of antibiotic resistance. A multiclass, confirmatory method for the determination of seventy-three antimicrobial agents (amphenicols, cephalosporins, diaminopyrimidines, lincosamides, macrolides, penicillins, pleuromutilins, quinolones, sulfonamides, and tetracyclines) with liquid chromatography high-resolution mass spectrometry was applied to 200 egg samples collected from 119 Italian farms during the years 2018–2021.
1. Introduction
For the last few decades, antibiotics have been widely administered in animal husbandry to treat and prevent diseases and to act as growth-promoting agents. Their residues can become part of the food chain through various environmental pathways (i.e., water, soil, plant, and aquaculture), affecting human health [1]. Particularly, sub-therapeutic consumption of drugs can activate allergic reactions and antibiotic resistance phenomena. However, it is noteworthy that in some European countries, a decline in antibiotics sales has been observed from 2010 to 2018 [2]. The European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) analyze annual data collected by the EU Member States on antimicrobial resistance in zoonotic and indicator bacteria. The last summary report of 31 January 2020 pointed out the growth of this issue [3]. For these reasons, the European Union has established maximum residue limits (MRLs) on animal-origin matrices and foodstuffs such as eggs [4]. Eggs are one of the most representative foods of the European diet due to their affordability and nutritive properties. For some antibiotics such as tetracyclines, i.e., chlortetracycline, oxytetracycline and tetracycline, the MRL in eggs is set at 200 µg kg−1; and 50, 25, and 1000 µg kg−1 for lincomycin (lincosamide), penicillin V (penicillin), and tiamulin (pleuromutilin), respectively. For erythromycin A, 150 µg kg−1 has been established, 200 µg kg−1 for tylvalosin and tylosin A (macrolides), and finally, the MRL of neomycin B (aminoglycoside) is set at 500 µg kg−1. Other regulated antibiotics such as amphenicols, cephalosporins, doxycycline (tetracyclines), several β-lactams, some macrolides, quinolones, and sulfonamides are prohibited in laying hens. The EU Member States implement yearly official monitoring programs in order to ensure the MRLs and the regular use of antimicrobial agents in farming are observed. Therefore, sensitive and reliable methods for the determination of antibiotics in eggs are required. To date, liquid chromatography coupled with tandem mass spectrometry and high-resolution mass spectrometry techniques (especially referring to hybrid instruments) represent the gold standards for the development of multiclass methods due to the selectivity and sensitivity that they can offer, despite generic sample preparation, which is mandatory for wide ranges of analytes [5]. Several research studies [5,6,7,8,9,10,11,12,13] have reported antibiotic residues in eggs. To the best of our knowledge, surveys with more than 150 samples have not previously been conducted. Thus, the aims of this study were to develop a confirmatory multiclass method for more than 70 of the regulated and most used antibiotics (except for aminoglycosides and colistin) in eggs, and apply it to 200 Italian, commercial egg samples produced with conventional and organic approaches, collected during the years 2018–2021. Moreover, an exposure assessment of Italian public health was evaluated based on the most recent survey of Italian food consumption [14,15,16].
2. Materials and Methods
2.1. Chemicals, Reagents, Stock, and Intermediate Solutions
Acetonitrile (ACN), LC-MS-grade methanol, EDTA disodium salt dihydrate, and ammonium acetate were supplied by Merck KGaA (Darmstadt, Germany). LC-MS-grade deionized water was purchased from Biosolve Chimie (Dieuze, France). Formic acid ≥98% was provided by Carlo Erba Reagents (Milano, Italy), and N,N’-dimethylformamide (DMF) was supplied by Fluka (Buchs, Switzerland). The 73 analytes are presented in Table S1. The majority were purchased from Merck KGaA. Florfenicol-d3, cefacetrile, ceftiofur-d3, cephapirin, desacetyl cephapirin, pirlimycin, neospiramycin, spiramycin I-d3, tildipirosin, tulathromycin A, tulathromycin marker (CP60,300), tylvalosin, penicillin G-d7, enrofloxacin-d5, and sulfachloropyridazine were obtained from TRC Inc. (Toronto, ON, Canada); cefazolin and lincomycin from USP Reference Standards (Maryland, USA). Ciprofloxacin, difloxacin, enrofloxacin, marbofloxacin, nalidixic acid, norfloxacin, oxolinic acid, sarafloxacin, tylosin-3-acetate, ampicillin, cloxacillin, nafcillin, sulfadiazine, sulfamerazine, sulfadimethoxine, sulfathiazole, chlortetracycline, doxycycline, methacycline, and tetracycline were provided by LGC Standards (London, UK). Sulfamethazine-13C6 was purchased from Cambridge Isotope Laboratories Inc. (Tewksbury, MA, USA). Lastly, 4-epichlortetracycline and 4-epioxytetracycline were provided by ACROS ORGANICS (Geel, Belgium). The details about the preparation of stock and intermediate solutions were reported elsewhere [17] and the stability of stock is shown in Table S2 [18,19].
