Mitigation Mechanism of Membrane Fouling in MnFeOx Functionalized Ceramic Membrane Catalyzed Ozonation Process for Treating Natural Surface Water
Abstract
:1. Introduction
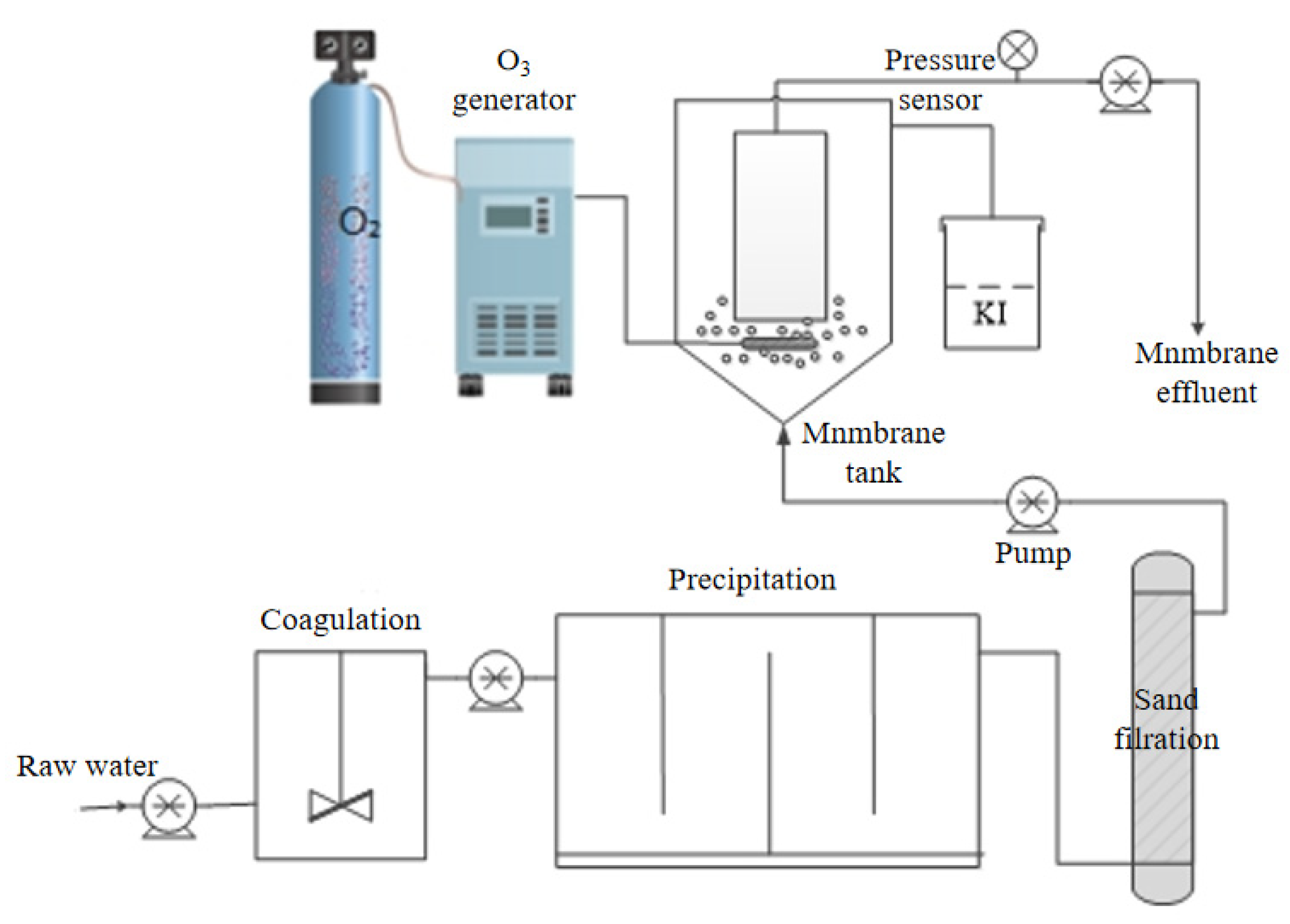
2. Materials and Methods
2.1. Materials
2.2. Process Parameter Setting
2.3. Characterization of Membrane Rejection Performance and Membrane Fouling
3. Results and Discussions
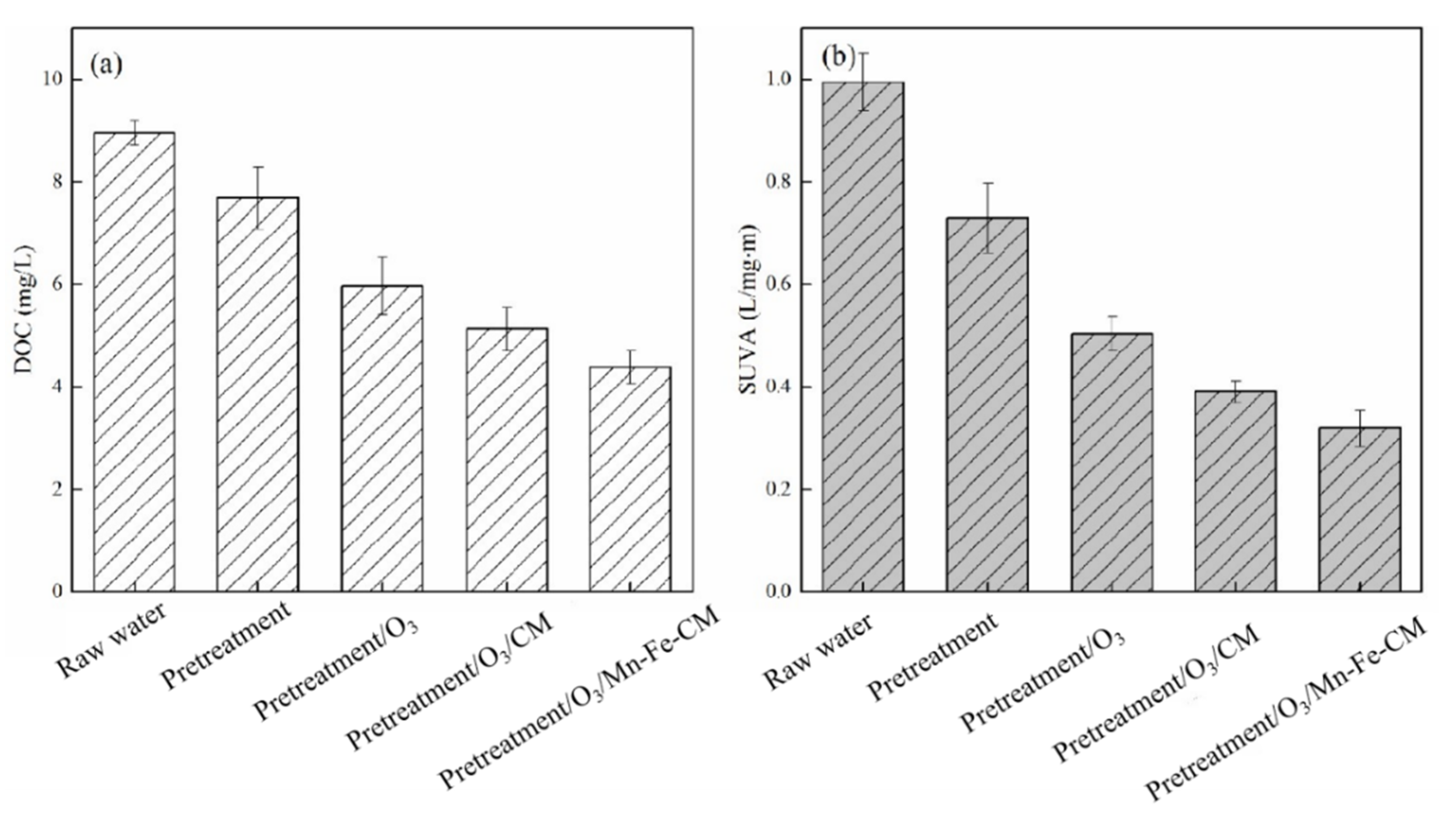
3.1. Effect of Different Treatment Process on the Removal of DOC, UV254 and Fluorescent Substances
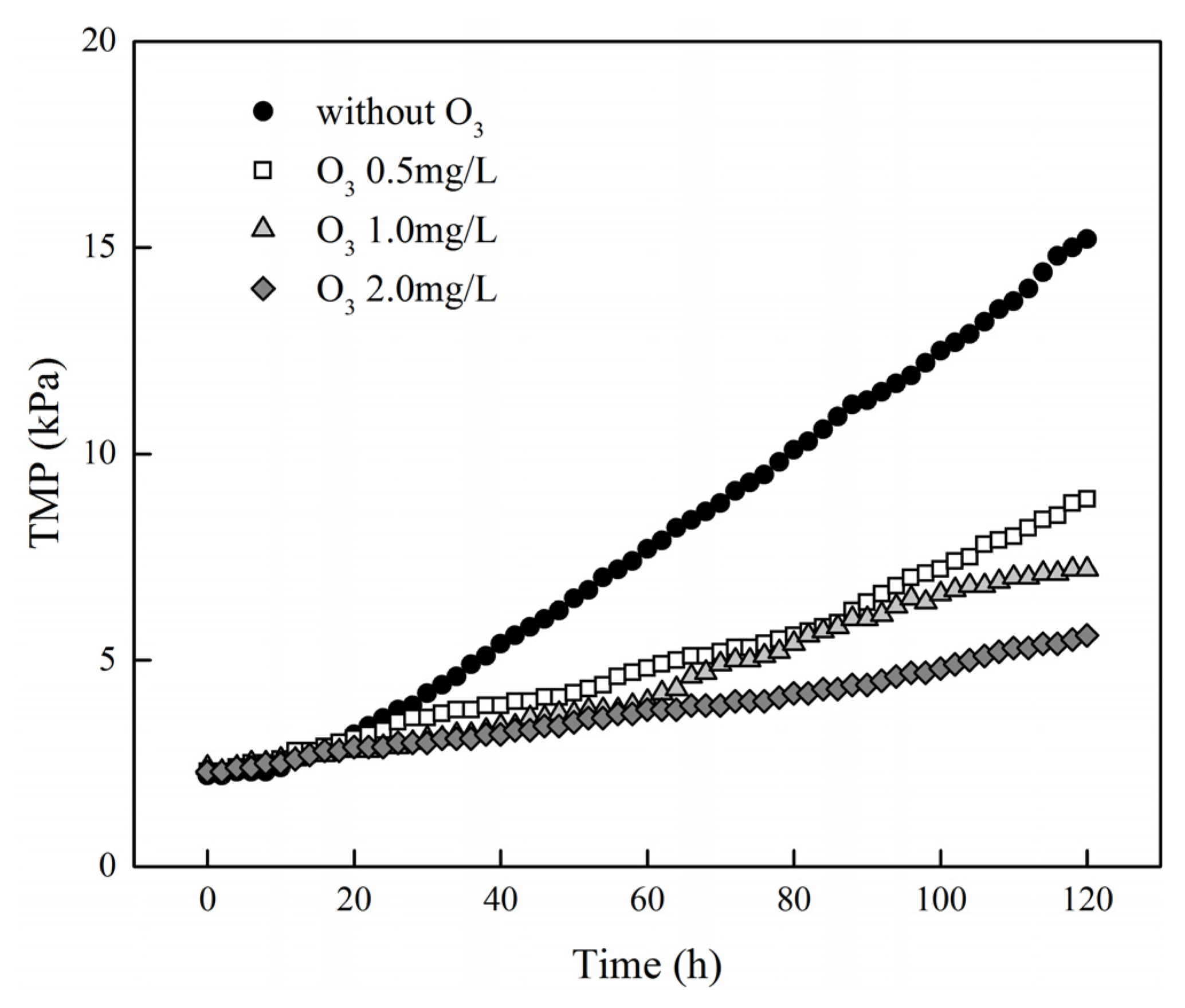
3.2. Effect of Ozone Concentration on Removal Performance and Transmembrane Pressure Variation
3.3. Analysis of Membrane Fouling during Long-Term Operation
3.3.1. Transmembrane Pressure Variation in Different Combined Treatment Process
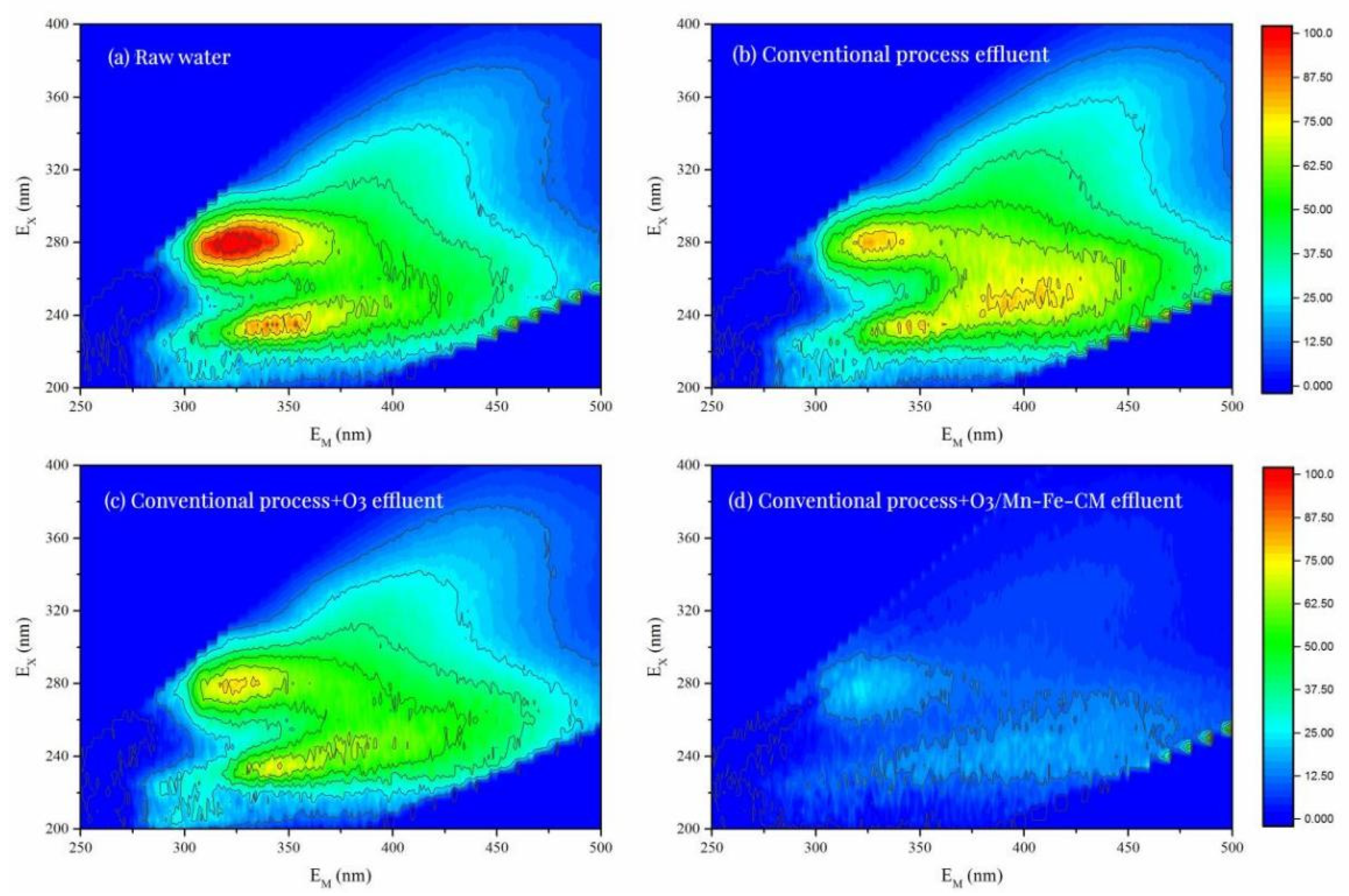
3.3.2. Characterization Analysis and the Anti-Membrane Fouling Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qiao, T.; Yu, Z.; Zhang, X.; Au, D. Occurrence and fate of pharmaceuticals and personal care products in drinking water in southern China. J. Environ. Monit. 2011, 13, 3097–3103. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Miao, H.; Wang, P.; Sun, F.; Li, X. Bi-metal oxide-modified flat-sheet ceramic membranes for catalytic ozonation of organic pollutants in wastewater treatment. Chem. Eng. J. 2012, 426, 131263. [Google Scholar] [CrossRef]
