Simultaneous Determination of 23 Mycotoxins in Broiler Tissues by Solid Phase Extraction UHPLC-Q/Orbitrap High Resolution Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrument Conditions
2.3. Sample Preparation
2.4. Method Validation
3. Results and Discussion
3.1. The Optimization of LC-HRMS Conditions
3.2. The Optimization of SPE Pretreatment
3.3. Matrix Effect
3.4. The Method Validation
3.5. The Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Zhang, Q.; Yan, Z.; Tan, Y.; Zhu, R.; Yu, D.; Yang, H.; Wu, A. Occurrence and Quantitative Risk Assessment of Twelve Mycotoxins in Eggs and Chicken Tissues in China. Toxins 2018, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Zhao, Z.; Wang, J.; Bai, B.; Wu, A.; Yan, L.; Song, S. A simple sample pretreatment method for multi-mycotoxin determination in eggs by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2015, 1417, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Zheng, N.; Guo, L.; Song, X.; Zhao, S.; Wang, J. Biological System Responses of Dairy Cows to Aflatoxin B1 Exposure Revealed with Metabolomic Changes in Multiple Biofluids. Toxins 2019, 11, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, S.Z.; Nisar, S.; Asi, M.R.; Jinap, S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control 2014, 43, 98–103. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, N.; Yang, L.; Deng, Y.; Wang, J.; Song, S.; Lin, S.; Wu, A.; Zhou, Z.; Hou, J. Multi-mycotoxin analysis of animal feed and animal-derived food using LC–MS/MS system with timed and highly selective reaction monitoring. Anal. Bioanal. Chem. 2015, 407, 7359–7368. [Google Scholar] [CrossRef]
- Mazur-Kuśnirek, M.; Antoszkiewicz, Z.; Lipiński, K.; Fijałkowska, M.; Purwin, C.; Kotlarczyk, S. The effect of polyphenols and vitamin E on the antioxidant status and meat quality of broiler chickens fed diets naturally contaminated with ochratoxin A. Arch. Anim. Nutr. 2019, 73, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, K.T.; Els, V.P.; Bart, H.; Evelyne, D.; Els, D. Carry-over of some Fusarium mycotoxins in tissues and eggs of chickens fed experimentally mycotoxin-contaminated diets. Food Chem. Toxicol. 2020, 145, 111715. [Google Scholar] [CrossRef]