2.2. Chromatographic Conditions
Chromatography was performed on a Thermo Ultimate 3000 High Performance Liquid Chromatography system (San Jose, CA, USA). Analytes were separated on a Poroshell 120 EC-C18 column (100 × 3.0 mm; 2.7 µm; Agilent Technologies, Santa Clara, CA, USA), connected to a Poroshell guard column (5 × 3.0 mm). HPLC eluent A was an aqueous solution containing 0.1% (v/v) formic acid and eluent B was methanol. The gradient was set as described elsewhere [17]. The injection volume was 5 µL.
2.3. MS Conditions
A Q-Orbitrap mass spectrometer (Thermo Scientific, San Jose, CA, USA) was equipped with a heated electrospray ionization (HESI-II) source. The parameters were set similarly to previously published work [17]. Particularly, the HESI-II and capillary temperatures were set at 320 and 300 °C, respectively, and the electrospray voltage at 3.60 kV (positive ionization mode). Sheath and auxiliary gas were 35 and 15 arbitrary units, respectively. The mass spectrometer was controlled by Xcalibur 3.0 software (Thermo Fisher Scientific, San Jose, CA, USA). The exact mass of the compounds was calculated using Qualbrowser in Xcalibur 3.0. Instrument calibration was performed for every analytical batch with a direct infusion of an LTQ Velos ESI Positive Ion Calibration Solution (Pierce Biotechnology Inc., Rockford, IL, USA). The individual compounds were infused with a syringe through a T union connected to an LC system with a mobile phase flow rate of 0.1 mL min−1 (50% eluent A). The product ions were found by increasing the collision energy (CE) using Q-Exactive Tune 2.3 software (Thermo Fisher Scientific, Waltham, MA, USA). After choosing the more intense product ions, fragmentation energies were optimized with spiked samples at 10 µg kg−1 using the selected gradient program. All Q Exactive parameters (resolution, AGC, and IT) were optimized to improve sensitivity and selectivity. MS acquisition was performed as described elsewhere [17], with some modifications to obtain the best instrumental signal mixing full scan/dd-MS2 and SIM experiments. The monitored adducts and product ions such as the collision energies are presented in Table S3.
2.4. Sample Preparation
One-half gram of homogenized eggs was weighed in a Falcon tube. The sample was spiked with internal standards (ISs), particularly, 15 µL of a solution containing the two labelled beta-lactams at 1 µg mL−1 (ceftiofur-d3 and penicillin G-d7), 15 µL of a solution of all other antibiotics ISs (florfenicol-d3, enrofloxacin-d5, spiramycin I-d3, sulfamethazine-d5 and methacycline) at the same concentration. Later, 900 µL of 0.15 M EDTA was added and the sample was extracted with 2.4 mL of acetonitrile. After shaking and centrifugation, a second extraction with 3 mL of acetonitrile was performed. The reunited extracts were evaporated and then redissolved in 1.5 mL of 200 mM ammonium acetate. After centrifugation, the sample was injected.
2.5. Method Validation
A full validation study was carried out in accordance with the performance criteria required by Commission Decision 2002/657/EC [20] and SANTE/12682/2019 [21] for quantitative confirmatory methods. The approach followed was based on [5]. Briefly, the analytes were validated at the spiking concentration levels, encompassing 3.3–100 µg kg−1; for tetracycline, oxytetracycline, chlortetracycline and their epimers, erythromycin A, tylosin A, tylvalosin, tylosin-3-acetate, the range was 3.3–1000 µg kg−1; and finally, for tiamulin, the interval was 3.3–3330 µg kg−1. Moreover, the analytes were successfully validated at the additional level of 2 µg kg−1 (data not shown).
2.6. Real Samples Analysis
The validated method was applied to 200 real egg samples taken from the Italian market during October 2018–June 2021. Each sample was placed in a plastic container and stored at −20 °C after homogenization.
2.7. Risk Exposure
The risk for Italian public health was determined based on food consumption data of [14]. The daily intake for the detected substance in eggs is related to the acceptable daily intake (ADI). To calculate the ADI percentage, the following Equation (1) [22] was used:
where C is the detected concentration of the antibiotic residue during the real sample analysis, E is the egg consumption per day, and w is the mean weight of the people. The updated ADI for the detected analyte was provided by [23].