- Song, Z.; Li, Y.; Wang, Z.; Wang, M.; Wang, Z.; Zhang, Y.; Sun, J.; Liu, C.; Liu, Y.; Xu, B.; et al. Effect of the coupling modes on EfOM degradation and fouling mitigation in ozonation-ceramic membrane filtration. Chem. Eng. J. 2022, 394, 124935. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. The effects of combined ozonation and filtration on disinfection by-product formation. Water Res. 2005, 39, 2839–2850. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Qu, F.; Ding, A.; Chang, H.Q.; Liu, B.; Tang, X.B.; Wu, D.J.; Li, G.B. Fabrication of Mn oxide incorporated ceramic membranes for membrane fouling control and enhanced catalytic ozonation of p-chloronitrobenzene. Chem. Eng. J. 2017, 308, 1010–1020. [Google Scholar] [CrossRef]
- Corneal, L.M.; Baumann, M.J.; Masten, S.J.; Davies, S.H.R.; Tarabara, V.V.; Byun, S. Mn oxide coated catalytic membranes for hybrid ozonation-membrane filtration: Membrane microstructural characterization. J. Membr. Sci. 2011, 369, 182–187. [Google Scholar] [CrossRef]
- Li, M.; Yang, K.; Huang, X.; Liu, S.; Jia, Y.; Gu, P.; Miao, H. Efficient degradation of trimethoprim by catalytic ozonation coupled with Mn/FeOx-functionalized ceramic membrane: Synergic catalytic effect and enhanced anti-fouling performance. J. Colloid. Interface Sci. 2022, 616, 440–452. [Google Scholar] [CrossRef]