- Buranatragool, K.; Poapolathep, S.; Isariyodom, S.; Imsilp, K.; Klangkaew, N.; Poapolathep, A. Dispositions and tissue residue of zearalenone and its metabolites α-zearalenol and β-zearalenol in broilers. Toxicol. Rep. 2015, 2, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; et al. A QuEChERS-Based Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of Nine Zearalenone-Like Mycotoxins in Pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Muhammad, I.; Li, R.; Jin, H.; Guo, Z.; Yang, Y.; Hamid, S.; Li, J.; Cheng, P.; Zhang, X. Development of a UPLC-FLD Method for Detection of Aflatoxin B1 and M1 in Animal Tissue to Study the Effect of Curcumin on Mycotoxin Clearance Rates. Front. Pharmacol. 2017, 8, 650. [Google Scholar] [CrossRef] [Green Version]
- D’Agnello, P.; Vita, V.; Franchino, C.; Urbano, L.; Curiale, A.; Debegnach, F.; Iammarino, M.; Marchesani, G.; Chiaravalle, A.; Pace, R. ELISA and UPLC/FLD as Screening and ConfirmatoryTechniques for T-2/Ht-2 Mycotoxin Determination in Cereals. Appl. Sci. 2021, 11, 1688. [Google Scholar] [CrossRef]
- McMaster, N.; Acharya, B.; Harich, K.; Grothe, J.; Mehl, H.L.; Schmale, D.G. Quantification of the Mycotoxin Deoxynivalenol (DON) in Sorghum Using GC-MS and a Stable Isotope Dilution Assay (SIDA). Food Anal. Methods 2019, 12, 2334–2343. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Mañes, J.; Berrada, H. Exposure assessment approach through mycotoxin/creatinine ratio evaluation in urine by GC–MS/MS. Food Chem. Toxicol. 2014, 72, 69–75. [Google Scholar] [CrossRef]
- Turkmen, Z.; Kurada, O. Rapid HPTLC determination of patulin in fruit-based baby food in Turkey. JPC—J. Planar Chromatogr.–Mod. TLC 2020, 33, 209–217. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Jedziniak, P. Development of a multi-mycotoxin LC-MS/MS method for the determination of biomarkers in pig urine. Mycotoxin Res. 2021, 37, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiu, J.; Qian, Y. Polyethyleneimine-modified magnetic carbon nanotubes as solid-phase extraction adsorbent for the analysis of multi-class mycotoxins in milk via liquid chromatography–tandem mass spectrometry. J. Sep. Sci. 2020, 44, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Malachová, A.; Sulyok, M.; Krska, R. Challenges and future directions in LC-MS-based multiclass method development for the quantification of food contaminants. Anal. Bioanal. Chem. 2020, 413, 25–34. [Google Scholar] [CrossRef] [PubMed]
- den Hollander, D.; Croubels, S.; Lauwers, M.; Caekebeke, N.; Ringenier, M.; De Meyer, F.; Reisinger, N.; Van Immerseel, F.; Dewulf, J.; Antonissen, G. Applied Research Note: Biomonitoring of mycotoxins in blood serum and feed to assess exposure of broiler chickens. J. Appl. Poult. Res. 2020, 30, 100111. [Google Scholar] [CrossRef]