3. Results and Discussion
3.1. Sample Preparation
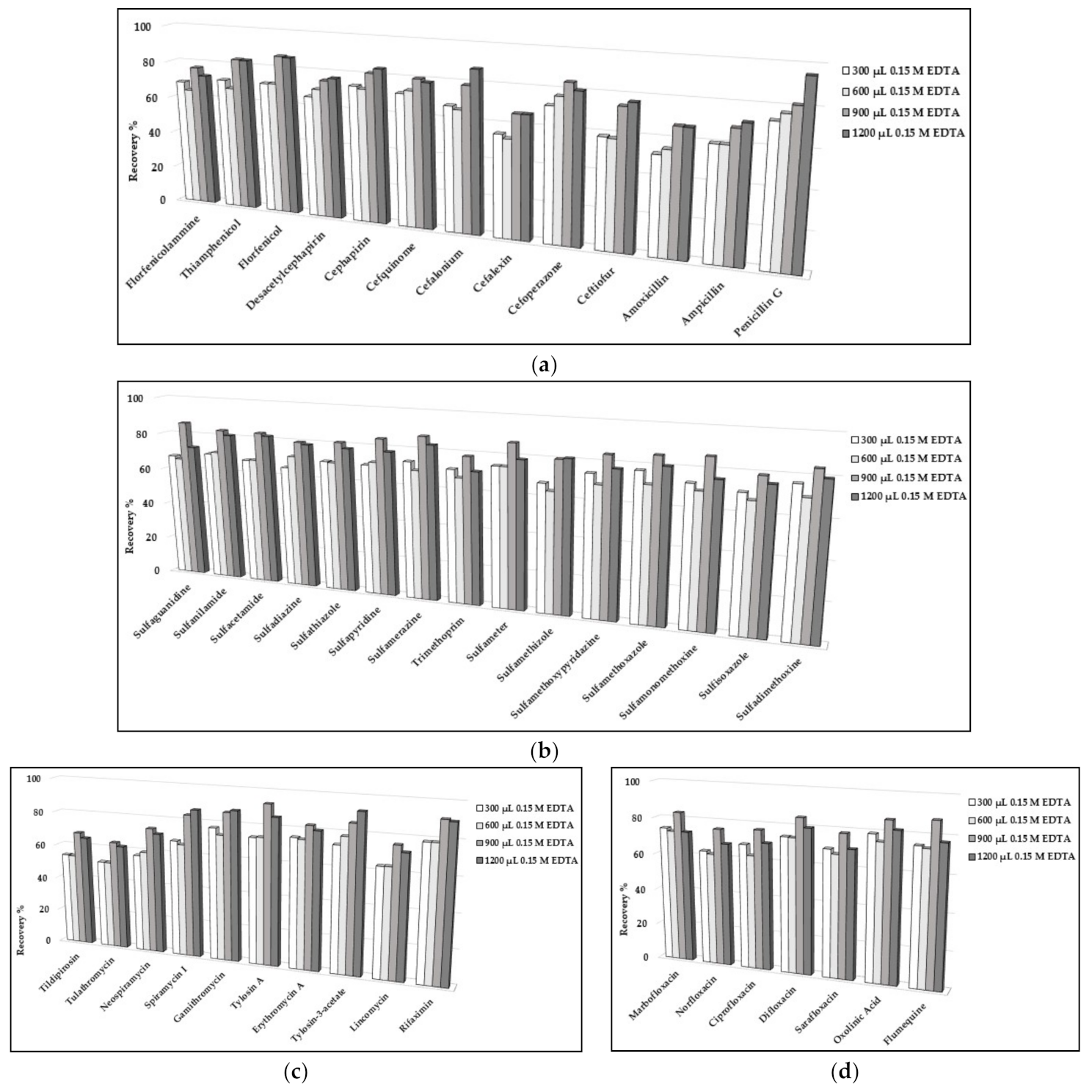
The sample extraction was optimized starting from [5,17] with some modifications. Despite the good performances of [5], a strong matrix effect was evident for several analytes. Therefore, the intent was the development of a sample protocol able to achieve a compromise between performance and cleanliness of the final extract. Due to the high concentrations of minerals in the matrix and the well-known chelating properties of quinolones, sulfonamides, and tetracyclines, three variables that affect the yield of extraction were investigated: volume of acetonitrile and volume and concentration of the EDTA solution. For reducing the concentration of the extracted interferent substances, the preliminary experiment was reducing the volume of acetonitrile during the first extraction, but more crucial (and just cited) substances demonstrated poor recoveries (data not shown). Consequently, experiments were carried out to explore the effects of a higher concentration and volume of EDTA. Particularly, Figure 1 presents the molecules for which the recoveries provided a rise ≥10% from 300 to 900 µL of 0.15 M EDTA. The highest tested volume (i.e., 1200 µL) demonstrated probably the worst performance for several analytes for the enhancement in polarity of the extraction mixture.
Figure 1.
Extraction efficiency of different volumes of EDTA solution evaluated in egg samples (n = 4 per experiment) spiked at 10 µg kg−1 for (a) amphenicols and beta-lactams, (b) sulfonamides and trimethoprim, (c) lincomycin, rifaximin and macrolides, and (d) quinolones.
