Apoptotic Cell Death via Activation of DNA Degradation, Caspase-3 Activity, and Suppression of Bcl-2 Activity: An Evidence-Based Citrullus colocynthis Cytotoxicity Mechanism toward MCF-7 and A549 Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Crude Extraction
2.2. In-Vitro Cytotoxicity of C. colocynthis Extracts
2.3. Flow Cytometry Based Mechanistic Study
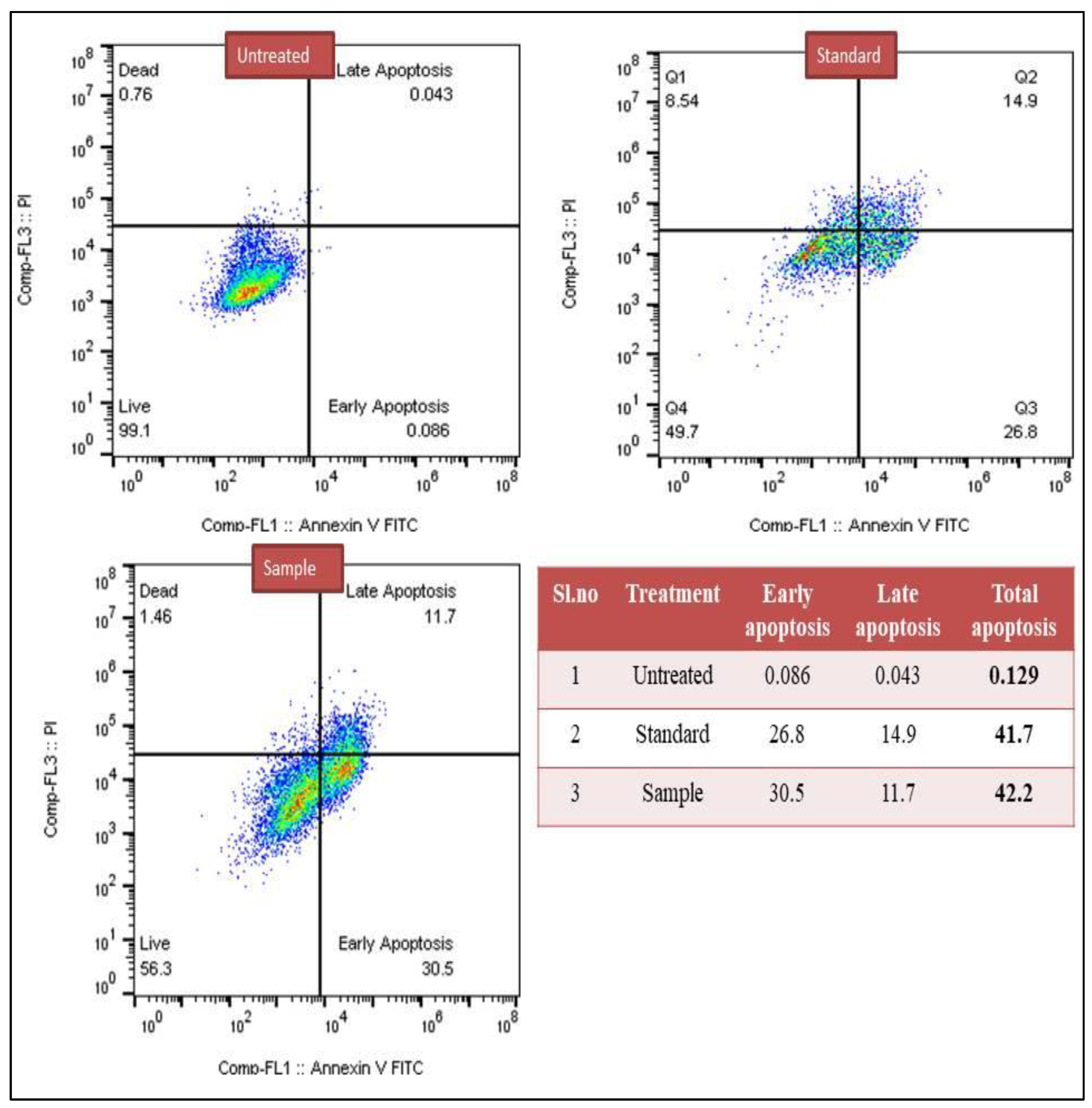
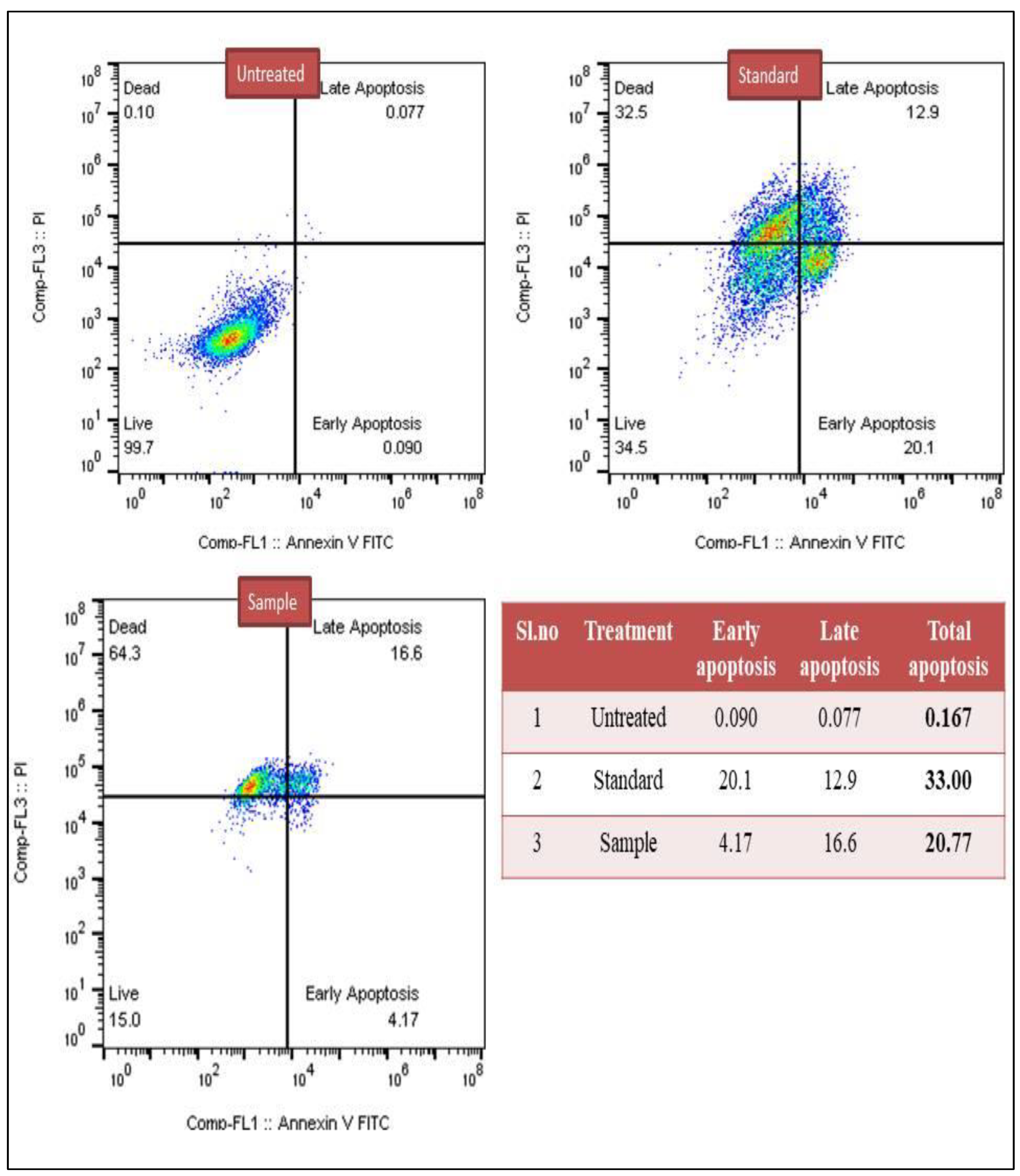
2.3.1. Detection of Early and Late Apoptosis through Binding of Annexin-V to Phosphatidylserine
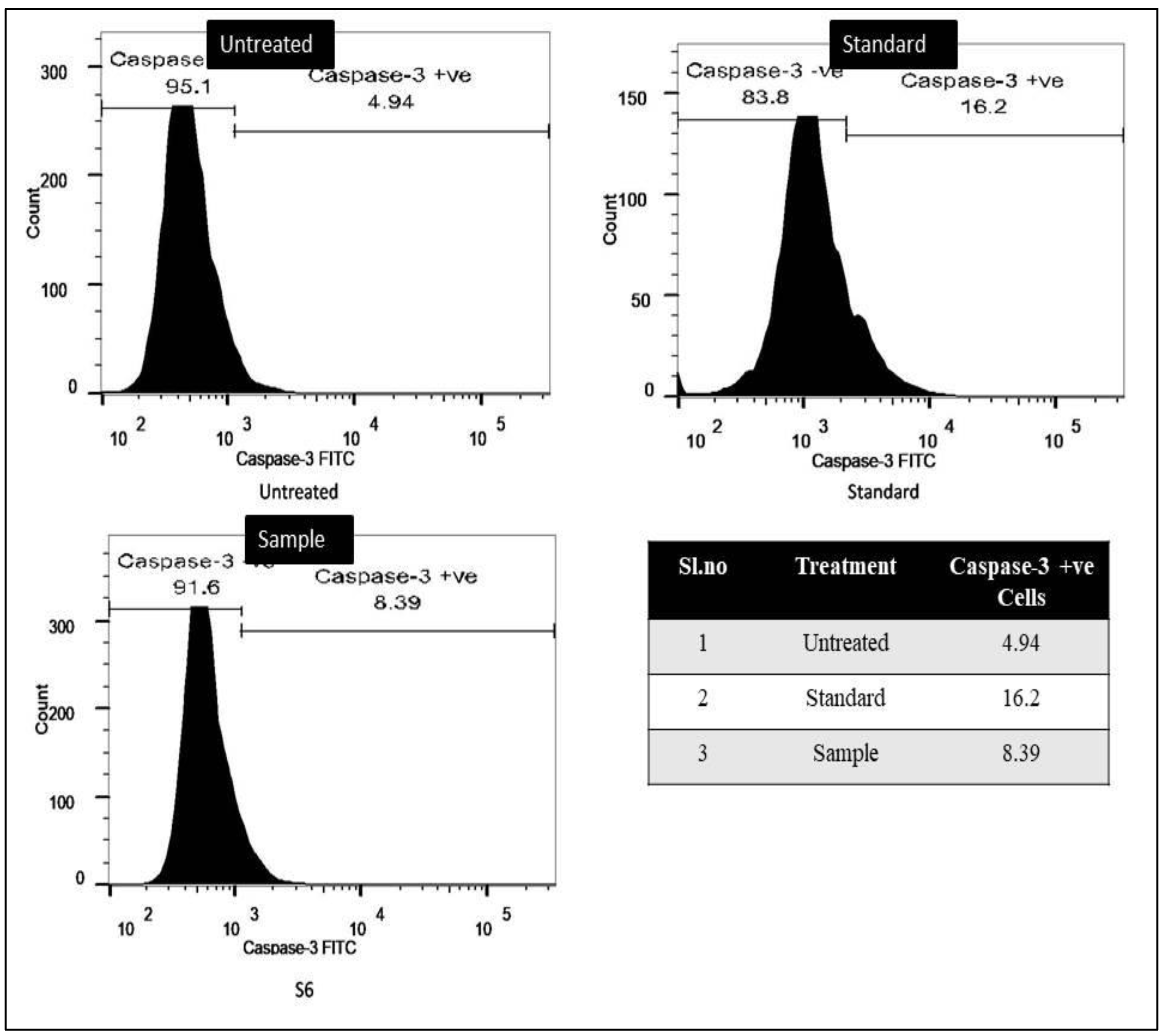
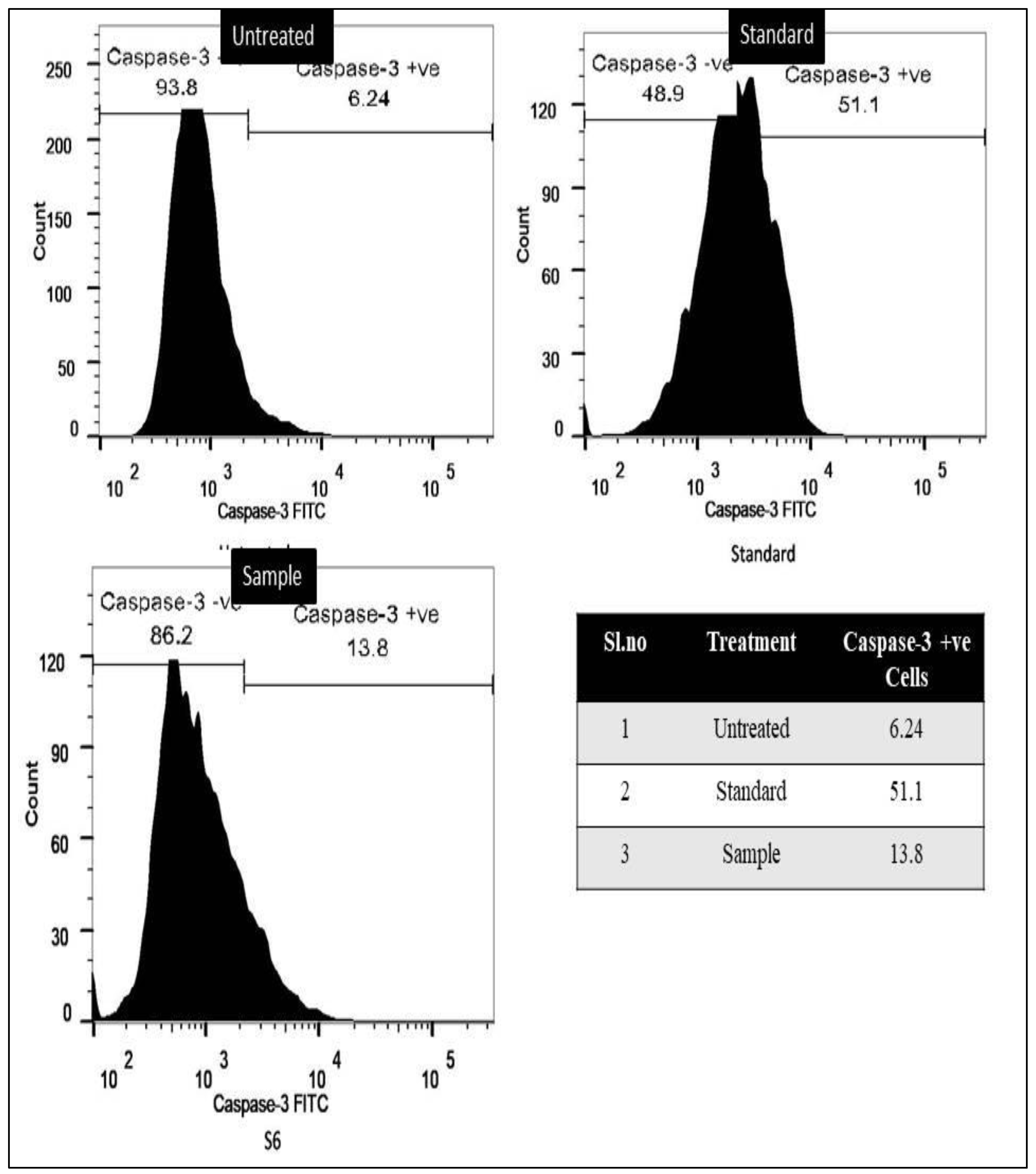
2.3.2. Caspase Activation Assay
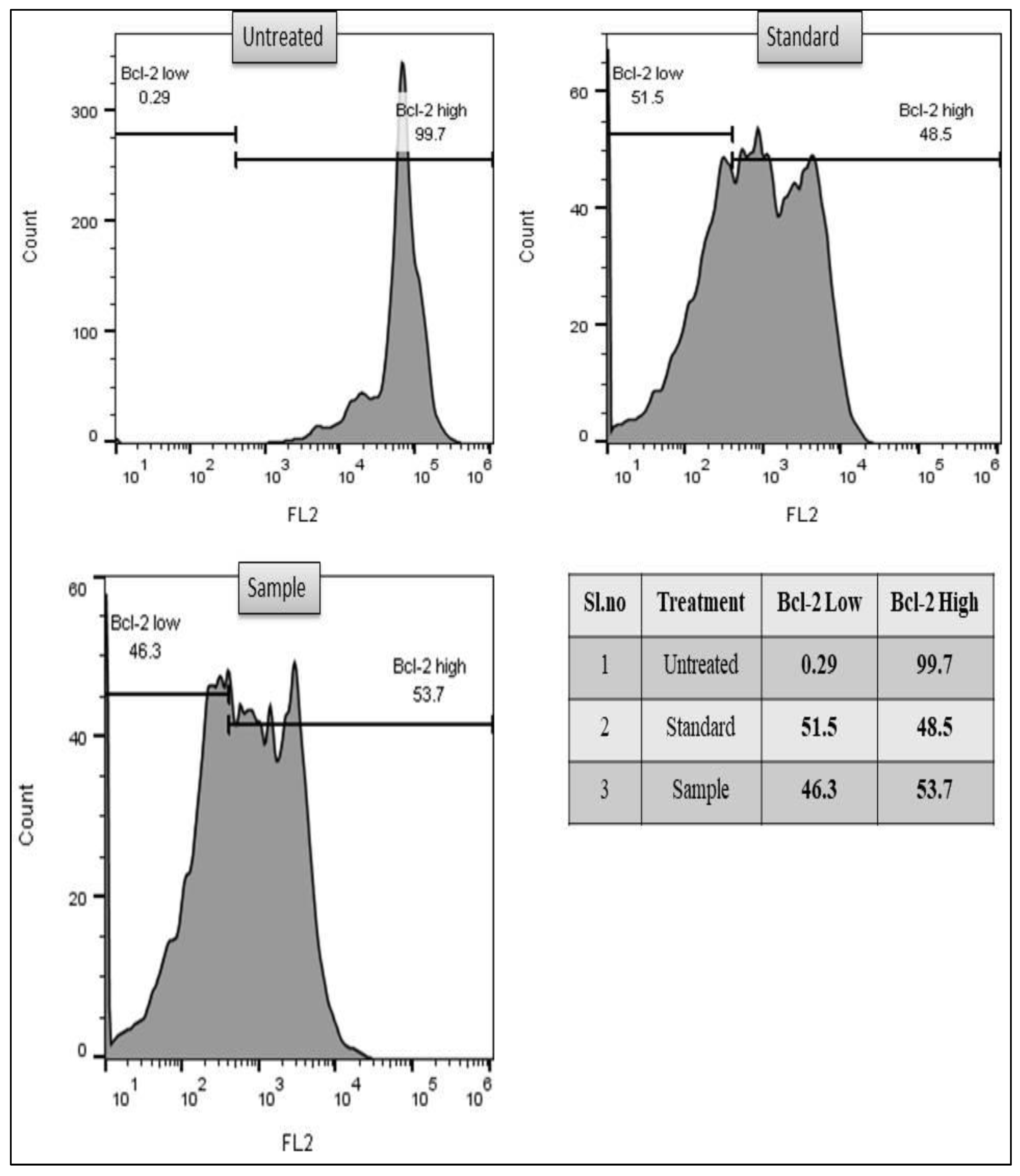
2.3.3. Anti Bcl-2 Activity
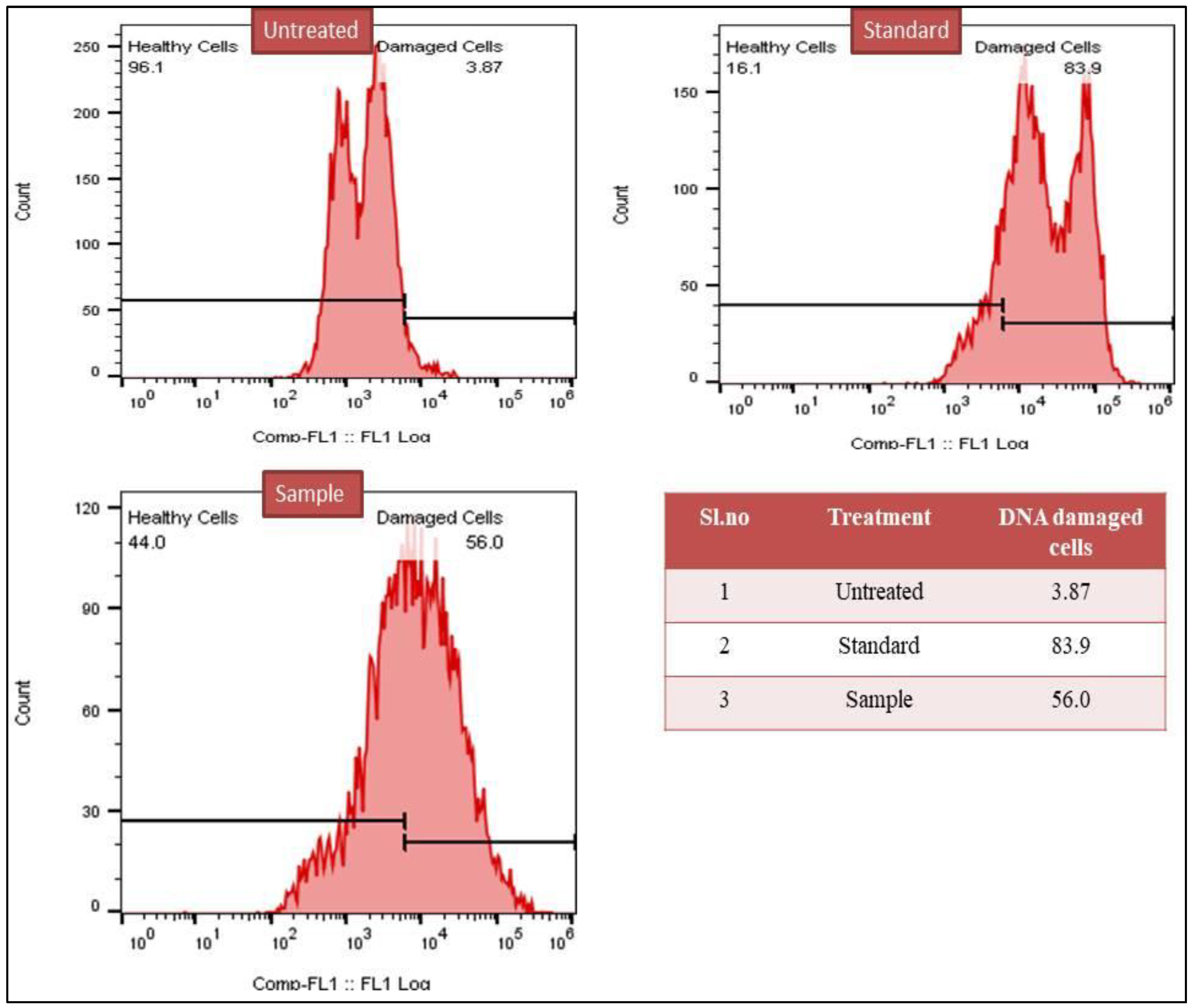
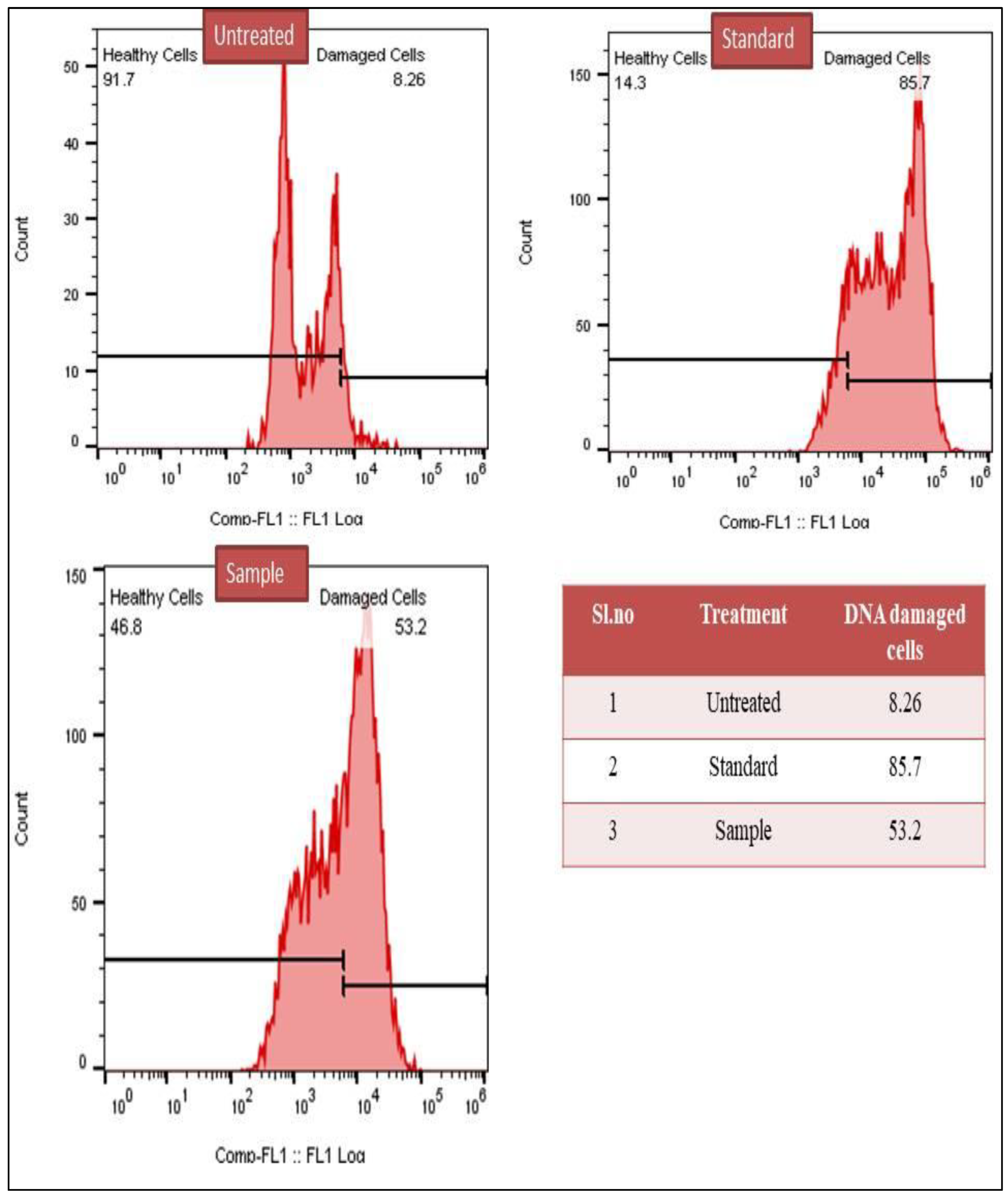
2.3.4. DNA Fragmentation Analysis
2.4. Phytochemical Analysis
2.4.1. Estimation of Total Phenols and Flavonoid Content
2.4.2. Gas Chromatography-Mass Spectrometry (GC-MS) Profiling
2.5. Statistical Analysis
3. Results
3.1. Cytotoxicity Study of C. colocynthis Extracts
3.2. Flow Cytometry-Based Mechanistic Pathway Studies
3.2.1. Detection of Apoptosis Based on Annexin-V Assay
3.2.2. Caspase-3 Marker Assay
3.2.3. Bcl-2 Marker Assay
3.2.4. TUNEL Assay for DNA Damage
3.3. Total Phenolic and Total Flavonoid Contents
3.4. Chromatography and Spectrometry GC–MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, W.S.; Almufareh, N.A.; Domiaty, D.M.; Albasher, G.; Alduwish, M.A.; Alkhalaf, H.; Almuzzaini, B.; Al-Marshidy, S.S.; Alfraihi, R.; Elasbali, A.M.; et al. Epidemiology of Cancer in Saudi Arabia Thru 2010–2019: A Systematic Review with Constrained Meta-Analysis. AIMS Public Health 2020, 7, 679–696. [Google Scholar] [CrossRef]
- Asiri, S.; Asiri, A.; Ulahannan, S.; Alanazi, M.; Humran, A.; Hummadi, A. Incidence Rates of Breast Cancer by Age and Tumor Characteristics among Saudi Women: Recent Trends. Cureus 2020, 12, e6664. [Google Scholar] [CrossRef] [Green Version]
- Yonbawi, A.R.; Abdallah, H.M.; Alkhilaiwi, F.A.; Koshak, A.E.; Heard, C.M. Anti-Proliferative, Cytotoxic and Antioxidant Properties of the Methanolic Extracts of Five Saudi Arabian Flora with Folkloric Medicinal Use: Aizoon Canariense, Citrullus Colocynthis, Maerua Crassifolia, Rhazya Stricta and Tribulus Macropterus. Plants 2021, 10, 2073. [Google Scholar] [CrossRef]
- Innocent, E. Trends and Challenges toward Integration of Traditional Medicine in Formal Health-Care System: Historical Perspectives and Appraisal of Education Curricula in Sub-Sahara Africa. J. Intercult. Ethnopharmacol. 2016, 5, 312–316. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Abbas, A.; Shehzad, A. Journal of Traditional and Complementary Medicine Role of Traditional Islamic and Arabic Plants in Cancer Therapy. J. Tradit. Chin. Med. Sci. 2017, 7, 195–204. [Google Scholar]
- Al-Jauziyah, Q. Healing with the Medicine of the Prophet, 2nd ed.; Manderola, R.J., Ed.; Dar-us-Salam for Publishing & Distribution: Riyadh, Saudi Arabia, 2003; ISBN 9789960892917. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [Green Version]
