Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands
Abstract
1. Introduction
2. Synergism in the Liquid-Liquid Extraction of Metal Ions
3. General Physicochemical Properties of Chelating and O-Donor Organo-Phosphorus Ligands
4. Presentation of Equilibrium Data and Some Abbreviations Used
5. Data Evaluation Criteria
- Communications with possibly correct data, but inadequate or poor descriptions of experimental conditions;
- Communications that reviewers could not access in the original version;
- Publications from the same research group as that of a cited study with stability constant data that completely duplicate the data in the cited study;
- Publications that need further independent evaluation (this situation includes the cases where two independent research groups offer data that formally met the requirements stated above, but owing to some hidden systematic errors exhibit very large numerical discrepancies);
- Publications that provide data for conditions that contrast with those used to obtain other data (e.g., high or low temperatures, “unusual” ionic strengths, mixed diluents, overly complicated metal mixed ligand complexes, effective or conditional stability constants, etc.). Another obvious reason for this is associated with an inadequate description of chemical equilibria in a particular solvent extraction system, especially implementing ionic liquids compounds;
- Publications that present only enthalpy values (e.g., [102]); and
- Publications that present only D values (e.g., [103]).
6. Combination of β-Diketone and Organophosphorus Ligands
| Cation | I (M) | Diluents/T °C | Equilibrium | logK | Refs. |
|---|---|---|---|---|---|
| Eu | 0.1 NaClO4 and pH = 3.4 | C6H6 | Eu(TTA)3 | −7.22 ± 0.05 | [130] |
| Eu(TTA)3·TOPO | −0.63 ± 0.02 | ||||
| Eu(TTA)3·2TOPO | 4.74 ± 0.02 | ||||
| 10 °C | Eu(TTA)3·2TOPO | 4.74 ± 0.02 | |||
| 25 °C | 4.74 ± 0.02 | ||||
| 35 °C | 4.74 ± 0.02 | ||||
| 45 °C | 4.75 ± 0.03 | ||||
| Tb | Tb(TTA)3 | −7.25 ± 0.05 | |||
| Tb(TTA)3·TOPO | −0.93 ± 0.03 | ||||
| Tb(TTA)3·2TOPO | 4.32 ± 0.02 | ||||
| 152,154Eu a | 0.1 chloroacetate buffer, pH 2.77 | C6H6, 30 °C | Eu(TTA)3·2TBP | 2.85 ± 0.07 | [131] |
| 40 °C | 2.67 ± 0.04 | ||||
| 50 °C | 2.54 ± 0.04 | ||||
| 60 °C | 2.27 ± 0.04 | ||||
| 30 °C | Eu(TTA)3·2TOPO | 3.30 ± 0.04 | |||
| 40 °C | 2.95 ± 0.05 | ||||
| 50 °C | 2.89 ± 0.03 | ||||
| 60 °C | 2.77 ± 0.06 |
7. Combination of 4-Acyl-Pyrazolones and Organophosphorus Ligand
8. Combination of 4-Acyl-5-Isoxazolones and Organophosphorus Ligands
| Cation | I (mol dm−3) | Diluents | Equilibrium | logK | Refs. |
|---|---|---|---|---|---|
| 0.2 NaNO3 | C6H6 | LnL3·2TBP L: 4-acyl-3-phenyl-5-isoxazolones | [149] | ||
| Pr | 4-acetyl | 3.34 P | |||
| Eu | 3.93 | ||||
| Yb | 3.25 | ||||
| Pr | 4-benzoyl | 6.01 | |||
| Eu | 6.40 | ||||
| Yb | 5.56 | ||||
| Pr | 4-(4-toluoyl)- | 5.41 | |||
| Eu | 6.15 | ||||
| Yb | 5.14 | ||||
| Pr | 4-(4-fluorobenzoyl)- | 5.89 | |||
| Eu | 6.27 | ||||
| Yb | 5.40 | ||||
| Pr | 4-(4-nitrobenzoyl)- | 5.93 | |||
| Eu | 6.38 | ||||
| Yb | 5.55 | ||||
| La | 0.01 NaClO4 | xylene | ML3 L: 3-phenyl-4-benzoyl-5-isoxazolone | −0.78 ± 0.02 | [150] |
| Eu | 0.26 ± 0.05 | ||||
| Lu | 0.48 ± 0.01 | ||||
| La | ML3·2TBP | 7.13 ± 0.02 | [150] | ||
| Eu | 8.47 ± 0.02 | ||||
| Lu | 8.53 ± 0.02 | ||||
| La | 0.1 NaClO4 | CHCl3 | LnL3 L: HPBI | −1.33 ± 0.05 | [98] |
| Nd | −0.54 ± 0.05 | ||||
| Eu | 0.06 ± 0.05 | ||||
| Ho | 0.36 ± 0.05 | ||||
| Lu | 0.70 ± 0.05 | ||||
| Lac | 0.1 MES | CHCl3 | LnL3; L: HPBI | −1.34 ± 0.1 | [60] |
| Eu | 0.85 ± 0.11 | ||||
| Lu | 0.73 ± 0.10 | ||||
| La | 0.1 MES | [C1C4im+][Tf2N−] | LnL4−; L: HPBI | 3.60 ± 0.02 | [60] |
| Eu | 5.12 ± 0.03 | ||||
| Lu | 5.07 ± 0.03 | ||||
| La | 0.1 NaClO4 | CHCl3 | LnL3∙2S; S: S1, (Figure 6) | 6.7 ± 0.05 | [98] |
| Nd | 7.98 ± 0.05 | ||||
| Eu | 8.60 ± 0.05 | ||||
| Ho | 8.96 ± 0.05 | ||||
| Lu | 9.46 ± 0.05 | ||||
| La | 0.1NaClO4 | C6H6 | LnL3∙S; L: HPBI; S: S7, (Figure 6) | 5.32 ± 0.05 | [151] |
| Ce | 5.56 ± 0.05 | ||||
| Pr | 5.74 ± 0.05 | ||||
| Nd | 5.85 ± 0.05 | ||||
| Sm | 6.04 ± 0.05 | ||||
| Eu | 6.16 ± 0.05 | ||||
| Gd | 6.26 ± 0.05 | ||||
| Tb | 6.36 ± 0.05 | ||||
| Dy | 6.53 ± 0.05 | ||||
| Ho | 6.65 ± 0.05 | ||||
| Er | 6.75 ± 0.05 | ||||
| Tm | 6.90 ± 0.05 | ||||
| Yb | 7.02 ± 0.05 | ||||
| Lu | 7.18 ± 0.05 | ||||
| La | 0.1 NaClO4 | CHCl3 | 3.18 ± 0.05 | ||
| Nd | 3.87 ± 0.05 | ||||
| Eu | 4.54 ± 0.05 | ||||
| Ho | 5.02 ± 0.05 | ||||
| Lu | 5.45 ± 0.05 | ||||
| La | 0.1 NaCl | C2H4Cl2 | −1.25 ± 0.05 | ||
| Nd | −0.46 ± 0.05 | ||||
| Eu | 0.16 ± 0.05 | ||||
| Ho | 0.82 ± 0.05 | ||||
| Lu | 1.24 ± 0.05 | ||||
| La | 0.1 NaCl | CCl4 | 0.82 ± 0.05 | ||
| Nd | 1.26 ± 0.05 | ||||
| Eu | 1.77 ± 0.05 | ||||
| Ho | 2.23 ± 0.05 | ||||
| Lu | 2.63 ± 0.05 |
9. Combination of Organic Acid and Organophosphorus Ligands
10. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Adunka, R.; Orna, M. Carl Auer von Welsbach: Chemist, Inventor, Entrepreneur; Spinger: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Evans, C. (Ed.) Episodes from the History of the Rare Earth Elements; Kluwer Academic Publisher: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Rydberg, J.; Cox, M.; Musikas, C.; Choppin, G. (Eds.) Solvent Extraction Principles and Practice; Marcel Deker: New York, NY, USA, 2004. [Google Scholar]
- Marcus, Y.; Kertes, A.S. (Eds.) Ion Exchange and Solvent Extraction of Metal Complexes; Willey Interscience: New York, NY, USA, 1969; pp. 815–858. [Google Scholar]
- Khopkar, S. Solvent Extraction-Separation of Elements with Liquid Ion Exchangers; New Age International: New Delhi, India, 2009. [Google Scholar]
- Moyer, B.A. Ion Exchange and Solvent Extraction: A Series of Advances; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2010; Volume 19. [Google Scholar]
- De Anil, K.; Shripad, M.; Khopkar, A.; Chalmers, R. Solvent Extraction of Metals; van Dyk, B., Nieuwoudt, I., Eds.; Van Nostrand Reinhold Company: Washington, DC, USA, 2001. [Google Scholar]
- Liu, T.; Chen, J. Extraction and separation of heavy rare earth elements: A review. Sep. Purif. Technol. 2021, 276, 119263. [Google Scholar] [CrossRef]
- Neves, H.; Ferreira, G.; Ferreira, G.; Lemos, L.; Rodrigues, G.; Leao, V.; Mageste, A. Liquid-liquid extraction of rare earth elements using systems that are more environmentally friendly: Advances, challenges and perspectives. Sep. Purif. Technol. 2022, 282(Part B), 120064. [Google Scholar] [CrossRef]
- Rice, N.; Irving, H.; Leonard, M. Nomenclature for liquid-liquid distribution (solvent extraction) (IUPAC Recommendations 1993). Pure Appl. Chem. 1993, 65, 2373. [Google Scholar] [CrossRef]
- Shimojo, K. Solvent extraction in analytical separation techniques. Anal. Sci. 2018, 34, 1345. [Google Scholar] [CrossRef] [PubMed]
- Nancollas, G.H.; Tomson, M.B. Guidelines for the determination of stability constants. Pure Appl. Chem. 1982, 54, 2675. [Google Scholar] [CrossRef]
- Chen, J. Application of Ionic Liquids on Rare Earth Green Separation and Utilization; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kolaric, Z. Ionic Liquids: How Far Do they Extend the Potential of Solvent Extraction of f-Elements? Solvent Extr. Ion Exch. 2013, 3, 24. [Google Scholar] [CrossRef]
- Marcus, Y. Ionic Liquid Properties. From Molten Salts to RTILs; Springer: Berlin, Germany, 2016. [Google Scholar]
- Atanassova, M. Solvent extraction of metallic species in ionic liquids: An overview of s-, p- and d-elements. J. Chem. Technol. Met. 2021, 3, 443. [Google Scholar]
- Petrova, M.A. The Crucial Performance of Mutual Solubility Among Water and Ionic Liquids in the Time of Liquid-Liquid Extraction of Metallic Species; Academic Solutions: Sofia, Bulgaria, 2020. [Google Scholar]
- Binnemans, K. Lanthanides and Actinides in Ionic Liquids. Chem. Rev. 2007, 107, 2592. [Google Scholar] [CrossRef] [PubMed]
- Billard, I. Ionic Liquids: New Hopes for Efficient Lanthanide/Actinide Extraction and Separation. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.C.G., Pecharsky, V., Eds.; Elsevier Science Publ. B.V.: Amsterdam, The Netherlands, 2013; Volume 43, Chapter 256, pp. 213–273. [Google Scholar]
- Handy, S. (Ed.) Application of Ionic Liquids in Science and Technology; InTech: Rijeka, Croatia, 2011; Available online: http://www.issp.ac.ru/ebooks/books/open/Applications_of_Ionic_Liquids_in_Science_and_Technology.pdf (accessed on 26 June 2022).
- Okamura, H.; Hirayama, N. Recent Progress in Ionic Liquid Extraction for the Separation of Rare Earth Elements. Anal. Sci. 2021, 37, 119. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Synergism as a phenomenon in solvent extraction of 4f-elements with calixarenes. RSC Adv. 2016, 6, 11303. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Dukov, I. The interaction of extractants during synergistic solvent extraction of metals. Is it an important reaction? RSC Adv. 2016, 6, 81250–81265. [Google Scholar] [CrossRef]
- Mathur, J.N. Synergism of trivalent actinides and lanthanides. Solv. Extr. Ion Exch. 1983, 1, 34. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. High Molecular Weight Amines and Quaternary Ammonium Salts as Synergistic Agents in the Solvent Extraction of Metal Ions with Chelating Extractants. In Handbook of Inorganic Chemistry Research; Morrison, D.A., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2010; Chapter 7. [Google Scholar]
- Freiser, H. Solvent extraction of tervalent lanthanides as chelates a systematic investigation of extraction equilibria. Solv. Extr. Ion Exch. 1988, 6, 1093–1108. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Kuang, S.; Liao, W. Separation of rare earths in chloride media by synergistic solvent extraction with mixture of HEHAMP and CA12 and stripping with HCl. Hydrometallurgy 2022, 213, 105912. [Google Scholar] [CrossRef]
- Healy, T.V. Synergistic adducts as coordination compounds, Solvent extraction research. In Proceedings of the Fifth International Conference On Solvent Extraction Chemistry, Jerusalem, Israel, 16–18 September 1968; Kerters, A.S., Marcus, Y., Eds.; Wiley Interscience: New York, NY, USA, 1969. [Google Scholar]
- Blake, C.A.; Baes, C.A.; Coleman, C.F. Solvent extraction of uranium and other metals by acidic and neutral organophosphorus compounds. In Proceedings of the Second International Conference on the Peaceful Uses of Atomic Energy, Geneva, Switzerland, 1–13 September 1958; IAEA: Vienna, Austria, 1959; Volume 28, p. 289. [Google Scholar]
- Healy, T.V. Synergism with Thenoyltrifluoracetone in the Solvent Extraction of Metallic Species. Nucl. Sci. Eng. 1963, 16, 413. [Google Scholar] [CrossRef]
- Nash, K.; Choppin, G. Separations Chemistry for Actinide Elements: Recent Developments and Historical Perspective. Sep. Sci. Technol. 1997, 32, 255–274. [Google Scholar] [CrossRef]
- Rare Earth Coordination Chemistry, Fundamentals and Applications; Wiley & Sons: Hoboken, NJ, USA, 2010.