3.2. Method Validation
Method validation was based on the approach followed by Paoletti et al. [5]. Particularly, the selectivity was evaluated, analyzing more than 20 blank samples during the validation study, and no peaks were found, considering the established criteria.
Good linearity was observed for all the analytes (deviations of back-calculated concentration ≤20%) [21]. It was judged by analyzing curves in matrix-matched calibration (MMC) samples and in solvent (i.e., 200 mM ammonium acetate) in the concentration range encompassing the lowest and highest validation level (3.3–100 µg kg−1), and to evaluate the matrix effect. The absolute matrix effect study demonstrated moderate ion suppression/ion enhancement, as the differences in the slopes in MMC standard and in solvent were below 29%, except for valnemulin (40%), in absolute value (Table 1).

Table 1.
Validation performances of the investigated analytes, sorted by class and elution order.
Moreover, the relative matrix effect (egg to egg) was evaluated comparing the recovery of spiked samples, calculated with different matrix-matched standards, and was considered negligible. This approach compensates for the ME on completion of the results [24,25].
Validation data in terms of recovery (trueness) and precision were calculated with the analyte concentrations of spiked samples, obtained from the linear regression equation of the matrix-matched calibration standards, and are shown in Table 1. Recoveries were in the range of 62% (tulathromycin A)–90% (tylvalosin), repeatability and within-laboratory reproducibility encompass the interval of 6.3% (chlortetracycline) –14% (gamithromycin and pirlimycin) (CVr,pooled), and 7.2% (enrofloxacin) –15% (gamithromycin and valnemulin) (CVwR, pooled), respectively [26].
The limits of detection (LOD) and quantification (LOQ) were primarily estimated on the basis of the recovery and precision observed at the first two validation levels (3.3 and 10 µg kg−1). However, prior to monitoring, an additional validation level (i.e., 2 µg kg−1) was tested, with the aim of evaluating the background contamination levels. The LOD and LOQ were equal to 2 µg kg−1 for all of the analytes, except for thiamphenicol, florfenicol, cefazolin, tulathromycin A, sulfacetamide, and sulfamerazine for which an LOQ of 10 µg kg−1 was fixed, as they showed poor precision and/or linearity.