- Zaid, H.; Rayan, A.; Said, O.; Saad, B. Cancer Treatment by Greco-Arab and Islamic Herbal Medicine. Open Nutraceuticals J. 2010, 3, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Al-Oqail, M.M.; Al-Sheddr, E.S.; Al-Rehaily, A.J.; Rahman, M.A. Diversity of Medicinal Plants in the Flora of Saudi Arabia 3: An Inventory of 15 Plant Families and Their Conservation Management. Int. J. Environ. 2014, 3, 312–320. [Google Scholar] [CrossRef]
- Al-Namazi, A.A.; Algarni, S.M.; Wan, J.S.H.; Al Mosallam, M.S.; Alotaibi, F. Floristic Composition of Jandaf Mountain as Biodiversity Hotspot Area in Southwestern Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 3654–3660. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.I.; Rathore, H.A. Abdur-Sattar, “Citrullus Colocynthis (L.) Schrad (Bitter Apple Fruit): A Review of Its Phytochemistry, Pharmacology, Traditional Uses and Nutritional Potential. J. Ethnopharmacol. 2014, 155, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.S.A.; Ghassan, M.A.; Sheikha, A. Evaluation of the Anticancer Activity and Fatty Acids Composition of ‘Handal’ (Citrullus Colocynthis L.) Seed Oil, a Desert Plant from South Jordan. Food Sci. Nutr. 2020, 9, 282–289. [Google Scholar]
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Citrullus Colocynthis Colocynth, Bitter Apple, Bitter Gourd. In Unconventional Oilseeds and Oil Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–105. ISBN 9780128094358. [Google Scholar]
- Karimabad, M.N.; Niknia, S.; Golnabadi, M.B.; Poor, S.F.; Hajizadeh, M.R.; Mahmoodi, M. Effect of Citrullus Colocynthis Extract on Glycated Hemoglobin Formation (in Vitro). Eurasian J. Med. 2020, 52, 47–51. [Google Scholar] [CrossRef]
- Shi, C.; Karim, S.; Wang, C.; Zhao, M.; Murtaza, G. A Review on Antidiabetic Activity of Citrullus Colocynthis Schrad. Acta Pol. Pharm. 2014, 71, 363–367. [Google Scholar]
- Pashmforosh, M.; Rajabi Vardanjani, H.; Rajabi Vardanjani, H.; Pashmforosh, M.; Khodayar, M.J. Topical Anti-Inflammatory and Analgesic Activities of Citrullus Colocynthis Extract Cream in Rats. Medicina 2018, 54, 51. [Google Scholar] [CrossRef] [Green Version]
- Hameed, B.; Ali, Q.; Hafeez, M.M.; Malik, A. Antibacterial and Antifungal Activity of Fruit, Seed and Root Extracts of Citrullus Colocynthis Plant. Biol. Clin. Sci. Res. J. 2020, 2020, 1–5. [Google Scholar] [CrossRef]
- Bourhia, M.; Bouothmany, K.; Bakrim, H.; Hadrach, S.; Salamatullah, A.M.; Alzahrani, A.; Khalil Alyahya, H.; Albadr, N.A.; Gmouh, S.; Laglaoui, A.; et al. Chemical Profiling, Antioxidant, Antiproliferative, and Antibacterial Potentials of Chemically Characterized Extract of Citrullus Colocynthis L. Seeds. Separations 2021, 8, 114. [Google Scholar] [CrossRef]
- Barzegar, M.H.; Khazali, H.; Kalantar, S.M.; Khoradmehr, A. Effect of Citrullus Colocynthis Hydro-Alcoholic Extract on Hormonal and Folliculogenesis Process in Estradiol Valerate-Induced PCOs Rats Model: An Experimental Study. Int. J. Reprod. Biomed. 2017, 15, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Morikawa, T.; Kobayashi, H.; Nakamura, A.; Matsuhira, K.; Nakamura, S.; Matsuda, H. Bioactive Saponins and Glycosides. XXVII. Structures of New Cucurbitane-Type Triterpene Glycosides and Antiallergic Constituents from Citrullus Colocynthis. Chem. Pharm. Bull. 2007, 55, 428–434. [Google Scholar] [CrossRef] [Green Version]