- Irving, H.; Edgington, D.N. Synergic effects in the solvent extraction of the actinides—I uranium(VI). J. Inorg. Nucl. Chem. 1960, 15, 158. [Google Scholar] [CrossRef]
- Gold, V. IUPAC Gold Book. In Compendium of Chemical Terminology; IUPAC: Zurich, Switzerland, 2014. [Google Scholar]
- Taube, M.; Siekierski, S. General remarks on synergic effects in the extraction of uranium and plutonium compounds. Nucleonica 1961, 6, 489. [Google Scholar]
- Tagushi, S.; Goto, K. Pre-Concentration Techniques for Trace Elements; Alfassi, Z.B., Waim, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 1989; p. 31. [Google Scholar]
- Caceci, M.; Chopin, G.; Liu, Q. Calorimetric studies of the synergistic effect. The reaction of UO22+, Th4+ and Nd3+ TTA complexes with TBP and TOPO in benzene. Solv. Extr. Ion Exch. 1985, 3, 605. [Google Scholar] [CrossRef]
- Wenquing, W.; Quingliang, L.; Caceci, M.; Chopin, G.; Yuwen, D.; Min, Y. Calorimetric studies of the synergistic effect: The reaction of UO2, Ln(PMBP)3 and Th(PMBP)4 with TOPO in nitrobenzene. Solv. Extr. Ion Exch. 1986, 4, 663. [Google Scholar] [CrossRef]
- Nash, K. Global 2001. In Proceedings of the International Conference on Back-End of the Fuel Cycle: From Research to Solution, France, Paris, 9–13 September 2001; p. 8. [Google Scholar]
- Atanassova, M.; Todorova, S.; Kurteva, V.; Todorova, N. Insights into the synergistic selectivity of 4f-ions implementing 4-acyl-5-pyrazolone and two new unsymmetrical NH-urea containing ring molecules in an ionic liquid. Sep. Purif. Technol. 2018, 204, 328. [Google Scholar] [CrossRef]
- Timmermans, F.; Katainem, J. Reflection Paper: Towards a Sustainable Europe by 2030; European Commision: Brussels, Belgium, 2019; ISBN 978-92-79-98963-6. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.; Feitzer, I.; Bennett, E.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Irving, H.M.N.H. Coordination compounds in analytical chemistry. Pure Appl. Chem. 1978, 50, 1129–1146. [Google Scholar] [CrossRef]
- Tschugaeff, L. Über ein neues, empfindliches Reagens auf Nickel. Ber. Der Dtsch. Chem. Ges. 1905, 38, 2520–2522. [Google Scholar] [CrossRef]
- Yudaev, P.; Kolpinskaya, N.; Chistayakov, E. Organophosphorous extractants for metals. Hydrometallurgy 2021, 201, 105558. [Google Scholar] [CrossRef]
- Atanassova, M.; Billard, I. Determination of pKaIL values of three chelating extractants in ILs: Consequences for the extraction of 4f elements. J. Solut. Chem. 2015, 44, 606. [Google Scholar] [CrossRef]
- Tasker, P.; Roach, B. Supramolecular Interactions in the Outer Coordination Spheres of Extracted Metal Ions. In Ion Exchange Solvent Extrartion: Supramolecular Aspects of Solvent Extraction; Moyer, B., Sengupta, A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Volume 21, Chapter 2, pp. 49–79. [Google Scholar]
- Dalton, R.; Price, R.; Quan, P.; Townson, B. Novel Solvent Extractants for Copper from Chloride Leach Solutions Derived from Sulphide Ores; Reagents in the Minerals Industry: London, UK; IMM: London, UK, 1984; p. 181. [Google Scholar]
- Binnemans, K. Chapter 225—Rare Earth Beta-Diketones. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Gschneider, K.A., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 35. [Google Scholar]
- Stary, J.; Liljenzin, J. Critical evaluation of equilibrium constants involving acetylacetone and its metal chelates. Pure Appl. Chem. 1982, 54, 2557. [Google Scholar] [CrossRef]
- Johansson, H.; Rydberg, J. Solvent Extraction Studies by the AKUFVE Method IV. Spectrophotometric determination of the distribution of acetylacetone. Acta Chem. Scand. 1969, 23, 2797. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V. Peculiar synergistic extraction behavior of Eu (III) in ionic liquids: Benzoylacetone and CMPO fusion. Sep. Purif. Technol. 2017, 183, 226. [Google Scholar] [CrossRef]
- Atanassova, M. Thenoyltrifluoroacetone: Preferable molecule for solvent extraction of metals-ancient twists to new approaches. Separations 2022, 9, 154. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Pettinari, R. Acylpyrazolone ligands: Synthesis, structures, metal coordination chemistry and applications. Coord. Chem. Rev. 2005, 249, 2909. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, R.; Pettinari, C. Recent advances in acylpyrazolone metal complexes and their potential applications. Coord. Chem. Rev. 2015, 303, 1. [Google Scholar] [CrossRef]
- Kurteva, V.B.; Petrova, M.A. Synthesis of 3-methyl-4-(4-methylbenzoyl)-1-phenyl-pyrazol-5-one: How to avoid o-acylation. J. Chem. Educ. 2015, 92, 382. [Google Scholar] [CrossRef]
- Zolotov, Y.; Kuzmin, N. Metal Extraction with Acylpyrazolones; Nauka: Moscow, Russia, 1977. [Google Scholar]
- Jensen, B.S. The synthesis of 1-phenyl-3-methyl-4-acyl-pyrazolones-5. Acta Chem. Scand. 1959, 13, 1668. [Google Scholar] [CrossRef]
- Atanassova, M. Synergistic solvent extraction and separation of lanthanide(III) ions with 4-benzoyl-3-phenyl-5-isoxazolone and the quaternary ammonium salt. Solv. Extr. Ion Exch. 2009, 27, 159. [Google Scholar] [CrossRef]
- Atanassova, M.; Okamura, H.; Eguchi, A.; Ueda, Y.; Sugita, T.; Shimojo, K. Extraction ability of 4-benzoyl-3-phenyl-5-isoxazolone towards 4f-ions into ionic and molecular media. Anal. Sci. 2018, 34, 973. [Google Scholar] [CrossRef]
- El-Nady, Y.A. Solvent extraction and its applications on ore processing and recovery of metals: Classical approach. Sep. Purif. Rev. 2017, 46, 195. [Google Scholar] [CrossRef]
- Kumar, P.; Vincent, T.; Khanna, A. Extraction of UO22+ into ionic liquid using TTA and TBP as extractants. Sep. Sci. Technol. 2015, 50, 2668. [Google Scholar] [CrossRef]
- Herbst, R.; Baron, P.; Nilsson, M. 6-Standard and advanced separation: PUREX process for nuclear fuel reprocessing. In Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Woodhead Publications Series in Energy: Cambridge, UK, 2011; pp. 141–175. [Google Scholar]
- Petrova, M.A.; Kurteva, V.B.; Lubenov, L.A. Synergistic effect in the solvent extraction and separation of lanthanoids by 4-(4-fluorobenzoyl)-3-methyl-1-phenyl-pyrazol-5-one in the presence of monofunctional neutral organophosphorus extractants. Ind. Eng. Chem. Res. 2011, 50, 12170. [Google Scholar] [CrossRef]
- El-Nady, Y.A. Lanthanum and neodymium from Egyptian monazite: Synergistic extractive separation using organophosphorus reagents. Hydrometallurgy 2012, 119–120, 23. [Google Scholar] [CrossRef]
- Gutsche, C. Calixarenes for separations. In ACS Symposium Series 757; ACS: Washington, DC, USA, 2000; Chapter 1. [Google Scholar]
- Le Saulnier, L.; Varbanov, S.; Scopelliti, R.; Elhabiri, M.; Bunzli, J.-C.G. Lanthanide complexes with a p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms. J. Chem. Soc. Dalton Trans. 1999, 22, 3919–3925. [Google Scholar] [CrossRef]
- Mandolini, L.; Ungaro, R. (Eds.) Calixarenes in Action; Imperial College Press: England, UK, 2000. [Google Scholar]
- Sliwa, W.; Kozlowski, C. Calixarenes and Resorcinarenes: Synthesis, Properties and Application; Wiley-VCH: Hoboken, NJ, USA, 2009. [Google Scholar]
- Johansson, L. The role of the perchlorate ion as ligand in solution. Coord. Chem. Rev. 1974, 12, 241–261. [Google Scholar] [CrossRef]
- Marcus, Y. Development and publication of solvent extraction methods. Talanta 1976, 23, 203–209. [Google Scholar] [CrossRef]
- Dukov, I.; Atanassova, M. Some aspects of synergistic metal extractions with chelating extractants and amines or quaternary ammonium salts. J. Univ. Chem. Technol. Met. 2003, 38, 7. [Google Scholar]
- Beck, M.T. Critical evaluation of equilibrium constants in solution. Stability constants of metal complexes. Pure Appl. Chem. 1977, 49, 127. [Google Scholar] [CrossRef]
- Nash, K. Aqueous complexes in separations of f-elements: Options and strategies for future development. Sep. Sci. Technol. 1999, 34, 911. [Google Scholar] [CrossRef]
- Sekine, T. Solvent extraction study of trivalent actinide and lanthanide complexes in aqueous solutions. III Oxalate complexes of La(III), Eu(III), Lu(III) and Am(III) in 1M NaClO4. Acta Chim. Scand. 1965, 19, 1476. [Google Scholar] [CrossRef]
- Sekine, T. Solvent extraction study of trivalent actinide and lanthanide complexes in aqueous solutions. II Sulfate complexes of La(III), Eu(III), Lu(III) and Am(III) in 1M NaClO4. Acta Chim. Scand. 1965, 19, 1469. [Google Scholar] [CrossRef][Green Version]
- Sekine, T. Solvent extraction study of trivalent actinide and lanthanide complexes in aqueous solutions. I Chloride complexes of La(III), Eu(III), Lu(III) and Am(III) in 4M NaClO4. Acta Chim. Scand. 1965, 19, 1435. [Google Scholar] [CrossRef]
- Sekine, T. Solvent extraction study of trivalent actinide and lanthanide complexes in aqueous solutions. IV Thiocyanate complexes of La(III), Eu(III), Lu(III) and Am(III) in 5M NaClO4. Acta Chim. Scand. 1965, 19, 1519. [Google Scholar] [CrossRef]
