Metal-Organic Frameworks in Bioanalysis: Extraction of Small Organic Molecules
Abstract
:1. Introduction
2. Preparation and Characterization Strategies for MOFs
3. Bioanalytical Applications
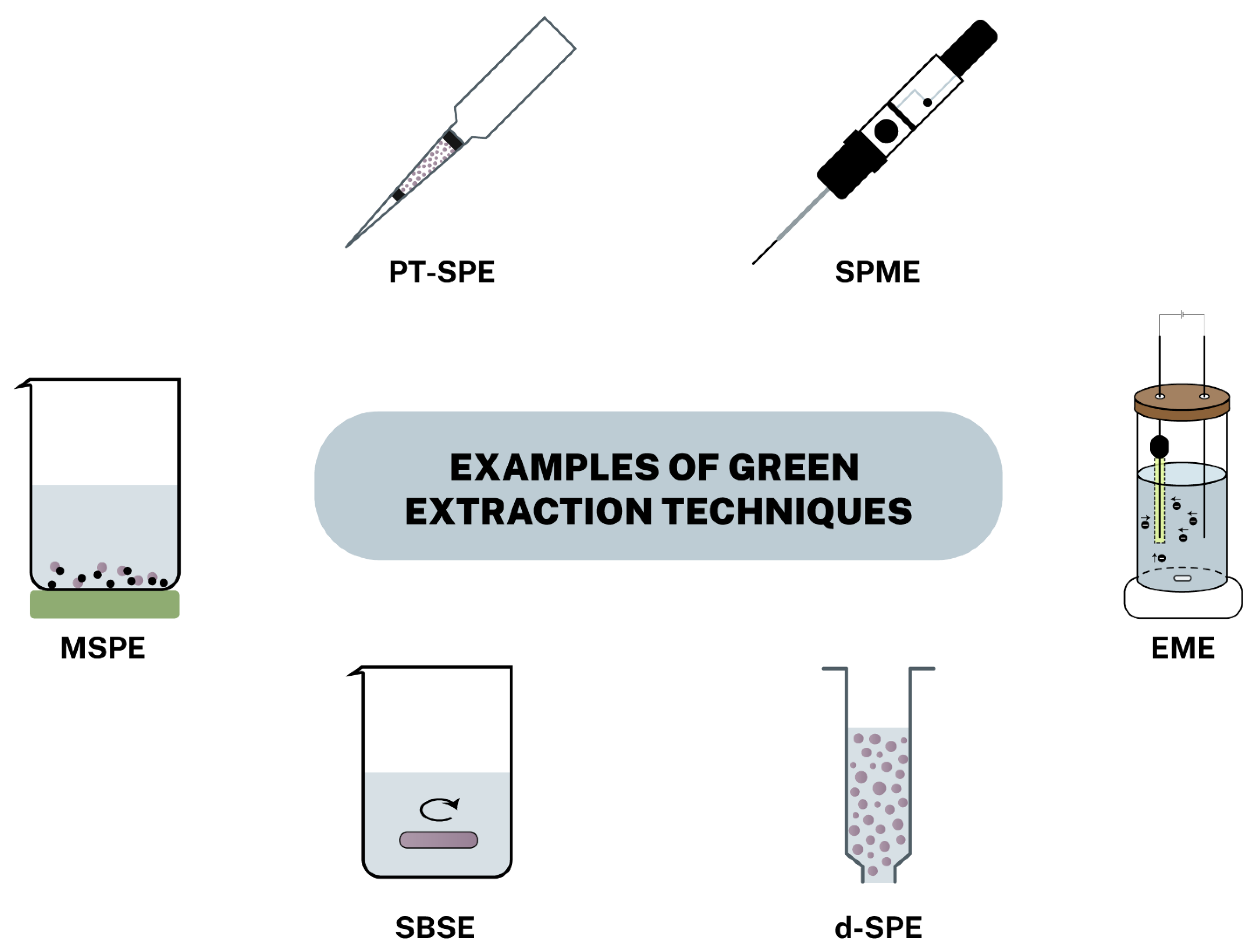
3.1. Dispersive Solid-Phase Extraction
3.2. Magnetic Solid-Phase Extraction
3.3. Solid-Phase Microextraction
3.4. Stir Bar Sorptive Extraction
3.5. Pipette Tip SPE
3.6. Other Extraction Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Bartlett, M.G. A review of sample preparation methods for quantitation of small-molecule analytes in brain tissue by liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal. Methods 2014, 6, 6183–6207. [Google Scholar] [CrossRef]
- Manousi, N.; Raber, G.; Papadoyannis, I. Recent Advances in Microextraction Techniques of Antipsychotics in Biological Fluids Prior to Liquid Chromatography Analysis. Separations 2017, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Drummer, O.H. Drug testing in oral fluid. Clin. Biochem. Rev. 2006, 27, 147–159. [Google Scholar]
- Kovatsi, L.; Titopoulou, A.; Tsakalof, A.; Samanidou, V. HPLC Analysis of Antipsychotic Asenapine in Alternative Biomatrices: Hair and Nail Clippings. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1666–1670. [Google Scholar] [CrossRef]
- Livadiotou, D.; Samanidou, V. Dried blood spot sampling for therapeutic drug monitoring. New Sampl. Strateg. Toxicol. Ther. Drug Monit. 2015, 67–78. [Google Scholar]
- Xu, R.N.; Fan, L.; Rieser, M.J.; El-Shourbagy, T.A. Recent advances in high-throughput quantitative bioanalysis by LC-MS/MS. J. Pharm. Biomed. Anal. 2007, 44, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M.; Bielenstein, M.; Arvidsson, T. Evaluation of solid-phase microextraction in combination with gas chromatography (SPME-GC) as a tool for quantitative bioanalysis. J. Microcolumn Sep. 2000, 12, 308–315. [Google Scholar] [CrossRef]
- Kindt, E.K.; Kurzyniec, S.; Wang, S.C.; Kilby, G.; Rossi, D.T. Quantitative bioanalysis of enantiomeric drugs using capillary electrophoresis and electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2003, 31, 893–904. [Google Scholar] [CrossRef]
- James, C.A.; Breda, M.; Baratt, S.; Casati, M.; Grassi, S.; Pellegatta, B.; Sarati, S.; Frigerio, E. Analysis of Drugs and Metabolites in Tissues and Other Solid Matrices. Chromatographia 2004, 59, S149–S156. [Google Scholar] [CrossRef]
- Kataoka, H. New trends in sample preparation for clinical and pharmaceutical analysis. TrAC Trends Anal. Chem. 2003, 22, 232–244. [Google Scholar] [CrossRef]
- Manousi, N.; Samanidou, V.F. Applications of gas chromatography for the analysis of tricyclic antidepressants in biological matrices. Separations 2019, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Samanidou, V.; Nika, M.; Papadoyannis, I. HPLC as a Tool in Medicinal Chemistry for the Monitoring of Tricyclic Antidepressants in Biofluids. Mini-Rev. Med. Chem. 2008, 8, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 44–62. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Liu, H.; Dasgupta, P.K. Analytical chemistry in a drop. solvent extraction in a microdrop. Anal. Chem. 1996, 68, 1817–1821. [Google Scholar] [CrossRef]
- Manousi, N.; Gomez-Gomez, B.; Madrid, Y.; Deliyanni, E.A.; Zachariadis, G.A. Determination of rare earth elements by inductively coupled plasma-mass spectrometry after dispersive solid phase extraction with novel oxidized graphene oxide and optimization with response surface methodology and central composite design. Microchem. J. 2020, 152, 104428. [Google Scholar] [CrossRef]
