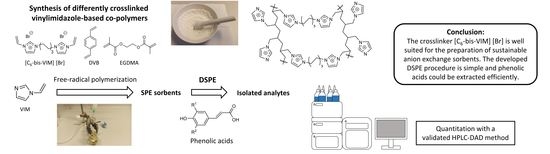
1. Introduction
Naturally occurring phenolic acids belong to the substance class of phenolic compounds, which is one of the largest groups of secondary plant metabolites biosynthesized by vegetables, fruits, cocoa, teas and other plants [
1]. Although the roles of phenolic acids in plants has not yet been completely clarified, they have been associated with various functions, including enzyme activity [
2], nutrient uptake [
3,
4], allelopathy [
5], as well as photosynthesis and protein synthesis [
6]. Besides their important functions in plants, phenolic acids have been associated with the nutritional, organoleptic and antioxidative properties of foods. In this context, their content and profile have been intensively investigated by the food industry, mainly due to their effect on maturation, preservation and enzymatic browning [
7,
8]. Since phenolic acids are widespread in plant-based foods, they account for almost one third of dietary phenols. Consequently, there is a growing awareness and interest in the antioxidant behavior and human health benefits associated with these compounds [
7,
9]. Countless scientific studies have already examined their effects on different oxidative stress related diseases, including cancer [
10,
11], diabetes [
12] and cardiovascular diseases [
13]. Nowadays, it is generally recognized that the uptake of phenolic acids in conjunction to a cereal, fruit and vegetable rich diet strongly contributes to human health and reduced disease risk [
14,
15,
16]. In this context, and due to the increasing industrial attention, efficient extraction and purification techniques regarding these compounds are in high demand [
17].
Extraction techniques in analytical sample preparation are typically used for isolation, matrix simplification, pre-concentration or solvent exchange to facilitate a successful detection of analytes [
18]. In this context, solid-phase extraction (SPE) has become an indispensable tool for both the laboratory and industrial fields [
19] and even though SPE has been utilized for many years, it is still a dynamic field of science which is heavily researched especially in the field of miniaturization, automation, material science and eco-friendly analytical processes [
18,
20]. An alternative to conventional SPE is dispersive solid-phase extraction (DSPE) in which the sorbent is directly dispersed into the sample matrix. The close contact between the sorbent and analytes enhances both the sorption and elution process and therefore increases the efficiency of the overall extraction procedure. Its simplicity especially makes DSPE a valuable tool for analytical sample preparation [
21,
22].
Since the interaction mechanism and thus the quality of the extraction is highly dependent on the choice of sorbent, constant progress in the synthesis of novel materials has been and is still a driving force in the development of SPE [
18,
23]. The first emerging sorbents were silica- or carbon-based; however, their numerous disadvantages promoted the development of porous polymers to overcome the limitations presented by those materials [
18,
24]. Unlike silica-based sorbents, they are stable at virtually any pH value, contain no troublesome silanol groups and generally exhibit higher surface areas. In comparison to carbon-based sorbents, porous polymers are superior for recovering organic compounds from aqueous samples [
24] and since their introduction, polymer-based materials have become an indispensable sorbent and have driven progress in the field of SPE. This is mainly due to their morphology and the possibility of incorporating various chemical functionalities into their porous framework, resulting in high retention capacities for different types of analytes and improved stability under different extraction conditions [
18]. At present, a large selection of sorbents are available on the market, both as bulk material and in the form of packed cartridges, discs, pipette tips and 96-wellplates, enabling the use of SPE for a wide range of applications [
25,
26]. In this regard, polymer-based sorbents still count as one of the most important SPE materials with progressive research being conducted over the last decades [
27,
28,
29,
30].
The structure of polymerizable compounds and the polymerization process are primarily responsible for the physical and chemical properties of the obtained polymer [
31]. There are several mechanisms for polymer synthesis with free-radical polymerization (FRP) being one of the most significant. Its robustness against impurities of the raw materials and the possibility to customize polymer properties by the choice of initiator and polymerization conditions are the main advantages over other processes. FRP is of enormous industrial importance, due to the large-scale production of vinyl polymers [
32,
33]. Furthermore, this method is also highly significant for the synthesis of efficient sorbents for SPE applications regarding scientific research. It is one of the most widely used techniques for the preparation of crosslinked polymers, both by academics and industrialists [
34].