- Torkey, H.M.; Abou-Yousef, H.M.; Azeiz, A.; Farid, H.E.A. Insecticidal Effect of Cucurbitacin E Glycoside Isolated from Citrullus Colocynthis against Aphis Craccivora. Aust. J. Basic Appl. Sci. 2009, 3, 4060–4066. [Google Scholar]
- Hussain, A.I.; Rathore, H.A.; Sattar, M.Z.A.; Chatha, S.A.S.; ud din Ahmad, F.; Ahmad, A.; Johns, E.J. Phenolic Profile and Antioxidant Activity of Various Extracts from Citrullus colocynthis (L.) from the Pakistani Flora. Ind. Crops Prod. 2013, 45, 416–422. [Google Scholar] [CrossRef]
- Chawech, R.; Jarraya, R.; Girardi, C.; Vansteelandt, M.; Marti, G.; Nasri, I.; Racaud-Sultan, C.; Fabre, N. Cucurbitacins from the Leaves of Citrullus colocynthis (L.) Schrad. Molecules 2015, 20, 18001–18015. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Fang, X.; He, C.; Li, P.; Xiao, F.; Wang, Y.; Chen, M. Cucurbitacins: A Systematic Review of the Phytochemistry and Anticancer Activity. Am. J. Chin. Med. 2015, 43, 1331–1350. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth Inhibitory Activity of Cucurbitacin Glucosides Isolated from Citrullus Colocynthis on Human Breast Cancer Cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef]
- Hajjar, D.; Kremb, S.; Sioud, S.; Emwas, A.H.; Voolstra, C.R.; Ravasi, T. Anti-Cancer Agents in Saudi Arabian Herbals Revealed by Automated High-Content Imaging. PLoS ONE 2017, 12, e0177316. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, K.; Sharma, A.; Kumar, S.; Gunjan, G.K.; Nag, A.; Mandal, C.C. Colocynth Extracts Prevent Epithelial to Mesenchymal Transition and Stemness of Breast Cancer Cells. Front. Pharmacol. 2017, 8, 593. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Bian, Z.G.; Zhang, Y.; Wang, J.H.; Kan, L.; Wang, X.; Niu, H.Y.; He, P. Cucurbitacin B Inhibits Proliferation and Induces Apoptosis via STAT3 Pathway Inhibition in A549 Lung Cancer Cells. Mol. Med. Rep. 2014, 10, 2905–2911. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Jajeh, F.; Khan, M.I.; Mukhtar, E.; Shabana, S.M.; Mukhtar, H. Sestrin-3 Modulation Is Essential for Therapeutic Efficacy of Cucurbitacin B in Lung Cancer Cells. Carcinogenesis 2016, 38, 184–195. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Wu, S.; Wang, X. Cucurbitacin I Induces pro-Death Autophagy in A549 Cells via the ERK-MTOR-STAT3 Signaling Pathway. J. Cell. Biochem. 2018, 119, 6104–6112. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Khan, S.; Kumar, S. Cucurbitacin B Alters the Expression of Tumor-Related Genes by Epigenetic Modifications in NSCLC and Inhibits NNK-Induced Lung Tumorigenesis. Cancer Prev. Res. 2015, 8, 552–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.C.; Tian, B.; Yang, Y.L.; Wang, Y.C.; Liu, S.; Urisman, A.; Yang, C.T.; Xu, Z.; Jablons, D.M.; You, L. Cucurbitacin E Inhibits the Yes associated Protein Signaling Pathway and Suppresses Brain Metastasis of Human Non small Cell Lung Cancer in a Murine Model. Oncol. Rep. 2019, 42, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yan, Q.; Peng, B. Use of Cucurbitacins for Lung Cancer Research and Therapy. Cancer Chemother. Pharmacol. 