- Filippou, O.; Deliyanni, E.A.; Samanidou, V.F. Fabrication and evaluation of magnetic activated carbon as adsorbent for ultrasonic assisted magnetic solid phase dispersive extraction of bisphenol A from milk prior to high performance liquid chromatographic analysis with ultraviolet detection. J. Chromatogr. A 2017, 1479, 20–31. [Google Scholar] [CrossRef]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Myridakis, A.; Stephanou, E.G.; Samanidou, V. Multiresidue LC-MS/MS analysis of cephalosporins and quinolones in milk following ultrasound-assisted matrix solid-phase dispersive extraction combined with the quick, easy, cheap, effective, rugged, and safe methodology. J. Sep. Sci. 2013, 36, 2020–2027. [Google Scholar] [CrossRef]
- Nazyropoulou, C.; Samanidou, V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 2015, 7, 2241–2250. [Google Scholar] [CrossRef]
- Luo, Y.B.; Ma, Q.; Feng, Y.Q. Stir rod sorptive extraction with monolithic polymer as coating and its application to the analysis of fluoroquinolones in honey sample. J. Chromatogr. A 2010, 1217, 3583–3589. [Google Scholar] [CrossRef]
- Shen, Q.; Dong, W.; Wang, Y.; Gong, L.; Dai, Z.; Cheung, H.Y. Pipette tip solid-phase extraction and ultra-performance liquid chromatography/mass spectrometry based rapid analysis of picrosides from Picrorhiza scrophulariiflora. J. Pharm. Biomed. Anal. 2013, 80, 136–140. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Yuan, D. Preparation of solid-phase microextraction fiber coated with single-walled carbon nanotubes by electrophoretic deposition and its application in extracting phenols from aqueous samples. J. Chromatogr. A 2009, 1216, 1305–1311. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Kechagia, M.; Samanidou, V.; Kabir, A.; Furton, K.G. One-pot synthesis of a multi-template molecularly imprinted polymer for the extraction of six sulfonamide residues from milk before high-performance liquid chromatography with diode array detection. J. Sep. Sci. 2018, 41, 723–731. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A.; Samanidou, V.F. Applications of metal-organic frameworks in food sample preparation. Molecules 2018, 23, 2896. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wu, Q.; Gao, J.; Li, H.; Dong, S.; Shi, X.; Zhao, L. Applications of covalent organic frameworks in analytical chemistry. TrAC Trends Anal. Chem. 2019, 113, 182–193. [Google Scholar] [CrossRef]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A.; Deliyanni, E.A. On the use of metal-organic frameworks for the extraction of organic compounds from environmental samples. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.R.; Li, J.R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.C. Potential applications of metal-organic frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 1–29. [Google Scholar] [CrossRef]
- Chowdhury, M.A. Metal-Organic-Frameworks as Contrast Agents in Magnetic Resonance Imaging. ChemBioEng Rev. 2017, 4, 225–239. [Google Scholar] [CrossRef]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Almeida Paz, F.A. Metal-organic frameworks: A future toolbox for biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z. Metal-organic frameworks as stationary phase for application in chromatographic separation. J. Chromatogr. A 2017, 1530, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.M.; Zhang, Z.J.; Wang, Z.Y.; Yuan, L.M. Chiral metal-organic frameworks for high-resolution gas chromatographic separations. J. Am. Chem. Soc. 2011. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of metal ions with metal–organic frameworks. Molecules 2019, 24, 4605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, W.; Li, X.; Bai, Y.; Liu, H. Applications of metal-organic frameworks as advanced sorbents in biomacromolecules sample preparation. TrAC Trends Anal. Chem. 2018, 109, 154–162. [Google Scholar] [CrossRef]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–organic frameworks as key materials for solid-phase microextraction devices—A review. Separations 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are metal-organic frameworks able to provide a new generation of solid-phase microextraction coatings?—A review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef]