Incorporating compounds that have at least two free-radically polymerizable double bonds, so-called crosslinking agents or crosslinkers, enables the synthesis of polymers with three-dimensional networks. By changing the monovinyl to divinyl molar ratios, the degree of cross-linking can be altered to form weakly to highly crosslinked materials, which directly affects the properties of the obtained polymer. Many commercially available polymers owe their value to their specific three-dimensional structures, which makes crosslinking an important tool for polymer tuning [
35]. Commonly used free-radically polymerizable crosslinking agents are divinylbenzene (DVB) or ethylene glycol dimethacrylate (EGDMA) [
33]. DVB, for example, is an important crosslinker for commercial SPE sorbents, with Oasis
® from Waters
TM and Strata
TM-X from Phenomenex
® being two of the most significant product lines regarding analytical sample preparation. EGDMA has become increasingly important in scientific research due to its higher polarity compared with DVB and the possibility to form hydrogen bonds with certain types of analytes [
36,
37,
38,
39]. A newly developed crosslinker that has been utilized for the synthesis of polymers is 3,3′-(hexane-1,6-diyl)bis(1-vinylimidazolium) bromide [C
6-bis-VIM] [Br]. Recent studies have shown that it can be used for the preparation of thermally and mechanically robust hydrogels [
40], inverse opal microspheres [
41], monolithic stationary phases [
42] as well as anion-exchange membranes [
43]; however, after a thorough search of the literature, it became apparent that [C
6-bis-VIM] [Br] has not yet been applied as a crosslinking agent for the preparation of SPE materials. Due to its structural properties, [C
6-bis-VIM] [Br] appeared to be well suited for the preparation of anion exchange sorbents and according to Wang et al., its synthesis is simple and feasible with high yields [
40].
Phenolic acids can be isolated by different SPE materials, such as mixed-mode, ion-exchange, or reversed-phase sorbents. Yılmaz et al. reported the extraction of phenolic acids using different commercially available sorbents including Chromabond
® C18, SAX and Oasis
® HLB [
44]. Furthermore, phenolic acids and flavonoids could be isolated and determined in honey by applying Bond Elut C18, Oasis
® HLB, Strata-X and Amberlite XAD-2 [
45]. Recent studies have shown that, in addition to commercially available sorbents, there is still great potential for the preparation of novel SPE materials to further improve the extraction of phenolic acids [
46,
47]. The aim of the presented study was to synthesize a novel [C
6-bis-VIM] [Br] crosslinked anion exchange polymer and subsequently develop an efficient extraction procedure for phenolic acids. Therefore, differently crosslinked polymers were prepared and compared with respect to their sorption capacity and analyte recoveries. The poly(n-VIM/C
6-bis-VIM) polymer was optimized regarding its monomer to crosslinker ratio and an efficient DSPE procedure was established. By adapting it to cartridges, the novel sorbent could be compared with the commercial anion exchange sorbent Oasis
® MAX from Waters
TM. Furthermore, the reusability of the sorbent was investigated. Based on the obtained results, the potential of the crosslinker [C
6-bis-VIM] [Br] for the preparation of efficient and sustainable anion exchange materials was clearly demonstrated. We hope that this study is a promising step towards increasing the attractiveness of this substance for polymer science and sustainable SPE applications in the future.
2. Materials and Methods
Ferulic acid (FeraA, 99%), cinnamic acid (CinA, ≥99%), caffeic acid (CaffA, ≥98%), chlorogenic acid (ChlorA, ≥95%), 2,3-dihydroxybenzoic acid (DHB, 99%), 1-vinylimidazole (VIM, ≥99%), divinylbenzene (DVB, 80%), 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH, 97%), trifluoroacetic acid (TFA, 99%), diethyl ether (Et2O, ≥99.9%), ethylene glycol dimethacrylate (EGDMA, 98%), and sodium phosphate monobasic monohydrate (NaH2PO4·H2O, ≥99.0%), as well as basic, activated aluminum oxide (Al2O3, approx. 150 mesh, standard grade) were all purchased from Sigma Aldrich (St. Louis, MI, USA), an affiliate of Merck (Darmstadt, Germany). Sodium phosphate dibasic dehydrate (Na2HPO4·2H2O, ≥99.5%) and hydrochloric acid (HCl, 37% in H2O) was obtained from Merck. The 1,6-dibromohexane (98%) was obtained from Acros Organics affiliate to Thermo Fisher Scientific (Waltham, MA, USA). The solvents methanol (MeOH, LC-MS grade), acetonitrile (ACN, LC-MS grade) and ethyl acetate (EtOAc, 99.5%) were purchased from Th. Geyer GmbH & Co. KG (Renningen, Germany) and 1-decanol (for synthesis) and 1-propanol (99.8%) from VWRTM (Radnor, PA, USA), and affiliate of AvantorTM (Radnor, PA, USA). Sodium hydroxide (NaOH, >99%), ammonium hydroxide solution (30% NH3 in water) and formic acid (FA, ≥98%) were obtained from Carl Roth® (Karlsruhe, Germany). Empty SPE cartridges (1 mL) and matching polyethylene frits with 20 µm porosity were obtained from Supelco® (Bellefonte, PA, USA). Pre-packed Oasis® MAX cartridges (30 mg) were purchased from WatersTM (Milford, MA, USA).