2021, 88, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Jan, S.U.; Faridullah, S.; Sherani, S.; Jahan, N. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra Intermedia Indigenous to Balochistan. Sci. World J. 2017, 2017, 5873648. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Davies, N.W. Gas Chromatographic Retention Indices of Monoterpenes and Sesquiterpenes on Methyl Silicon and Carbowax 20M Phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Irawan, C.; Rochaeni, H.; Sulistiawaty, L.; Roziafanto, A.N. Phytochemical Screening, LC-MS Studies and Antidiabetic Potential of Methanol Extracts of Seed Shells of Archidendron Bubalinum (Jack) I.C. Nielson (Julang Jaling) from Lampung, Indonesia. Pharmacogn. J. 2018, 10, s77–s82. [Google Scholar] [CrossRef] [Green Version]
- Crowley, L.C.; Marfell, B.J.; Scott, A.P.; Waterhouse, N.J. Quantitation of Apoptosis and Necrosis by Annexin V Binding, Propidium Iodide Uptake, and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, db.prot087288. [Google Scholar] [CrossRef] [PubMed]
- Moraes, V.W.R.; Caires, A.C.F.; Paredes-Gamero, E.J.; Rodrigues, T. Organopalladium Compound 7b Targets Mitochondrial Thiols and Induces Caspase-Dependent Apoptosis in Human Myeloid Leukemia Cells. Cell Death Dis. 2013, 4, e658. [Google Scholar] [CrossRef] [Green Version]
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of Apoptosis by TUNEL Assay. Methods Mol. Biol. 2012, 887, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinaria Ornata Suppresses LPS-Induced Inflammatory Response in RAW 264.7 Macrophages and Sprague Dawley Rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.; Mantawy, E.; El-Bakly, W.; Abdel-Mottaleb, Y.; Azab, S. Methyl Palmitate: The Naturally Occurring Cardioprotective Agent. Arch. Pharm. Sci. Ain Shams Univ. 2020, 9, 47–62. [Google Scholar] [CrossRef]
- Breeta, R.D.I.E.; Grace, V.M.B.; Wilson, D.D. Methyl Palmitate-A Suitable Adjuvant for Sorafenib Therapy to Reduce in Vivo Toxicity and to Enhance Anti-Cancer Effects on Hepatocellular Carcinoma Cells. Basic Clin. Pharmacol. Toxicol. 2021, 128, 366–378. [Google Scholar] [CrossRef]
- Ahamed, A.; Panneerselvam, A.; Alaklabi, A.; Arif, I.A.; Ambikapathy, V.; Thajuddin, N. Molecular Perspective and Anticancer Activity of Medicinal Plants. Saudi J. Biol. Sci. 2020, 27, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lendzion, K.; Gornowicz, A.; Strawa, J.W.; Bielawska, K.; Czarnomysy, R.; Popławska, B.; Bielawski, K.; Tomczyk, M.; Miltyk, W.; Bielawska, A. LC-PDA-MS and GC-MS Analysis of Scorzonera Hispanica Seeds and Their Effects on Human Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 11584. [Google Scholar] [CrossRef]
- Atolani, O.; Olatunji, G.A.; Fabiyi, O.A.; Adeniji, A.J.; Ogbole, O.O. Phytochemicals from Kigelia Pinnata Leaves Show Antioxidant and Anticancer Potential on Human Cancer Cell Line. J. Med. Food 2013, 16, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Win, D.T. Oleic Acid-The Anti-Breast Cancer Component in Olive Oil. AU Jt 2005, 9, 75–78. [Google Scholar]