Consequently, the validated method was fit for purpose, and on July 2021, the laboratory accredited it [27,28].
3.3. Real Samples Analysis
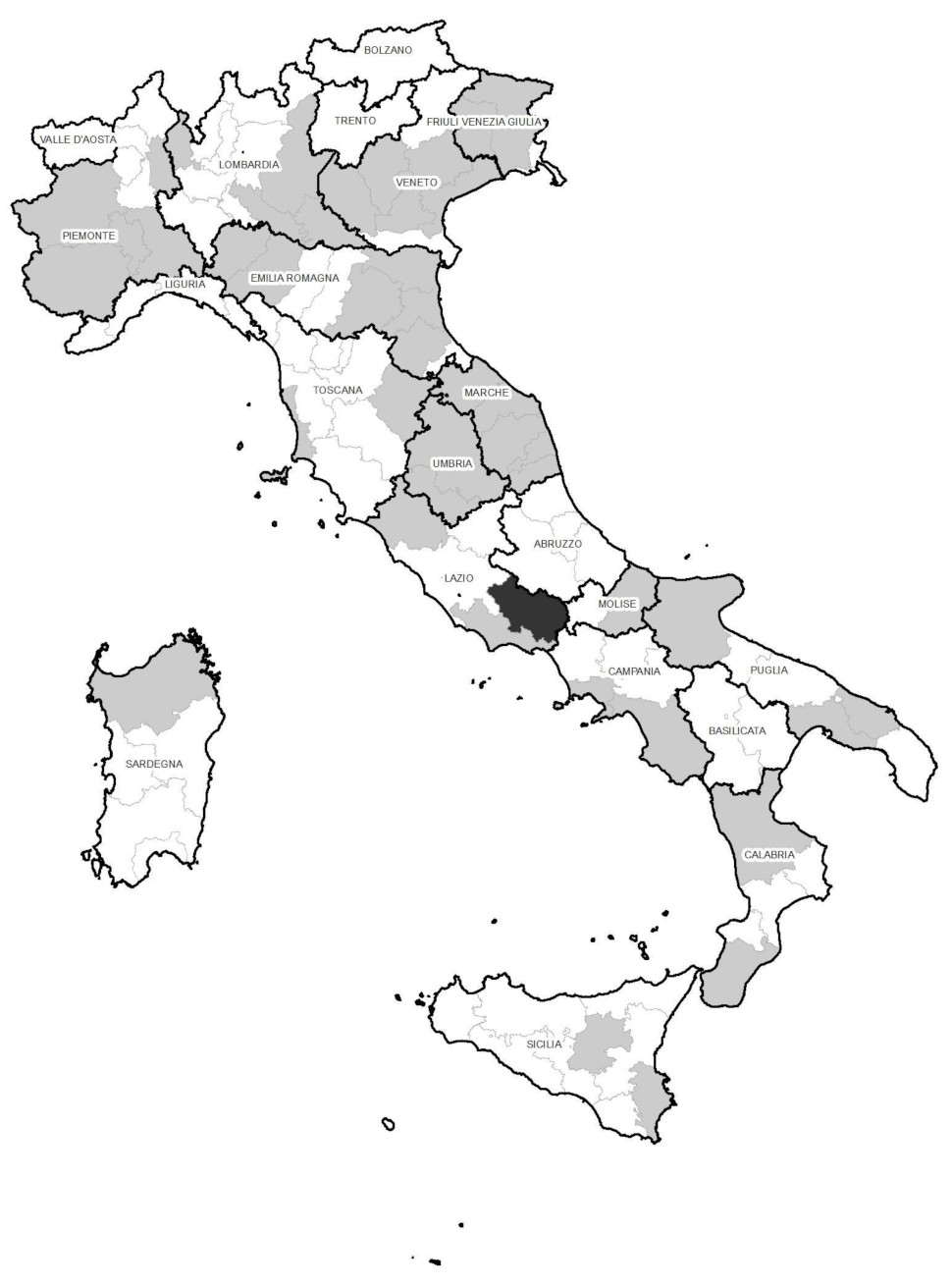
The validated method was employed for the determination of antibiotic residues in 200 egg samples. They belonged to farms spread out throughout most of Italy and encompass the three farming methods, i.e., 60 organic, 73 free-range, and 67 barn. The samples were randomly collected by supermarkets during 2018 (n = 27), 2019 (n = 100), 2020 (n = 45), and 2021 (n = 28), and came from 119 farms located in 45 provinces (Figure 2).
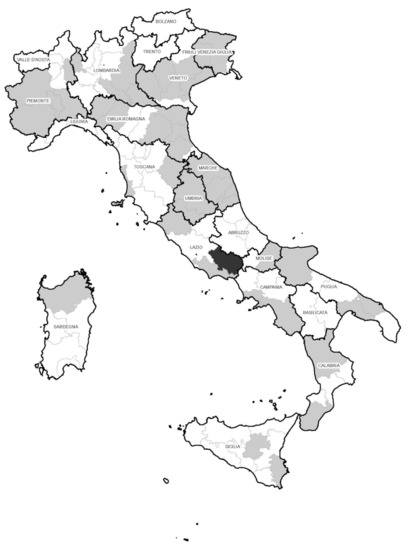
Figure 2.
Sites (provinces) of farms where no residue (grey) and one residue (black) was detected during the survey.
An internal quality control was implemented for the analytical batches by adding the internal standards solution to each sample prior to extraction. These ISs were not used with quantitative aims; rather, they were used to check the yield of the process. Moreover, a blank and at least a spiked muscle at 10 µg kg−1 was analyzed to verify the absence of a false positive/negative result. Lastly, a matrix-matched calibration standard was prepared, adding the analytes immediately prior to LC injection.
Suspected samples were newly analyzed by twice performing ad hoc spiked sample and matrix-matched calibration curves based on the preliminary analysis.
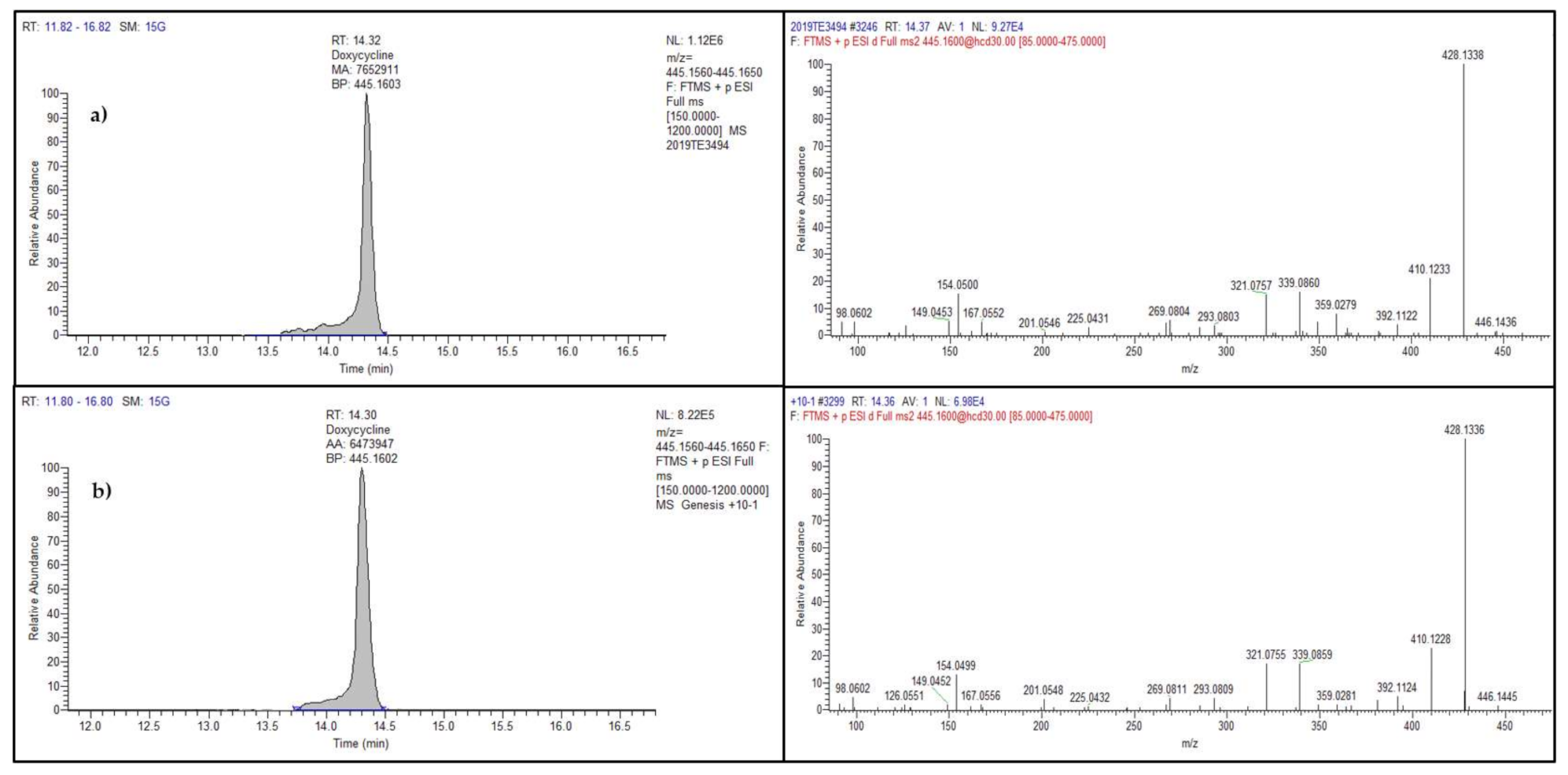
Among the 200 samples, antibiotic residue was detected in only one sample, collected in 2019, representing 0.5%. Particularly, doxycycline, belonging to the tetracycline family and banned in eggs, was found at 22 µg kg−1. Figure 3 shows the full scan chromatograms and the MS2 spectra of the incurred and a spiked sample.
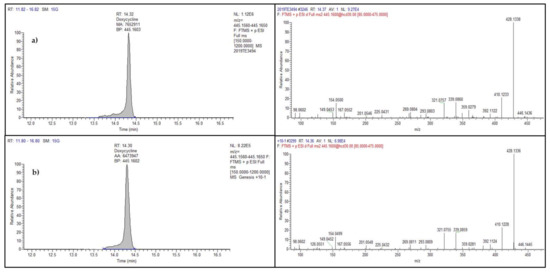
Figure 3.
Chromatograms (left) and product ion spectra (right) of doxycycline in the real egg sample at 22 µg kg−1 (a) and in a spiked sample at 10 µg kg−1 (b).