- Wan, Y.; Xie, P.; Wang, Z.; Wang, J.; Ding, J.; Raf, D.; Bart, V.B. Application of UV/chlorine pretreatment for controlling ultrafiltration (UF) membrane fouling caused by different natural organic fractions. Chemosphere 2021, 263, 127993. [Google Scholar] [CrossRef]
- Guo, Y.; Song, Z.L.; Xu, B.B.; Li, Y.N.; Qi, F.; Croue, J.P.; Yuan, D.H. A novel catalytic ceramic membrane fabricated with CuMn2O4 particles for emerging UV absorbers degradation from aqueous and membrane fouling elimination. J. Hazard. Mater. 2018, 344, 1229–1239. [Google Scholar] [CrossRef]
- Corneal, L.M.; Masten, S.J.; Davies, S.H.R.; Tarabara, V.V.; Byun, S.; Baumann, M.J. AFM, SEM and EDS characterization of manganese oxide coated ceramic water filtration membranes. J. Membr. Sci. 2010, 360, 292–302. [Google Scholar] [CrossRef]
- Chen, W.; Paul, W.; Jerry, A.L.; Karl, B. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 24, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Stéphane, V.; Onita, D.B.; Benoit, B. Ozone Chemically Enhanced Backwash for Ceramic Membrane Fouling Control in Cyanobacteria-Laden Water. Membranes 2020, 10, 213. [Google Scholar] [CrossRef]
- Xueke, L.; Ruirui, L.; Bao, Z.; Ting, R.; Guibin, J. Characterization of Carbonyl Disinfection By-Products During Ozonation, Chlorination, and Chloramination of Dissolved Organic Matters. Environ. Sci. Technol. 2020, 54, 2218–2227. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef] [Green Version]