- Wang, Y.; Rui, M.; Lu, G. Recent applications of metal–organic frameworks in sample pretreatment. J. Sep. Sci. 2018, 41, 180–194. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Termopoli, V. Metal–Organic Frameworks in Solid-Phase Extraction Procedures for Environmental and Food Analyses. Chromatographia 2019, 82, 1191–1205. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; González-Hernández, P.; Pino, V.; Pasán, J.; Afonso, A.M. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. TrAC Trends Anal. Chem. 2017, 90, 114–134. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-organic frameworks in green analytical chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K.H. Metal-organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. TrAC Trends Anal. Chem. 2017, 97, 65–82. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Li, H.; Bai, Y.; Liu, H. Metal-organic frameworks as advanced sorbents in sample preparation for small organic analytes. Coord. Chem. Rev. 2019, 397, 1–13. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Wang, X.; Ye, N. Recent advances in metal-organic frameworks and covalent organic frameworks for sample preparation and chromatographic analysis. Electrophoresis 2017, 38, 3059–3078. [Google Scholar] [CrossRef]
- Bashir, K.; Chen, G.; Han, J.; Shu, H.; Cui, X.; Wang, L.; Li, W.; Fu, Q. Preparation of magnetic metal organic framework and development of solid phase extraction method for simultaneous determination of fluconazole and voriconazole in rat plasma samples by HPLC. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1152, 122201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiao, Z.; Yao, W. A simple solvothermal process for fabrication of a metal-organic framework with an iron oxide enclosure for the determination of organophosphorus pesticides in biological samples. J. Chromatogr. A 2014. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Chen, Y.J.; Jiang, J.Q.; Yan, X.P. Metal-organic frameworks for efficient enrichment of peptides with simultaneous exclusion of proteins from complex biological samples. Chem. Commun. 2011, 47, 4787–4789. [Google Scholar] [CrossRef]
- Yu, L.Q.; Su, F.H.; Ma, M.Y.; Lv, Y.K. Metal-organic frameworks for the sorption of acetone and isopropanol in exhaled breath of diabetics prior to quantitation by gas chromatography. Microchim. Acta 2019, 186, 588. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, S.; Jia, J.; Xu, F.; Long, Z.; Hou, X. Ultrasensitive determination of inorganic arsenic by hydride generation-atomic fluorescence spectrometry using Fe3O4@ZIF-8 nanoparticles for preconcentration. Microchem. J. 2016, 124, 578–583. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [Green Version]
- Ge, D.; Lee, H.K. Water stability of zeolite imidazolate framework 8 and application to porous membrane-protected micro-solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2011, 1218, 8490–8495. [Google Scholar] [CrossRef]
- Parisis, N.A.; Giokas, D.L.; Vlessidis, A.G.; Evmiridis, N.P. Concentration of organic compounds in natural waters with solid-phase dispersion based on advesicle modified silica prior to liquid chromatography. J. Chromatogr. A 2005, 1097, 17–24. [Google Scholar] [CrossRef]
