Comparative Extraction of Aluminum Group Metals Using Acetic Acid Derivatives with Three Different-Sized Frameworks for Coordination
Abstract
1. Introduction
2. Experimental
2.1. Reagents
2.1.1. 1,1,1-Tris(carboxymethoxymethyl)-9-decene (2)
2.1.2. 10-Undecenoxyacetic acid (5)
2.2. Distribution Study
3. Results and Discussion
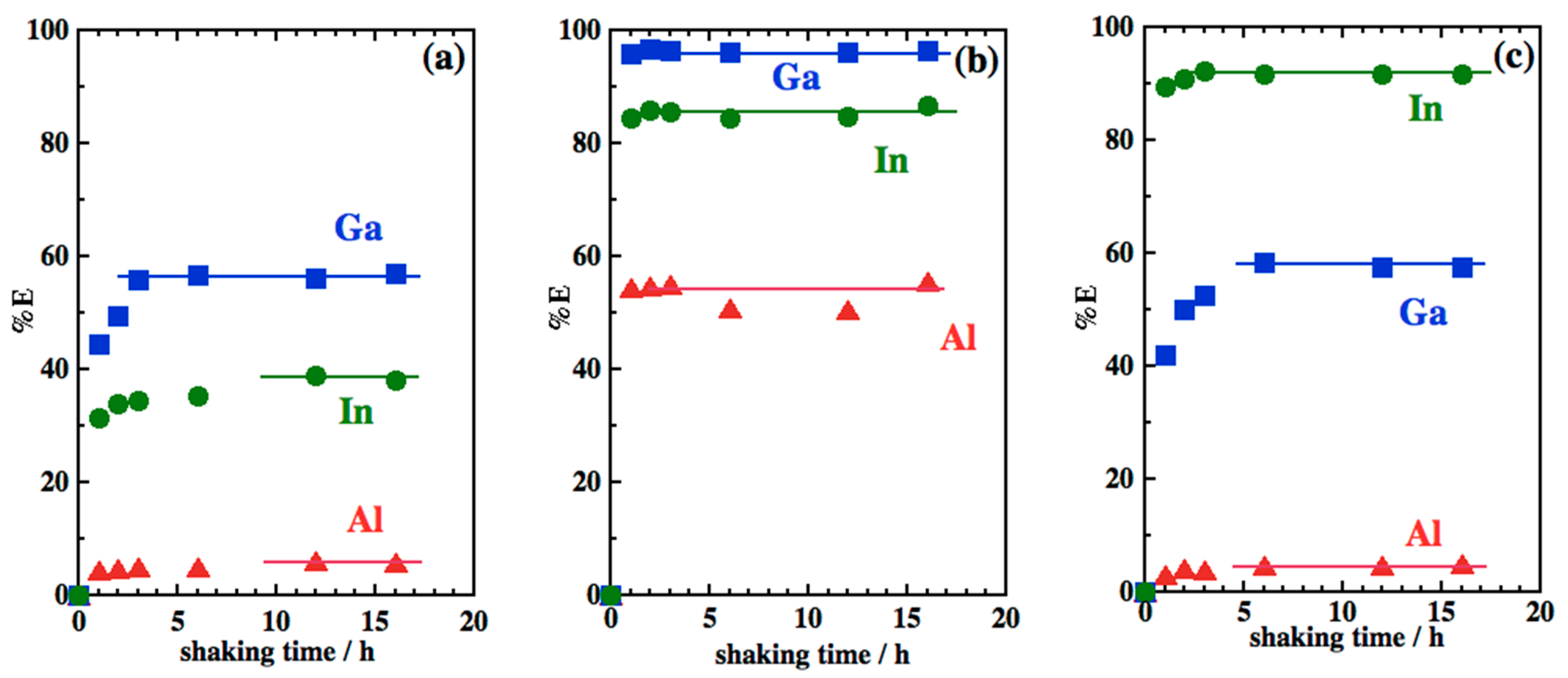
3.1. Extraction Rate
3.2. Structural Effect
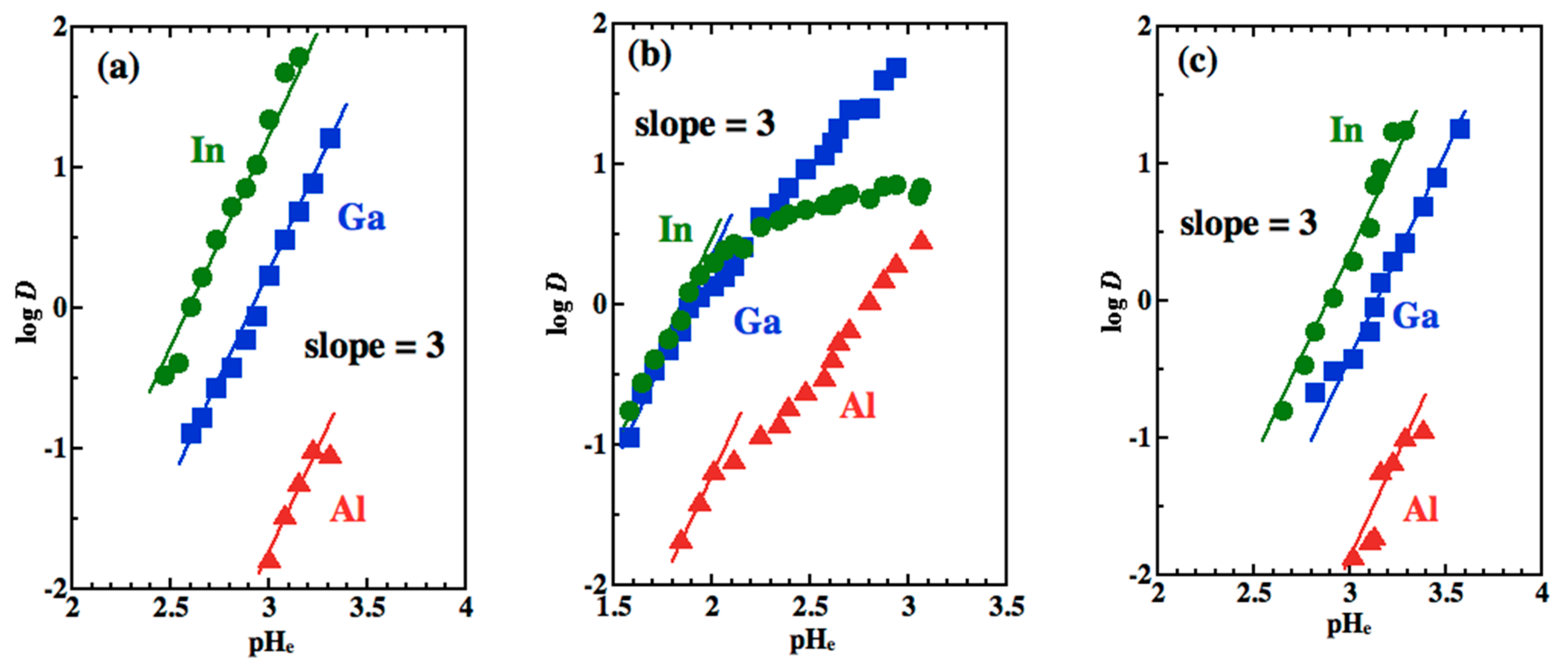
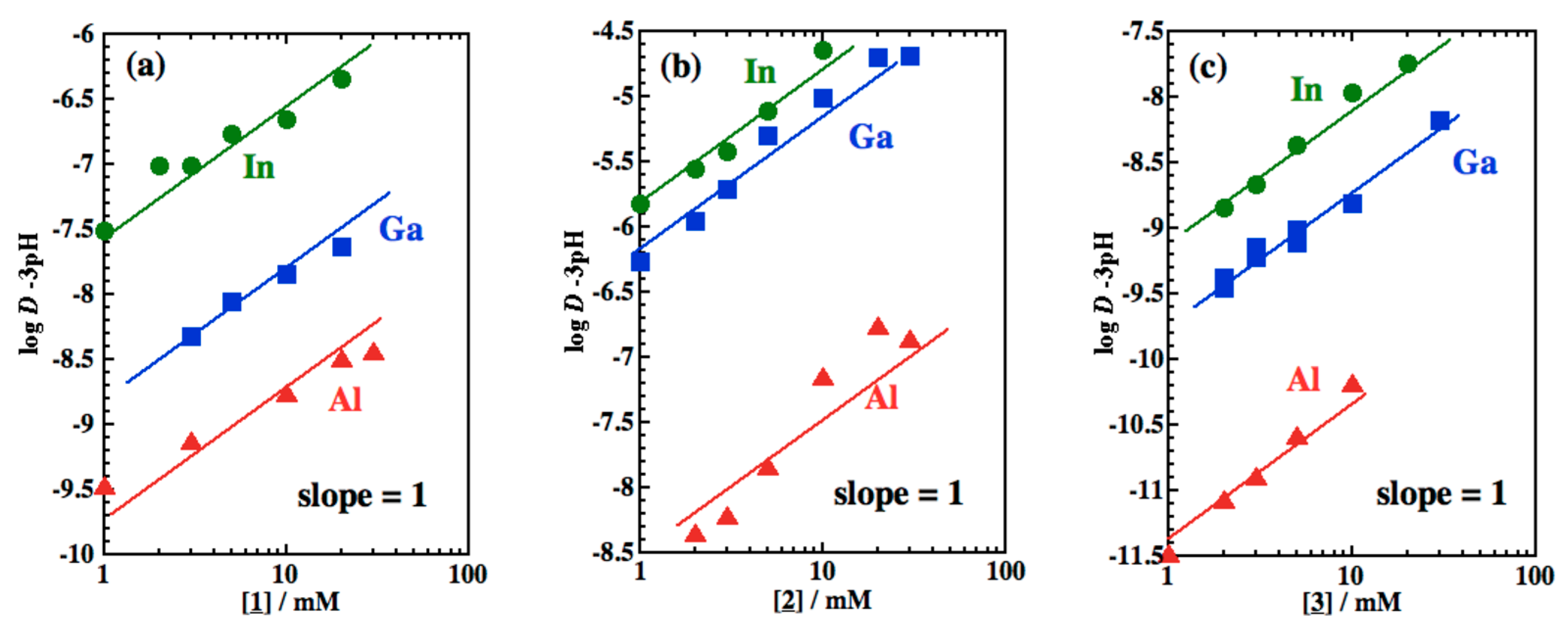
3.3. Slope Analysis and Extraction Reactions
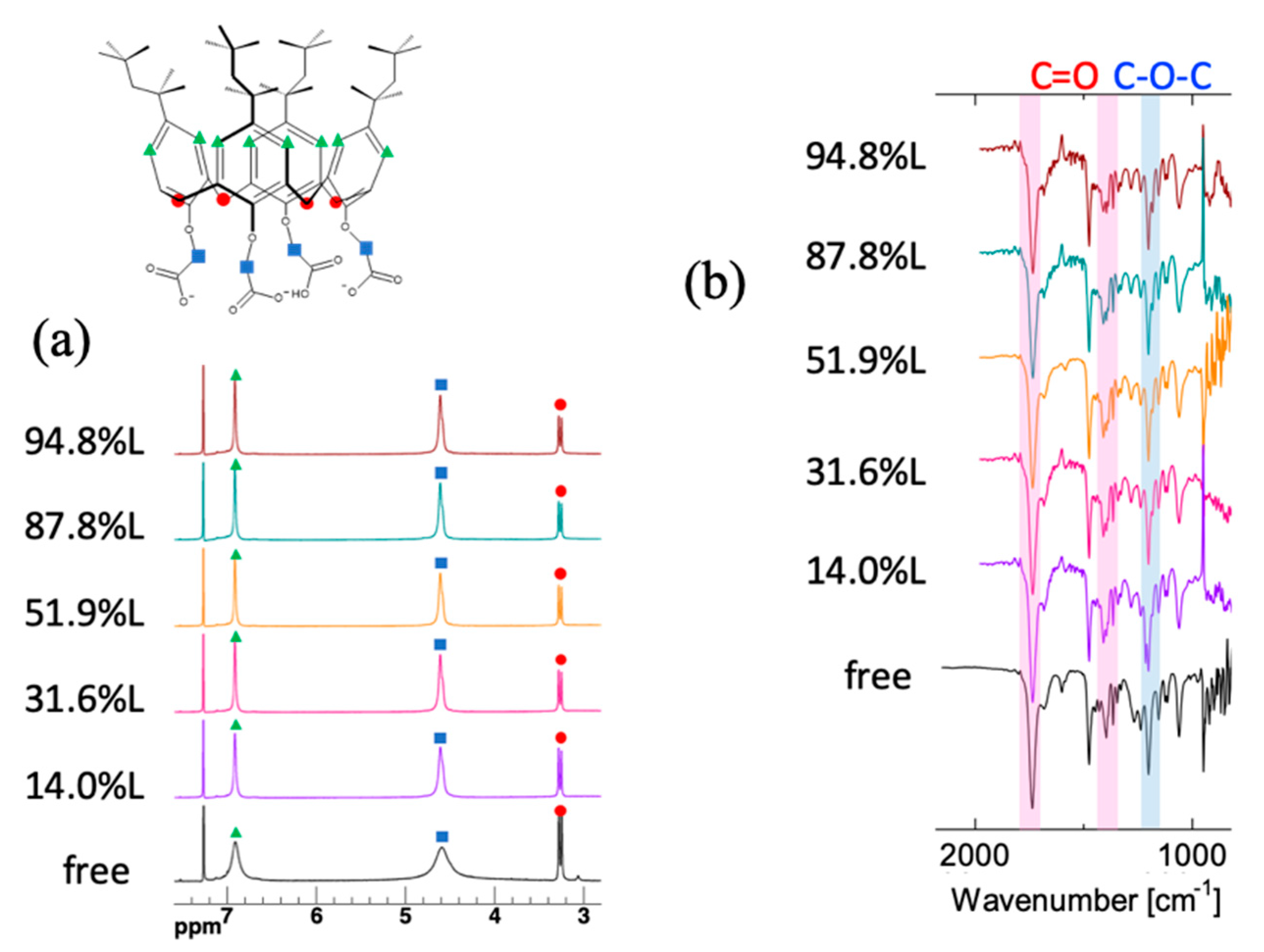
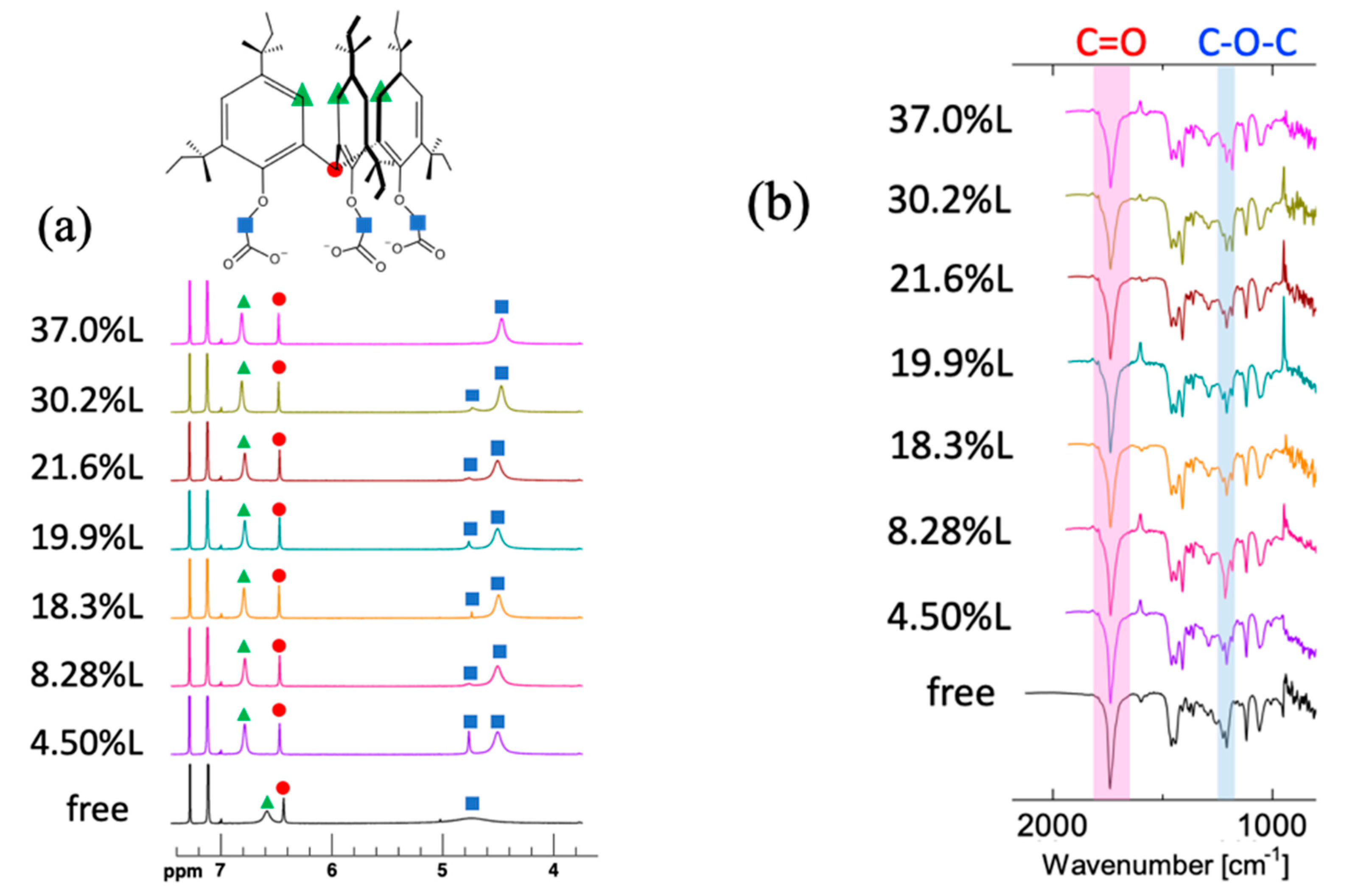
3.4. Spectroscopic Studies on the Ga3+- and In3+-Loaded Reagents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Cram, D.J.; Cram, J.M. Host-guest chemistry. Science 1974, 183, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 7017–7036. [Google Scholar] [CrossRef]
- Lehn, J.-M. Cryptates: Inclusion complexes of macropolycyclic receptor molecules. Pure Appl. Chem. 1978, 50, 871–892. [Google Scholar] [CrossRef]
- Cram, D.J.; Kaneda, T.; Helgeson, R.C.; Lein, G.M. Spherands-ligands whose binding of cations relieves enforced electron-electron repulsions. J. Am. Chem. Soc. 1979, 101, 6752–6754. [Google Scholar] [CrossRef]
- Cram, D.J.; Kaneda, T.; Lein, G.M.; Helgeson, R.C. A spherand containing an enforced cavity that selectively binds lithium and sodium ions. J. Chem. Soc. Chem. Commun. 1979, 948b–950. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Muthukrishnan, R. Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem. 1978, 43, 4905–4906. [Google Scholar] [CrossRef]
- Yoshio, M.; Noguchi, H. Crown ethers for chemical analysis: A review. Anal. Lett. 1982, 15, 1197–1276. [Google Scholar] [CrossRef]
- Vögtle, F.; Weber, E. (Eds.) Host Guest Complex Chemistry III; Topics in Current Chemistry Series; Springer: Berlin, Germany, 1984; Volume 121, pp. 1–104. [Google Scholar]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 1991, 91, 1721–2085. [Google Scholar] [CrossRef]