- Rao, A.; Srivastava, S.J.; Kannaujiya, A.K.; Singh, V.K. Structural Information of Natural Product Metabolites in Bryophytes. In Evolutionary Diversity as a Source for Anticancer Molecules; Singh, D., Ed.; Elsevier: San Diego, CA, USA, 2021; pp. 209–231. [Google Scholar]
- Yang, Y.; Fu, C.; Zhou, F.; Luo, X.; Li, J.; Zhao, J.; He, J.; Li, X.; Li, J. Chemical Composition, Antioxidant and Antitumor Activities of Sub-Fractions of Wild and Cultivated Pleurotus Ferulae Ethanol Extracts. PeerJ 2018, 6, e6097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Hady, H.; El-Wakil, E.A.; Abdel-Gawad, M. GC-MS Analysis, Antioxidant and Cytotoxic Activities of Mentha Spicata. Eur. J. Med. Plants 2018, 26, 1–12. [Google Scholar] [CrossRef]
- Nakagawa, M.; Yamaguchi, T.; Fukawa, H.; Ogata, J.; Komiyama, S.; Akiyama, S.; Kuwano, M. Potentiation by Squalene of the Cytotoxicity of Anticancer Agents against Cultured Mammalian Cells and Murine Tumor. Jpn. J. Cancer Res. 1985, 76, 315–320. [Google Scholar]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and Pharmacological Activities of Squalene and Related Compounds: Potential Uses in Cosmetic Dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ma, X.; Sun, J.; Ding, G.; Cui, Q.; Miao, Y.; Hou, Y.; Jiang, M.; Bai, G. Active Fragments-Guided Drug Discovery and Design of Selective Tropane Alkaloids Using Ultra-High Performance Liquid Chromatography-Quadrupole Time-of-Flight Tandem Mass Spectrometry Coupled with Virtual Calculation and Biological Evaluation. Anal. Bioanal. Chem. 2017, 409, 1145–1157. [Google Scholar] [CrossRef]
- Dai, X.; Wang, X.; Yang, C.; Huang, M.; Zhou, Z.; Qu, Y.; Cui, X.; Liu, R.; Chen, C. Human Fibroblasts Facilitate the Generation of IPSCs-Derived Mammary-like Organoids. Stem Cell Res. Ther. 2022, 13, 377. [Google Scholar] [CrossRef]
- Bharadvaja, N. Medicinal Plants in the Management of Cancer: A Review. Int. J. Complement. Altern. Med. 2017, 9, 00291. [Google Scholar] [CrossRef] [Green Version]
- Chiang, E.P.I.; Tsai, S.Y.; Kuo, Y.H.; Pai, M.H.; Chiu, H.L.; Rodriguez, R.L.; Tang, F.Y. Caffeic Acid Derivatives Inhibit the Growth of Colon Cancer: Involvement of the PI3-K/Akt and AMPK Signaling Pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of Oxidative Stress in the Development and Progression of Breast Cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of Antioxidant Supplementation on Chemotherapeutic Toxicity: A Systematic Review of the Evidence from Randomized Controlled Trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, D.; Manjusha; Saroha, K.; Singh, N.; Vashishta, B. Antioxidant and Free Radical Scavenging Potential of Citrullus colocynthis (L.) Schrad. Methanolic Fruit Extract. Acta Pharm. 2008, 58, 215–220. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and Molecular Targeting Therapy in Cancer. Biomed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, J.; Tait, S.W.G. Mitochondrial Apoptosis: Killing Cancer Using the Enemy Within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, X. Endoplasmic Reticulum-Mitochondria Tethering in Neurodegenerative Diseases. Transl. Neurodegener. 