- Román, I.P.; Chisvert Alberto, A.; Canals, A. Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography-mass spectrometry for UV-filter determination in water samples. J. Chromatogr. A 2011, 1218, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Rocío-Bautista, P.; Martínez-Benito, C.; Pino, V.; Pasán, J.; Ayala, J.H.; Ruiz-Pérez, C.; Afonso, A.M. The metal-organic framework HKUST-1 as efficient sorbent in a vortex-assisted dispersive micro solid-phase extraction of parabens from environmental waters, cosmetic creams, and human urine. Talanta 2015, 139, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Li, S.; Li, S.; Wang, Y.; Zhao, P.; Zhang, X.; Hou, X. A combination of computational−experimental study on metal-organic frameworks MIL-53(Al) as sorbent for simultaneous determination of estrogens and glucocorticoids in water and urine samples by dispersive micro-solid-phase extraction coupled to UPLC-MS/MS. Talanta 2018, 180, 358–367. [Google Scholar] [CrossRef]
- Qu, F.; Xia, L.; Wu, C.; Liu, L.; Li, G.; You, J. Sensitive and accurate determination of sialic acids in serum with the aid of dispersive solid-phase extraction using the zirconium-based MOF of UiO-66-NH2 as sorbent. RSC Adv. 2016, 6, 64895–64901. [Google Scholar] [CrossRef]
- Mohammadi, F.; Shabani, A.M.H.; Dadfarnia, S.; Ansari, M.; Asgharinezhad, A.A. Dispersive solid-phase extraction of buprenorphine from biological fluids using metal-organic frameworks and its determination by ultra-performance liquid chromatography. J. Sep. Sci. 2020, 43, 3045–3052. [Google Scholar] [CrossRef] [PubMed]
- Taghvimi, A.; Tabrizi, A.B.; Dastmalchi, S.; Javadzadeh, Y. Metal organic framework based carbon porous as an efficient dispersive solid phase extraction adsorbent for analysis of methamphetamine from urine matrix. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1109, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Rosenberg, E.; Deliyanni, E.; Zachariadis, G.A.; Samanidou, V. Magnetic Solid-Phase Extraction of Organic Compounds Based on Graphene Oxide Nanocomposites. Molecules 2020, 25, 1148. [Google Scholar] [CrossRef] [Green Version]
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Du, J.; Wu, D.; Liu, J.; Li, N.; Sun, Z.; Li, G.; Wu, Y. Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC Trends Anal. Chem. 2018, 108, 154–166. [Google Scholar] [CrossRef]
- Maya, F.; Palomino Cabello, C.; Frizzarin, R.M.; Estela, J.M.; Turnes Palomino, G.; Cerdà, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. TrAC Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Dargahi, R.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Hashemzadeh, A.; Amini, M.M. Dispersive magnetic solid-phase extraction of phthalate esters from water samples and human plasma based on a nanosorbent composed of MIL-101(Cr) metal–organic framework and magnetite nanoparticles before their determination by GC–MS. J. Sep. Sci. 2018, 41, 948–957. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, M.; Ji, Q.; Wang, M.; Wang, Q.; Wang, X.; Hao, Y. Fast magnetic solid-phase extraction using an Fe3O4-NH2@MOF material for monohydroxy polycyclic aromatic hydrocarbons in urine of coke-oven workers. Anal. Methods 2020, 12, 2872–2880. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lv, B.; Jiang, H.; Yuan, P.; Zhu, H.; Gao, B. Determination of diuretics in human urine using HPLC coupled with magnetic solid-phase extraction based on a metal–organic framework. Biomed. Chromatogr. 2020, 34, e4876. [Google Scholar] [CrossRef] [PubMed]
- Rahimpoor, R.; Bahrami, A.; Nematollahi, D.; Ghorbani Shahna, F.; Farhadian, M. Sensitive determination of urinary muconic acid using magnetic dispersive-solid-phase extraction by magnetic amino-functionalised UiO. Int. J. Environ. Anal. Chem. 2020, 00, 1–14. [Google Scholar] [CrossRef]