- Vögtle, F. Crown ethers, cryptands, podands and spherands. In Supramolecular Chemistry: An Introduction; John Wiley & Sons Ltd.: Chichester, UK, 1993; Chapter 2.2; pp. 27–83. [Google Scholar]
- Cram, D.J.; Cram, J.M. Spherands, spheraplexes, and their relatives. In Container Molecules and Their Guests, Monographs; Stoddart, J.F., Ed.; RSC: Cambridge, UK, 1997; Chapter 2; pp. 20–48. [Google Scholar]
- Arnaud-Neu, F.; Mckervey, M.A.; Schwing-Weill, M.-J. Metal-ion complexation by narrow rim carbonyl derivatives. In Calixarenes 2001; Asfari, Z., Böhmer, V., Harrowfield, J.M., Vicens, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; Chapter 21; pp. 385–406. [Google Scholar]
- Pedersen, C.J.; Frensdorff, H.K. Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. 1972, 11, 16–25. [Google Scholar] [CrossRef]
- Ohto, K. Research on various structural effects of calix[4]arene derivatives on extractive separation behavior of metal ions. J. Ion Exch. 2019, 30, 17–28. (In Japanese) [Google Scholar] [CrossRef]
- Ohto, K.; Furugou, H.; Morisada, S.; Kawakita, H.; Isono, K.; Inoue, K. Stepwise recovery of molybdenum, tungsten, and vanadium by stripping using an amino-type “Trident” molecule by stripping. Solvent Extr. Ion Exch. 2021, 39, 512–532. [Google Scholar] [CrossRef]
- Ohto, K. Review on adsorbents incorporating calixarene derivatives used for metals recovery and hazardous ions removal: The concept of adsorbent design and classification of adsorbents. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 175–194. [Google Scholar] [CrossRef]
- Ohto, K. Molecular design and metal extraction behavior of calixarene compounds as host extractants. In Ion Exchange and Solvent Extraction. Supramolecular Aspects of Solvent Extraction; Moyer, B.A., Ed.; CPC Press: Boca Raton, FL, USA, 2013; Volume 21, Chapter 3; pp. 81–127. [Google Scholar]