- Kang, I.; Yoon, S.; Lee, C. Comparison of the filtration characteristics of organic and inorganic membranes in a membrane-coupled anaerobic bioreactor. Water Res. 2002, 36, 1803–1813. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, Y.; Wang, Y.; Tong, X.; Zhao, X.; Ikuno, N.; Hu, H. Membrane fouling potential of the denitrification filter effluent and the control mechanism by ozonation in the process of wastewater reclamation. Water Res. 2020, 173, 115591. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yang, H.; Xie, Y. Effects of organic fouling and cleaning on the retention of pharmaceutically active compounds by ceramic nanofiltration membranes. J. Membr. Sci. 2018, 563, 734–742. [Google Scholar] [CrossRef]
- Regula, C.E.; Carretier, Y.; Wyart, G.; Gesan, G.A.; Vincent, D.; Boudot, P. Chemical cleaning/disinfection and ageing of organic UF membranes: A review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Ding, S.; Hou, L. Dynamics of biofouling development on the conditioned membrane and its relationship with membrane performance. J. Membr. Sci. 2016, 514, 264–273. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Hazarika, S. In situ Assembly of Functionalized Single-Walled Carbon Nanotube with partially reduced Graphene oxide Nanocomposite Membrane for Chiral Separation of β-substituted-α-amino acids. Sep. Purif. Technol. 2022, 283, 120201. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, B.; Qi, F. A novel ceramic membrane coated with MnO2-Co3O4 nanoparticles catalytic ozonation for benzophenone-3 degradation in aqueous solution: Fabrication, characterization and performance. Chem. Eng. J. 2016, 287, 381–389. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Nanofiltration of trace organic chemicals: A comparison between ceramic and polymeric membranes. Sep. Purif. Technol. 2014, 136, 258–264. [Google Scholar] [CrossRef]
- Mansas, C.; Mendret, J.; Brosillon, S.; Ayral, A. Coupling catalytic ozonation and membrane separation: A review. Sep. Purif. Technol. 2020, 236, 116221. [Google Scholar] [CrossRef]
- Zhou, Y.; Ren, S.; Wang, M.; Yang, J.; Chen, Z.; Chen, L. Mn and Fe oxides co-effect on nanopolyhedron CeO2 catalyst for NH3-SCR of NO. J. Energy Inst. 2021, 99, 97–104. [Google Scholar] [CrossRef]
- Geluwe, S.; Braeken, L.; Bruggen, B. Ozone oxidation for the alleviation of membrane fouling by natural organic matter: A review. Water Res. 2011, 45, 3551–3570. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.; Gogoi, M.; Borah, A.; Sarmah, H.; Ingole, P.G.; Hazarik, S. Functionalized activated carbon and carbon nanotube hybrid membrane with enhanced antifouling activity for removal of cationic dyes from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100492. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Liu, B.; Zhong, X. Natural organic matter removal and flux decline with PEG-TiO2-doped PVDF membranes by integration of ultrafiltration with photocatalysis. J. Membr. Sci. 2012, 405–406, 48–56. [Google Scholar] [CrossRef]