- Sekine, T.; Sakairi, M.; Shimada, F.; Hasegawa, Y. Use of the synergistic solvent extraction system for the determination of the stability constants of metal complexes. Bull. Chem. Soc. Jpn. 1965, 38, 847. [Google Scholar] [CrossRef]
- Quinn, J.; Soldenhoff, K.; Stevens, G.; Lengkeek, N. Solvent extraction of rare earth elements using phosphonic/phosphinic acid mixtures. Hydrometallurgy 2015, 157, 298. [Google Scholar] [CrossRef]
- Hokura, A.; Sekine, T. Gradual destruction of synergistic enhancement of copper(II) extraction into carbon tetrachloride with 2-thenoyltrifluoroacetone and trioctylphosphine oxide. Solv. Extr. Res. Dev. Jpn. 1995, 2, 102. [Google Scholar]
- Sekine, T.; Saitou, T.; Iga, H. Association of β-diketones with trioctylphosphine oxide in solvent extraction systems. Bull. Chem. Soc. Jpn. 1983, 56, 700. [Google Scholar] [CrossRef]
- Bogdanov, M.; Svinyarov, I. Distribution of N-methylimidazole in ionic liquids/organic solvents systems. Processes 2017, 5, 52. [Google Scholar] [CrossRef]
- Arrachart, G.; Coturier, J.; Dourdain, S.; Levard, C.; Pellet-Rostaing, S. Recovery of rare earths elements using ionic solvents. Processes 2021, 9, 1202. [Google Scholar] [CrossRef]
- Dwamena, A. Recent advances in hydrophobic deep eutectic solvents for extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Sunder, G.; Adhikari, S.; Rahanifar, A.; Poudel, A.; Kirchhoff, J. Evolution of environmentally friendly strategies for metal extraction. Separations 2020, 7, 4. [Google Scholar] [CrossRef]
- Ruiz-Angel, M.; Carda-Broch, S. Rcent advances on ionic liquids uses in separation techniques. Separations 2022, 9, 96. [Google Scholar] [CrossRef]
- Chatzimitkos, T.; Anagnostou, P.; Constantinou, I.; Dakidi, K.; Stalikas, C. Magnetic ionic liquids in sample preparation: Recent advances and future trends. Separations 2021, 8, 153. [Google Scholar] [CrossRef]
- Lukomska, A.; Wisniewska, A.; Dabrowski, Z.; Lach, J.; Wrobel, K.; Kolasa, D.; Domanska, U. Recovery of metals from electronic waste-printed circuit boards by ionic liquids, DESs and organophosphorus-based acid extraction. Molecules 2021, 27, 4984. [Google Scholar] [CrossRef] [PubMed]
- Lanaridi, O.; Platzer, S.; Nischkaner, W.; Limbeck, A.; Schnurch, M.; Bica-Schroder, K. A combined deep eutectic solvent—Ionic liquid process for the extraction and separation of platinium group metals (Pt, Pd, Rh). Molecules 2021, 26, 7204. [Google Scholar] [CrossRef]
- Kiss, T.; Sovago, I.; Gergely, A. Critical survey of stability constants of complexes of glycine. Pure Appl. Chem. 1991, 63, 597. [Google Scholar] [CrossRef]
- Smith, R.M.; Martell, A.E.; Chen, Y. Critical evaluation of stability constants for nucleotide complexes with protons and metal ions and the accompanying enthalpy changes. Pure Appl. Chem. 1991, 63, 1015. [Google Scholar] [CrossRef]
- Yamauchi, O.; Odani, A. Stability constants of metal complexes of amino acids with charged side chains-Part I: Positively charged side chains (Technical Report). Pure Appl. Chem. 1996, 68, 469. [Google Scholar] [CrossRef]
- Lajunen, L.H.J.; Portanova, R.; Piispanen, J.; Tolazzi, M. Critical evaluation of stability constants for alpha-hydroxycarboxylic acid complexes with protons and metal ions and the accompanying enthalpy changes Part I: Aromatic ortho-hydroxycarboxylic acids (Technical Report). Pure Appl. Chem. 1997, 69, 37. [Google Scholar] [CrossRef]
- Popov, K.I.; Rönkkömäki, H.; Lajunen, L.H.J. Critical evaluation of stability constants of phosphonic acids (IUPAC technical report). Pure Appl. Chem. 2001, 73, 1641. [Google Scholar] [CrossRef]
- Anderegg, G.; Arnaud-Neu, F.; Delgado, R.; Felcman, J.; Popov, K. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications* (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1445. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Delgado, R.; Chaves, S. Critical evaluation of stability constants and thermodynamic functions of metal complexes of crown ethers (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 71. [Google Scholar] [CrossRef]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S.; Dukov, I. Complexation of trivalent lanthanoids ions with 4-benzoyl-3-phenyl-5-isoxazolone and p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms in solution during synergistic solvent extraction and structural study of solid complexes by IR and NMR. Polyhedron 2010, 29, 655. [Google Scholar] [CrossRef]
- Anderegg, G. Critical survey of stability constants of NTA complexes. Pure Appl. Chem. 1982, 54, 2693. [Google Scholar] [CrossRef]
- Kolarik, Z. Critical evaluation of some equilibrium constants involving acidic organophosphorus extractants. Pure Appl. Chem. 1982, 54, 2593. [Google Scholar] [CrossRef]
- Powell, K.; Brown, P.; Byrne, R.; Gajda, T.; Hefter, G.; Sjöberg, S.; Wanner, H. Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+– Cl−, OH−, CO32–, SO42–, and PO43– aqueous systems (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 739. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, D.; Anitha, M.; Sharma, J.; Hubli, R.; Singh, H. New synergistic solvent mixture of DNPPA and bidentate octyl (phenyl) CMPO for enhanced extraction of uranium (VI) from phosphoric acid medium. Hydrometallurgy 2014, 147–148, 95–102. [Google Scholar] [CrossRef]
- Krea, M.; Khalif, H. Liquid–liquid extraction of uranium and lanthanides from phosphoric acid using a synergistic DOPPA–TOPO mixture. Hydrometallurgy 2000, 58, 215. [Google Scholar] [CrossRef]
- Marcus, Y. Diluent effects in solvent extraction. Solv. Extr. Ion Exch. 1989, 7, 567. [Google Scholar] [CrossRef]
- Stary, J.; Freiser, H. Equilibrium Constants of liquid-Liquid Distribution Reactions. Part IV: Chelating Extractants; IUPAC Chemical Data Series-No 18; Pergamon Press: Oxford, UK, 1978. [Google Scholar]
- Sekine, T.; Ono, M. Studies of the liquid-liquid partition systems. IV. The solvent extraction study of europium(III) adduct chelate complexes with six acetylacetone derivatives and tributylphosphate. Bull. Chem. Soc. Jpn. 1965, 38, 2087. [Google Scholar] [CrossRef]
- Atanasova, M.; Kurteva, V. Synergism in the solvent extraction of Europium (III) with thenoyltrifluoroacetone and CMPO in methylimidazolium ionic liquids. J. Solut. Chem. 2019, 48, 15. [Google Scholar] [CrossRef]
- Atanassova, M. Worthy extraction and uncommon selectivity of 4f-ions in ionic liquid medium: 4-Acylpyrazolones and CMPO. ACS Sustain. Chem. Eng. 2016, 4, 2366. [Google Scholar]
- Okamura, H.; Hirayama, N.; Morita, K.; Shimojo, K.; Naganawa, H.; Imura, H. Synergistic effect of 18-crown-6 derivatives on chelate extraction of lanthanoids (III) into an ionic liquid with 2-thenoyltrifluoroacetone. Anal. Sci. 2010, 26, 607. [Google Scholar] [CrossRef]
- Irving, H.; Edgington, D. Synergic effects in the solvent extraction of the actinides-IV. J. Inorg. Nucl. Chem. 1961, 21, 169. [Google Scholar] [CrossRef]
- Akiba, K. Some regularities in the formation constants of TBP adducts of europium tris-TTA chelate. J. Inorg. Nucl. Chem. 1973, 35, 2525. [Google Scholar] [CrossRef]
- Farbu, L.; Alstad, J.; Augustson, J.H. Synergistic solvent extraction of rare earth metal ions with thenoyltrifluoroacetone mixed with tributylphosphate. J. Inorg. Nucl. Chem. 1974, 36, 2091. [Google Scholar] [CrossRef]
- Sekine, T.; Koizumi, A.; Sakairi, M. Studies of scandium in various solutions. III The solvent extraction and complex formation of scandium(III) with acetylacetone and thenoyltrifluoroacetone. Bull. Chem. Soc. Jpn. 1966, 39, 2681. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. V-adduct formation of some tris-tta complexes. J. Inorg. Nucl. Chem. 1967, 29, 1481. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D. Solvent extraction of metal ions with mixed ligands. IV-Extraction of Eu(III) with chelating acids and neutral adduct-forming ligands. J. Inorg. Nucl. Chem. 1967, 29, 1475. [Google Scholar] [CrossRef]
- Akiba, K.; Wada, M.; Kanno, T. Effect of diluent on synergic extraction of europium by thenoyltrifluoroacetone and tri-n-octylphosphine oxide. J. Inorg. Nucl. Chem. 1981, 43, 1031. [Google Scholar] [CrossRef]
- Favaro, D.I.; Atalla, L.T. Interaction effect between thenoyltrifluoroacetone and tri-n-octylphosphine oxide in the synergistic extraction of trivalent lanthanides. Determination of the composition of the extracted species of the extracted species. J. Radioanal. Nucl. Chem. Art. 1987, 111, 81. [Google Scholar] [CrossRef]
- Aly, H.F.; Khalifa, S.M.; Zakareia, N. Periodic variations of lanthanides synergic extraction by thenoyltrifluoroacetone and triphenylphosphine oxide mixture. Solv. Extr. Ion Exch. 1984, 2, 887. [Google Scholar] [CrossRef]
- Okamura, H.; Mizuno, M.; Hirayama, N.; Shimojo, K.; Naganawa, H.; Imura, H. Synergistic enhancement of the extraction and separation efficiencies of lanthanoid (III) ions by the formation of charged adducts in an ionic liquid. Ind. Eng. Chem. Res. 2020, 59, 329. [Google Scholar] [CrossRef]