- Ohto, K.; Ueda, Y.; Sathuluri, R.R.; Kawakita, H.; Morisada, S.; Inoue, K. Silver extraction and recovery with macrocyclic and tripodal compounds. In Silver Recovery from Assorted Spent Sources: Toxicology of Silver Ion; Sabir, S., Ed.; World Scientific: New York, NY, USA, 2018; Chapter 9; pp. 275–301. [Google Scholar]
- Yamaguma, R.; Yamashita, A.; Kawakita, H.; Miyajima, T.; Takemura, C.; Ohto, K.; Iwachido, N. Selective extraction of precious metal ions with novel trident molecules with pyridyl groups. Sep. Sci. Technol. 2012, 47, 1303–1309. [Google Scholar] [CrossRef]
- Ohto, K.; Yamashita, A.; Ueda, Y.; Yamaguma, R.; Morisada, S.; Kawakita, H. Extractive removal of formaldehyde by using imination reaction with amine type of trident molecule. Solvent Extr. Res. Dev. Jpn. 2014, 21, 173–180. [Google Scholar] [CrossRef][Green Version]
- Ohto, K.; Furugou, H.; Yoshinaga, T.; Morisada, S.; Kawakita, H.; Inoue, K. Precious Metal Extraction with Thiol and Dithioether Derivatives of Trident Molecule. Solvent Extr. Res. Dev. Jpn. 2017, 24, 77–88. [Google Scholar] [CrossRef][Green Version]
- Ohto, K.; Fuchiwaki, N.; Yoshihara, T.; Chetry, A.B.; Morisada, S.; Kawakita, H. Extraction of scandium and other rare earth elements with a tricarboxylic acid derivative of tripodal pseudcalix[3]arene prepared from a new phenolic tripodal framework. Sep. Purif. Technol. 2019, 226, 259–266. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Alfantazi, A.M.; Moskalyk, R.R. Processing of indium: A review. Miner. Eng. 2003, 16, 687–694. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Ye, Q.; Xiang, X.; Xie, B. Novel recycle technology for recovering rare metals (Ga, In) fromwaste light-emitting diodes. J. Hazard. Mater. 2015, 299, 388–394. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X.; et al. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- Pradhan, D.; Panda, S.; Sukla, L.B. Recent advances in indium metallurgy: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 167–180. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Kucuker, M.A.; Kuchta, K.; et al. Recent advances on hydrometallurgical recovery of critical and precious elements from end of life electronic wastes—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Xiao, Y.; Fan, Y. Recovery of gallium from Bayer liquor: A review. Hydrometallurgy 2012, 125, 115–124. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A review on separation of gallium and indium from leach liquors by solvent extraction and ion exchange. Miner. Process. Extr. Metall. Rev. 2019, 40, 278–291. [Google Scholar] [CrossRef]
- Nishihama, S.; Hino, A.; Hirai, T.; Komasawa, I. Extraction and separation of gallium and indium from aqueous chloride solution using several organophosphorus compounds as extractants. J. Chem. Eng. Jpn. 1998, 31, 818–827. [Google Scholar] [CrossRef]
- Hirayama, N.; Horita, Y.; Oshima, S.; Kubono, K.; Kokusen, H.; Honjo, T. Selective extraction of gallium from aluminum and indium using tripod phenolic ligands. Talanta 2001, 53, 857–862. [Google Scholar] [CrossRef]
- Koshimoto, A.; Oshima, T.; Ohto, K.; Baba, Y. Synthesis of phosphonic acid extractants and selective extraction of In(III) and Ga(III) from acidic media containing Zn(II). Solvent Extr. Res. Dev. Jpn. 2011, 18, 137–147. [Google Scholar] [CrossRef][Green Version]
- Sasaki, Y.; Oshima, T.; Baba, Y. Mutual separation of indium(III), gallium(III) and zinc(II) with alkylated aminophosphonic acids with different basicities of amine moiety. Sep. Purif. Technol. 2017, 173, 37–43. [Google Scholar] [CrossRef]
- Sasaki, Y.; Oshima, T.; Baba, Y. Synthesis of aminophosphonic acid extractants and the effect of the alkyl chain on their extraction selectivities for indium(III), gallium(III), and zinc(II). Solvent Extr. Res. Dev. Jpn. 2018, 25, 11–21. [Google Scholar] [CrossRef]
- Sasaki, Y.; Uto, M.; Oshima, T.; Baba, Y. Synthesis of a carboxylic acid extractant containing an amino group and its selective extraction of In(III) and Ga(III). Solvent Extr. Res. Dev. Jpn. 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Sasaki, Y.; Matsuo, N.; Oshima, T.; Baba, Y. Selective extraction of In(III), Ga(III) and Zn(II) using a novel extractant with phenylphosphinic acid. Chin. J. Chem. Eng. 2016, 24, 232–236. [Google Scholar] [CrossRef]