- Nakhjavan, B.; Ahmed, N.S.; Khosravifard, M. Development of an Improved Method of Sample Extraction and Quantitation of Multi-Mycotoxin in Feed by LC-MS/MS. Toxins 2020, 12, 462. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, L.; Graziani, G.; Gaspari, A.; Izzo, L.; Tolosa, J.; Rodríguez-Carrasco, Y.; Ritieni, A. Target Analysis and Retrospective Screening of Multiple Mycotoxins in Pet Food Using UHPLC-Q-Orbitrap HRMS. Toxins 2019, 11, 434. [Google Scholar] [CrossRef] [Green Version]
- Sun, F.; Tan, H.; Li, Y.; De Boevre, M.; Zhang, H.; Zhou, J.; Li, Y.; Yang, S. An integrated data-dependent and data-independent acquisition method for hazardous compounds screening in foods using a single UHPLC-Q-Orbitrap run. J. Hazard. Mater. 2020, 401, 123266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cao, Y.-M.; Miao, S.; Lan, L.; Chen, M.; Li, W.-T.; Mao, X.-H.; Ji, S. Qualitative screening and quantitative determination of 569 pesticide residues in honeysuckle using ultrahigh-performance liquid chromatography coupled to quadrupole-Orbitrap high resolution mass spectrometry. J. Chromatogr. A 2019, 1606, 460374. [Google Scholar] [CrossRef]
- Alaboudi, A.R.; Osaili, T.M.; Otoum, G. Quantification of mycotoxin residues in domestic and imported chicken muscle, liver and kidney in Jordan. Food Control. 2021, 132, 108511. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Jia, Q.; Zhang, L.; Zhang, W.; Mu, P.; Jia, Y.; Qian, Y.; Qiu, J. Simultaneous determination of 58 pesticides and relevant metabolites in eggs with a multi-functional filter by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1593, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Arce-López, B.; Lizarraga, E.; Flores-Flores, M.; Irigoyen, Á.; González-Peñas, E. Development and validation of a methodology based on Captiva EMR-lipid clean-up and LC-MS/MS analysis for the simultaneous determination of mycotoxins in human plasma. Talanta 2020, 206, 120193. [Google Scholar] [CrossRef] [PubMed]

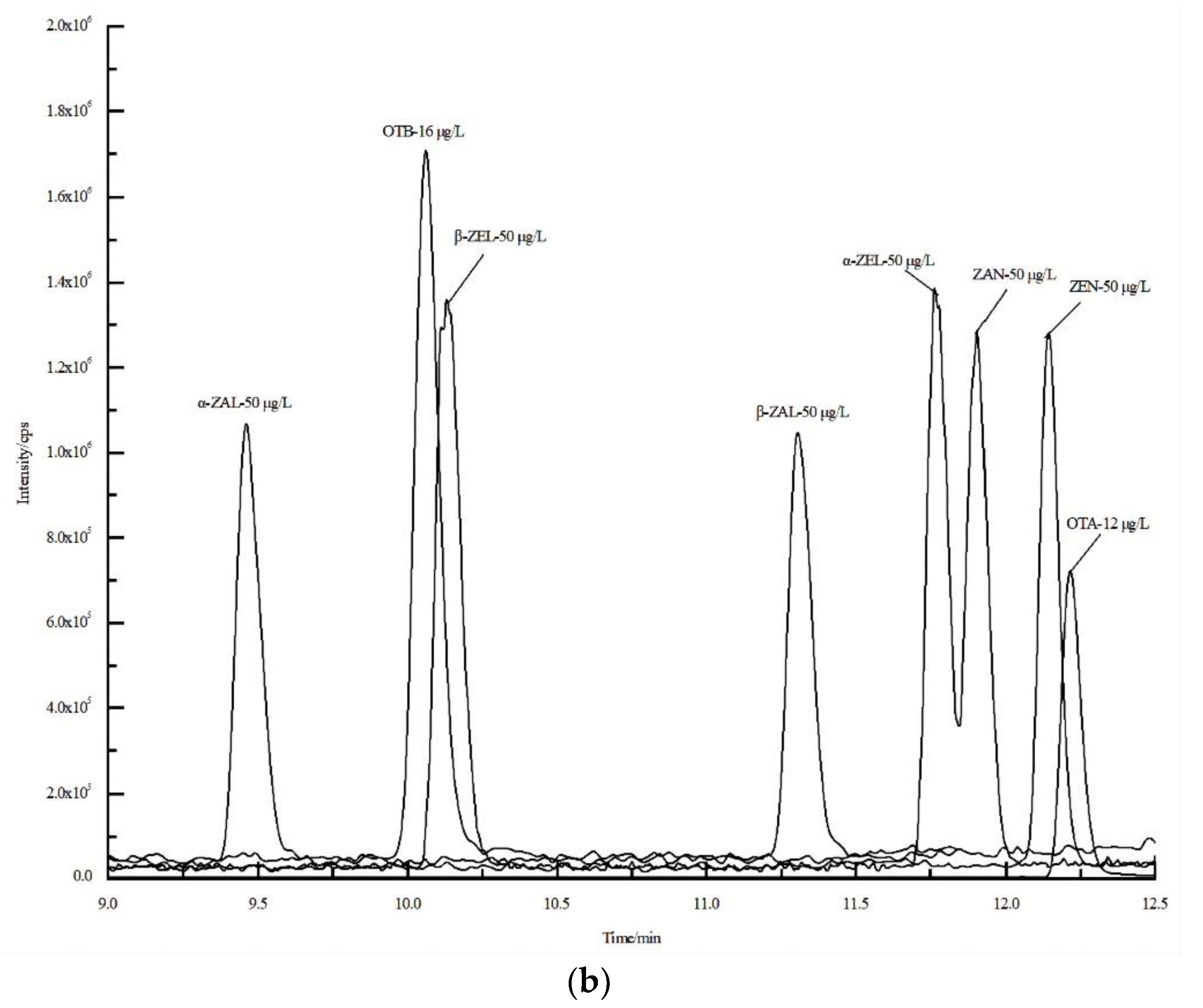
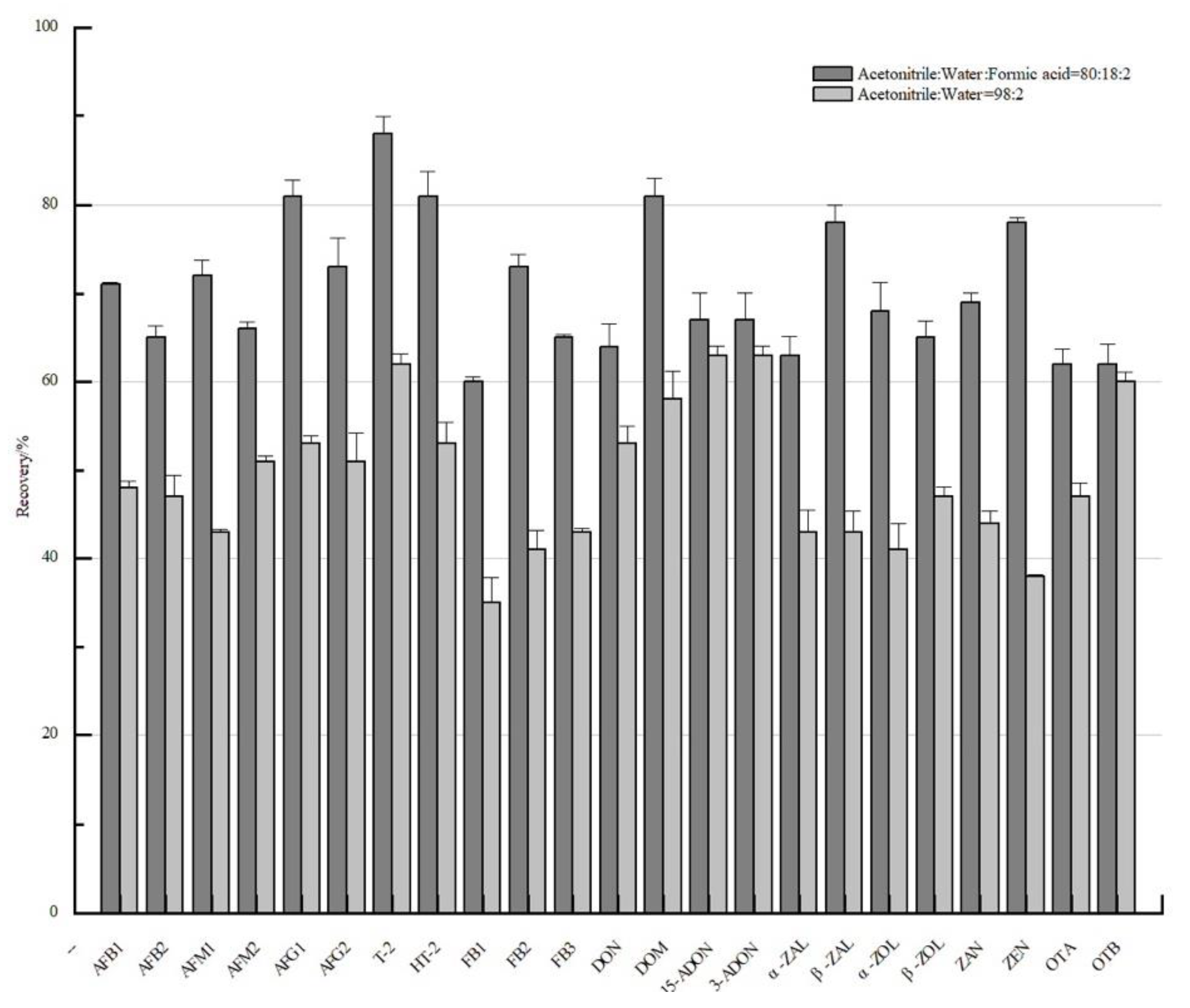
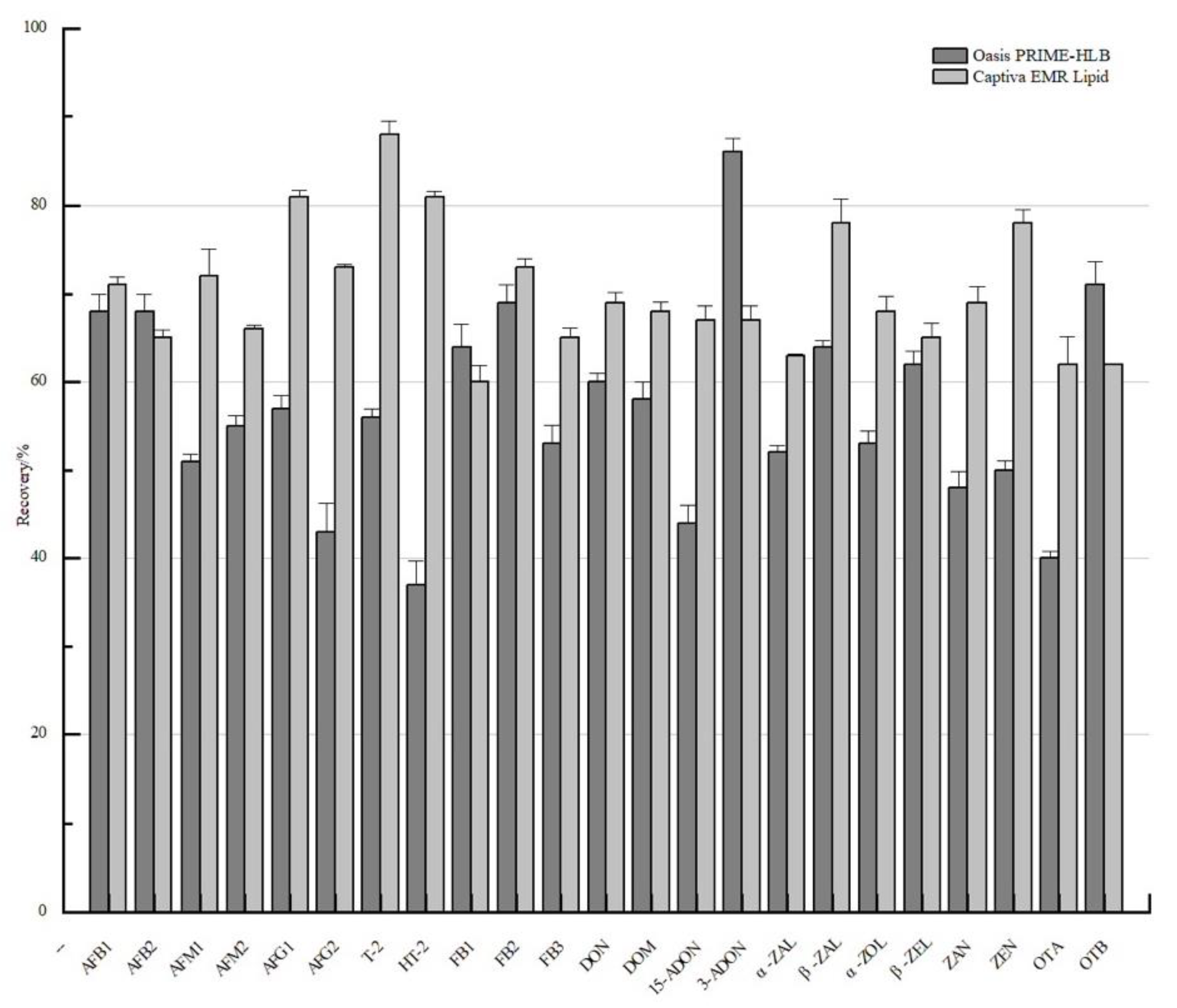
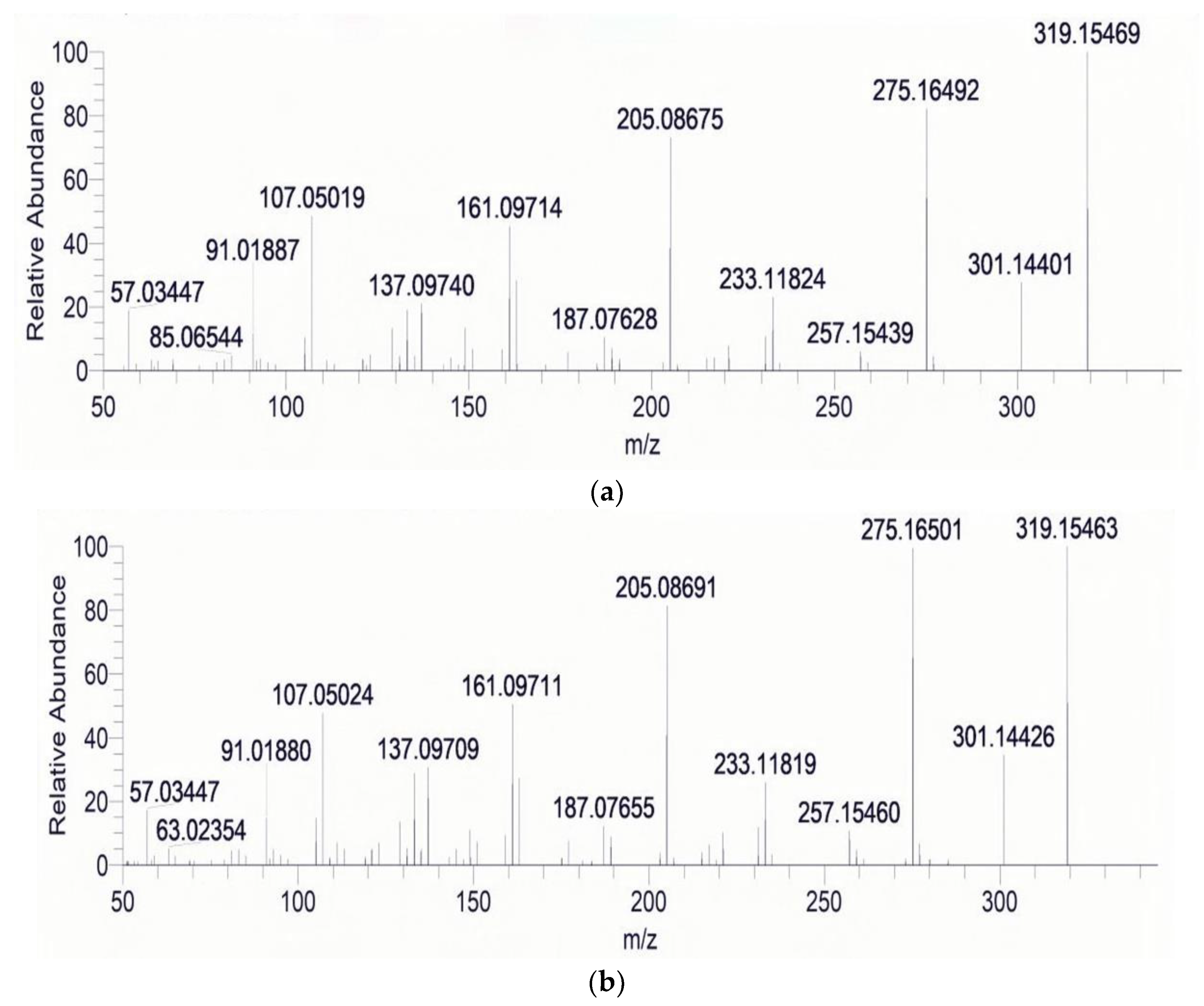
| Acquisition Mode | Time (min) | Gradient (%) | Acquisition Mode | Time (min) | Gradient (%) | ||
|---|---|---|---|---|---|---|---|