- Shigematsu, T.; Tabushi, M.; Matsui, M.; Honjyo, T. The synergistic effect in solvent extraction-the correlation of the ionic radius of rare earth elements with the stability constants of rare earth benzoyltrifluoroacetonate aducts with n-hexyl alcohol, TBP and TOPO. Bull. Chem. Soc. Jpn. 1967, 40, 2807. [Google Scholar] [CrossRef]
- Atanassova, M.; Kaloyanova, S.; Deligeorgiev, T. Application of 4,4,4-trifluoro-1-(biphenyl-4-yl)butane-1,3-dione as a chelating extractant in the solvent extraction and separation of light lanthanoids in combination with phosphine oxides. Acta Chim. Slovenika 2010, 57, 821. [Google Scholar]
- Umetani, S.; Kawase, Y.; Le, Q.; Matsui, M. The synergistic extraction of lanthanides with trifluoroacetylcycloalkanones and trioctylphosphine oxide. Chem. Lett. 1997, 26, 771–772. [Google Scholar] [CrossRef]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Shivachev, B.; Varbanov, S.; Dukov, I. Effect of p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms as synergistic agent in the solvent extraction of trivalent lanthanoids with 4,4,4-trifluoro-1-(2-thienyl)1,3-butanedione and structural study of solid complexes by IR, NMR and X-ray. Polyhedron 2008, 27, 3306. [Google Scholar] [CrossRef]
- Atanassova, M.; Vassilev, N.; Dukov, I. p-Tert-butylcalix[4]arene tetrkis (N,N-dimethylacetamide) as a second ligand in the complexation of trivalent lanthanoids with thenoyltrifluoroacetone in solution and investigation of a solid Eu(III) complex. Sep. Purif. Technol. 2011, 78, 214. [Google Scholar] [CrossRef]
- Atanassova, M. Lanthanoid complexes with thenoyltrifluoroacetone and 4-tert-butylcalix[4]arene-teraacetic acid tetraethylester: Synergistic extraction and separation. Microchim. Acta 2011, 174, 175. [Google Scholar] [CrossRef]
- Hatakeyama, M.; Nishiyama, Y.; Nagatani, H.; Okamura, H.; Imura, H. Synergistic extraction equilibrium of lanthanide(III) ions with benzoylacetone and a neutral ligand in an ionic liquid. Solv. Extr. Res. Dev. Jpn. 2018, 25, 79. [Google Scholar] [CrossRef]
- Choppin, G.; Morgenstern, A. Thermodynamics of solvent extraction. Solv. Extr. Ion Exch. 2000, 18, 1029. [Google Scholar] [CrossRef]
- Atanassova, M.; Jordanov, V.; Dukov, I. Effect of the quaternary ammonium salt Aliquat 336 on the solvent extraction of lanthanoid (III) ions with thenoyltrifluoroacetone. Hydrometallurgy 2002, 63, 41–47. [Google Scholar] [CrossRef]
- Atanasova, M.; Dukov, I. Crown ethers as synergistic agents in the solvent extraction of trivalent lanthanoids with thenoyltrifluoroacetone. Sep. Sci. Technol. 2005, 40, 1104–1113. [Google Scholar] [CrossRef]
- Kandil, A.; Farah, K. Thermodynamic studies of TOPO adducts of europium and terbium tris-tta chelates. J. Inorg. Nucl. Chem. 1980, 42, 1491. [Google Scholar] [CrossRef]
- Mathur, J.; Pai, S.; Khopkar, P.; Subramanian, M. Thermodynamics of synergistic extraction of europium(III) by mixtures of thenoyltrifluoroacetone and some neutral oxo-donors. J. Inorg. Nucl. Chem. 1977, 39, 653. [Google Scholar] [CrossRef]
- Sasayama, K.; Umetani, S.; Matsui, M. The substituent effect on the synergistic extraction of europium and scandium with 1-phenyl-3-methyl-4-acylpyrazol-5-one and tri-n-octylphosphine oxide. Anal. Chim. Acta 1983, 149, 253. [Google Scholar] [CrossRef]
- Umetani, S.; Freiser, H. Mixed-ligand chelate extraction of lanthanides with 1-phenyl-3-methyl-4-(trifluoroacetyl)-5-pyrazolone and some phosphine oxide compounds. Inorg. Chem. 1987, 26, 3179. [Google Scholar] [CrossRef]
- Santhi, P.B.; Reddy, M.L.P.; Ramamohan, T.R.; Damodaran, A.D. Synergistic solvent extraction of trivalent lanthanides and actinide by muxtures of 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5 and neutral oxo-donors. Solv. Extr. Ion Exch. 1994, 12, 633. [Google Scholar] [CrossRef]
- Reddy, M.L.P.; Damodaran, A.D.; Mathur, J.N.; Murali, M.S.; Iyer, R.H. Mixed-ligand chelate extraction of trivalent lanthanides and actinides with 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5 and dihexyl-N,N-diethylcarbamoylmethyl phosphonate. J. Radioanal. Nucl. Chem. 1995, 198, 367. [Google Scholar] [CrossRef]
- Sujatha, S.; Reddy, M.L.P.; Luxmi Varma, R.; Ramamohan, T.R.; Prasada Rao, T.; Iyer, C.S.P.; Damodaran, A.D. Synergistic solvent extraction of trivalent lanthanides by mixtures of 1-phenyl-3-methyl-4-trifluoroacetl-pyrazolone-5 and neutral oxo-donors. J. Chem. Eng. Jpn. 1996, 29, 187. [Google Scholar] [CrossRef][Green Version]
- Mukai, H.; Umetani, S.; Matsui, M. The synergistic extraction of rare earth metals with ortho-substituted 1-phenyl-3-methyl-4-aroyl-5-pyrazolones and trioctylphosphine oxide. Anal. Sci. 1997, 13, 145. [Google Scholar] [CrossRef]
- Mukai, H.; Umetani, S.; Matsui, M. Steric effect of ortho substituents of 1-phenyl-3-methyl-4-aroylpyrazol-5-ones on the synergic extraction of scandium and lanthanum with tri-n-octylphosphine oxide. Solv. Extr. Ion Exch. 2003, 21, 73. [Google Scholar] [CrossRef]
- Pavithran, R.; Reddy, M.L.P. Steric effects of polymethylene chain of 4-acylbis(pyrazolones) on the solvent extraction of trivalent lanthanoids: Synergistic effect with mono and bifunctional neutral organophosphorus extractants. Anal. Chim. Acta 2005, 536, 219. [Google Scholar] [CrossRef]
- Atanassova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S.; Dukov, I. Effect of p-tert-butylcalix[4]arene fitted with phosphinoyl pendant arms as synergistic agent in the solvent extraction and separation of some trivalent lanthanoids with 4-benzoyl-3-methyl-1-phenyl-5-pyrazolone. J. Inclus. Phenom. Macrocycl. Chem. 2007, 58, 173. [Google Scholar] [CrossRef]
- Tashev, E.; Atanassova, M.; Varbanov, S.; Tosheva, T.; Shenkov, S.; Chauvin, A.; Dukov, I. Synthesis of octa(1,1,3,3-tetramethylbutyl)octakis(dimethylphosphinoylmethyleneoxy)calix[8]arene and its application in the synergistic solvent extraction and separation of lanthanoids. Sep. Purif. Technol. 2008, 64, 170. [Google Scholar] [CrossRef]
- Atanassova, M.; Vassilev, N.; Tashev, E.; Lachkova, V.; Varbanov, S. Coordination chemistry of a para-tert-octylcalix[4]arene fitted with phosphinoyl pendant arms towards 4f-elements: Extraction, synergism, separation. Sep. Sci. Technol. 2016, 51, 49. [Google Scholar] [CrossRef]
- Varbanov, S.; Tashev, E.; Vassilev, N.; Atanassova, M.; Lachkova, V.; Tosheva, T.; Shenkov, S.; Dukov, I. Synthesis, characterization and implementation as a synergistic agent in the solvent extraction of lanthanoids. Polyhedron 2017, 134, 135. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Varbanov, S.; Dukov, I. Behaviour of mixed systems based on para-substituted 4-aroyl-5-pyrazolones in the presence of phosphorus containing calix[4]arene towards lanthanoids: Synergistic solvent extraction and separation. Sep. Purif. Technol. 2012, 95, 58. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Billard, I. Comparing extraction, synergism and separation of lanthanoids using acidic and neutral compounds in chloroform and one ionic liquid: Is the latter always “better”? RSC Adv. 2014, 4, 38820. [Google Scholar] [CrossRef]
- Atanassova, M.; Kurteva, V.; Lubenov, L.; Varbanov, S.; Billard, I. Are fancy acidic or neutral ligands really needed for synergism in ionic liquids? A comparative study of lanthanoid extraction in CHCl3 and an ionic liquid. New, J. Chem. 2015, 39, 7932. [Google Scholar] [CrossRef]
- Kertes, A. The chemistry of solvent extraction. In Recent Advances in Liquid-Liquid Extraction, 1st ed.; Hadson, C., Ed.; Elsevier: Amsterdam, The Netherlands, 1971; Chapter 2. [Google Scholar]
- Sinha, S. Structure and Bonding 30. Rare Earths; Springer: Berlin/Heidelberg, Germany, 1976. [Google Scholar]
- Odashima, T.; Satoh, S.; Sato, T.; Ishii, H. Solvent extraction of some trivalent lanthanoids with 4-acyl-3-phenyl-5-isoxazolones. Solv. Extr. Ion Exch. 1995, 13, 845. [Google Scholar] [CrossRef]
- Reddy, M.L.P.; Luxmi Varma, R.; Ramamohan, T.R.; Prasada Rao, T.; Iyer, C.S.P.; Damodaran, A.D.; Mathur, J.N.; Murali, M.S.; Iyer, R.H. Mixed-ligand chelate extraction of trivalent lanthanides and actinides with 3-phenyl-4-benzoyl-5-isoxazolone and neutral oxo-donors. Radiochim. Acta 1995, 69, 55. [Google Scholar] [CrossRef]
- Petrova, M.; Lachkova, V.; Vassilev, N.; Varbanov, S. Effect of diluents on the synergistic solvent extraction and separation of trivalent lanthanoids with 4-benzoyl-3-phenyl-5-isoxazolone and tert-butylcalix[4]arene tetrakis(N,N-dimethyl acetamide) and structural study of Gd(III) solid complex by IR and NMR. Ind. Eng. Chem. Res. 2010, 49, 6189. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Q.; Bao, B. Solvent extraction of Pr and Nd(III) from chloride-acetate medium by 8-hydroquinoline with and without 2-ethylhexyl phosphoric acid mono-2-ethylhexyl ester as an added synergist in heptane diluent. Hydrometallurgy 2007, 88, 210. [Google Scholar] [CrossRef]
- Reddy, M.; Ramamohan, T.; Iyer, C. Enhanced extraction and separation of trivalent lanthanides and yttrium with bis-(2,4,4-trimethylpentyl)phosphinic acid and trialkyl phosphine oxide. Solv. Extr. Res. Dev. Jpn. 1998, 5, 1–15. [Google Scholar]