- Safari, M.; Shahlaei, M.; Yamini, Y.; Shakorian, M.; Arkan, E. Magnetic framework composite as sorbent for magnetic solid phase extraction coupled with high performance liquid chromatography for simultaneous extraction and determination of tricyclic antidepressants. Anal. Chim. Acta 2018, 1034, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Khezeli, T.; Daneshfar, A. Dispersive micro-solid-phase extraction of dopamine, epinephrine and norepinephrine from biological samples based on green deep eutectic solvents and Fe3O4@MIL-100 (Fe) core-shell nanoparticles grafted with pyrocatechol. RSC Adv. 2015, 5, 65264–65273. [Google Scholar] [CrossRef]
- Bahrani, S.; Ghaedi, M.; Dashtian, K.; Ostovan, A.; Mansoorkhani, M.J.K.; Salehi, A. MOF-5(Zn)-Fe2O4 nanocomposite based magnetic solid-phase microextraction followed by HPLC-UV for efficient enrichment of colchicine in root of colchicium extracts and plasma samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1067, 45–52. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, W.; Fu, D.; Zhang, C.; Zhao, H. Fabrication of magnetic zinc adeninate metal–organic frameworks for the extraction of benzodiazepines from urine and wastewater. J. Sep. Sci. 2018, 41, 1864–1870. [Google Scholar] [CrossRef]
- Pourbahman, F.; Zeeb, M.; Monzavi, A.; Homami, S.S. Simultaneous trace monitoring of prokinetic drugs in human plasma using magnetic dispersive micro-solid phase extraction based on a new graphene oxide/metal–organic framework-74/Fe3O4/polytyramine nanoporous composite in combination with HPLC. Chem. Pap. 2019, 73, 3135–3150. [Google Scholar] [CrossRef]
- Peng, J.; Tian, H.; Du, Q.; Hui, X.; He, H. A regenerable sorbent composed of a zeolite imidazolate framework (ZIF-8), Fe3O4 and graphene oxide for enrichment of atorvastatin and simvastatin prior to their determination by HPLC. Microchim. Acta 2018, 185, 2–10. [Google Scholar] [CrossRef]
- Hua, X.; Gao, G.; Pan, S. High-affinity graphene oxide-encapsulated magnetic Zr-MOF for pretreatment and rapid determination of the photosensitizers hematoporphyrin and hematoporphyrin monomethyl ether in human urine prior to UPLC-HRMS. Anal. Bioanal. Chem. 2018, 410, 7749–7764. [Google Scholar] [CrossRef]
- Bitas, D.; Samanidou, V. Molecularly imprinted polymers as extracting media for the chromatographic determination of antibiotics in milk. Molecules 2018, 23, 316. [Google Scholar] [CrossRef] [Green Version]
- Asfaram, A.; Ghaedi, M.; Dashtian, K. Rapid ultrasound-assisted magnetic microextraction of gallic acid from urine, plasma and water samples by HKUST-1-MOF-Fe3O4-GA-MIP-NPs: UV–vis detection and optimization study. Ultrason. Sonochem. 2017, 34, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Hao, L.; Wang, J.; Wang, C.; Wu, Q.; Wang, Z. Magnetic porous carbon derived from a metal–organic framework as a magnetic solid-phase extraction adsorbent for the extraction of sex hormones from water and human urine. J. Sep. Sci. 2016, 39, 3571–3577. [Google Scholar] [CrossRef]
- Wu, W.; Lin, F.; Yang, X.; Wang, B.; Lu, X.; Chen, Q.; Ye, F.; Zhao, S. Facile synthesis of magnetic carbon nanotubes derived from ZIF-67 and application to magnetic solid-phase extraction of profens from human serum. Talanta 2020, 207, 120284. [Google Scholar] [CrossRef]
- Chen, C.; Liang, X.; Wang, J.; Yang, S.; Yan, Z.; Cai, Q.; Yao, S. Development of a highly robust solid phase microextraction fiber based on crosslinked methyl methacrylate-polyhedral oligomeric silsesquioxane hybrid polymeric coating. Anal. Chim. Acta 2013, 792, 45–51. [Google Scholar] [CrossRef]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Preparation and characterization of magnetic metal–organic framework nanocomposite as solid-phase microextraction fibers coupled with high-performance liquid chromatography for determination of non-steroidal anti-inflammatory drugs in biological fluids an. Microchem. J. 2019, 144, 270–284. [Google Scholar] [CrossRef]