- Borah, A.; Gogoi, M.; Goswami, R.; Sarmah, H.; Hazarik, K.K.; Hazarika, S. Thin film nanocomposite membrane incorporated with clay-ionic liquid framework for enhancing rejection of epigallocatechin gallate in aqueous media. J. Environ. Chem. Eng. 2022, 10, 107423. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Borah, A.; Sarmah, H.; Rajguru, P.; Hazarika, S. Amide functionalized DWCNT nanocomposite membranes for chiral separation of the racemic DOPA. Sep. Purif. Technol. 2021, 279, 119704. [Google Scholar] [CrossRef]
- Bhuyan, C.; Konwar, A.; Bora, P.; Rajguru, P.; Hazarika, S. Cellulose nanofiber-poly(ethylene terephthalate) nanocomposite membrane from waste materials for treatment of petroleum industry wastewater. J. Hazard. Mater. 2023, 442, 129955. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.N.; Hu, J.Y.; Tao, Y.; Zhu, J.; Zhang, X.H. Effect of ozone on the performance of a hybrid ceramic membrane-biological activated carbon process. J. Environ. Sci. 2014, 26, 783–791. [Google Scholar] [CrossRef]
- Zhu, B.; Hu, Y.X.; Kennedy, S.; Milne, N.; Morris, G.; Jin, W.Q.; Gray, S.; Duke, M. Dual function filtration and catalytic breakdown of organic pollutants in wastewater using ozonation with titania and alumina membranes. J. Membr. Sci. 2011, 378, 61–72. [Google Scholar] [CrossRef]