- Turanov, A.; Karandashev, V. Synergistic solvent extraction of lanthanides(III) with mixtures of tetraphenylmethylenediphosphine dioxide and picrolonic acid from HCl solution. Solv. Extr. Ion Exch. 2017, 35, 104–116. [Google Scholar] [CrossRef]
- Sekine, T.; Koike, Y.; Hasegawa, Y. Studies of Actinium(III) in Various Solutions. II. Distribution Behavior of Lanthanum(III) and Actinium(III) in Some Chelate Extraction Systems. Bull. Chem. Soc. Jpn. 1969, 42, 432. [Google Scholar] [CrossRef]
- Sekine, T.; Dyrssen, D.J. Solvent extraction of metal ions with mixed ligands—III: Adduct formation of Eu(III) and Th(IV) chelates with TTA and IPT. Inorg. Nucl. Chem. 1967, 29, 1457. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Healy, T.V. Synergism in the solvent extraction of di-, tri- and tetravalent metal ions—V: Infra-red studies of the isolated complexes. J. Inorg. Nucl. Chem. 1962, 24, 1463. [Google Scholar] [CrossRef]
- Healy, T.V.; Ferraro, J.R. Synergism in the solvent extraction of di, tri and tetravalent metal ions—IV: Absorption spectral studies of the synergistic complexes. J. Inorg. Nucl. Chem. 1962, 24, 1449. [Google Scholar] [CrossRef]
- El-Naggar, H.A. Extraction of Eu(III) and Co(II) by dihexyl N,N-diethyl carbamoyl methyl phosphonate (DHDECMP) and thenoyl trifluoroacetone (HTTA). J. Radioanal. Nucl. Chem. 2001, 249, 601. [Google Scholar] [CrossRef]
- Murthy, K.S.R.; Krupadam, R.J.; Anjaneyuly, Y. Lanthanum and neodymium from egyptian monazite: Synergistic extractive separation using organophosphorus reagents. Proc. Ind. Acad. Sci. Chem. Sci. 1998, 110, 83. [Google Scholar] [CrossRef]
- Umetani, S.; Kihara, S.; Matsui, M. Adduct formation properties of some polydentate phosphine oxides in the synergistic extraction of metals with 4-acyl-5-pyrazolone derivatives. In Proceedings of the ISEC’90, Kyoto, Japan, 6–21 July 1990; Sekine, T., Ed.; Solvent Extraction 1990. Elsevier Science Publishers BV: Amsterdam, The Netherlands, 1992; pp. 309–314. [Google Scholar]
- Navratil, O. Synergistic effects in liquid-liquid extraction of some heavy metals by 1-phenyl-3-methyl-4-benzoyl-pyrazol-5-one. In Proceedings of International Solvent Extraction Conference; Lyon, France, 8–14 September 1974, Chemical Society: London, UK, 1974; pp. 2585–2592. [Google Scholar]
- Meera, R.; Luxmi Varma, R.; Reddy, M.L. Enhanced extraction of thorium(IV) and uranium(VI) with 1-phenyl-3-methyl-4-pivaloyl-5-pyrazolone in the presence of various neutral organophosphorus extractants. P. Radiochim. Acta 2004, 92, 17. [Google Scholar] [CrossRef]
- Mathur, J.N.; Khopkar, P.K. Extraction of trivalent actinides with some substituted pyrazolones and their synergistic mixtures with tri-n-octylphosphine oxide in chloroform. Polyhedron 1984, 3, 1125. [Google Scholar] [CrossRef]
- Turanov, A.; Karandashev, V.; Kharlamov, A.; Bondarenko, N. Synergistic solvent extraction of lanthanides(III) with mixtures of 4-benzoyl-3-methyl-1-phenylpyrazol-5-one and some novel carbamoyl- and phosphorylmethoxymethylphosphine oxides. Solv. Extr. Ion Exch. 2014, 32, 492. [Google Scholar] [CrossRef]
- Jia, Q.; Liao, W.; Li, D.; Niu, C. Synergistic extraction of lanthanum (III) from chloride medium by mixtures of 1-phenyl-3-methyl-4-benzoyl-pyrazalone-5 and triisobutylphosphine sulphide. Anal. Chim. Acta 2003, 477, 251–256. [Google Scholar] [CrossRef]
- Smith, B.F.; Jarvinen, G.; Jones, M.; Hay, P. The synthesis and actinide and lanthanide complexation of “soft” donor ligands: Comparison between 4-benzoyl-2,4-dihidro-5-methyl-2-phenyl-3H-pyrazol-3-thione (HBMPPT) and 4-thiobenzoyl-2,4-dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one (HTBMPP) with tri-n-octylphosphine oxide (TOPO) synergist for Am( III) and Eu( III) extraction. Solv. Extr. Ion Exch. 1989, 7, 749–765. [Google Scholar]
- Shmidt, V.S. Some problems of the development of the physicochemical principles of modern extraction technology. Uspekhi Khimii 1987, 56, 1387. (In Russian) [Google Scholar] [CrossRef]
- Dhami, P.; Chitnis, R.; Gopalakrishnan, V.; Watta, P.; Ramanujan, A.; Banri, A. Studies on the partitioning of actinides from high level waste using a mixture of Hdehp and Cmpo as extractant. Sep. Sci. Technol. 2001, 36, 325. [Google Scholar] [CrossRef]
- Zhang, P.; Kimura, T. Complexation of Eu(III) with dibutyl phosphate and tributyl phosphate. Solv. Extr. Ion Exch. 2006, 24, 149. [Google Scholar] [CrossRef]
- Bakos, L.; Szabo, E.; Andras, L.; Iyer, N. Synergistic and antagonistic effects in organic solvent extraction of various metals. In Proceedings of the Symposium on Coordination Chemistry, Tihany, Hungary, 15 September 1964; Beck, M., Ed.; Academiai Kiado: Budapest, Hungary, 1965; pp. 241–254. [Google Scholar]
- Singh, S.K.; Dhami, P.S.; Dakshinamoorthy, A.; Sundersanan, M. Studies on the recovery of uranium from phosphoric acid medium using synergistic mixture of 2-ethyl hexyl hydrogen 2-ethyl hexyl phosphonate and octyl(phenyl)-N,N-diisobutyl Carbamoyl Methyl Phosphine Oxide. Sep. Sci. Technol. 2009, 44, 491–505. [Google Scholar] [CrossRef]
- Kondo, K.; Watanabe, T.; Matsumoto, M.; Kamio, E.J. Mechanism of synergistic extraction of samarium using alkylphosphoric acid as main extractant. Chem. Eng. Jpn. 2009, 42, 563. [Google Scholar] [CrossRef]
- Geist, A.; Weigl, M.; Gompper, K. Minor actinide partitioning by liquid–liquid extraction: Using a synergistic mixture of bis(chlorophenyl)-dithiophosphinic acid and topo in a hollow fiber module for americium(ii)–lanthanides(iii) separation. Sep. Sci. Technol. 2002, 37, 3369. [Google Scholar] [CrossRef]
- Ionova, G.; Ionov, S.; Rabbe, C.; Hill, C.; Madic, C.; Guillaumont, R.; Krupa, J.C. Mechanism of trivalent actinide/lanthanide separation using bis(2,4,4-trimethylpentyl) dithiophosphinic acid (cyanex 301) and neutral o-bearing co-extractant synergistic mixtures. Solv. Extr. Ion Exch. 2001, 19, 391. [Google Scholar] [CrossRef]
- Jia, Q.; Tong, J.; Li, Z.; Zhao, W.; Li, H.; Meng, J. Solvent extraction of rare earth elements with mixtures of sec-octylphenoxy acetic acid and bis(2,4,4-trimethylpentyl) dithiophosphinic acid. Sep. Purif. Technol. 2009, 64, 345. [Google Scholar] [CrossRef]
- Noro, J.; Sekine, T. Solvent Extraction of Europium(III) with 1-Naphthoic Acid into Chloroform in the Absence and Presence of Tetrabutylammonium Ions or Trioctylphosphine Oxide. Bull. Chem. Soc. Jpn. 1993, 66, 2242. [Google Scholar] [CrossRef]
- Tong, S.; Zhao, X.; Song, N.; Jia, Q.; Zhau, W.; Liao, W. Solvent extraction study of rare earth elements from chloride medium by mixtures of sec-nonylphenoxy acetic acid with Cyanex301 or Cyanex302. Hydrometallurgy 2009, 100, 15. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Jia, R.J. Separation of Am from lanthanides by a synergistic mixture of purified Cyanex 301 and TBP. Radioanal. Nucl. Chem. 2002, 251, 487–492. [Google Scholar] [CrossRef]
- Wu, D.B.; Wei, L.; Li, D. The extraction and separation of Ho, Y, and Er (III) with the mixtures of Cyanex 302 and another organic extractant. Sep. Sci. Technol. 2007, 42, 847–864. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Zhang, Z.; Liao, W. Synergistic solvent extraction of heavy rare earths from chloride media using mixture of HEHHAP and Cyanex272. Hydrometallurgy 2020, 191, 105240. [Google Scholar] [CrossRef]
- Santhi, P.B.; Reddy, M.L.P.; Damodaran, A.D. Liquid-liquid extraction of yttrium (III) with mixtures of organophosphorus extractants: Theoretical analysis of extraction behaviour. Hydrometallurgy 1991, 27, 169. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Bian, X.; Zeng, W. Synergistic extraction and separation of lanthanum (III) and cerium (III) using a mixture of 2-ethylhexylphosphonic mono-2-ethylhexyl ester and di-2-ethylhexyl phosphoric acid in the presence of two complexing agents containing lactic acid and citric acid. Hydrometallurgy 2014, 149, 238. [Google Scholar] [CrossRef]
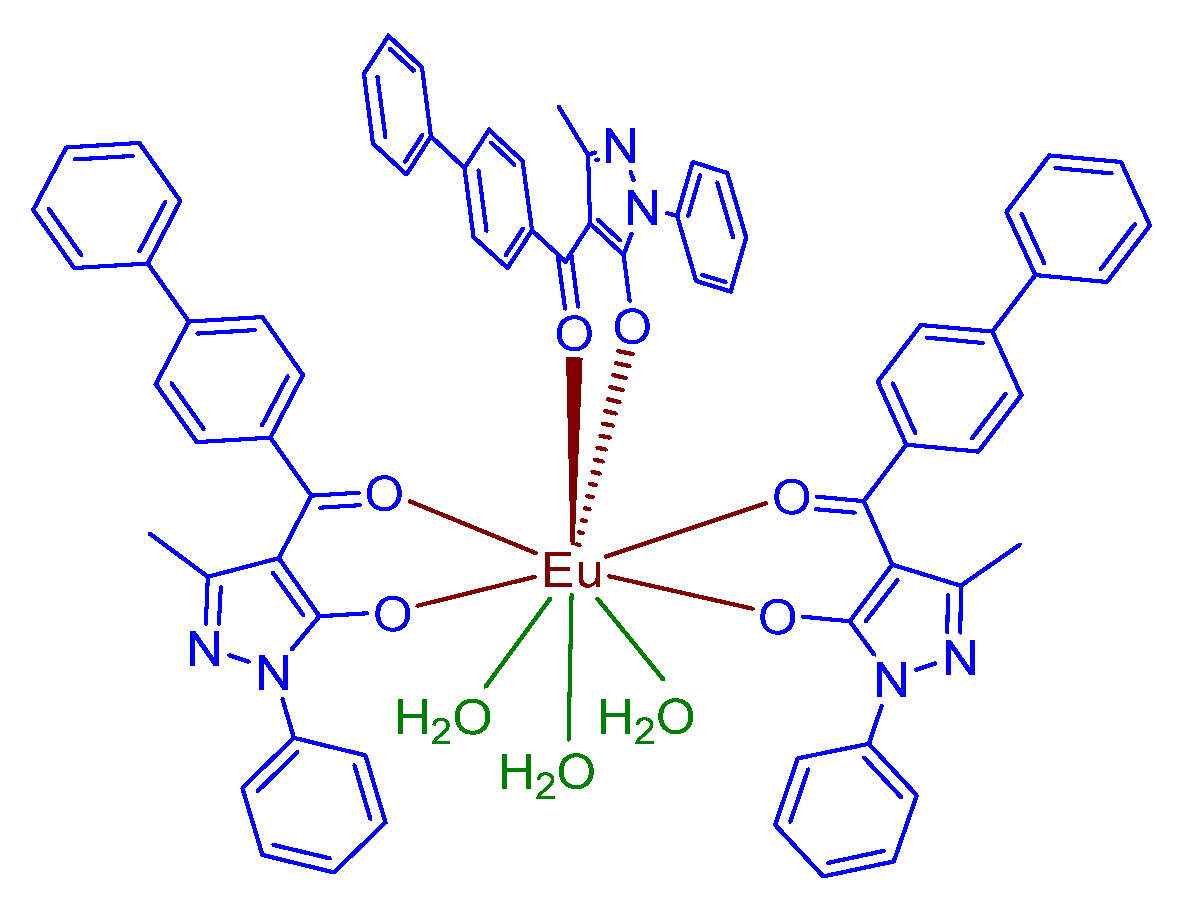
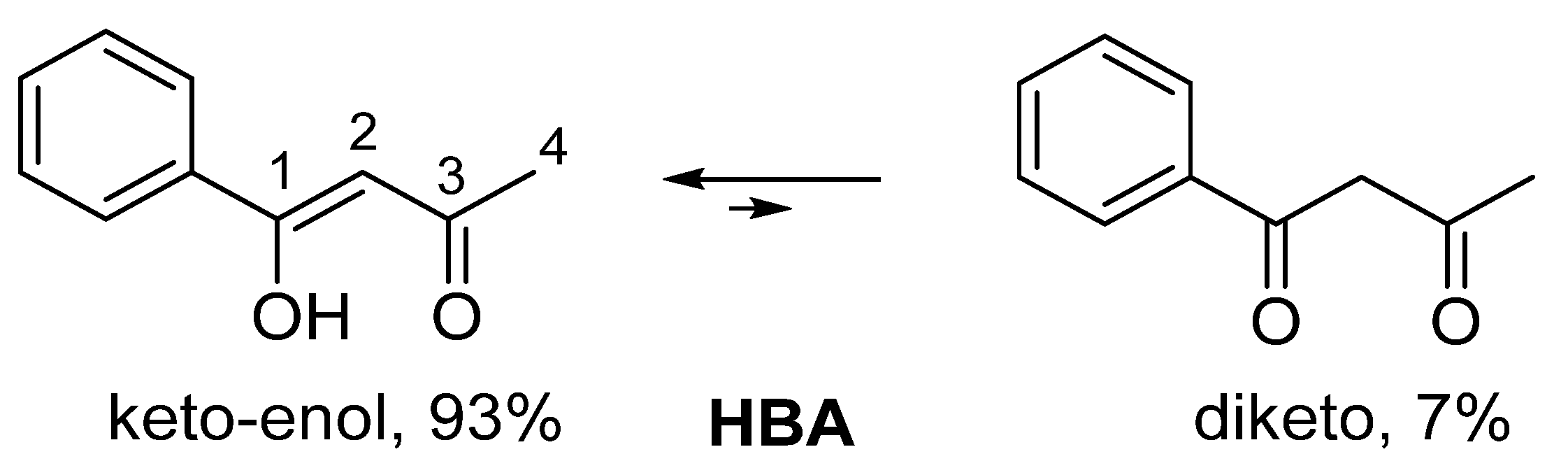
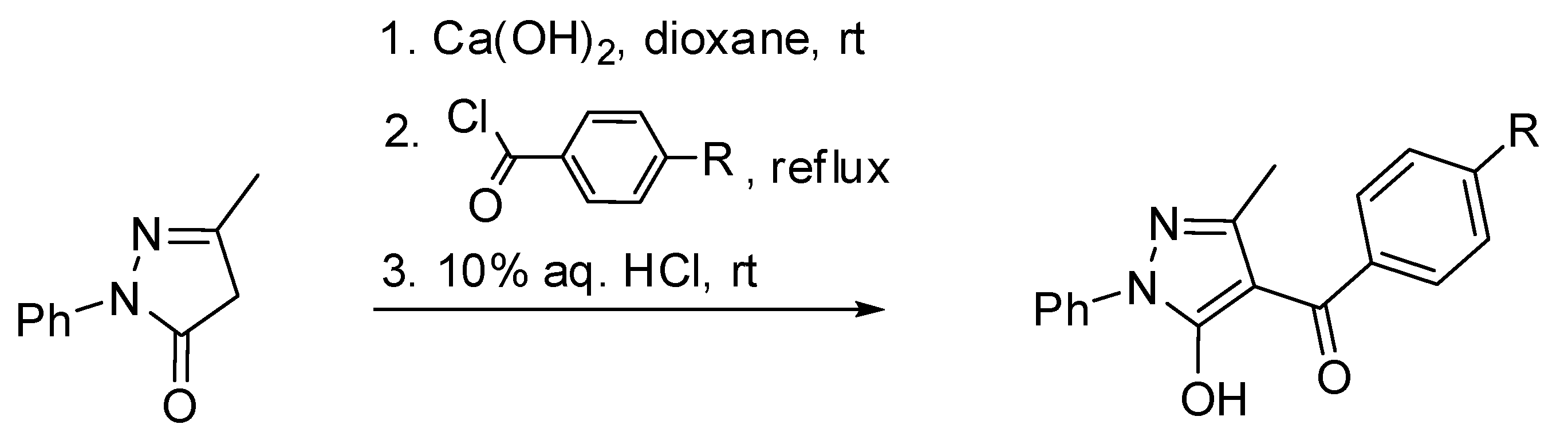
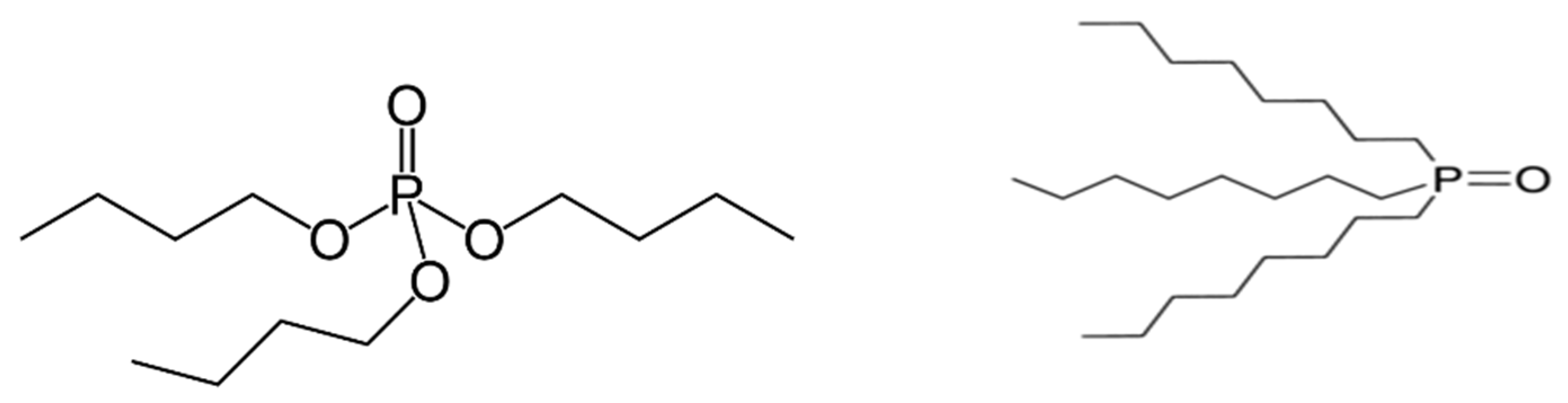
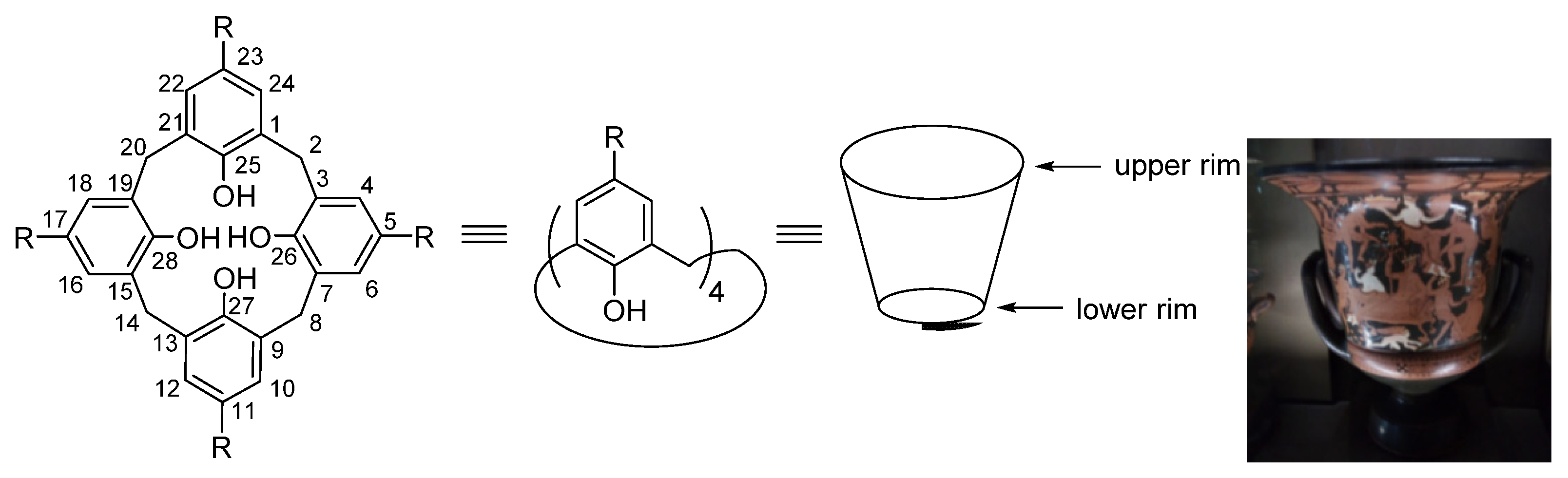
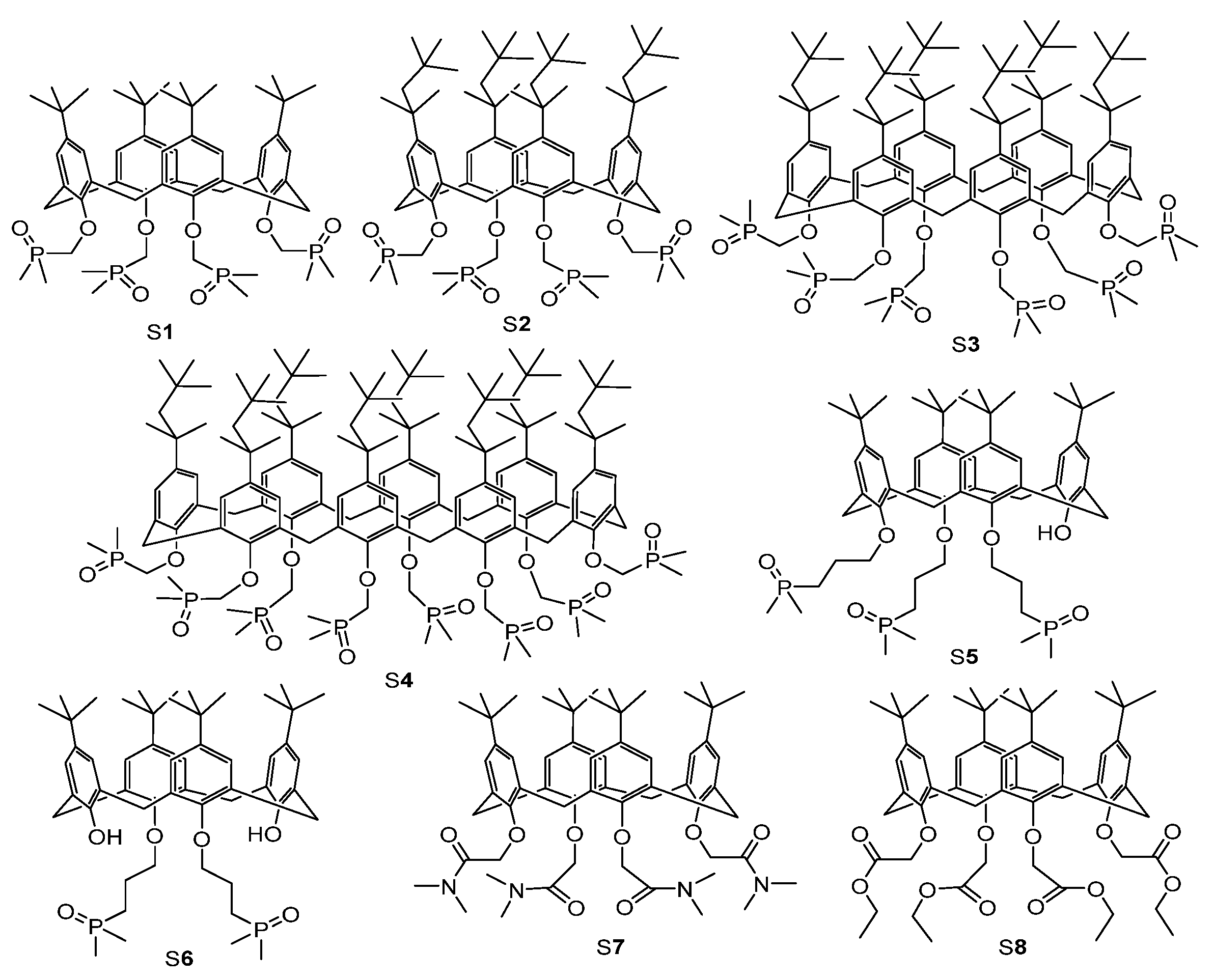


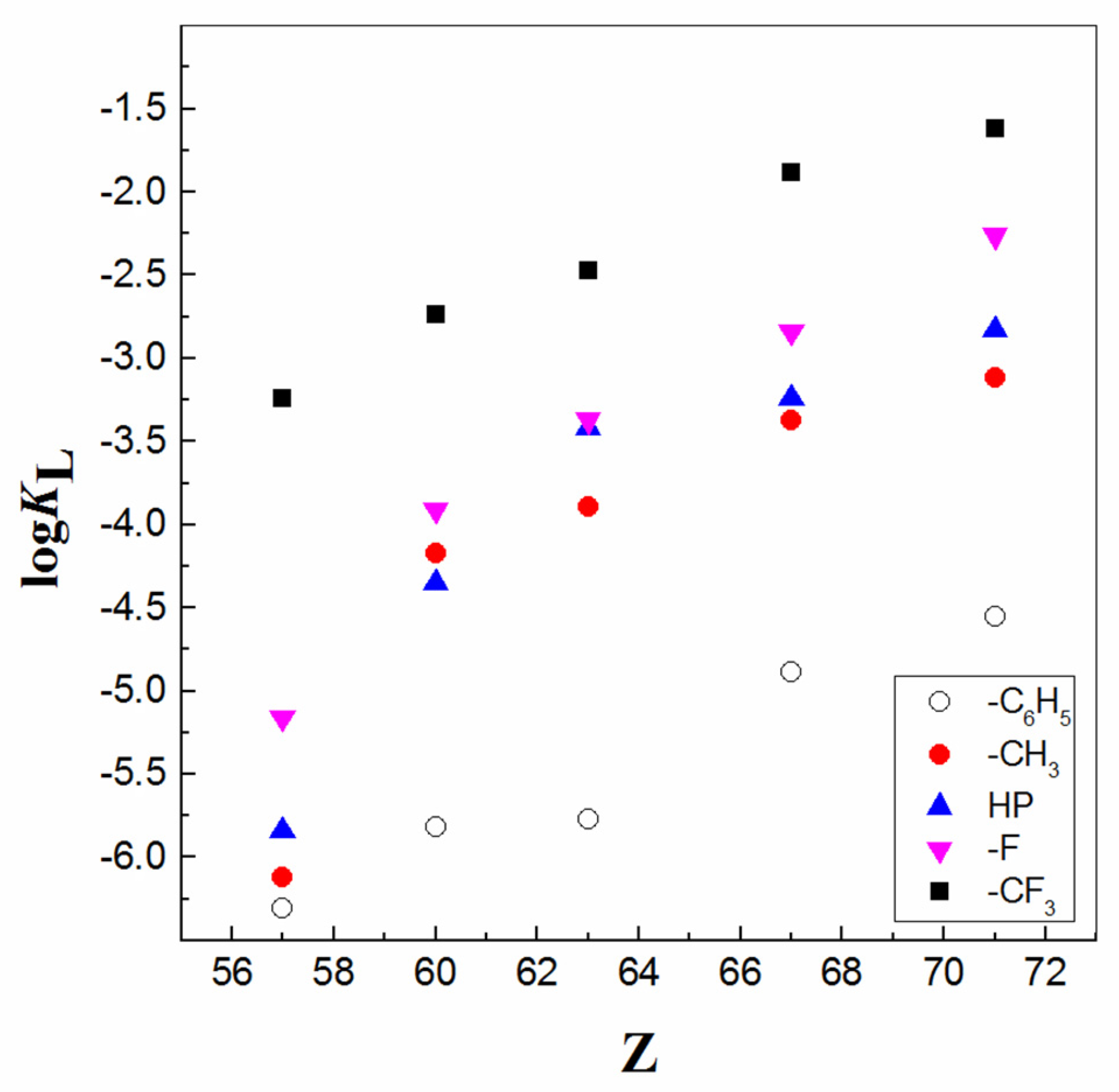
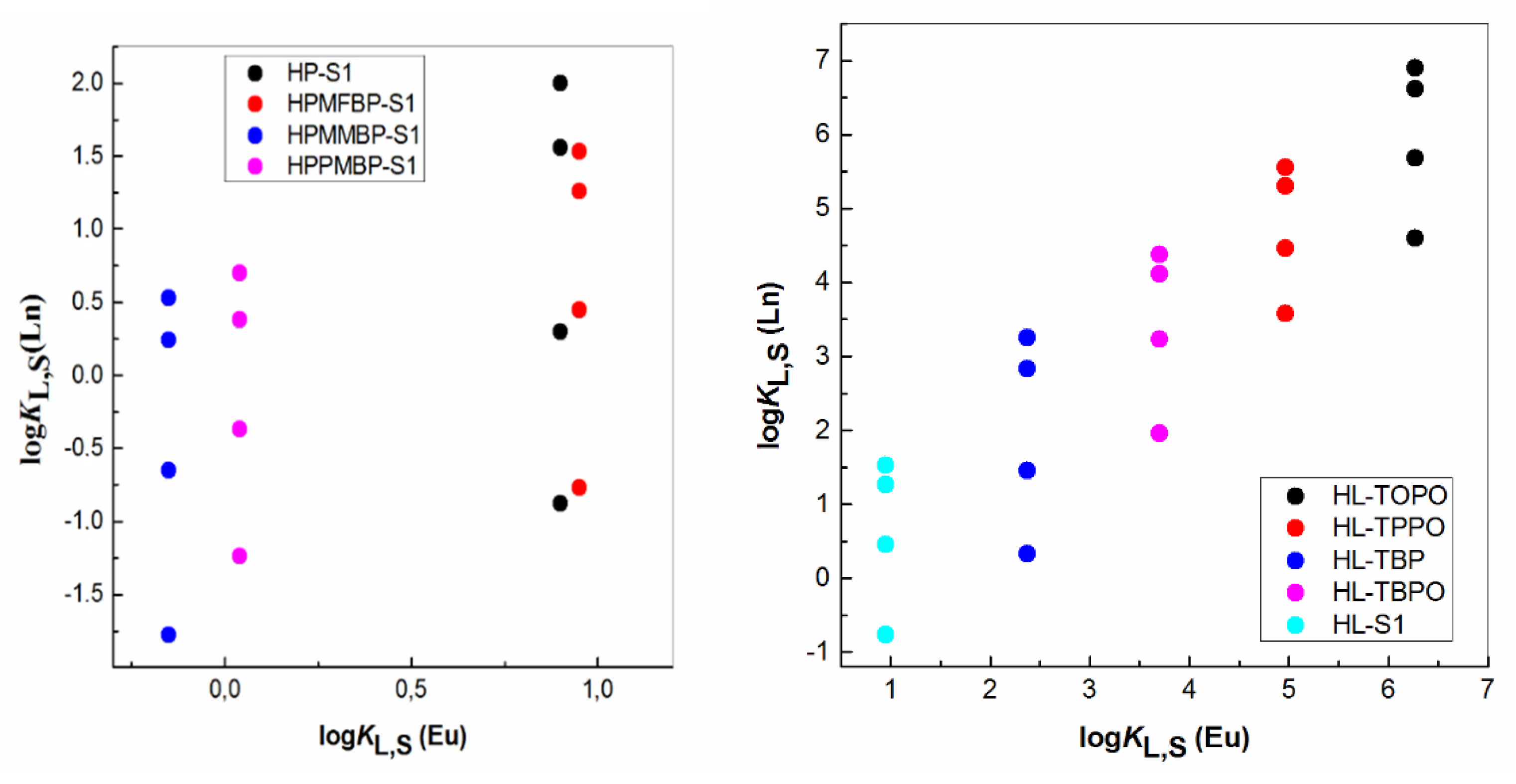
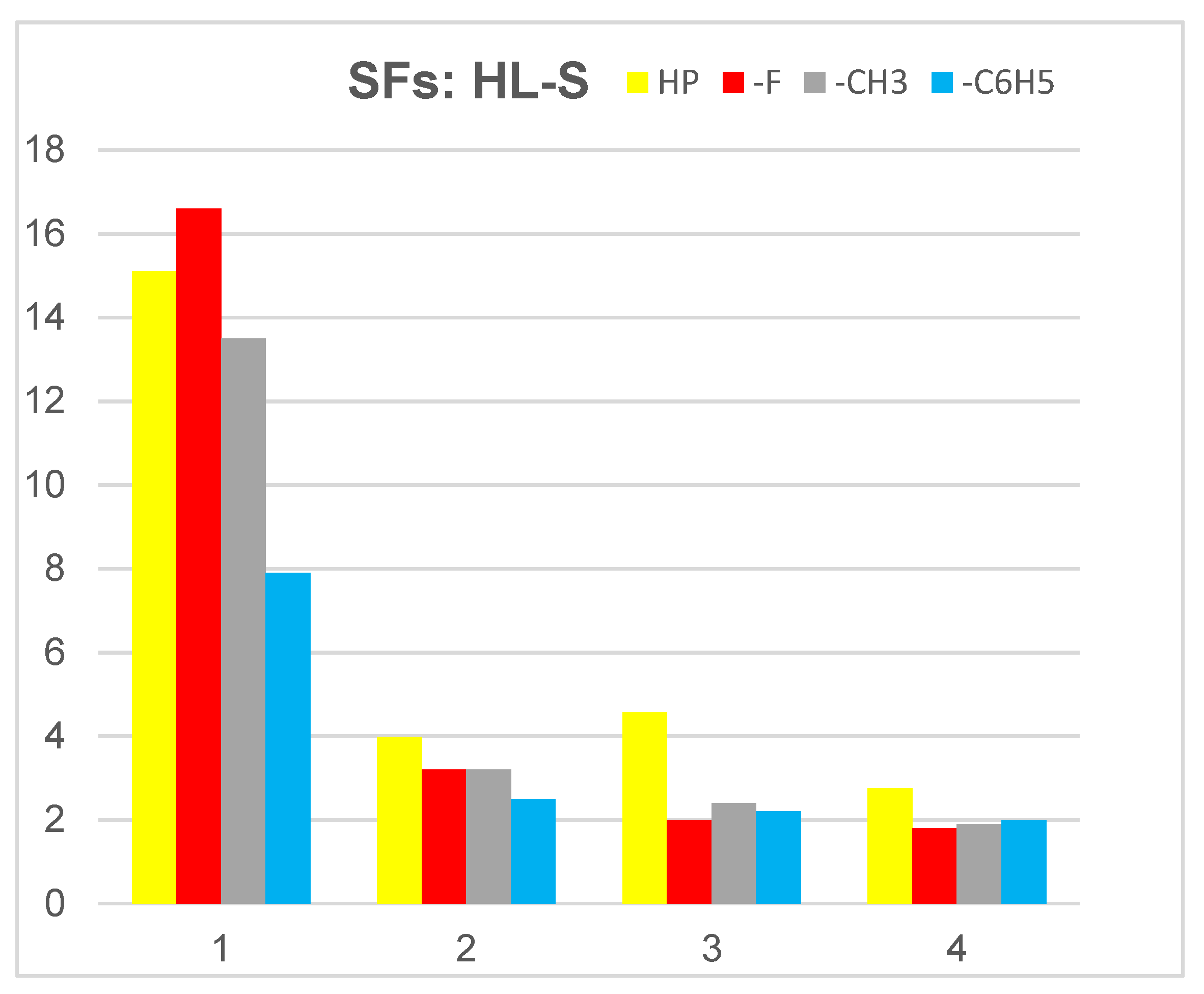

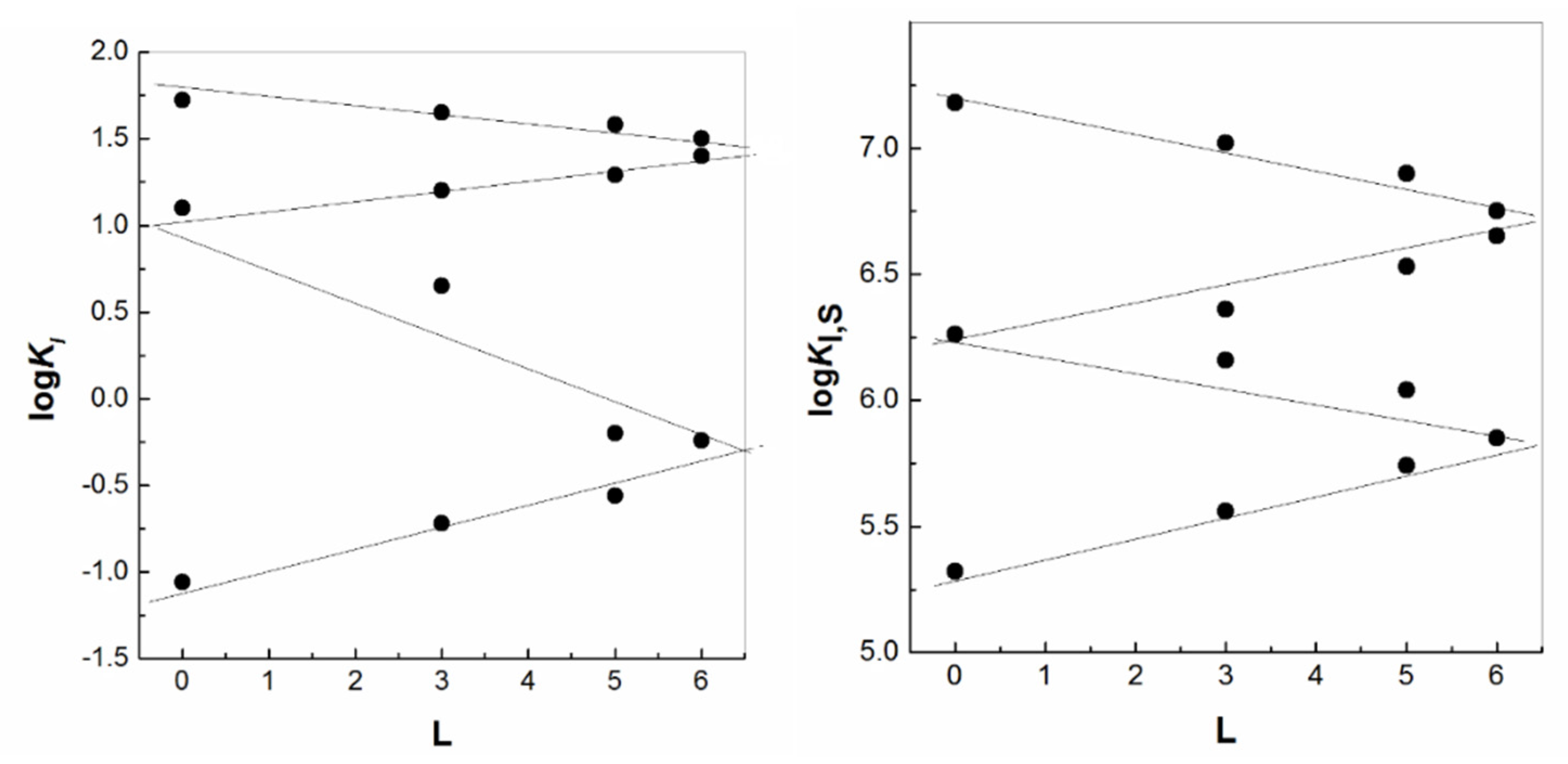

| IUPAC Ligand Name a | Acronym | Other Names b |
|---|---|---|
| 2,4-pentanedione | HA | acetylacetone |
| 1-phenyl-1,3-butanedione | HBA | benzoylacetone |
| 4,4,4-trifluoro-1-(2-thienyl)-1,3-butanedione | HTTA | 2-thenoyltrifluoroacetone |
| 4,4,4-trifluoro-1-phenyl-1,3-butanedione | HBFA | benzoyltrifluoroacetone |
| HFTA | froyltrifluoroacetone | |
| 1,1,1-trifluoro-2,4-pentdione | HTAA | trifluoroacetylacetone |
| 4-benzoyl-3-mehyl-1-phenyl-pyrazolin-5-one | HPMBP, HP | 1-phenyl-3-mehyl-4-benzoyl-pyrazol-5 |
| 3-methyl-1-phenyl-4-(4-trifluoromethylbenzoyl)-pyrazol-5-one | HPMTFBP | |
| 3-methyl-1-phenyl-4-(4-phenylbenzoyl)-pyrazol-5-one | HPMPBP | |