- Miura, R.; Tokumaru, M.; Oshima, T.; Baba, Y. Selective extraction of indium(III) and gallium(III) with 2-ethylhexyl thioglycolate. Solvent Extr. Res. Dev. Jpn. 2017, 24, 123–130. [Google Scholar] [CrossRef][Green Version]
- Adhikari, B.B.; Gurung, M.; Kawakita, H.; Ohto, K. Complexation and extraction behavior of trivalent indium with multiple proton ionizable p—t-butylcalix[5]arene pentacaarboxylic acid derivative: A new efficient solvent extraction reagent for indium. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 479–487. [Google Scholar] [CrossRef]
- Kawashima, M.; Kawakita, H.; Ohto, K.; Shiwa, Y. Aluminum group extraction with benzoic acid—Acetic acid crossed type calix[4]arene derivatives. Solvent Extr. Res. Dev. Jpn. 2012, 19, 41–50. [Google Scholar] [CrossRef]
- Chetry, A.B.; Adhikari, B.B.; Morisada, S.; Kawakita, H.; Ohto, K. Selective extraction of Ga(III) with p-t-butylcalix[4]arene tetrahydroxamic acid. Solvent Extr. Res. Dev. Jpn. 2015, 22, 25–35. [Google Scholar] [CrossRef]
- Pang, G.; Morisada, S.; Kawakita, H.; Hanamoto, T.; Umecky, T.; Ohto, K.; Song, X.-M. Allosteric extraction of a second gallium anion assisted by the first, loaded onto a fluorinated secondary amide reagent. Sep. Purif. Technol. 2021, 278, 119036. [Google Scholar] [CrossRef]
- Ohto, K.; Yano, M.; Inoue, K.; Yamamoto, T.; Goto, M.; Nakashio, F.; Shinkai, S.; Nagasaki, T. Solvent extraction of trivalent rare earth metal ions with carboxylate derivatives of calixarenes. Anal. Sci. 1995, 11, 893–902. [Google Scholar] [CrossRef]
- Ohto, K.; Nakagawa, H.; Furutsuka, H.; Shinohara, T.; Nakamura, T.; Oshima, T.; Inoue, K. Solvent extraction of rare earths with a novel phosphonate extractant providing a narrow coordination site. Solvent Extr. Res. Dev. Jpn. 2004, 11, 121–134. [Google Scholar]