| A | B | A | B | ||||
| Positive mode | 1 | 70 | 30 | Negative mode | 1 | 97 | 3 |
| 6.5 | 45 | 55 | 2 | 45 | 55 | ||
| 8.5 | 45 | 55 | 9 | 30 | 70 | ||
| 10 | 20 | 80 | 10 | 1 | 99 | ||
| 12 | 20 | 80 | 11 | 1 | 99 | ||
| 12.1 | 70 | 30 | 11.1 | 97 | 3 | ||
| 16.1 | 70 | 30 | 15 | 97 | 3 | ||
| Comment | Ion Mode | Measured Mass (m/z) | Characteristic Ion 1 (m/z) | Characteristic Ion 2 (m/z) | RT/min |
|---|---|---|---|---|---|
| AFB1 | [M + H]+ | 313.07111 | 285.07611 | 270.05267 | 9.68 |
| AFB2 | [M + H]+ | 315.08661 | 287.09186 | 259.06024 | 9.22 |
| AFG1 | [M + H]+ | 329.06577 | 243.06554 | 283.06055 | 8.42 |
| AFG2 | [M + H]+ | 331.08191 | 313.07135 | 245.08141 | 7.86 |
| AFM1 | [M + H]+ | 329.06577 | 273.07593 | 259.06036 | 7.68 |
| AFM2 | [M + H]+ | 331.08008 | 273.07608 | 285.07596 | 6.70 |
| T-2 | [M + NH4]+ | 484.25464 | 305.13736 | 185.09566 | 12.34 |
| HT-2 | [M + NH4]+ | 442.24233 | 263.12665 | 235.10591 | 11.90 |
| FB1 | [M + H]+ | 722.39337 | 704.38312 | 352.32013 | 11.75 |
| FB2 | [M + H]+ | 706.3985 | 336.32513 | 688.38812 | 13.15 |
| FB3 | [M + H]+ | 706.3985 | 336.32523 | 688.38812 | 12.82 |
| DON | [M + H]+ | 297.13287 | 249.11194 | 203.10658 | 2.35 |
| DOM | [M + H]+ | 281.13724 | 235.10661 | 137.05975 | 4.18 |
| 15-ADON | [M + H]+ | 339.14368 | 323.12293 | 137.05972 | 6.28 |
| 3-ADON | [M + H]+ | 339.14368 | 231.10149 | 279.12253 | 6.28 |
| α-ZAL | [M − H]− | 323.17032 | 277.18048 | 303.15970 | 9.45 |
| β-ZAL | [M − H]− | 323.17041 | 277.1806 | 303.15982 | 11.29 |
| α-ZOL | [M − H]− | 319.15454 | 275.16489 | 160.01656 | 11.76 |
| β-ZOL | [M − H]− | 319.15463 | 275.16495 | 160.01651 | 10.13 |
| ZAN | [M − H]− | 319.1546 | 275.16501 | 205.08682 | 11.91 |
| ZEN | [M − H]− | 317.13907 | 131.05017 | 175.03992 | 12.14 |
| OTA | [M − H]− | 402.07407 | 358.08435 | 231.01634 | 12.23 |
| OTB | [M − H]− | 368.11105 | 324.12436 | 280.09824 | 10.06 |
| Mycotoxins | Matrix Effect/% | ||
|---|---|---|---|
| Breast | Gizzard | Liver | |
| AFB1 | 88.64 | 71.29 | 59.10 |
| AFB2 | 88.14 | 40.46 | 55.95 |
| AFG1 | 90.35 | 62.03 | 85.96 |
| AFG2 | 78.85 | 66.55 | 70.11 |
| AFM1 | 80.98 | 59.78 | 87.02 |
| AFM2 | 87.94 | 55.50 | 87.72 |
| T-2 | 48.11 | 32.62 | 42.23 |
| HT-2 | 46.24 | 35.27 | 46.98 |
| FB1 | 15.96 | 16.00 | 46.82 |
| FB2 | 38.38 | 20.79 | 54.17 |
| FB3 | 48.29 | 73.76 | 39.81 |
| DON | 34.31 | 10.32 | 36.97 |
| DOM | 90.11 | 76.82 | 67.87 |