| 3-methyl-4-(4-methylbenzoyl)-1-phenyl-pyrazol-5-one | HPMMBP | |
| 4-(4-fluorobenzoyl)-3-methyl-1-phenyl-pyrazol-5-one | HPMFBP | |
| 4-benzoyl-3-phenyl-5-isoxazolone | HPBI | 4-benzoyl-3-phenyl-5-isoxazolone |
| HQ | 8-hydroxyquinoline | |
| tri-n-butyl phosphate | TBP | tributyl phosphate |
| tri-n-butylphosphine oxide | TBPO | tributylphosphine oxide |
| tri-n-octylphosphine oxide | TOPO | |
| TPPO | triphenylphosphine oxide | |
| dihexyl-N,N-diethylcarbamoylmethyl phosphonate | CMP | |
| N,N-diisobutyl-2-[octyl(phenyl)phosphoryl]acetamide | CMPO | octyl(phenyl)-N,N-diisobutylcarbaoylmethyl phosphine oxide |
| 5,11,17,23-tert-butyl-25,26,27,28-tetrakis(dimethylphosphinoylmethoxy)calix[4]arene | S1 | |
| 5,11,17,23-tetra(para-tert-octyl)-25,26,27,28-tetrakis(dimethylphosphinoylmethoxy)calix[4]arene | S2 | |
| 5,11,17,23,29,35-hexa(1,1,3,3-tetramethyl-butyl)-37,38,39,40,41,42-hexakis (dimethylphosphinoylmethyleneoxy)-calix[6]arene | S3 | |
| 5,11,17,23,29,35,41,47-octa (1,1,3,3-tetramethylbutyl)-49,50,51,52,53,54,55,56-octakis(dimethylphosphinoylmethyleneoxy)cali[8]arene | S4 | |
| 5,11,17,23-tetra-tert-butyl-25,26,27-tris(dimethylphosphinoylpropoxy)-28-hydroxy-calix[4]arene | S5 | |
| 5,11,17,23-tetra-tert-butyl-25,27-bis(dimethylphosphinoylpropoxy)-26,28-dihydroxy-calix[4]arene | S6 | |
| 5,11,17,23-tert-butyl-25,26,27,28-tetrakis(2-dimethylamino-2-oxoethyl)calix[4]arene | S7 | tert-butylcalix[4]arene tetrakis(N,N-dimethylacetamide) |
| 5,11,17,23-tert-butyl-25,26,27,28-tetrakis(2-ethoxy-2-oxoethyl)calix[4]arene | S8 | 4-tert-butylcalix[4]arene-tetraacetic acid tetraethyl ester |
| methyltri-n-octylammonium chloride, tricaprylylmethylammonium chloride | QCl, QClO4 | Aliquat 336 |
| di-n-butyl sulfoxide | DBSO | dibutyl sulfoxide |
| 3-(2-ethylhexylsulfinylmethyl)heptane | B2EHSO | bis-2-ethylhexyl sulfoxide |
| 2H-chromen-2-one | coumarin, 1,2-benzopyrone, 1-benzopyran-2-one | |
| naphtalen-1-ol | α-naphtol, 1-hydroxynapthalene | |
| 2-[2-(dioctylamino)-2-oxoethoxy]-N,N-dioctylacetamide | TODGA | N,N,N’,N’-tetraoctyldiglycilamide |
| Cation | I (mol dm−3) | Diluents | Equilibrium | LogK | Refs. |
|---|---|---|---|---|---|
| Eu | cyclohexane | Eu(TTA)3 | −7.66 P | [110] | |
| Eu(TTA)3·2TBP | 1.78 P | ||||
| 152,154Eu | 0.1 NaClO4 | n-hexane | Eu(TTA)3·TBP | 5.87 P | [111] |
| n-heptane | 6.27 P | ||||
| cyclohexane | 6.08 P | ||||
| methylenechloride | 3.32 P | ||||
| chloroform | 3.40 P | ||||
| benzene | 4.70 P | ||||
| CCl4 | 5.05 P | ||||
| bromoform | 3.66 P | ||||
| toluene | 4.84 P | ||||
| isopropylbenzene | 4.98 P | ||||
| chlorobenzene | 4.48 P | ||||
| o-dichlorobenzene | 4.30 P | ||||
| n-hexane | Eu(TTA)3·2TBP | 10.78 P | |||
| n-heptane | 11.14 P | ||||
| cyclohexane | 1.96 P | ||||
| methylene chloride | 5.24 P | ||||
| chloroform | 5.20 P | ||||
| benzene | 8.00 P | ||||
| CCl4 | 8.40 P | ||||
| bromoform | 5.62 P | ||||
| toluene | 7.98 P | ||||
| isopropylbenzene | 8.56 P | ||||
| chlorobenzene | 7.26 P | ||||
| o-dichlorobenzene | 7.20 P | ||||
| Ce | 1 NaClO4 | CCl4 | Ln(TTA)3 | −3.89 ± 0.10 P | [112] |
| Pr | −3.74 ± 0.10 P | ||||
| Nd | −3.45 ± 0.09 P | ||||
| Pm | −2.92 ± 0.07 | ||||
| Sm | −3.06 ± 0.10 P | ||||
| Gd | −2.44 ± 0.07 | ||||
| Tb | −2.48 ± 0.04 | ||||
| Dy | −2.56 ± 0.06 | ||||
| Ho | −2.39 ± 0.05 | ||||
| Er | −2.23 ± 0.05 | ||||
| Tm | −2.01 ± 0.07 | ||||
| Yb | −1.79 ± 0.04 | ||||
| Lu | −1.62 ± 0.04 | ||||
| Y | −2.32 ± 0.09 P | ||||
| Pr | −0.59 ± 0.06 | ||||
| Nd | −0.55 ± 0.08 | ||||
| Pm | −0.17 ± 0.07 | ||||
| Sm | 0.05 ± 0.06 | ||||
| Gd | 0.14 ± 0.07 | ||||
| Tb | 0.04 ± 0.05 | ||||
| Dy | 0.03 ± 0.08 | ||||
| Ho | 0.09 ± 0.07 | ||||
| Er | −0.16 ± 0.1 P | ||||
| Tm | −0.17 ± 0.13 P | ||||
| Yb | −0.33 ± 0.11 P | ||||
| Lu | −0.67 ± 0.16 P | ||||
| Y | −0.24 ± 0.11 P | ||||
| Sc | 0.1 NaClO4 | CHCl3 | Sc(TTA)3 | −1.30 P | [113] |
| Sc | 0.1 NaClO4 | CHCl3 | Sc(acac)3 | −6.35 P | [113] |
| Sc | 1 NaClO4 | CCl4 | Ln(TTA)3 | −0.81 P | [114] |
| La | −10.95 P | ||||
| Eu | −8.57 P | ||||
| Lu | −7.43 P | ||||
| Sc | 1 NaClO4 | CCl4 | Ln(TTA)3·TBP | 3.44 P | [114] |
| La | 4.83 ± 0.27 P | ||||
| Eu | 5.15 ± 0.013 | ||||
| Lu | 5.69 ± 0.05 | ||||
| La | Ln(TTA)3·2TBP | 9.33 ± 0.11 P | |||
| Eu | 8.89 ± 0.08 | ||||
| Lu | 6.67 ± 0.29 P | ||||
| Eu | 0.1 NaClO4 | CHCl3 | Eu(TTA)3·TBP | 3.63 P | [115] |
| Eu(TTA)3·2TBP | 5.40 P | ||||
| CCl4 | Eu(TTA)3·TBP | 5.36 P | |||
| Eu(TTA)3·2TBP | 8.96 P | ||||
| CHCl3 | Eu(TTA)3·TOPO | 5.40 P | |||
| Eu(TTA)3·2TOPO | 7.60 P | ||||
| CCl4 | Eu(TTA)3·TOPO | 7.49 P | |||
| Eu(TTA)3·2TOPO | 12.26 P | ||||
| Tm | C6H6 | Tm(TTA)3 | −6.96 P | [28] | |
| Tm(TTA)3·TBP | −2.61 P | ||||
| Tm(TTA)3·TOPO | −0.01 P | ||||
| Tm(TTA)3·2TBP | −0.34 P | ||||
| cyclohexane | Tm(TTA)3 | −5.60 P | |||
| Tm(TTA)3·2TBP | 2.62 P | ||||
| Tm(TTA)3·2TOPO | 5.72 P | ||||
| Eu | cyclohexane | Eu(TTA)3 | −7.66 P | ||
| Eu(TTA)3·2TBP | 1.78 P | ||||
| 152,154Eu | 0.1 NaClO4 | pentane | Eu(TTA)3·TOPO | 8.71 P | [116] |
| hexane | 8.56 P | ||||
| heptane | 8.68 P | ||||
| cyclohexane | 8.40 P | ||||
| isopropylbenzene | 7.84 P | ||||
| CCl4 | 8.04 P | ||||
| toluene | 7.40 P | ||||
| benzene | 7.19 P | ||||
| chloroform | 4.95 P | ||||
| chlorobenzene | 6.97 P | ||||
| methylene chloride | 5.91 P | ||||
| o-dichlorobenzene | 7.21 P | ||||
| bromoform | 6.00 P | ||||
| Eu(TTA)3·2TOPO | |||||
| pentane | 6.37 P | ||||
| hexane | 6.40 P | ||||
| heptane | 6.08 P | ||||
| cyclohexane: | 6.06 P | ||||
| isopropylbenzene | 5.20 P | ||||
| CCl4 | 4.84 P | ||||
| toluene | 4.62 P | ||||
| benzene | 4.59 P | ||||
| chloroform | 2.73 P | ||||
| chlorobenzene | 4.37 P | ||||
| methylene chloride | 4.23 P | ||||
| o-dichlorobenzene | 4.45 P | ||||
| bromoform | 3.04 P | ||||
| 169Yb | 1 NaNO3 | cyclohexane | Ln(TTA)3·TOPO | 2.4 ± 0.1 P | [117] |
| Ln(TTA)3·2TOPO | 6.88 ± 0.04 | ||||
| 140La | Ln(TTA)3·TOPO | −0.3 ± 0.2 | |||
| Ln(TTA)3·2TOPO | 4.67 ± 0.07 | ||||
| La | 0.2 NaClO4 | benzene | Ln(TTA)3 | −10.25 ± 0.01 | [118] |
| Ce | −9.04 ± 0.01 | ||||
| Pr | −8.41 ± 0.02 | ||||
| Nd | −8.33 ± 0.02 | ||||
| Sm | −7.99 ± 0.02 | ||||
| Eu | −7.79 ± 0.02 | ||||
| Gd | −7.70 ± 0.01 | ||||
| Tb | −7.21 ± 0.01 | ||||
| Dy | −6.98 ± 0.02 | ||||
| Ho | −6.88 ± 0.01 | ||||
| Er | −6.69 ± 0.01 | ||||
| Tm | −6.55 ± 0.01 | ||||
| Yb | −6.43 ± 0.01 | ||||
| La | Ln(TTA)3·2TPPO | −3.34 ± 0.01 | |||
| Ce | −3.14 ± 0.01 | ||||
| Pr | −2.99 ± 0.01 | ||||
| Nd | −2.94 ± 0.01 | ||||
| Sm | −2.72 ± 0.01 | ||||
| Eu | −2.65 ± 0.01 | ||||
| Gd | −2.60 ± 0.01 | ||||
| Tb | −2.54 ± 0.01 | ||||
| Dy | −2.50 ± 0.01 | ||||
| Ho | −2.46 ± 0.01 | ||||
| Er | −2.42 ± 0.02 | ||||
| Tm | −2.40 ± 0.01 | ||||
| Yb | −2.36 ± 0.02 | ||||
| La | 0.1–1 (Na,Li)Cl 0.01 acetate buffer | [C1C4im+][Tf2N−] | Ln(TTA)3 | −7.48 ± 0.07 | [119] |
| Nd | −6.21 ± 0.09 P | ||||
| Eu | −5.51 ± 0.03 | ||||
| Dy | −5.29 ± 0.07 | ||||
| Lu | −4.94 ± 0.06 | ||||
| La | 0.1–1 (Na,Li)Cl 0.01 acetate buffer | [C1C4im+][Tf2N−] | Ln(TTA)4− | −10.02 ± 0.06 | [119] |
| Nd | −8.37 ± 0.09 P | ||||
| Eu | −8.14 ± 0.05 | ||||
| Dy | −7.22 ± 0.08 | ||||
| Lu | −7.78 ± 0.15 P | ||||
| La | 0.1–1 (Na,Li)Cl 0.01 acetate buffer | [C1C4im+][Tf2N−] | Ln(TTA)3·2TOPO | −0.74 ± 0.18 P | [119] |
| Nd | 0.65 ± 0.17 P | ||||
| Nd | Ln(TTA)2·2TOPO | 3.00 ± 0.09 P | |||
| La | Ln(TTA)2·3TOPO | 4.60 ± 0.05 | |||
| Nd | 5.55 ± 0.15 P | ||||
| Eu | 7.22 ± 0.07 | ||||
| Dy | 7.69 ± 0.05 | ||||
| Lu | 8.08 ± 0.07 | ||||
| Lu | Ln(TTA)·3TOPO | 8.27 ± 0.14 P |
| Cation | I (mol dm−3) | Diluents | Equilibrium | logK | Refs. |
|---|---|---|---|---|---|
| Eu | 0.1 NaClO4 | CHCl3 | EuL3 | [106] | |
| L: acetylacetone | − | ||||
| L: benzoylacetone | −19 P | ||||
| L: trifluoroacetylacetone | − | ||||
| L: benzoyltrifluoroacetone | −9.47 P | ||||
| L: froyltrifluoroacetone | −8.73 P | ||||
| L: thenoyltrifluoroacetone | −8.68 P | ||||
| EuL3·TBP | |||||
| L: acetylacetone | 1.90 P | ||||
| L: benzoylacetone | 1.60 P | ||||
| L: trifluoroacetylacetone | 3.32 P | ||||
| L: benzoyltrifluoroacetone | 3.64 P | ||||
| L: froyltrifluoroacetone | 3.50 P | ||||
| L: thenoyltrifluoroacetone | 3.34 P | ||||
| EuL3·2TBP | |||||
| L: acetylacetone | − | ||||
| L: benzoylacetone | − | ||||
| L: trifluoroacetylacetone | 4.64 P | ||||
| L: benzoyltrifluoroacetone | 5.28 P | ||||
| L: froyltrifluoroacetone | 5.00 P | ||||
| L: thenoyltrifluoroacetone | 5.28 P | ||||
| 0.1 NaClO4 | C6H6 | LnL3, L: benzoyltrifluoroacetone | [120] | ||
| La | −11.6 P | ||||
| Eu | −8.9 P | ||||
| Tb | −8.8 P | ||||
| Lu | −7.7 P | ||||
| La | LnL3·TBP | 4.38 P | |||
| Eu | 4.55 P | ||||
| Tb | 4.50 P | ||||
| Lu | 4.70 P | ||||
| La | LnL3·2TBP | 7.80 P | |||
| Eu | 7.40 P | ||||
| Tb | 7.30 P | ||||
| Lu | 6.00 P | ||||
| La | LnL3·TOPO | 7.00 P | |||
| Eu | 6.85 P | ||||
| Tb | 6.90 P | ||||
| Lu | 7.50 P | ||||
| La | LnL3·2TOPO | 12.30 P | |||
| Eu | 11.70 P | ||||
| Tb | 11.20 P | ||||
| Lu | − | ||||
| La | 0.1 NaCl | C6H6 | LnL3; L: 4,4,4-trifluoro-1-(biphenyl-4-yl)butane-1,3-dione | −6.40 ± 0.05 | [121] |