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villa-Pulgarín, J.A.; Gajate, C.; Botet, J.; Jimenez, A.; Justies, N.; Varela-M, R.E.; Cuesta-Marbán, Á.; Müller, I.; Modolell, M.; Revuelta, J.L.; et al. Mitochondria and Lipid Raft-Located FOF1-ATP Synthase as Major Therapeutic Targets in the Antileishmanial and Anticancer Activities of Ether Lipid Edelfosine. PLoS Negl. Trop. Dis. 2017, 11, e0005805. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, Q.; Zhu, Z.; Xu, H.; Ding, F.; Wang, M.; Du, S.; Du, Y.; Yan, Z. BHX, a Novel Pyrazoline Derivative, Inhibits Breast Cancer Cell Invasion by Reversing the Epithelial-Mesenchymal Transition and down-Regulating Wnt/β-Catenin Signalling. Sci. Rep. 2017, 7, 9153. [Google Scholar] [CrossRef] [Green Version]
- Danial, N.N.; Korsmeyer, S.J. Cell Death. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef] [Green Version]
- Zaman, S.; Wang, R.; Gandhi, V. Targeting the Apoptosis Pathway in Hematologic Malignancies. Leuk. Lymphoma 2014, 55, 1980–1992. [Google Scholar] [CrossRef]
- Liao, Y.; Xu, Y.; Cao, M.; Huan, Y.; Zhu, L.; Jiang, Y.; Shen, W.; Zhu, G. Luteolin Induces Apoptosis and Autophagy in Mouse Macrophage ANA-1 Cells via the Bcl-2 Pathway. J. Immunol. Res. 2018, 2018, 4623919. [Google Scholar] [CrossRef]
- Choi, J.B.; Kim, J.H.; Lee, H.; Pak, J.N.; Shim, B.S.; Kim, S.H. Reactive Oxygen Species and P53 Mediated Activation of P38 and Caspases Is Critically Involved in Kaempferol Induced Apoptosis in Colorectal Cancer Cells. J. Agric. Food Chem. 2018, 66, 9960–9967. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Waterhouse, N.J. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, db.prot087312. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Yadav, R.; Jain, S.; Vaidya, A. Caspase-3: A Primary Target for Natural and Synthetic Compounds for Cancer Therapy. Chem. Biol. Drug Des. 2021, 98, 144–165. [Google Scholar] [CrossRef] [PubMed]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012, 2012, 524308. [Google Scholar] [CrossRef] [Green Version]
- Hyun, H.B.; Lee, W.S.; Go, S.I.; Nagappan, A.; Park, C.; Han, M.H.; Hong, S.H.; Kim, G.; Kim, G.Y.; Cheong, J.; et al. The Flavonoid Morin from Moraceae Induces Apoptosis by Modulation of Bcl-2 Family Members and Fas Receptor in HCT 116 Cells. Int. J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Huang, H.; Liu, L.; Sun, Y. Malvidin Induced Anticancer Activity in Human Colorectal HCT-116 Cancer Cells Involves Apoptosis, G2/M Cell Cycle Arrest and Upregulation of P21WAFI. Int. J. Clin. Exp. Med. 2018, 11, 1734–1741. [Google Scholar]
- Safari, F.; Rabieepor, M.; Jamalomidi, F.; Baghaeifar, Z.; Khodaei, L. Evaluation of Anti-Cancer and pro-Apoptotic Activities of Iranian Green Tea Extract against A549, PC3, and MCF-7 Cancer Cell Lines. Int. J. Basic Sci. Med. 2019, 4, 113–118. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Wang, Q.; Deng, S.; Huang, M.; Ma, X.; Song, P.; Du, J.; Huang, Y.; Wen, Y.; et al. Inhibitory Effect of Kurarinone on Growth of Human Non-Small Cell Lung Cancer: An Experimental Study Both in Vitro and in Vivo Studies. Front. Pharmacol. 2018, 9, 252. [Google Scholar] [CrossRef]