Interestingly, this positive sample belonged to the free-range farming method, showing a case of illicit use.
3.4. Risk Exposure
The calculation of the daily intake and the consequent ADI percentage was based on egg consumption presented in the most recent published survey of the Italian diet [14]. Table 2 shows the data in detail: ADI percentage values were calculated on the basis of the mean and 99th percentile daily intake, encompassing age and sex categories. The ages ranged from infants (0–2.9 years), children (3–9.9 years), teenagers (10–17.9 years), and adults (18–64.9 years) to the elderly (≥65 years).

Table 2.
Risk exposure based on the Italian diet.
The ADI values related to the contaminated sample with doxycycline did not pose any risk for human health; even taking into account the 99th percentile, the ADI percentage reached 2.9 and 3.6% for infants and children, respectively. Despite the noncompliance of the positive sample, these values are not dangerous because they are far below 100%. In fact, the sample is considered toxicologically acceptable.
4. Conclusions
The developed and validated multiclass method for the determination of 73 antibiotic residues was applied to 200 egg samples collected between 2018 and 2021 from the Italian market. The monitoring showed the presence of antibiotic residues in 0.5% of the cases and the same percentage of noncompliant samples. This value doubles (1%), when only taking 2019 into account.
Doxycycline, found in an egg sample in this survey, is more lipophilic than the other tetracyclines, and causes long-term persistence in eggs and animal tissues, which is why the EU banned its use in laying hens [4]. Nevertheless, some incidences of this antibiotic residue in food occurred during the yearly monitoring plans of European Member States. It is important to note, in this respect, that the latest monitoring reports of veterinary medicinal products in live animals and animal products of EFSA (2018 and 2019) declared 0.19% and 0.17% of non-compliant samples as positive for the B1 substance group, respectively [29,30].