| Ce | −5.59 ± 0.05 | ||||
| Pr | −5.17 ± 0.05 | ||||
| Nd | −4.76 ± 0.05 | ||||
| Sm | −4.36 ± 0.05 | ||||
| Eu | −4.06 ± 0.05 | ||||
| Gd | −3.75 ± 0.05 | ||||
| La | LnL3·2TOPO | 1.82 ± 0.05 | |||
| Ce | 2.57 ± 0.05 | ||||
| Pr | 2.92 ± 0.05 | ||||
| Nd | 3.12 ± 0.05 | ||||
| Sm | 3.33 ± 0.05 | ||||
| Eu | 3.50 ± 0.05 | ||||
| Gd | 3.76 ± 0.05 | ||||
| La | LnL3·2TBPO | −0.36 ± 0.05 | |||
| Ce | 0.32 ± 0.05 | ||||
| Pr | 0.58 ± 0.05 | ||||
| Nd | 1.02 ± 0.05 | ||||
| Sm | 1.35 ± 0.05 | ||||
| Eu | 1.62 ± 0.05 | ||||
| Gd | 1.97 ± 0.05 | ||||
| La | LnL3·TPPO | 0.92 ± 0.05 | |||
| Ce | 1.09 ± 0.05 | ||||
| Pr | 1.35 ± 0.05 | ||||
| Nd | 1.63 ± 0.05 | ||||
| Sm | 2.14 ± 0.05 | ||||
| Eu | 2.34 ± 0.05 | ||||
| Gd | 2.52 ± 0.05 | ||||
| La | 0.1 NaClO4 0.01 CH3COONa | CHCl3 | LnL3 L:2-trifluoroacetyl-cyclopentanone | −13.26 P | [122] |
| Pr | −11.80 P | ||||
| Eu | −10.81 P | ||||
| Ho | −10.34 P | ||||
| Yb | −9.62 P | ||||
| Pr | LnL3; L:2-trifluoroacetyl-cyclohaxanone | −17.27 P | |||
| Eu | −16.52 P | ||||
| Ho | −15.74 P | ||||
| Yb | −14.61 P | ||||
| Pr | LnL3 L:2-trifluoroacetyl-cycloheptanone | −17.55 P | |||
| Eu | −16.23 P | ||||
| Ho | −15.60 P | ||||
| Yb | −14.56 P | ||||
| La | ML3·TOPO L:2-trifluoroacetyl-cyclopentanone | −6.19 P | |||
| Pr | −5.52 P | ||||
| Eu | −5.25 P | ||||
| Ho | −4.41 P | ||||
| Yb | −4.48 P | ||||
| Pr | ML3·TOPO L:2-trifluoroacetyl-cyclohaxanone | −11.51 P | |||
| Eu | −10.68 P | ||||
| Ho | −9.22 P | ||||
| Yb | −8.25 P | ||||
| Pr | ML3·TOPO L:2-trifluoroacetyl-cycloheptanone | −11.13 P | |||
| Eu | −9.87 P | ||||
| Ho | −9.22 P | ||||
| Yb | −7.90 P | ||||
| La | 0.1 NaCl | CHCl3 | LnL3, L: HTTA | −11.06 ± 0.05 | [123] |
| Nd | −10.12 ± 0.05 | ||||
| Eu | −8.68 ± 0.05 | ||||
| Ho | −8.56 ± 0.05 | ||||
| Lu | −8.15 ± 0.05 | ||||
| La | 0.1 NaCl | CHCl3 | LnL3∙2S; S: S1, (Figure 6) | −3.22 ± 0.06 | |
| Nd | −1.89 ± 0.06 | ||||
| Eu | −1.07 ± 0.06 | ||||
| Ho | −0.50 ± 0.06 | ||||
| Lu | 0.08 ± 0.06 | ||||
| La | 0.1 NaClO4 | CCl4 | LnL3; L: HTTA | −10.50 ± 0.05 | [124] |
| Ce | −9.99 ± 0.05 | ||||
| Pr | −9.53 ± 0.05 | ||||
| Nd | −9.35 ± 0.05 | ||||
| Sm | −8.68 ± 0.05 | ||||
| Eu | −8.55 ± 0.05 | ||||
| Gd | −8.40 ± 0.05 | ||||
| Tb | −8.22 ± 0.05 | ||||
| Dy | −7.98 ± 0.05 | ||||
| Ho | −7.87 ± 0.05 | ||||
| Er | −7.76 ± 0.05 | ||||
| Tm | −7.40 ± 0.05 | ||||
| Yb | −7.14 ± 0.05 | ||||
| Lu | −6.99 ± 0.05 | ||||
| La | LnL3·2S; S: S7, (Figure 6) | 1.29 ± 0.05 | |||
| Ce | 1.74 ± 0.05 | ||||
| Pr | 1.90 ± 0.05 | ||||
| Nd | 2.10 ± 0.05 | ||||
| Sm | 2.38 ± 0.05 | ||||
| Eu | 2.61 ± 0.05 | ||||
| Gd | 2.78 ± 0.05 | ||||
| Tb | 2.92 ± 0.05 | ||||
| Dy | 3.03 ± 0.05 | ||||
| Ho | 3.11 ± 0.05 | ||||
| Er | 3.23 ± 0.05 | ||||
| Tm | 3.33 ± 0.05 | ||||
| Yb | 3.47 ± 0.05 | ||||
| Lu | 3.60 ± 0.05 | ||||
| La | 0.1 NaClO4 | CCl4 | Ln(TTA)3·S; S: S8, (Figure 6) | −5.69 ± 0.05 | [125] |
| Ce | −5.41 ± 0.05 | ||||
| Pr | −5.22 ± 0.05 | ||||
| Nd | −5.08 ± 0.05 | ||||
| Sm | −4.84 ± 0.05 | ||||
| Eu | −4.65 ± 0.05 | ||||
| Gd | −4.50 ± 0.05 | ||||
| Tb | −4.34 ± 0.05 | ||||
| Dy | −4.15 ± 0.05 | ||||
| Ho | −3.94 ± 0.05 | ||||
| Er | −3.76 ± 0.05 | ||||
| Tm | −3.59 ± 0.05 | ||||
| Yb | −3.38 ± 0.05 | ||||
| Lu | −3.28 ± 0.05 | ||||
| La | 1 × 10−2 buffer | [C1C4im+][Tf2N−] | Ln(ba)2+; L: benzoylacetone | −12.65 ± 0.05 | [126] |
| Nd | −11.11 ± 0.08 | ||||
| Eu | −10.85 ± 0.05 | ||||
| Dy | −10.08 ± 0.08 | ||||
| Lu | −9.68 ± 0.21 | ||||
| La | LnL3; L: benzoylacetone | −18.03 ± 0.03 | |||
| Nd | −16.69 ± 0.14 | ||||
| Eu | −15.61 ± 0.06 | ||||
| Dy | −15.35 ± 0.15 | ||||
| Lu | −13.62 ± 0.13 | ||||
| La | 1 × 10−2 buffer | [C1C4im+][Tf2N−] | LnL2(TOPO)2+ L: benzoylacetone | −5.69 ± 0.07 | [126] |
| Nd | −3.93 ± 0.05 | ||||
| Eu | −2.66 ± 0.06 | ||||
| Dy | −1.93 ± 0.14 | ||||
| La | LnL(TOPO)4+; L: benzoylacetone | 3.85 ± 0.05 | |||
| Nd | 5.37 ± 0.05 | ||||
| Eu | 6.01 ± 0.06 | ||||
| Dy | 7.11 ± 0.17 | ||||
| Lu | 9.26 ± 0.05 |
| Cation | I (mol dm−3) | Diluents | Equilibrium | logK | Refs. |
|---|---|---|---|---|---|
| Eu | 0.1 NaClO4 | C6H6 | EuL3 L: 1-phenyl-3-methyl-4-acylpyrazol-5-one | [132] | |
| R: phenyl (see Figure 4) | −4.50 P | ||||
| R: 2-chlorophenyl | −2.55 P | ||||
| R: 2,4-dichlorophenyl | −2.28 P | ||||
| R: 4-chlorophenyl | −3.45 P | ||||
| R: 2-methylphenyl | −3.12 P | ||||
| R: 3-methylphenyl | −3.90 P | ||||
| R: 4-methylphenyl | −4.32 P | ||||
| R: 2-naphthyl | −3.75 P | ||||
| R: cyclohexyl | −6.00 P | ||||
| R: n-heptyl | −6.00 P | ||||
| R: methyl | −6.12 P | ||||
| R: trifluoromethyl | −2.61 P | ||||
| Sc | 0.1 NaClO4 | C6H6 | EuL3 2-methylphenyl | −4.26 P | [132] |
| R: 3-methylphenyl | −3.54 P | ||||
| R: 4-methylphenyl | −3.12 P | ||||
| La | 0.1 NaClO4 | CHCl3 | LnL3 L: 1-phenyl-3-methyl-4-(trifluoroacetyl)-5-pyrazolone | −6.18 P | [133] |
| Pr | −4.98 P | ||||
| Eu | −3.78 P | ||||
| Ho | −3.36 P | ||||
| Yb | −3.15 P | ||||
| La | LnL3·2TOPO | 4.20 P | [133] | ||
| Pr | 5.19 P | ||||
| Eu | 6.06 P | ||||
| Ho | 6.08 P | ||||
| Yb | 5.68 P | ||||
| La | LnL3·CMPO | 1.47 P | [133] | ||
| Pr | 2.61 P | ||||
| Eu | 3.43 P | ||||
| Ho | 3.54 P | ||||
| Yb | 3.42 P | ||||
| La | LnL3·MBDPO (methylenebis) (diphenylphosphine oxide) | 3.81 P | [133] | ||
| Pr | 4.89 P | ||||
| Eu | 5.64 P | ||||
| Ho | 5.52 P | ||||
| Yb | 5.34 P | ||||
| La | LnL2(ClO4)·2MBDPO | 8.10 P | [133] | ||
| Pr | 8.96 P | ||||
| Eu | 9.36 P | ||||
| Ho | 8.74 P | ||||
| Yb | 8.15 P | ||||
| 140La a | 0.01 chloroacetate buffer pH = 2.7 | xylene | ML3·HL L: 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5 | −4.41 ± 0.09 | [134] |
| 152,154Eu | −2.11 ± 0.04 | ||||
| 177Lu | −1.86 ± 0.04 | ||||
| 140La | 0.01 chloroacetate buffer pH = 2.7 | ML3·CMPO L: 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5 | −0.49 ± 0.01 | [134] | |
| 152,154Eu | 1.75 ± 0.04 | ||||
| 177Lu | 1.92 ± 0.04 | ||||
| La b | 0.01 chloroacetate buffer solutions, pH = 2.70 | xylene | ML3·HL L: 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5 | −4.41 ± 0.09 P | [135] |
| Eu | −2.11 ± 0.04 | ||||
| Lu | −1.86 ± 0.04 | ||||
| La | 0.01 chloroacetate buffer solutions, pH = 2.70 | xylene | ML3·CMP L: 1-phenyl-3-methyl-4-benzoyl-pyrazolone-5 | −0.76 ± 0.01 | [135] |
| Eu | 1.42 ± 0.01 | ||||
| Lu | 1.27 ± 0.01 | ||||
| 147Nd | 0.01 chloroacetate buffer pH = 2.7 | CHCl3 | LnL3 L: 1-phenyl-3-methyl-4-trifluoroacetl-pyrazolone-5 | 1.98 × 10−4 | [136] |
| 152,154Eu | 1.11 × 10−3 | ||||
| 177Lu | 2.74 × 10−3 | ||||
| 147Nd | LnL3·TPPO | 1.49 × 101 [(mol dm3)−1] | [136] | ||
| 152,154Eu | 1.5 × 102 [(mol dm3)−1] | ||||
| 177Lu | 4.7 × 102 [(mol dm3)−1] | ||||
| 47Nd | LnL3·2TPPO | 9.30 × 101 [(mol dm3)−2] | |||
| 152,154Eu | 5.47 × 105 [(mol dm3)−2] | ||||
| 177Lu | 2.7 × 106 [(mol dm3)−2] | ||||
| Lu | 0.1 NaClO4 | C6H6 | LuL3 L: ortho-substituted 1-phenyl-3-methyl-4-aroyl-5-pyrazolones | [137] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | −3.90 P | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | −2.67 P | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | −3.36 P | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | −2.31 P | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | −2.85 P | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | −1.74 P | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | −2.16 P | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | −2.58 P | ||||
| Lu | 0.1 NaClO4 | C6H6 | LuL3·TOPOL: ortho-substituted 1-phenyl-3-methyl-4-aroyl-5-pyrazolones | [137] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | 6.69 P | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | 6.50 P | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | 6.03 P | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | 6.70 P | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | 7.06 P | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | 6.65 P | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | 6.58 P | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | 6.97 P | ||||
| Lu | 0.1 NaClO4 | C6H6 | LuL3·2TOPO L: ortho-substituted 1-phenyl-3-methyl-4-aroyl-5-pyrazolones | [137] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | 2.15 P | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | 1.52 P | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | 2.43 P | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | 1.49 P | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | 2.22 P | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | 1.85 P | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | 1.71 P | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | 2.48 P | ||||
| La c | 0.1 NaClO4 | C6H6 | ML3 L: ortho-substitited 4-aroylpyrazol-5-ones | [138] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | −7.29 ± 0.03 | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | −6.48 ± 0.09 P | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | −7.05 ± 0.11 P | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | − | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | −6.33 ± 0.08 | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | −5.64 ± 0.10 P | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | −5.46 ± 0.01 | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | −5.85 ± 0.06 | ||||