- Lu, H.F.; Chie, Y.J.; Yang, M.S.; Lee, C.S.; Fu, J.J.; Yang, J.S.; Tan, T.W.; Wu, S.H.; Ma, Y.S.; Ip, S.W.; et al. Apigenin Induces Caspase-Dependent Apoptosis in Human Lung Cancer A549 Cells through Bax- and Bcl-2-Triggered Mitochondrial Pathway. Int. J. Oncol. 2010, 36, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive Effect of Quercetin, a Natural Dietary Flavonoid on Prostate Cancer in in Vivo Model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Shim, H.Y.; Park, J.H.; Paik, H.D.; Nah, S.Y.; Kim, D.S.H.L.; Han, Y.S. Acacetin-Induced Apoptosis of Human Breast Cancer MCF-7 Cells Involves Caspase Cascade, Mitochondria-Mediated Death Signaling and SAPK/JNK1/2-c-Jun Activation. Mol. Cells 2007, 24, 95–104. [Google Scholar] [PubMed]
- Ferenc, P.; Solár, P.; Kleban, J.; Mikes, J.; Fedorocko, P. Down-Regulation of Bcl-2 and Akt Induced by Combination of Photoactivated Hypericin and Genistein in Human Breast Cancer Cells. J. Photochem. Photobiol. B 2010, 98, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Do TUNEL and Other Apoptosis Assays Detect Cell Death in Preclinical Studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef] [PubMed]
- Alshabi, A.M.; Vastrad, B.; Shaikh, I.A.; Vastrad, C. Exploring the Molecular Mechanism of the Drug-Treated Breast Cancer Based on Gene Expression Microarray. Biomolecules 2019, 9, 282. [Google Scholar] [CrossRef] [PubMed]

| Concentration in µg/mL | Percentage (%) of Cell Viability a | |||
|---|---|---|---|---|
| Hexane | Ethyl Acetate | Methanol | Aqueous | |
| 50 | 97.98 ± 0.033 | 88.55 ± 0.005 | 85.26 ± 0.009 | 72.85 ± 0.021 |
| 100 | 87.56 ± 0.010 | 72.72 ± 0.018 | 74.22 ± 0.023 | 67.25 ± 0.020 |
| 150 | 77.65 ± 0.006 | 63.21 ± 0.001 | 68.00 ± 0.013 | 62.23 ± 0.003 |
| 200 | 71.11 ± 0.0045 | 58.68 ± 0.022 | 56.23 ± 0.01 | 53.12 ± 0.015 |
| 250 | 63.58 ± 0.007 | 52.65 ± 0.002 | 41.93 ± 0.005 | 48.26 ± 0.006 |
| Concentration in µg/mL | Percentage (%) of Cell Viability a | |||
|---|---|---|---|---|
| Hexane Extract | Ethyl Acetate Extract | Methanol Extract | Aqueous Extract | |
| 50 | 46.99 ± 0.002 | 37.81 ± 0.022 | 43.57 ± 0.003 | 49.39 ± 0.012 |
| 100 | 38.17 ± 0.018 | 32.17 ± 0.010 | 27.13 ± 0.002 | 44.71 ± 0.005 |
| 150 | 28.03 ± 0.015 | 22.50 ± 0.010 | 22.80 ± 0.015 | 36.01 ± 0.010 |
| 200 | 22.26 ± 0.009 | 18.18 ± 0.013 | 17.52 ± 0.009 | 29.71 ± 0.014 |
| 250 | 7.86 ± 0.002 | 10.86 ± 0.011 | 9.90 ± 0.006 | 18.72 ± 0.026 |
| Concentration in µg/mL | Percentage of Cell Viability a | |||
|---|---|---|---|---|
| Hexane Extract | Ethyl Acetate Extract | Methanol Extract | Aqueous Extract | |
| 50 | 61.36 ± 0.005 | 65.21 ± 0.020 | 55.00 ± 0.022 | 66.99 ± 0.005 |
| 100 | 57.05 ± 0.002 | 54.40 ± 0.017 | 47.82 ± 0.013 | 61.81 ± 0.009 |
| 150 | 51.49 ± 0.018 | 37.12 ± 0.008 | 36.59 ± 0.018 | 50.20 ± 0.008 |
| 200 | 42.57 ± 0.010 | 22.72 ± 0.007 | 30.66 ± 0.033 | 41.96 ± 0.006 |