Particularly, in 2018, doxycycline was found in Italy and Spain for a total of two egg samples (2.5% and 0.4%, respectively); the Italian percentage was calculated on a limited number of samples (i.e., 40). In 2019, doxycycline was not found in European eggs and, unfortunately, the total number of egg samples analyzed was not reported in the document. However, this survey suggests that the number of the monitored samples in Italy should be increased to offer a better overview of egg contamination and, especially, to find cases of illicit use of veterinary drugs.
In conclusion, the results of this wide survey are reassuring in relation to Italian public health, considering the acceptable toxicological level.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/separations8090148/s1, Table S1: List of the 73 investigated analytes, Table S2: Individual stock solutions of analytes (storage temperature: −20 °C), Table S3: UHPLC-Q-Orbitrap parameters of the 73 analytes and 7 ISs.
Author Contributions
G.S. (Giorgio Saluti), conceptualization, formal analysis, methodology, project administration, supervision, writing—original draft preparation, and writing—review and editing; M.N.C., validation, and investigation; F.C., validation and investigation; M.R., investigation; G.D., conceptualization, funding acquisition, methodology, project administration, and resources; G.S. (Giampiero Scortichini), conceptualization, methodology, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by Italian Ministry of Health (Ricerca Corrente IZS AM 06/20 RC “Scenario of Italian dietary exposure to priority contaminants”).
Acknowledgments
The authors thankfully acknowledge Sandro Pelini (Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale”) for the IT support, Giannina Chessa (Istituto Zooprofilattico Sperimentale della Sardegna “G. Pegreffi”) and Pasquale Gallo (Istituto Zooprofilattico Sperimentale del Mezzogiorno) for the donation of some egg samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, J.; Cui, Y.; Tao, Y.; Huang, L.; Peng, D.; Xie, S.; Wang, X.; Liu, Z.; Chen, D.; Yuan, Z. Multiclass method for the quantification of 92 veterinary antimicrobial drugs in livestock excreta, wastewater, and surface water by liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2016, 39, 4086–4095. [Google Scholar] [CrossRef]
- European Medicines Agency. Sales of veterinary antimicrobial agents in 31 European countries in 2017: Trends from 2010–2017, Ninth ESVAC Rep. EMA/294674/2019. (2019) 106. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2017_en.pdf (accessed on 9 July 2021).
- EFSA; European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2017/2018; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef] [Green Version]
- European Commission. REGULATION (EU) No 37/2010 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin (Text with EEA Relevance); European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Paoletti, F.; Sdogati, S.; Barola, C.; Giusepponi, D.; Moretti, S.; Galarini, R. Development and validation of a multiclass confirmatory method for the determination of over 60 antibiotics in eggs using liquid-chromatography high-resolution mass spectrometry. Food Control 2021, 127, 108109. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Laganà, A. Multiclass screening method based on solvent extraction and liquid chromatography–tandem mass spectrometry for the determination of antimicrobials and mycotoxins in egg. J. Chromatogr. A 2012, 1268, 84–90. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Liu, Y.; Du, Z.; Feng, Y.; Jia, L.; Zhang, J. Extraction optimization of sixteen cephalosporins in milk by filtered solid phase extraction and ultra high pressure liquid chromatography coupled to tandem mass spectrometry. Anal. Methods 2017, 9, 1282–1289. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Okihashi, M.; Harada, K.; Konishi, Y.; Uchida, K.; Do, M.H.N.; Bui, L.T.; Nguyen, T.D.; Phan, H.B.; Bui, H.D.T.; et al. Detection of antibiotics in chicken eggs obtained from supermarkets in Ho Chi Minh City, Vietnam. J. Environ. Sci. Health Part B 2017, 52, 430–433. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, J.-J.; Cong, J.-M.; Cai, Z.-X.; Zhang, J.-S.; Wang, J.-L.; Ren, Y.-P. Optimization for quick, easy, cheap, effective, rugged and safe extraction of mycotoxins and veterinary drugs by response surface methodology for application to egg and milk. J. Chromatogr. A 2018, 1532, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, X.; Han, M.; Qiu, S.; Hou, X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019, 276, 419–426. [Google Scholar] [CrossRef]
- Hou, X.; Xu, X.; Xu, X.; Han, M.; Qiu, S. Application of a multiclass screening method for veterinary drugs and pesticides using HPLC-QTOF-MS in egg samples. Food Chem. 2020, 309, 125746. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, W.; Li, Y.; Liu, L. Antibiotic residues in poultry food in Fujian Province of China. Food Addit. Contam. Part B 2020, 13, 177–184. [Google Scholar] [CrossRef]