- Wu, M.; Ai, Y.; Zeng, B.; Zhao, F. In situ solvothermal growth of metal-organic framework-ionic liquid functionalized graphene nanocomposite for highly efficient enrichment of chloramphenicol and thiamphenicol. J. Chromatogr. A 2016, 1427, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kissoudi, M.; Samanidou, V. Recent advances in applications of ionic liquids in miniaturized microextraction techniques. Molecules 2018, 23, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, N.; Gu, Z.Y.; Wang, H.F.; Yan, X.P. Metal-organic-framework-based tandem molecular sieves as a dual platform for selective microextraction and high-resolution gas chromatographic separation of n -alkanes in complex matrixes. Anal. Chem. 2011, 83, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Manousi, N.; Tzanavaras, P.D.; Zacharis, C.K. Bioanalytical HPLC Applications of In-Tube Solid. Molecules 2020, 25, 2096. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Song, C.; Liao, J.; Huang, Z.; Li, G. Water stable metal-organic framework packed microcolumn for online sorptive extraction and direct analysis of naproxen and its metabolite from urine sample. J. Chromatogr. A 2013, 1294, 17–24. [Google Scholar] [CrossRef]
- Lyu, D.Y.; Yang, C.X.; Yan, X.P. Fabrication of aluminum terephthalate metal-organic framework incorporated polymer monolith for the microextraction of non-steroidal anti-inflammatory drugs in water and urine samples. J. Chromatogr. A 2015, 1383, 1–7. [Google Scholar] [CrossRef]
- Luo, X.; Li, G.; Hu, Y. In-tube solid-phase microextraction based on NH2-MIL-53(Al)-polymer monolithic column for online coupling with high-performance liquid chromatography for directly sensitive analysis of estrogens in human urine. Talanta 2017, 165, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kremser, A.; Jochmann, M.A.; Schmidt, T.C. PAL SPME Arrow—Evaluation of a novel solid-phase microextraction device for freely dissolved PAHs in water. Anal. Bioanal. Chem. 2016, 408, 943–952. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Lin, X.; Li, T.; Pang, T.; Dong, Y.; Zhuo, R.; Wang, Q.; Cao, Y.; Gan, N. A solid phase microextraction Arrow with zirconium metal–organic framework/molybdenum disulfide coating coupled with gas chromatography–mass spectrometer for the determination of polycyclic aromatic hydrocarbons in fish samples. J. Chromatogr. A 2019, 1592, 9–18. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Manousi, N.; Zachariadis, G.A. Recent Advances in the Extraction of Polycyclic Aromatic Hydrocarbons from Environmental Samples. Molecules 2020, 25, 2182. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, W.; Liao, X.; Wang, X.; Chen, Z. Covalent immobilization of metal organic frameworks onto chemical resistant poly(ether ether ketone) jacket for stir bar extraction. Anal. Chim. Acta 2018, 1025, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Ghoreishi, S.M.; Shahin, M.; Azamati, M. Zeolitic imidazole framework templated synthesis of nanoporous carbon as a coating for stir bar sorptive extraction of fluorouracil and phenobarbital in human body fluids. Microchem. J. 2019, 146, 798–806. [Google Scholar] [CrossRef]
- Rezaei Kahkha, M.R.; Oveisi, A.R.; Kaykhaii, M.; Rezaei Kahkha, B. Determination of carbamazepine in urine and water samples using amino-functionalized metal–organic framework as sorbent. Chem. Cent. J. 2018, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordin, D.C.M.; Alves, M.N.R.; De Campos, E.G.; De Martinis, B.S. Disposable pipette tips extraction: Fundamentals, applications and state of the art. J. Sep. Sci. 2016, 39, 1168–1172. [Google Scholar] [CrossRef]
- Rezaei Kahkha, M.R.; Kaykhaii, M.; Sargazi, G.; Kahkha, B.R. Determination of nicotine in saliva, urine and wastewater samples using tantalum metal organic framework pipette tip micro-solid phase extraction. Anal. Methods 2019, 11, 6168–6175. [Google Scholar] [CrossRef]