- Eigen, M. Fast elementary steps in chemical reaction mechanisms. Pure Appl. Chem. 1963, 6, 97–115. [Google Scholar] [CrossRef]
- Ohto, K.; Ota, H.; Inoue, K. Solvent extraction of rare earths with a calix[4]arene compound containing phosphonate groups introduced onto upper rim. Solvent Extr. Res. Dev. Jpn. 1997, 4, 167–182. [Google Scholar]
- Ohto, K.; Murakami, E.; Shinohara, T.; Shiratsuchi, K.; Inoue, K.; Iwasaki, M. Selective extraction of silver(I) over palladium(II) with ketonic derivatives of calixarenes from highly concentrated nitric acid. Anal. Chim. Acta 1997, 341, 275–283. [Google Scholar] [CrossRef]
- Ohto, K.; Yamaga, H.; Murakami, E.; Inoue, K. Specific extraction behavior of amide derivative of calix[4]arene for silver(I) and gold(III) ions from highly acidic chloride media. Talanta 1997, 44, 1123–1130. [Google Scholar] [CrossRef]
- Ohto, K.; Fujimoto, Y.; Inoue, K. Stepwise extraction of two lead ions with a single molecule of calix[4]arene tetracarboxylic Acid. Anal. Chim. Acta 1999, 387, 61–69. [Google Scholar] [CrossRef]
- Ohto, K.; Ishibashi, H.; Kawakita, H.; Inoue, K.; Oshima, T. Allosteric coextraction of sodium and metal ions with calix[4]arene derivatives 1 Role of the first-extracted sodium ion as an allosteric trigger for self-coextraction of sodium ions with calix[4]arene tetracarboxylic acid. J. Incl. Phenom. Macrocycl. Chem. 2009, 65, 111–120. [Google Scholar] [CrossRef]
- Yoneyama, T.; Sadamatsu, H.; Kuwata, S.; Kawakita, H.; Ohto, K. Allosteric coextraction of sodium and metal ions with calix[4]arene derivatives 2: First numerical evaluation for the allosteric effect on alkali metal extraction with crossed carboxylic acid type calix[4]arenes. Talanta 2012, 88, 121–128. [Google Scholar] [CrossRef]
- Sadamatsu, H.; Morisada, S.; Kawakita, H.; Ohto, K. Allosteric coextraction of sodium and metal ions with calix[4]arene derivatives 3. Effect of propyl groups on size-discrimination for the second coextracted ion. Solvent Extr. Ion Exch. 2015, 33, 264–277. [Google Scholar] [CrossRef]