| La | 0.1 NaClO4 | C6H6 | ML3·TOPO / ML3·2TOPO L: ortho-substitited 4-aroylpyrazol-5-ones | [138] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | 6.40 ± 0.06/ 3.70 ± 0.08 | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | 5.97 ± 0.08/ 3.65 ± 0.09 | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | 5.52 ± 0.04/ 3.73 ± 0.04 | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | − | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | 6.41 ± 0.05/ 3.73 ± 0.06 | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | 5.68 ± 0.09/ 3.82 ± 0.11 P | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | 5.67 ± 0.09/ 3.68 ± 0.11 P | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | 6.07 ± 0.06/ 3.82 ± 0.08 | ||||
| Sc | 0.1 NaClO4 | C6H6 | ML3 L: ortho-substitited 4-aroylpyrazol-5-ones | [138] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | 3.48 ± 0.04 | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | 4.32 ± 0.05 | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | 3.39 ± 0.04 | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | 4.20 ± 0.04 | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | 3.63 ± 0.03 | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | 4.20 ± 0.04 | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | 4.35 ± 0.02 | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | 3.69 ± 0.04 | ||||
| Sc | 0.1 NaClO4 | C6H6 | ML3·TOPO / ML3·2TOPO L: ortho-substitited 4-aroylpyrazol-5-ones | [138] | |
| 1-phenyl-3-methyl-4-benzoylpyrazol-5-one | 2.81 ± 0.41/ 3.66 ± 0.41 P | ||||
| 1-phenyl-3-methyl-4-(2-toluoyl)pyrazol-5-one | 2.84 ± 0.22/ 3.19 ± 0.22 P | ||||
| 1-phenyl-3-methyl-4-(2-methoxybenzoyl)pyrazol-5-one | 2.71 ± 0.22/ 3.29 ± 0.22 P | ||||
| 1-phenyl-3-methyl-4-(2-trifluoromethylbenzoyl)pyrazol-5-one | 3.38 ± 0.06/ 2.91 ± 0.06 | ||||
| 1-phenyl-3-methyl-4-(2-fluorobenzoyl)pyrazol-5-one | 3.90 ± 0.08/ 3.04 ± 0.09 P | ||||
| 1-phenyl-3-methyl-4-(2-chlorobenzoyl)pyrazol-5-one | 3.78 ± 0.05/ 2.75 ± 0.06 | ||||
| 1-phenyl-3-methyl-4-(2-bromobenzoyl)pyrazol-5-one | 3.66 ± 0.03/ 2.54 ± 0.04 | ||||
| 1-phenyl-3-methyl-4-(2,6-difluorobenzoyl)pyrazol-5-one | 4.23 ± 0.05/ 2.78 ± 0.06 | ||||
| La | 0.1 NaCl | C6H6 | LnL3·HL L: 4-(4-fluorobenzoyl)-3-methyl-1-phenyl-pyrazol-5-one | −4.74 ± 0.05 | [64] |
| Nd | −2.76 ± 0.05 | ||||
| Eu | −2.37 ± 0.05 | ||||
| Ho | −2.06 ± 0.05 | ||||
| Lu | −1.58 ± 0.05 | ||||
| La | 0.1 NaCl | C6H6 | LnL3·2TOPO L: HPMFBP | 4.60 ± 0.05 | [64] |
| Nd | 5.68 ± 0.05 | ||||
| Eu | 6.27 ± 0.05 | ||||
| Ho | 6.62 ± 0.05 | ||||
| Lu | 6.90 ± 0.05 | ||||
| La | 0.1 NaCl | C6H6 | LnL3·2TPPO L: HPMFBP | 3.58 ± 0.05 | [64] |
| Nd | 4.46 ± 0.05 | ||||
| Eu | 4.97 ± 0.05 | ||||
| Ho | 5.30 ± 0.05 | ||||
| Lu | 5.56 ± 0.05 | ||||
| La | 0.1 NaCl | C6H6 | LnL3·2TBP L: HPMFBP | 0.33 ± 0.05 | [64] |
| Nd | 1.45 ± 0.05 | ||||
| Eu | 2.37 ± 0.05 | ||||
| Ho | 2.83 ± 0.05 | ||||
| Lu | 3.25 ± 0.05 | ||||
| La | 0.1 NaCl | C6H6 | LnL3·2TBPO L: HPMFBP | 1.96 ± 0.05 | [64] |
| Nd | 3.23 ± 0.05 | ||||
| Eu | 3.70 ± 0.05 | ||||
| Ho | 4.12 ± 0.05 | ||||
| Lu | 4.38 ± 0.05 | ||||
| Nd | 1 NaNO3 | CHCl3 | 4-sebacoylbis(1-phenyl-3-methyl-5-pyrazolone) | −5.64 ± 0.04 | [139] |
| Eu | −5.21 ± 0.03 | ||||
| Tm | −4.79 ± 0.04 | ||||
| Nd | TOPO | −2.90 ± 0.03 | |||
| Eu | −2.36 ± 0.02 | ||||
| Tm | −1.15 ± 0.03 | ||||
| Nd | TBP | −4.32 ± 0.02 | |||
| Eu | −3.76 ± 0.03 | ||||
| Tm | −2.80 ± 0.02 | ||||
| Nd | CMPO | −2.92 ± 0.02 | |||
| Eu | −2.40 ± 0.03 | ||||
| Tm | −1.60 ± 0.02 |
| Cation | I (mol dm−3) | Diluents | Equilibrium | logK | Refs. |
|---|---|---|---|---|---|
| La | 0.1 NaCl | CHCl3 | LnL3∙HL L: HPMBP | −5.84 ± 0.05 | [140] |
| Nd | −4.35 ± 0.05 | ||||
| Eu | −3.42 ± 0.05 | ||||
| Ho | −3.24 ± 0.05 | ||||
| Lu | −2.83 ± 0.05 | ||||
| La | LnL3∙S S: S1, (Figure 6) | −0.88 ± 0.05 | |||
| Nd | 0.30 ± 0.05 | ||||
| Eu | 0.90 ± 0.05 | ||||
| Ho | 1.56 ± 0.05 | ||||
| Lu | 2.00 ± 0.05 | ||||
| La a | 0.1 NaCl | CHCl3 | LnL3·S L: HPMBP; S: S4, (Figure 6) | −0.73 ± 0.07 | [141] |
| Nd | 0.42 ± 0.07 | ||||
| Eu | 1.07 ± 0.07 | ||||
| Ho | 1.48 ± 0.07 | ||||
| Lu | 1.94 ± 0.07 | ||||
| La | 0.1 NaCl | CHCl3 | LnL3·S L: HPMBP; S: S2, (Figure 6) | −0.73 ± 0.05 | [142] |
| Nd | 0.64 ± 0.05 | ||||
| Eu | 1.60 ± 0.05 | ||||
| Ho | 2.08 ± 0.05 | ||||
| Lu | 2.48 ± 0.05 | ||||
| La | 0.1 NaCl | CHCl3 | LnL3·S L: HPMBP; S: S3, (Figure 6) | 2.15 ± 0.05 | [143] |
| Nd | 2.93 ± 0.05 | ||||
| Eu | 3.37 ± 0.05 | ||||
| Ho | 3.76 ± 0.05 | ||||
| Lu | 4.23 ± 0.05 | ||||
| La | 0.1 NaCl | CHCl3 | LnL3·HL L: HPMFBP | −5.16 ± 0.05 | [144] |
| Nd | −3.91 ± 0.05 | ||||
| Eu | −3.37 ± 0.05 | ||||
| Ho | −2.84 ± 0.05 | ||||
| Lu | −2.62 ± 0.05 | ||||
| La | LnL3·S S: S1, (Figure 6) | −0.77 ± 0.05 | |||
| Nd | 0.45 ± 0.05 | ||||
| Eu | 0.95 ± 0.05 | ||||
| Ho | 1.26 ± 0.05 | ||||
| Lu | 1.53 ± 0.05 | ||||
| La | LnL3·HL; L: HPMMBP | −6.12 ± 0.05 | |||
| Nd | −4.17 ± 0.05 | ||||
| Eu | −3.89 ± 0.05 | ||||
| Ho | −3.37 ± 0.05 | ||||
| Lu | −3.12 ± 0.05 | ||||
| La | LnL3·S; S: S1, (Figure 6) | −1.78 ± 0.05 | |||
| Nd | −0.65 ± 0.05 | ||||
| Eu | −0.15 ± 0.05 | ||||
| Ho | 0.24 ± 0.05 | ||||
| Lu | 0.53 ± 0.05 | ||||
| La | 0.1 NaCl | CHCl3 | LnL3·HL;L:HPMTFBP | −3.24 ± 0.05 | [145] |
| Nd | −2.74 ± 0.05 | ||||
| Eu | −2.47 ± 0.05 | ||||
| Ho | −1.88 ± 0.05 | ||||
| Lu | −1.62 ± 0.05 | ||||
| La | LnL3·S; S: S5, (Figure 6) | 1.22 ± 0.05 | |||
| Nd | 2.16 ± 0.05 | ||||
| Eu | 2.54 ± 0.05 | ||||
| Ho | 2.86 ± 0.05 | ||||
| Lu | 3.14 ± 0.05 | ||||
| La | LnL3·S; S: S6, (Figure 6) | 1.14 ± 0.05 | |||
| Nd | 2.02 ± 0.05 | ||||
| Eu | 2.40 ± 0.05 | ||||
| Ho | 2.67 ± 0.05 | ||||
| Lu | 2.97 ± 0.05 | ||||
| La | 0.1 NaCl | CHCl3 | LnL3·HL L: HPMPBP | −6.21 ± 0.05 | [146] |
| Nd | −5.84 ± 0.05 | ||||
| Eu | −5.57 ± 0.05 | ||||
| Ho | −4.97 ± 0.05 | ||||
| Lu | −4.58 ± 0.05 | ||||
| La | LnL3·S; S: S1, (Figure 6) | −1.27 ± 0.05 | |||
| Nd | −0.37 ± 0.05 | ||||
| Eu | 0.03 ± 0.05 | ||||
| Ho | 0.38 ± 0.05 | ||||
| Lu | 0.69 ± 0.05 |
| Cation | I (mol dm−3) | Diluents | Equilibrium | logK | Refs. |
|---|---|---|---|---|---|
| Pr | 0.1 NaCl/acetate medium | heptane | LnL3 L: 8-hydroxyquinoline | −11.52 ± 0.14 | [152] |
| Nd | LnL3 | −11.54 ± 0.14 | |||
| Ln | 1 NaNO3 | xylene | M = La, Nd, Eu: M(BMPP)3·3HBTMPP where bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272, HBTMPP) M(BTMPP)2(NO3)2(TRPO); M = Y, Ho, Tm, Lu: M(BTMPP)3(HBTMPP)(TRPO) where trialkylphosphine oxide (Cyanex 923, TRPO) | [153] | |
| La | M(BMPP)3·3HBTMPP | −6.73 ± 0.04 | |||
| Nd | −5.50 ± 0.03 | ||||
| Eu | −4.05 ± 0.02 | ||||
| Ho | −2.5 ± 0.02 | ||||
| Tm | −1.71 ± 0.02 | ||||
| Lu | −1.27 ± 0.02 | ||||
| Y | −2.24 ± 0.02 | ||||
| La | M(BTMPP)2(NO3)2(TRPO) | −0.75 ± 0.03 | |||
| Nd | 0.11 ± 0.02 | ||||
| Eu | 0.55 ± 0.03 | ||||
| Ho | M(BTMPP)3(HBTMPP)(TRPO) | −1.74 ± 0.03 | |||
| Tm | −0.97 ± 0.03 | ||||
| Lu | −0.36 ± 0.04 | ||||
| Y | −1.93 ± 0.02 | ||||
| La | 1,2-dichloro-ethane | LnL3·S L: picrolonic acid S: tetraphenylmethylene-diphosphine dioxide | 9.21 ± 0.04 | [154] | |
| Ce | 9.83 ± 0.05 | ||||
| Pr | 10.04 ± 0.05 | ||||
| Nd | 10.06 ± 0.05 | ||||
| Sm | 10.34 ± 0.05 | ||||
| Eu | 10.29 ± 0.05 | ||||
| Gd | 10.06 ± 0.05 | ||||
| Tb | 10.14 ± 0.05 | ||||
| Dy | 10.02 ± 0.05 | ||||
| Ho | 9.91 ± 0.05 | ||||
| Er | 9.73 ± 0.05 | ||||
| Tm | 9.64 ± 0.05 | ||||
| Yb | 9.51 ± 0.05 | ||||
| Lu | 9.31 ± 0.04 | ||||
| La | LnL3·2S; L: picrolonic acid S: tetraphenylmethylene-diphosphine dioxide | 12.80 ± 0.06 | |||
| Ce | 12.98 ± 0.06 | ||||
| Pr | 12.92 ± 0.06 | ||||
| Nd | 12.81 ± 0.06 | ||||
| Sm | 12.95 ± 0.06 | ||||
| Eu | 12.85 ± 0.06 | ||||
| Gd | 12.46 ± 0.06 | ||||
| Tb | 12.59 ± 0.06 | ||||
| Dy | 12.45 ± 0.06 | ||||
| Ho | 12.20 ± 0.06 | ||||
| Er | 12.06 ± 0.06 | ||||
| Tm | 11.91 ± 0.06 | ||||
| Yb | 11.72 ± 0.06 | ||||
| Lu | 11.48 ± 0.06 | ||||
| La | LnL3∙2S S: 5,11,17,23-tetra-tert-butyl-25,26,27,28-tetrakis-(dimethylphosphinoylmethoxy)calix[4]arene | 6.7 ± 0.05 | |||
| Nd | 7.98 ± 0.05 | ||||
| Eu | 8.60 ± 0.05 | ||||
| Ho | 8.96 ± 0.05 | ||||
| Lu | 9.46 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atanassova, M. Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands. Separations 2022, 9, 371. https://doi.org/10.3390/separations9110371
Atanassova M. Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands. Separations. 2022; 9(11):371. https://doi.org/10.3390/separations9110371
Chicago/Turabian StyleAtanassova, Maria. 2022. "Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands" Separations 9, no. 11: 371. https://doi.org/10.3390/separations9110371
APA StyleAtanassova, M. (2022). Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands. Separations, 9(11), 371. https://doi.org/10.3390/separations9110371