- Rahimpoor, R.; Bahrami, A.; Nematollahi, D.; Ghorbani Shahna, F.; Farhadian, M. Application of zirconium-based metal–organic frameworks for micro-extraction by packed sorbent of urinary trans, trans-muconic acid. J. Iran. Chem. Soc. 2020, 17, 2345–2358. [Google Scholar] [CrossRef]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Microextraction by packed sorbent (MEPS). TrAC Trends Anal. Chem. 2015, 67, 34–44. [Google Scholar] [CrossRef]
- Drouin, N.; Kubáň, P.; Rudaz, S.; Pedersen-Bjergaard, S.; Schappler, J. Electromembrane extraction: Overview of the last decade. TrAC Trends Anal. Chem. 2019, 113, 357–363. [Google Scholar] [CrossRef]
- Fakhari, A.R.; Asadi, S.; Kosalar, H.M.; Sahragard, A.; Hashemzadeh, A.; Amini, M.M. Metal-organic framework enhanced electromembrane extraction-a conceptual study using basic drugs as model substances. Anal. Methods 2017, 9, 5646–5652. [Google Scholar] [CrossRef]
- Gao, G.; Li, S.; Li, S.; Zhao, L.; Wang, T.; Hou, X. Development and application of vortex-assisted membrane extraction based on metal–organic framework mixed-matrix membrane for the analysis of estrogens in human urine. Anal. Chim. Acta 2018, 1023, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Arabsorkhi, B.; Sereshti, H.; Abbasi, A. Electrospun metal-organic framework/polyacrylonitrile composite nanofibrous mat as a microsorbent for the extraction of tetracycline residue in human blood plasma. J. Sep. Sci. 2019, 42, 1500–1508. [Google Scholar] [CrossRef]
- Asiabi, M.; Mehdinia, A.; Jabbari, A. Preparation of water stable methyl-modified metal—organic framework-5/polyacrylonitrile composite nanofibers via electrospinning and their application for solid-phase extraction of two estrogenic drugs in urine samples. J. Chromatogr. A 2015, 1426, 24–32. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzdeh, H.; Seidi, S.; Jalilian, N. Preparation of electrospun polyacrylonitrile/Ni-MOF-74 nanofibers for extraction of atenolol and captopril prior to HPLC-DAD. Microchim. Acta 2020, 187, 508. [Google Scholar] [CrossRef]




| Analyte | Sample Matrix | MOF Sorbent | Analytical Technique | Sorbent Amount (mg) | Extraction Recovery (%) | LODs (ng mL−1) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|
| d-SPE | ||||||||
| Parabens | Water, urine and cosmetics | HKUST-1 | HPLC-DAD | 150 | - | 0.1–0.6 | - | [59] |
| Estrogens and glucocorticoids | Water, urine | MIL-53(Al) | UHPLC-MS/MS | 8 | - | 0.005–1.8 | ≤10 times | [60] |
| Sialic acids | Serum | UiO-66-NH2 | HPLC-FLD | 8 | - | 0.11–0.16 | ≤8 times | [61] |
| Buprenorphine | Urine, plasma | ZIF-67 | HPLC-UV | 10 | 96.2 | 0.15 | - | [62] |
| Methamphetamine | Urine | ZIF-8 derived carboxylated carbon porous | HPLC-UV | 40 | - | 10 | ≤8 times | [63] |
| MSPE | ||||||||
| Phthalate esters | Human plasma | Fe3O4@MIL-101(Cr) | GC-MS | 15 | - | 0.15 × 10−3 | - | [68] |
| Monohydroxy polycyclic aromatic hydrocarbons | Urine | Fe3O4-NH2@MIL-101(Cr) | HPLC-FLD | 0.5 | - | 0.016–0.042 | ≤10 times | [69] |
| OPPs | Human urine, hair | Fe3O4/MIL-101(Fe) | GC-FPD | 20 | >76.8 | 0.21–2.28 | ≥10 times | [51] |
| Diuretics | Urine | Fe3O@UiO-66-OH | HPLC-UV | 10 | 90.3–103.0 | 0.08–0.23 | - | [70] |
| Urinary muconic acid | Urine | Fe3O4-SiO2-NH2@UiO-66 | HPLC-UV | 20 | 96–98 | 0.001 | ≤times | [71] |
| Tricyclic antidepressants | Urine, plasma | Fe3O4@TMU-10 | HPLC-UV | 5 | 57.33–66.66 | 2–4 | ≥5 times | [72] |
| Dopamine, epinephrine and norepinephrine | Urine, serum | Fe3O4@MIL-100(Fe) | HPLC-UV | 22 | 91.4–103.4 | 0.22–0.36 | ≥6 times | [73] |
| Colchicine | Root of colchicium extracts and plasma | MOF-5(Zn)-Fe2O4 | HPLC-UV | 2.5 | - | 0.13 | - | [74] |
| Benzodiazepines | Urine, wastewater | Bio-MOF-1 | HPLC-MS | 15 | 84.1–94.4 | 0.71–2.49 × 10−3 | ≤10 times | [75] |