- Ohto, K.; Yoshinaga, T.; Furugou, H.; Morisada, S.; Kawakita, H.; Inoue, K. Silver extraction with sulfide type trident compounds. Solvent Extr. Res. Dev. Jpn. 2018, 25, 71–78. [Google Scholar] [CrossRef]
- Suzuki, S.; Kato, M.; Nakajima, S. Application of electrogenerated triphenylmethyl anion as a base for alkylation of arylacetic esters and arylacetonitriles and isomerization of allylbenzenes. Can. J. Chem. 1994, 72, 357–361. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Terrier, F.; Xie, H.-Q.; Lelievre, J.; Boubaker, T.; Farrell, P.G. Ionization of nitrotriphenylmethanes. Remarkable kinetic evidence for steric inhibition to resonance and F-strain. J. Chem. Soc. Perkin Trans. 2 1990, 11, 1899–1903. [Google Scholar] [CrossRef]
- Olmstead, M.M.; Power, P.P. The isolation and x-ray structures of lithium crown ether salts of the free phenyl carbanions [CHPh2]- and [CPh3]-. J. Am. Chem. Soc. 1985, 107, 2174–2175. [Google Scholar] [CrossRef]
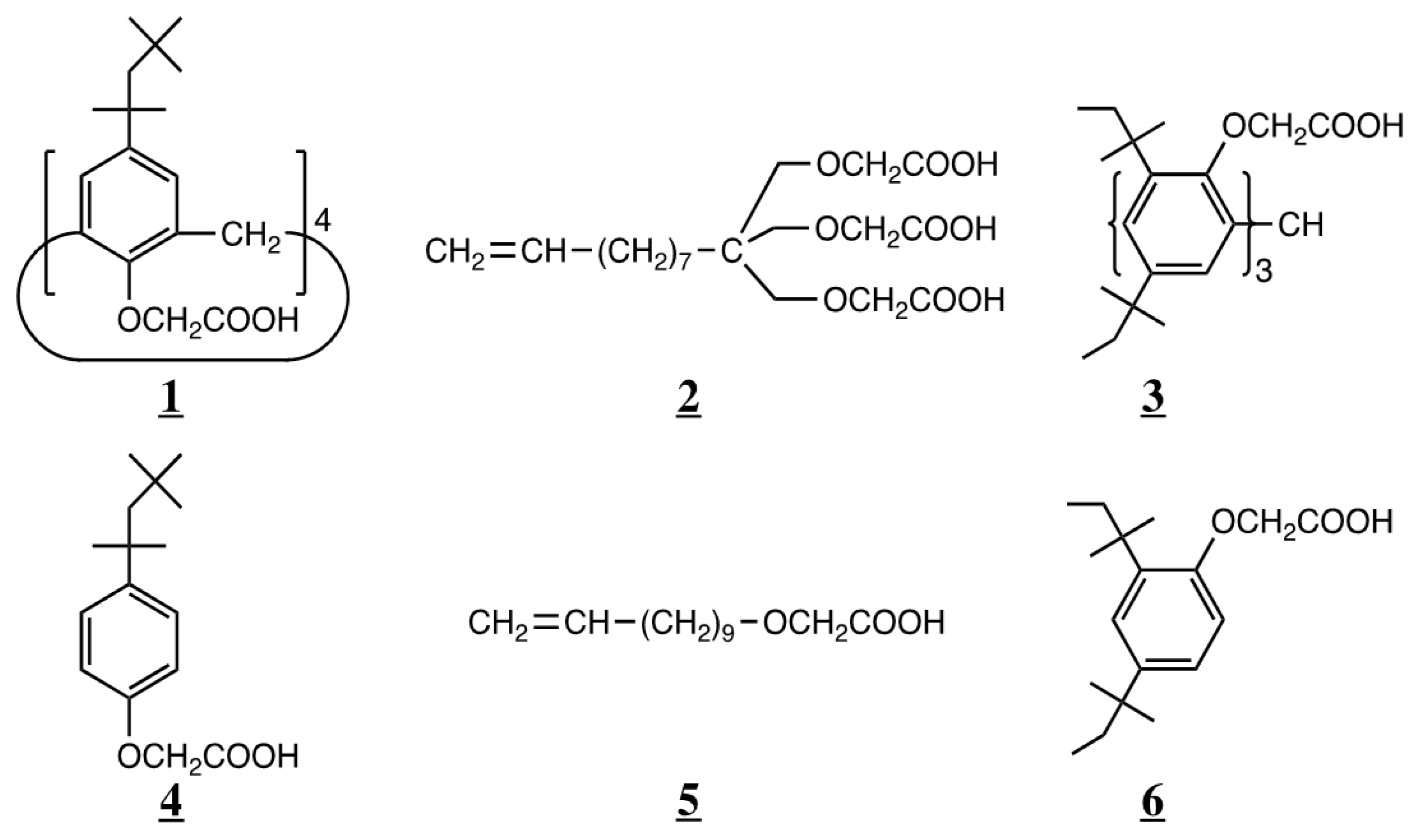







| Calix[4]arene (Cone) | “Trident” Molecule | Trihydroxytriphenylmethane | |
|---|---|---|---|
| Modified group | Active phenols | Non-reactive alchohols | Active phenols |
| Coordination site (Site size) | small at lower rim (2.0 Å at distal position) | Small and narrow (1.7 Å between O to O) | Small and narrow (less than 2.0 Å) |
| Inversion (Fixing) | Probable (controllable) | None (already 3-D controlled) | None |
| Rigidity | Rigid | Relatively rigid | Rigid |
| Polyfunctionality | e.g., Tetradentate | e.g., Tridentate | e.g., Tridentate |
| Symmetry | e.g., C2, C4 symmetry | e.g., C3 symmetry | e.g., C3 symmetry |
| Spectroscopic transparency | UV absorptive | UV Transparent | UV absorptive |
| Lipophilicity | Poor | Poor | High |
| Extractant | Half pH Values, pH1/2 | ∆pH1/2 | ∆pH1/2 | |||||
|---|---|---|---|---|---|---|---|---|
| Al | Ga | In | Al | Ga | In | Al-Ga | Ga-In | |