| 15-ADON | 87.14 | 63.23 | 57.97 |
| 3-ADON | 87.14 | 58.27 | 62.28 |
| α-ZAL | 21.54 | 40.17 | 23.75 |
| β-ZAL | 36.61 | 46.91 | 48.26 |
| α-ZOL | 32.47 | 47.98 | 37.62 |
| β-ZOL | 37.78 | 57.52 | 12.29 |
| ZAN | 40.42 | 26.37 | 50.35 |
| ZEN | 44.22 | 44.32 | 44.01 |
| OTA | 30.35 | 32.43 | 58.76 |
| OTB | 49.42 | 32.21 | 29.26 |
| Mycotoxins | Added Concentration (μg/kg) | Chicken Liver | Chicken Gizzard | Chicken Breast Meat | |||
|---|---|---|---|---|---|---|---|
| Recovery/% | RSD/% | Recovery/% | RSD/% | Recovery/% | RSD/% | ||
| AFB1 | 7.5 | 85 | 0.3 | 64 | 0.8 | 67 | 10.1 |
| 15 | 67 | 5.4 | 65 | 1.7 | 79 | 9.0 | |
| 37.5 | 69 | 11.9 | 69 | 0.4 | 80 | 5.0 | |
| AFB2 | 1.875 | 68 | 1.3 | 62 | 2.4 | 101 | 3.6 |
| 3.75 | 65 | 2.6 | 68 | 3.2 | 77 | 10.9 | |
| 9.375 | 73 | 7.6 | 81 | 1.4 | 69 | 3.9 | |
| AFG1 | 7.5 | 61 | 1.8 | 84 | 0.3 | 73 | 2.8 |
| 15 | 62 | 3.4 | 71 | 1.5 | 84 | 8.6 | |
| 37.5 | 61 | 1.7 | 61 | 1.7 | 70 | 8.6 | |
| AFG2 | 1.875 | 69 | 0.8 | 67 | 0.6 | 67 | 1.3 |
| 3.75 | 67 | 4.1 | 61 | 1.3 | 72 | 10.5 | |
| 9.375 | 63 | 4.1 | 66 | 12.5 | 73 | 5.5 | |
| AFM1 | 12.5 | 67 | 1.7 | 70 | 0.9 | 64 | 2.0 |
| 25 | 92 | 3.1 | 66 | 5.1 | 65 | 8.1 | |
| 62.5 | 65 | 3.3 | 68 | 3.2 | 63 | 4.1 | |
| AFM2 | 10 | 78 | 3.3 | 87 | 6.2 | 63 | 2.0 |
| 20 | 91 | 1.3 | 89 | 8.9 | 62 | 2.0 | |
| 50 | 88 | 5.4 | 82 | 3.6 | 66 | 6.1 | |
| T-2 | 37.5 | 75 | 5.9 | 80 | 9.2 | 70 | 10.9 |
| 75 | 97 | 9.4 | 67 | 12.6 | 67 | 9.0 | |
| 187.5 | 67 | 2.4 | 67 | 4.1 | 75 | 4.0 | |
| HT-2 | 52.5 | 87 | 2.7 | 75 | 2.4 | 87 | 2.3 |
| 105 | 75 | 8.2 | 88 | 4.1 | 71 | 13.5 | |
| 262.5 | 93 | 3.1 | 86 | 3.4 | 72 | 3.5 | |
| FB1 | 12.5 | 69 | 0.5 | 62 | 2.9 | 66 | 3.4 |
| 25 | 73 | 3.0 | 77 | 1.2 | 65 | 7.2 | |
| 62.5 | 75 | 6.9 | 81 | 6.2 | 77 | 4.2 | |
| FB2 | 12.5 | 102 | 1.4 | 66 | 2.2 | 64 | 8.0 |
| 25 | 68 | 3.9 | 67 | 3.6 | 64 | 6.5 | |
| 62.5 | 87 | 2.2 | 86 | 6.0 | 72 | 7.5 | |
| FB3 | 12.5 | 64 | 4.4 | 65 | 1.4 | 64 | 6.9 |
| 25 | 76 | 9.5 | 69 | 0.3 | 65 | 9.7 | |
| 62.5 | 66 | 5.1 | 64 | 4.9 | 65 | 7.7 | |
| DON | 350 | 67 | 1.2 | 64 | 2.6 | 66 | 1.1 |
| 700 | 68 | 2.8 | 67 | 3.8 | 68 | 7.9 | |
| 1750 | 67 | 2.7 | 69 | 4.6 | 76 | 5.8 | |
| DOM | 300 | 65 | 1.8 | 66 | 1.3 | 65 | 1.4 |
| 600 | 67 | 3.6 | 81 | 1.9 | 81 | 7.8 | |
| 1500 | 75 | 1.1 | 75 | 2.1 | 69 | 1.0 | |
| 15-ADON | 300 | 63 | 3.1 | 69 | 1.1 | 94 | 9.2 |
| 600 | 68 | 7.9 | 74 | 3.1 | 111 | 5.3 | |
| 1500 | 68 | 3.2 | 69 | 5.3 | 71 | 4.3 | |