| Prokinetic drugs | Plasma | GO/MOF-74/Fe3O4/polytyramine | HPLC-UV | 15 | 88.0–90.0 | 0.4–1.1 | ≤15 times | [76] |
| Atorvastatin and simvastatin | Urine | Composite sorbent of ZIF-8, Fe3O4 and GO | HPLC-DAD | 25 | - | 0.116–0.387 | ≥7 times | [77] |
| Hematoporphyrin and hematoporphyrin monomethyl ether | Urine | GO-Mag@Zr-MOF | UPLC-HRMS | 5 | >98 | 0.036–0.042 | ≤8 times | [78] |
| Gallic acid | Urine, plasma and water | HKUST-1-MOF-Fe3O4-GA-MIP-NPs | UV-Vis | 1.6 | 98.13 (average) | 1.377 | ≥6 cycles | [80] |
| Sex hormones | Urine | MIL-53(Al) derived carbon | HPLC-UV | 15 | >94.6 | 0.1–0.3 | ≥10 times | [81] |
| Profens | Serum | Carbon nanotubes derived from ZIF-67 | HPLC-UV | 20 | - | 0.60 × 10−3 | ≥10 times | [82] |
| SPME | ||||||||
| NSAIDs | Serum, plasma, urine, tablet | Magnetic Copper 1,3,5-tricarboxylate MOF | HPLC-UV | - | 94.0–102.0 | 0.03–0.05 | ≥110 times | [84] |
| Chloramphenicl and thiamphenicol | Milk, honey, urine, serum | MOF-5 | GC-FID | - | - | 14.8–19.5 × 10−3 | ≤150 times | [85] |
| n-alkanes | Petroleum-based fuel and human serum | ZIF-8 | GC-MS | - | - | 0.46–1.06 × 10−3 | - | [87] |
| Naproxen and its metabolite | Huma Urine | MIL-101(Cr) | HPLC-FLD | - | >85.3 | 11–34 × 10−3 | - | [89] |
| NSAIDs | Urine | MIL-53(Al) | HPLC-DAD | 40 | 77.3–104 | 0.12–0.24 | ≤120 times | [90] |
| Estrogens | Urine | NH2-MIL-53(Al) | HPLC-UV/FLD | - | 75.1–120 | 0.002–0.040 | - | [91] |
| PT-SPE | ||||||||
| Parabens | Cosmetics and rabbit plasma | MIL-68 | HPLC-MS/MS | - | - | 0.001–0.002 | - | [96] |
| Fluorouracil and phonobarbital | Urine and plasma | ZIF-67 | HPLC-UV | - | - | 0.21–1.4 | ≤70 times | [97] |
| SBSE | ||||||||
| Carbamazepine | Urine | UiO-66-NH2 | HPLC-UV | 5 | - | 0.05 | ≥8 times | [98] |
| Nicotine | Saliva, urine and wastewater | Tantalum MOF | HPLC-UV | 5 | 92.8–111.3 | 0.7 | ≤11 times | [100] |
| Others | ||||||||
| Trans-muconic acid | Urine | UiO-66 (1), UiO-66-NH2 (2), UiO-66-NH2@Fe3O4-SiO2 (3) | HPLC-UV | ≈3 | 86.0 (1), 95.0 (2), 98.5 (3) | 0.005 (1), 0.005 (2), 0.003 (3) | ≤70 times | [101] |
| Basic drugs | Urine, plasma | MIL-101(Cr) | CE-UV | - | 66–95 | 0.61–2.7 | - | [104] |
| Estrogens | Urine | MIL-53(Al) | HPLC-FLD | - | 80.5–91.8 | 0.005–1.0 | ≥20 times | [105] |
| Tetracycline | Human plasma | Copper 1,3,5-tricarboxylate MOF | HPLC-UV | 10 | >90 | 2.4 | ≥12 times | [106] |
| Estrogenic drugs | Urine | Methyl modified MOF-5 | HPLC-UV | 5 | 85.6–94.8 | 0.02 | ≤15 times | [107] |
| Atenolol and captopril | Urine, plasma | Ni-MOF-74 | HPLC-DAD | 15 | 77.5–87.5 | 0.13–0.15 | ≥50 times | [108] |
| Acetone, isopropanol | Exhaled breath | UiO-66 | GC-FID | 50 | 89.1–106.7 | 0.79–0.84 | ≥120 times | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manousi, N.; Plastiras, O.-E.; Kalogiouri, N.P.; Zacharis, C.K.; Zachariadis, G.A. Metal-Organic Frameworks in Bioanalysis: Extraction of Small Organic Molecules. Separations 2021, 8, 60. https://doi.org/10.3390/separations8050060
Manousi N, Plastiras O-E, Kalogiouri NP, Zacharis CK, Zachariadis GA. Metal-Organic Frameworks in Bioanalysis: Extraction of Small Organic Molecules. Separations. 2021; 8(5):60. https://doi.org/10.3390/separations8050060
Chicago/Turabian StyleManousi, Natalia, Orfeas-Evangelos Plastiras, Natasa P. Kalogiouri, Constantinos K. Zacharis, and George A. Zachariadis. 2021. "Metal-Organic Frameworks in Bioanalysis: Extraction of Small Organic Molecules" Separations 8, no. 5: 60. https://doi.org/10.3390/separations8050060
APA StyleManousi, N., Plastiras, O.-E., Kalogiouri, N. P., Zacharis, C. K., & Zachariadis, G. A. (2021). Metal-Organic Frameworks in Bioanalysis: Extraction of Small Organic Molecules. Separations, 8(5), 60. https://doi.org/10.3390/separations8050060