| 1 | 3.58 | 2.92 | 2.60 | 0.29 | 0.30 | 0.52 | 0.66 | 0.32 |
| 4 | 3.87 | 3.22 | 3.12 | 0.65 | 0.10 | |||
| 2 | 2.41 | 1.89 | 1.85 | >1.49 | 1.34 | 1.23 | 0.52 | 0.04 |
| 5 | >3.90 | 3.23 | 3.08 | >0.67 | 0.15 | |||
| 3 | 3.63 | 3.14 | 2.89 | >0.22 | 0.64 | 0.86 | 0.49 | 0.25 |
| 6 | >3.85 | 3.78 | 3.75 | >0.07 | 0.03 | |||
| Extractant | Extraction Equilibrium Constants, Kex1 and Kex2 | Separation Factors, ß | |||
|---|---|---|---|---|---|
| Al | Ga | In | Ga/Al | In/Ga | |
| 1 | 3.64 × 10−9 | 3.48 × 10−7 | 3.17 × 10−6 | 95.6 | 9.11 |
| 2 | 1.18 × 10−5 | 4.28 × 10−4 | 5.64 × 10−4 | 36.3 | 1.32 |
| 3 | 2.58 × 10−9 | 7.60 × 10−8 | 4.28 × 10−7 | 29.5 | 5.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohto, K.; Fuchiwaki, N.; Furugou, H.; Morisada, S.; Kawakita, H.; Wenzel, M.; Weigand, J.J. Comparative Extraction of Aluminum Group Metals Using Acetic Acid Derivatives with Three Different-Sized Frameworks for Coordination. Separations 2021, 8, 211. https://doi.org/10.3390/separations8110211
Ohto K, Fuchiwaki N, Furugou H, Morisada S, Kawakita H, Wenzel M, Weigand JJ. Comparative Extraction of Aluminum Group Metals Using Acetic Acid Derivatives with Three Different-Sized Frameworks for Coordination. Separations. 2021; 8(11):211. https://doi.org/10.3390/separations8110211
Chicago/Turabian StyleOhto, Keisuke, Nako Fuchiwaki, Hiroaki Furugou, Shintaro Morisada, Hidetaka Kawakita, Marco Wenzel, and Jan J. Weigand. 2021. "Comparative Extraction of Aluminum Group Metals Using Acetic Acid Derivatives with Three Different-Sized Frameworks for Coordination" Separations 8, no. 11: 211. https://doi.org/10.3390/separations8110211
APA StyleOhto, K., Fuchiwaki, N., Furugou, H., Morisada, S., Kawakita, H., Wenzel, M., & Weigand, J. J. (2021). Comparative Extraction of Aluminum Group Metals Using Acetic Acid Derivatives with Three Different-Sized Frameworks for Coordination. Separations, 8(11), 211. https://doi.org/10.3390/separations8110211