| 3-ADON | 300 | 63 | 3.1 | 69 | 1.1 | 93 | 3.1 |
| 600 | 68 | 7.9 | 73 | 3.1 | 111 | 5.8 | |
| 1500 | 68 | 3.2 | 69 | 5.3 | 78 | 3.8 | |
| α-ZAL | 12.5 | 95 | 2.1 | 77 | 2.5 | 67 | 7.3 |
| 25 | 64 | 5.3 | 87 | 1.1 | 86 | 5.1 | |
| 62.5 | 86 | 1.6 | 88 | 2.7 | 63 | 4.1 | |
| β-ZAL | 12.5 | 94 | 2.0 | 76 | 2.4 | 65 | 6.6 |
| 25 | 66 | 5.3 | 87 | 1.1 | 80 | 3.8 | |
| 62.5 | 83 | 1.5 | 88 | 2.5 | 62 | 3.8 | |
| α-ZOL | 12.5 | 86 | 3.2 | 83 | 3.0 | 62 | 4.5 |
| 25 | 64 | 2.3 | 85 | 1.3 | 72 | 5.5 | |
| 62.5 | 66 | 2.0 | 81 | 1.8 | 64 | 6.5 | |
| β-ZOL | 12.5 | 71 | 1.9 | 79 | 3.1 | 66 | 8.4 |
| 25 | 75 | 9.4 | 95 | 1.0 | 83 | 6.7 | |
| 62.5 | 87 | 3.4 | 95 | 0.8 | 65 | 5.7 | |
| ZAN | 12.5 | 76 | 5.1 | 85 | 2.4 | 64 | 7.7 |
| 25 | 71 | 10.0 | 69 | 2.0 | 76 | 5.5 | |
| 62.5 | 72 | 4.0 | 69 | 1.9 | 62 | 3.5 | |
| ZEN | 12.5 | 84 | 0.6 | 78 | 0.2 | 64 | 5.0 |
| 25 | 96 | 2.1 | 68 | 0.4 | 77 | 3.6 | |
| 62.5 | 79 | 8.2 | 71 | 0.6 | 68 | 4.6 | |
| OTA | 3 | 83 | 3.7 | 74 | 3.5 | 70 | 6.5 |
| 6 | 69 | 3.7 | 102 | 2.3 | 85 | 5.5 | |
| 15 | 95 | 5.1 | 61 | 1.4 | 66 | 7.5 | |
| OTB | 3.75 | 66 | 2.3 | 66 | 1.1 | 77 | 6.1 |
| 7.5 | 66 | 8.1 | 73 | 0.9 | 72 | 5.7 | |
| 18.75 | 70 | 5.9 | 71 | 0.7 | 89 | 4.7 | |
| Mycotoxins | Chicken Breast | Chicken Gizzard | Chicken Liver | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Linear Range (μg/L) | Linear Equation | Correlation Coefficient (R2) | LOD (μg/kg) | LOQ (μg/kg) | Linear Range (μg/L) | Linear Equation | Correlation Coefficient (R2) | LOD (μg/kg) | LOQ (μg/kg) | Linear Range (μg/L) | Linear Equation | Correlation Coefficient (R2) | LOD (μg/kg) | LOQ (μg/kg) | |
| AFB1 | 15–600 | Y = 1.774 × 107X + 1.826 × 108 | 0.994 | 2.5 | 7.5 | 10–400 | Y = 6.18 × 107X − 5.973 × 107 | 0.994 | 2 | 6 | 10–400 | Y = 1.115 × 107X + 1.125 × 108 | 0.99 | 1.6 | 5 |
| AFB2 | 3.75–150 | Y = 3.381 × 106X + 2.306 × 106 | 0.999 | 0.6 | 1.8 | 2.5–100 | Y = 5.679 × 106X − 1.568 × 107 | 0.995 | 0.5 | 1.5 | 2.5–100 | Y = 2.18 × 106X − 5.715 × 106 | 0.999 | 0.4 | 1.2 |
| AFG1 | 15–600 | Y = 1.189 × 107X + 4.353 × 106 | 0.999 | 2.5 | 7.5 | 10–400 | Y = 7.338 × 106X − 1.415 × 107 | 0.998 | 2 | 6 | 10–400 | Y = 8.478 × 106X + 7.596 × 107 | 0.993 | 2 | 6 |
| AFG2 | 3.75–150 | Y = 2.401 × 107X − 3.781 × 104 | 0.999 | 0.6 | 1.8 | 2.5–100 | Y = 7.178 × 107X − 1.472 × 107 | 0.995 | 0.5 | 1.5 | 2.5–100 | Y = 8.034 × 106X + 1.568 × 107 | 0.994 | 0.5 | 1.5 |