- Hu, M.; Ben, Y.; Wong, M.H.; Zheng, C. Trace Analysis of Multiclass Antibiotics in Food Products by Liquid Chromatography-Tandem Mass Spectrometry: Method Development. J. Agric. Food Chem. 2021, 69, 1656–1666. [Google Scholar] [CrossRef]
- Leclercq, C.; Arcella, D.; Piccinelli, R.; Sette, S.; Le Donne, C.; Turrini, A. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: Main results in terms of food consumption. Public Health Nutr. 2009, 12, 2504–2532. [Google Scholar] [CrossRef] [Green Version]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C. The third Italian National Food Consumption Survey, INRAN-SCAI 2005–06—Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Mistura, L.; Ferrari, M.; Leclercq, C. The third National Food Consumption Survey, INRAN-SCAI 2005–06: Major dietary sources of nutrients in Italy. Int. J. Food Sci. Nutr. 2013, 64, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Dusi, G.; Giusepponi, D.; Pellicciotti, S.; Rossi, R.; Saluti, G.; Cruciani, G.; Galarini, R. Screening and confirmatory method for multiclass determination of 62 antibiotics in meat. J. Chromatogr. A 2016, 1429, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, A.; Feuerrigel, P.; Ahmed, H.F.; Moulin, J.; Beck, A.; Mujahid, C.; Bessaire, T.; Savoy, M.-C.; Mottier, P. Stability study of veterinary drugs in standard solutions for LC-MS/MS screening in food. Food Addit. Contam. Part A 2018, 35, 696–706. [Google Scholar] [CrossRef]
- Jang, J.-W.; Lee, K.-S.; Kwon, K.; Bae, S.-C.; Kim, H.S. Simultaneous determination of thirteen quinolones in livestock and fishery products using ultra performance LC with electrospray ionization tandem mass spectrometry. Food Sci. Biotechnol. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- 96/23/EC Commission Decision, 96/23/EC COMMISSION DECISION of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Notified under Document Number C(2002) 3044)(Text with EEA Relevance) (2002/657/EC), 96/23/EC Commission Decision. (2002) 29. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2002:221:0008:0036:EN:PDF (accessed on 9 April 2021).
- European Commission. Analytical Quality Control and Method Validation for Pesticide Residues Analysis in Food and Feed (SANTE/12682/2019), Sante/12682/2019. (2019) 1–48. Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 9 April 2021).
- Wang, C.; Li, X.; Yu, F.; Wang, Y.; Ye, D.; Hu, X.; Zhou, L.; Du, J.; Xia, X. Multi-class analysis of veterinary drugs in eggs using dispersive-solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2021, 334, 127598. [Google Scholar] [CrossRef]
- EMEA. Summary of the Scientific Discussion for the Establishment of MRLs. European Agency for the Evaluation of Medicinal Products Committee for Veterinary Medicinal Products, 44 (2015) 1–7. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2015/02/WC500183123.pdf (accessed on 9 July 2021).
- Khaled, A.; Belinato, J.R.; Pawliszyn, J. Rapid and high-throughput screening of multi-residue pharmaceutical drugs in bovine tissue using solid phase microextraction and direct analysis in real time-tandem mass spectrometry (SPME-DART-MS/MS). Talanta 2020, 217, 121095. [Google Scholar] [CrossRef]
- Khaled, A.; Gómez-Ríos, G.A.; Pawliszyn, J. Optimization of Coated Blade Spray for Rapid Screening and Quantitation of 105 Veterinary Drugs in Biological Tissue Samples. Anal. Chem. 2020, 92, 5937–5943. [Google Scholar] [CrossRef]
- Galarini, R.; Moretti, S.; Saluti, G. Quality Assurance and Validation General Considerations and Trends. In Chromatographic Analysis of the Environment: Mass Spectrometry Based Approaches, 4th ed.; CRC Press: Boca Raton, FL, USA, 2017; p. 45. Available online: https://www.taylorfrancis.com/books/9781315316208/chapters/10.1201/9781315316208-10 (accessed on 10 July 2019).
- UNI CEI EN ISO/IEC 17025:2018, (n.d.). Available online: http://store.uni.com/catalogo/uni-cei-en-iso-iec-17025-2018?josso_back_to=http://store.uni.com/josso-security-check.php&josso_cmd=login_optional&josso_partnerapp_host=store.uni.com (accessed on 23 August 2021).
- Banche Dati—Accredia—Laboratori di Prova, (n.d.). Available online: https://services.accredia.it/accredia_labsearch.jsp?ID_LINK=293&area=7&numeroaccr=0111&classification=A&isRestricted=false&dipartimento=L (accessed on 23 August 2021).
- European Food Safety Authority. Report for 2018 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2020, 17. [Google Scholar] [CrossRef]
- European Food Safety Authority. Report for 2019 on the results from the monitoring of veterinary medicinal product residues and other substances in live animals and animal products. EFSA Support. Publ. 2021, 18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).