| 250 | 36.25 ± 0.008 | 16.48 ± 0.009 | 20.52 ± 0.012 | 29.41 ± 0.020 |
| Extract/Standard | IC50 (μg/mL) a | ||
|---|---|---|---|
| L929 Cell Line | MCF-7 Cell Line | A549 Cell Line | |
| Hexane | 324.11 ± 0.14 | 148.01 ± 1.10 | 36.80 ± 0.89 |
| Ethyl acetate | 249.97 ± 0.26 | 108.15 ± 0.85 | 39.48 ± 0.74 |
| Methanol | 222.29 ± 0.45 | 81.08 ± 0.45 | 17.84 ± 0.19 |
| Aqueous | 235.48 ± 0.89 | 150.43 ± 0.32 | 56.44 ± 0.77 |
| Cisplatin (standard) | 10.17 ± 0.14 | 2.68 ± 0.09 | 12.66 ± 0.06 |
| Peak | Compound | tR | Base Peak m/z | Nature | Uses |
|---|---|---|---|---|---|
| 1 | Neophytadiene | 26.629 | 68.05 | Sesquiterpene | Anti-inflammatory and antimicrobial [47] |
| 2 | Methyl palmitate | 28.393 | 74.05 | Fatty acid methyl ester | Cardioprotective, anticancer. [48,49] |
| 3 | Methyl linoleate | 31.618 | 67.05 | Fatty acid methyl ester | Cytotoxic, antibacterial [50,51] |
| 4 | Oleic acid methyl ester | 31.725 | 55.05 | Fatty acid methyl ester | Antioxidant, anticancer [52,53] |
| 5 | Isophytol acetate | 31.969 | 71.05 | diterpene | Anticancer, flavour and fragrance agents [54] |
| 6 | Methylstearate | 32.177 | 74.05 | Fatty acid methyl ester | Antioxidant, antitumor, cytotoxic [55,56] |
| 7 | Squalene | 43.216 | 69.05 | Triterpene | Anticancer, cosmetics, flavour and fragrance [57,58,59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, I.A.; Alshabi, A.M.; Alkahtani, S.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. Apoptotic Cell Death via Activation of DNA Degradation, Caspase-3 Activity, and Suppression of Bcl-2 Activity: An Evidence-Based Citrullus colocynthis Cytotoxicity Mechanism toward MCF-7 and A549 Cancer Cell Lines. Separations 2022, 9, 411. https://doi.org/10.3390/separations9120411
Shaikh IA, Alshabi AM, Alkahtani SA, Orabi MAA, Abdel-Wahab BA, Walbi IA, Habeeb MS, Khateeb MM, Shettar AK, Hoskeri JH. Apoptotic Cell Death via Activation of DNA Degradation, Caspase-3 Activity, and Suppression of Bcl-2 Activity: An Evidence-Based Citrullus colocynthis Cytotoxicity Mechanism toward MCF-7 and A549 Cancer Cell Lines. Separations. 2022; 9(12):411. https://doi.org/10.3390/separations9120411
Chicago/Turabian StyleShaikh, Ibrahim Ahmed, Ali Mohamed Alshabi, Saad Ahmed Alkahtani, Mohamed A. A. Orabi, Basel A. Abdel-Wahab, Ismail A. Walbi, Mohammed Shafiuddin Habeeb, Masood Medleri Khateeb, Arun K. Shettar, and Joy H. Hoskeri. 2022. "Apoptotic Cell Death via Activation of DNA Degradation, Caspase-3 Activity, and Suppression of Bcl-2 Activity: An Evidence-Based Citrullus colocynthis Cytotoxicity Mechanism toward MCF-7 and A549 Cancer Cell Lines" Separations 9, no. 12: 411. https://doi.org/10.3390/separations9120411
APA StyleShaikh, I. A., Alshabi, A. M., Alkahtani, S. A., Orabi, M. A. A., Abdel-Wahab, B. A., Walbi, I. A., Habeeb, M. S., Khateeb, M. M., Shettar, A. K., & Hoskeri, J. H. (2022). Apoptotic Cell Death via Activation of DNA Degradation, Caspase-3 Activity, and Suppression of Bcl-2 Activity: An Evidence-Based Citrullus colocynthis Cytotoxicity Mechanism toward MCF-7 and A549 Cancer Cell Lines. Separations, 9(12), 411. https://doi.org/10.3390/separations9120411