| AFM1 | 25–1000 | Y = 1.189 × 107X + 2.323 × 107 | 0.999 | 4.1 | 12.5 | 12.5–500 | Y = 2.956 × 107X − 1.481 × 107 | 0.997 | 3 | 9 | 25–1000 | Y = 9.161 × 106X − 3.877 × 107 | 0.998 | 4.1 | 12.5 |
| AFM2 | 20–800 | Y = 3.995 × 106X + 3.346 × 106 | 0.999 | 3.33 | 10 | 10–400 | Y = 8.977 × 106X − 9.662 × 104 | 0.998 | 2.6 | 8 | 20–800 | Y = 3.253 × 106X − 1.48 × 107 | 0.999 | 3.33 | 10 |
| T-2 | 75–3000 | Y = 5.261 × 106X − 8.745 × 105 | 0.999 | 12.5 | 37.5 | 37–1500 | Y = 3.966 × 106X + 6.703 × 106 | 0.999 | 11 | 33 | 75–3000 | Y = 3.699 × 106X + 3.321 × 106 | 0.99 | 12.5 | 37.5 |
| HT-2 | 105–4200 | Y = 6.596 × 105X − 4.328 × 105 | 0.999 | 17.5 | 52.5 | 52–2100 | Y = 1.019 × 106X − 9.894 × 105 | 0.992 | 16 | 48 | 105–4200 | Y = 1.244 × 106X − 1.584 × 106 | 0.999 | 17.5 | 52.5 |
| FB1 | 12.5–500 | Y = 1.496 × 107X − 2.147 × 107 | 0.997 | 3 | 9 | 12.5–500 | Y = 1.781 × 107X − 2.425 × 107 | 0.99 | 3 | 9 | 25–1000 | Y = 2.81 × 107X − 1.435 × 107 | 0.995 | 4.17 | 12.5 |
| FB2 | 12.5–500 | Y = 1.239 × 107X − 1.408 × 107 | 0.997 | 3 | 9 | 12.5–500 | Y = 1.539 × 107X − 1.507 × 107 | 0.994 | 3.3 | 10 | 25–1000 | Y = 2.66 × 106X − 1.205 × 107 | 0.992 | 4.17 | 12.5 |
| FB3 | 12.5–500 | Y = 1.781 × 107X − 1.608 × 107 | 0.999 | 3 | 9 | 12.5–500 | Y = 4.025 × 107X + 2.219 × 107 | 0.994 | 3.3 | 10 | 25–1000 | Y = 2.959 × 106X + 941 × 106 | 0.997 | 4.17 | 12.5 |
| DON | 700–28,000 | Y = 1.773 × 105X + 1.106 × 108 | 0.991 | 130 | 350 | 350–14,000 | Y = 1.986 × 105X + 1.826 × 108 | 0.993 | 100 | 300 | 700–28,000 | Y = 2.81 × 105X + 3.715 × 107 | 0.994 | 130 | 350 |
| DOM | 600–24,000 | Y = 7.032 × 105X + 4.598 × 108 | 0.996 | 100 | 300 | 300–12,000 | Y = 3.986 × 105X + 2.436 × 108 | 0.995 | 93 | 280 | 300–12,000 | Y = 4.19 × 105X + 2.715 × 108 | 0.991 | 93 | 280 |
| 15-AC-DON | 600–24,000 | Y = 3.089 × 106X + 1.774 × 108 | 0.999 | 100 | 300 | 300–12,000 | Y = 8.444 × 106X + 3.126 × 107 | 0.993 | 93 | 280 | 300–12,000 | Y = 7.35 × 106X + 5.715 × 107 | 0.992 | 93 | 280 |
| 3-AC-DON | 600–24,000 | Y = 3.089 × 106X + 1.774 × 108 | 0.999 | 100 | 300 | 300–12,000 | Y = 7.544 × 106X + 3.126 × 107 | 0.993 | 93 | 280 | 300–12,000 | Y = 8.19 × 106X + 5.715 × 107 | 0.996 | 93 | 280 |
| α-ZAL | 12.5–500 | Y = 1.464 × 107X − 4.241 × 107 | 0.993 | 3.75 | 11 | 25–1000 | Y = 2.452 × 107X − 2.507 × 107 | 0.99 | 4.1 | 12.5 | 12.5–500 | Y = 1.183 × 107X + 1.52 × 106 | 0.999 | 3.75 | 11 |