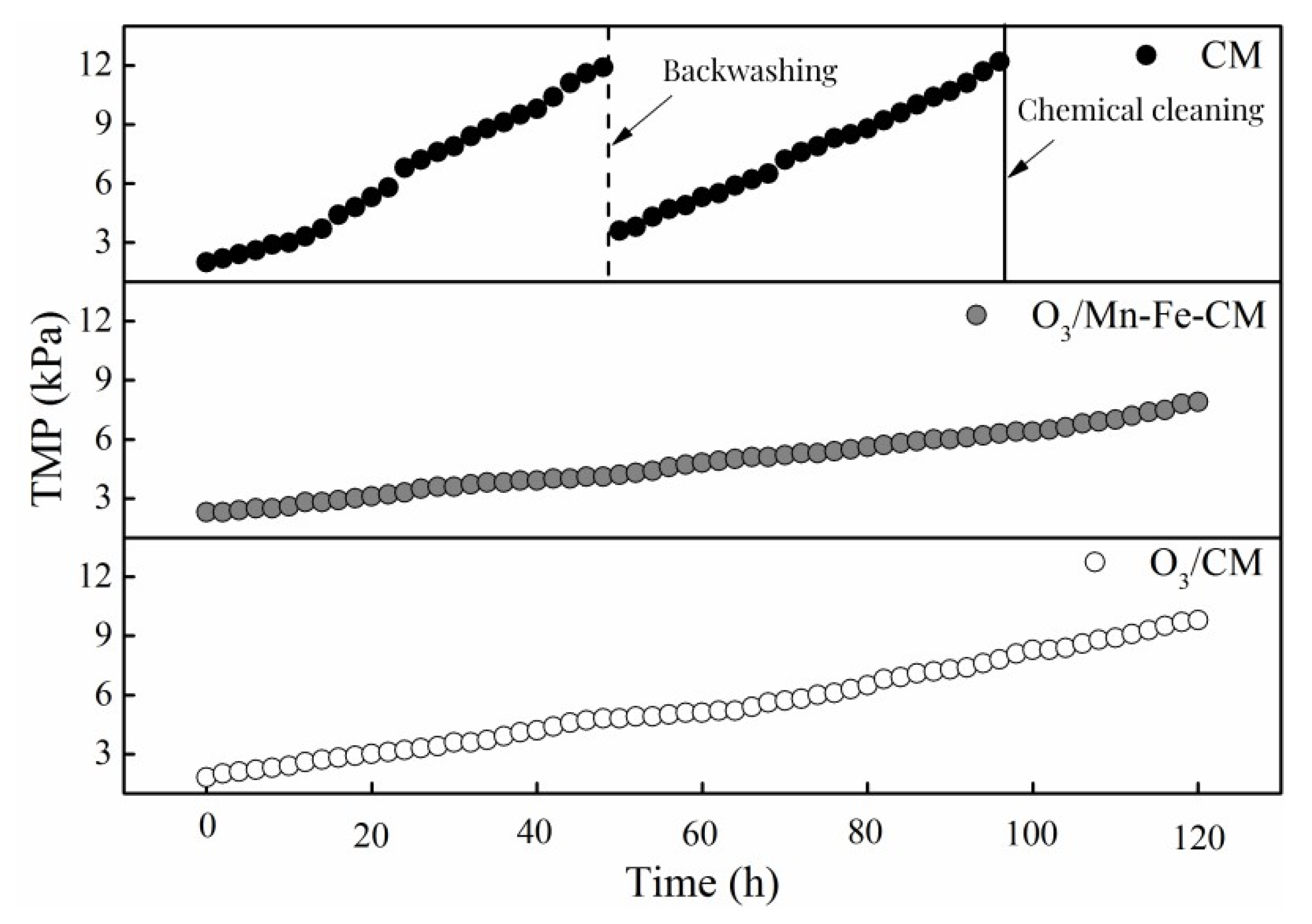
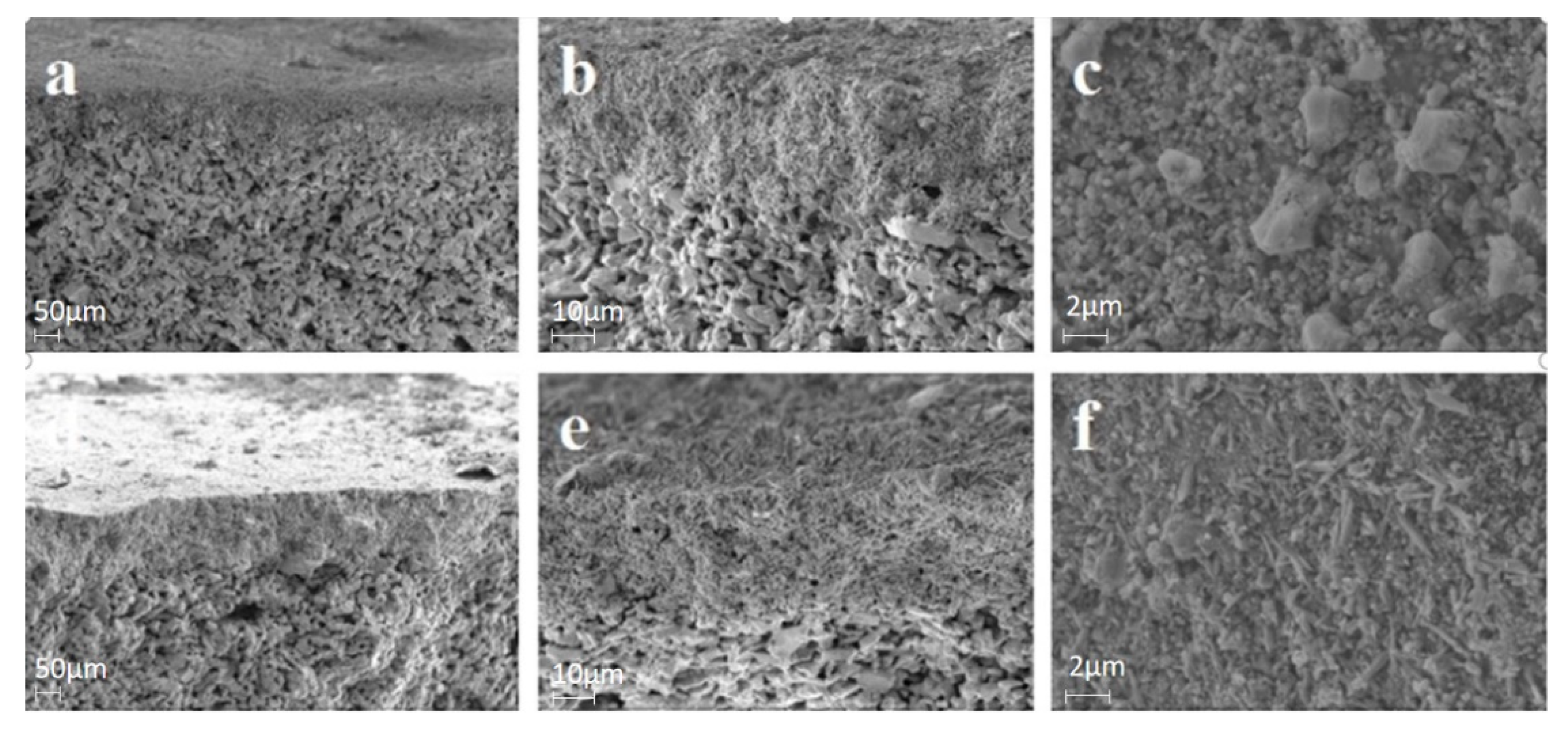
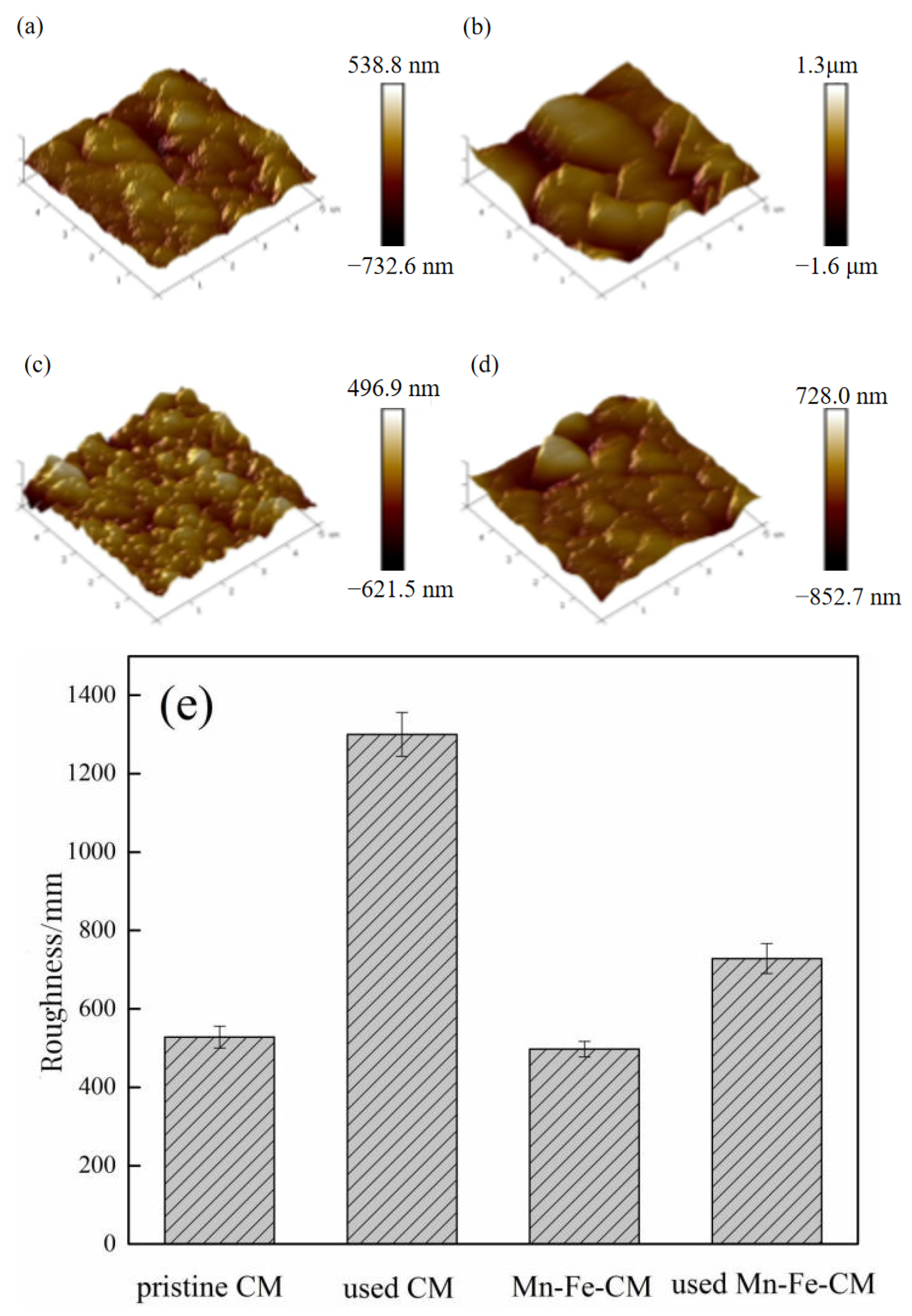
| Index | Raw Water | 0 mg/L | 0.5 mg/L | 1.0 mg/L | 2.0 mg/L |
|---|---|---|---|---|---|
| pH | 7.39 | 7.32 | 7.21 | 7.05 | 6.86 |
| DOC (mg/L) | 7.57 | 5.98 | 4.28 | 3.42 | 3.05 |
| SUVA (L/mg·m) | 1.45 | 1.42 | 0.63 | 0.41 | 0.25 |
| NH4+-N (mg/L) | 0.35 | 0.28 | 0.25 | 0.19 | 0.12 |
| Fe (mg/L) | 0.05 | 0.05 | 0.06 | 0.07 | 0.09 |
| Mn (mg/L) | 0.03 | 0.03 | 0.04 | 0.06 | 0.08 |
| Systems | DOC Removal | SUVA Removal | TMP Variation | Membrane Flux Recovery with Simple Hydraulic Flushing |
|---|---|---|---|---|
| CM (DOC = 9.0 mg·L−1, SUVA = 1.0 L/mg·m, The ozone concentration of 0.5 mg·L−1) | 5.3% | 6.1% | ||
| O3/CM [7] (DOC = 9.0 mg·L−1, SUVA = 1.0 L/mg·m, The ozone concentration of 0.5 mg·L−1) | 4.6% | 43.0% | Increased 45.4% after 120 h operation | 82.4% recovery after 120 min operation |
| O3/Mn-Fe-CM (DOC = 9.0 mg·L−1, SUVA = 1.0 L/mg·m, The ozone concentration of 0.5 mg·L−1) | 46.7% | 58.6% | 93.0% recovery after 120 min operation | |
| Pretreatment/ O3/Mn-Fe-CM (DOC = 9.0 mg·L−1, SUVA = 1.0 L/mg·m, The ozone concentration of 2 mg·L−1) | 60.0% | 82.8% | Increased 40.0% after 120 h operation | 95.7% recovery after 120 min operation |
| O3/CuMn2O4 /CM [9] (DOC= 10 mg·L−1, SUVA = 0.9 L/mg·m, The ozone concentration of 2 mg·L−1) | 10.0% | 81.1% | 85.1% recovery after 120 min operation | |