| β-ZAL | 12.5–500 | Y = 1.832 × 107X − 5.799 × 107 | 0.991 | 3 | 9 | 25–1000 | Y = 3.451 × 107X − 2.62 × 107 | 0.99 | 4.1 | 12.5 | 12.5–500 | Y = 3.541 × 107X − 2.079 × 107 | 0.996 | 3.75 | 11 |
| α-ZEL | 12.5–500 | Y = 2.086 × 107X − 6.629 × 107 | 0.993 | 3 | 9 | 25–1000 | Y = 4.01 × 107X − 1.722 × 107 | 0.999 | 4.1 | 12.5 | 12.5–500 | Y = 3.344 × 100X − 2.162 × 107 | 0.993 | 3.75 | 11 |
| β-ZEL | 12.5–500 | Y = 1.891 × 107X − 5.887 × 107 | 0.992 | 3 | 9 | 25–1000 | Y = 4.45 × 107X − 1.826 × 1026 | 0.994 | 4.1 | 12.5 | 12.5–500 | Y = 2.156 × 107X − 3.531 × 107 | 0.991 | 3.75 | 11 |
| ZAN | 12.5–500 | Y = 2.555 × 107X − 7.456 × 107 | 0.993 | 3 | 9 | 25–1000 | Y = 3.474 × 107X − 1.004 × 108 | 0.995 | 4.1 | 12.5 | 12.5–500 | Y = 5.146 × 107X − 2.078 × 106 | 0.997 | 3.75 | 11 |
| ZEN | 12.5–500 | Y = 2.361 × 107X − 6.68 × 107 | 0.994 | 3 | 9 | 25–1000 | Y = 1.636 × 107X − 5.971 × 107 | 0.9958 | 4.1 | 12.5 | 12.5–500 | Y = 4.224 × 107X − 3.686 × 106 | 0.9956 | 3.75 | 11 |
| OTA | 3–240 | Y = 4.359 × 106X − 7.068 × 106 | 0.992 | 0.6 | 2 | 3–240 | Y = 6.459 × 106X − 4.288 × 106 | 0.9989 | 0.6 | 2 | 6–480 | Y = 1.057 × 107X − 8.878 × 1025 | 0.9926 | 1 | 3 |
| OTB | 7.5–600 | Y = 4.373 × 106X − 1.36 × 107 | 0.991 | 1.25 | 3.75 | 3.75–300 | Y = 6.785 × 106X − 1.826 × 106 | 0.9943 | 1 | 3 | 3.75–300 | Y = 3.38 × 106X − 5.715 × 1026 | 0.9918 | 1 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; He, Z.; Mu, L.; Xie, Y.; Wang, L. Simultaneous Determination of 23 Mycotoxins in Broiler Tissues by Solid Phase Extraction UHPLC-Q/Orbitrap High Resolution Mass Spectrometry. Separations 2021, 8, 236. https://doi.org/10.3390/separations8120236
Yang Y, He Z, Mu L, Xie Y, Wang L. Simultaneous Determination of 23 Mycotoxins in Broiler Tissues by Solid Phase Extraction UHPLC-Q/Orbitrap High Resolution Mass Spectrometry. Separations. 2021; 8(12):236. https://doi.org/10.3390/separations8120236
Chicago/Turabian StyleYang, Youyou, Zhuolin He, Lei Mu, Yunfeng Xie, and Liang Wang. 2021. "Simultaneous Determination of 23 Mycotoxins in Broiler Tissues by Solid Phase Extraction UHPLC-Q/Orbitrap High Resolution Mass Spectrometry" Separations 8, no. 12: 236. https://doi.org/10.3390/separations8120236
APA StyleYang, Y., He, Z., Mu, L., Xie, Y., & Wang, L. (2021). Simultaneous Determination of 23 Mycotoxins in Broiler Tissues by Solid Phase Extraction UHPLC-Q/Orbitrap High Resolution Mass Spectrometry. Separations, 8(12), 236. https://doi.org/10.3390/separations8120236