| O3/CMF/ BAC [32] (DOC = 2.3–4.9 mg·L−1, SUVA = 0.058–0.114 L/mg·m, The ozone concentration of 2 mg·L−1) | 47.5% | 73.3% | Increased 30.0% after 120 h operation | 74.0% recovery after 60 min operation |
| O3/TiO2-CM [33] (DOC = 5.0 mg·L−1, SUVA = 1.0 L/mg·m, The ozone concentration of 8 mg L−1) | 44.0% | 80.0% | Increased 40.0% after 60 h operation | 70.0% recovery after 10 min operation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Chi, Y.; Jia, Y.; Li, M.; Yang, Y.; Yao, H.; Yang, K.; Zhang, Z.; Ren, X.; Gu, P.; et al. Mitigation Mechanism of Membrane Fouling in MnFeOx Functionalized Ceramic Membrane Catalyzed Ozonation Process for Treating Natural Surface Water. Separations 2022, 9, 372. https://doi.org/10.3390/separations9110372
Guo H, Chi Y, Jia Y, Li M, Yang Y, Yao H, Yang K, Zhang Z, Ren X, Gu P, et al. Mitigation Mechanism of Membrane Fouling in MnFeOx Functionalized Ceramic Membrane Catalyzed Ozonation Process for Treating Natural Surface Water. Separations. 2022; 9(11):372. https://doi.org/10.3390/separations9110372
Chicago/Turabian StyleGuo, Hui, Yanxiao Chi, Yifan Jia, Manman Li, Yuxuan Yang, Haiyong Yao, Kunlun Yang, Zengshuai Zhang, Xueli Ren, Peng Gu, and et al. 2022. "Mitigation Mechanism of Membrane Fouling in MnFeOx Functionalized Ceramic Membrane Catalyzed Ozonation Process for Treating Natural Surface Water" Separations 9, no. 11: 372. https://doi.org/10.3390/separations9110372