Water was obtained from a Milli-Q water purification system from Merck Millipore (Burlington, MA, USA). The phenolic acid standard stock solution mixture contained 500 mg L−1 of each analyte, including FerA, CinA, CaffA, ChlorA and DHB in 20 vol.% MeOH in water. Working standards were daily prepared by diluting the standard stock solution with 20 vol.% MeOH in water. All stock solutions were stored at 4 °C. Three phosphate buffers (0.1 M) with a pH 6.6, 7.0 and 7.7 were prepared in 5 vol.% MeOH in water by dissolving certain quantities of Na2HPO4·2H2O and NaH2PO4·H2O. The pH was adjusted by adding HCl or NaOH solution.
The DVB was extracted three times with 10% (w/v) NaOH solution and distilled under vacuum. The EGDMA was extracted with activated basic Al2O3 prior its use.
2.1. Instrumentation and Chromatographic Separation
HPLC analyses were performed on a 1100 Series HPLC Value System from Agilent (Santa Clara, CA, USA) equipped with a 1100 Series diode array detector (DAD). Analyte separation was performed with an Excel 5 C18 analytical column from ACE (Aberdeen, UK) with the dimensions 150 × 4.6 mm. The mobile phase consisted of 0.1 vol.% TFA in water (eluent A) and MeOH (eluent B). A gradient program with the following settings was applied (min/vol.% of eluent B): 0/10, 14/80, 14.1/99, 16/99, 16.1/10, 20/10. The injection temperature was set to 10 °C and the injection volume was 5 μL with a needle-wash in MeOH. The mobile-phase flow-rate was 700 µL min−1 and the temperature of the column oven was set to 40 °C. The detection of analytes was performed at 218 nm.
DSPE experiments were conducted on a ThermoMixer® C and the evaporation of solvents was performed using a Concentrator plus, both from Eppendorf AG (Hamburg, Germany). Drying of the synthesized crosslinker and polymers was accomplished in a Vacutherm vacuum drying oven from Thermo Fisher Scientific. The pH value of the phosphate buffer solutions was checked with a pH meter SevenEasyTM from Mettler Toledo (Columbus, OH, USA). BET measurements were performed utilizing a NOVA 2000e Surface Area and Pore Size Analyzer from Quantachrome Instruments (Boynton Beach, FL, USA) and the software, Quantachrome NovaWin. Prior to the measurement, the sample was heated to 120 °C in vacuum for 30 min followed by N2 adsorption at 77 K (five points from 0.05 to 0.30 p/p0). Images of the polymer surface were made by scanning electron microscopy (SEM) on a JSM-6010LV from JEOL (Freising, Germany). Prior to measurements the samples were sputtered with gold and measurements were conducted at high vacuum with an excitation voltage of 15 kV. NMR spectra were recorded with a Avance DPX 300 MHz spectrometer from Bruker (Billerica, MA, USA) equipped with a 5 mm broadband probe at 25 °C. ATR-FTIR measurements were performed using a Spectrum 100 equipped with a Universal ATR Sampling Accessory with the software Spectrum (version 2.0.0.0) from PerkinElmer (Waltham, MA, USA). Prior to measurements, the samples were dried in a vacuum centrifuge for 24 h with the mode setting V-AQ. Six scans were carried out with a wavelength range of 4000–650 cm−1 and a resolution of 4 cm−1.
2.2. Method Validation
The HPLC-DAD method was validated according to the “Society of Toxicological and Forensic Chemistry” (GTFCh) to examine its applicability towards the analysis of phenolic acids [
48]. A linear regression model was established to investigate the linearity and precision of the detection method. Analyte stabilities during the HPLC-DAD measurements and storage period were monitored. Furthermore, the limit of detection (LOD) and limit of quantification (LOQ) were determined.
2.2.1. Linearity of Calibration
Two dilution series from 25 to 250 mg L−1 and 250 to 500 mg L−1 were prepared by diluting the standard stock solution with 20 vol.% MeOH in water. Three calibration sets for each dilution series were prepared and each standard concentration was measured three times.
2.2.2. Repeatability
Two phenolic acid standards (50 and 500 mg L−1) were measured using the same HPLC-DAD method and the same instrumentation for a period of 8 days. Each standard was therefore divided into three aliquots, stored at 4 °C and measured once a day.
2.2.3. Processed Sample Stability
Stabilities of phenolic acids at two concentrations (50 and 500 mg L−1) were investigated over a period of 19 h in the autosampler at 10 °C. Each standard was divided into three separate vials and measured every hour.
2.2.4. Long-Term Stability
Phenolic acid stabilities at two concentrations (50 and 500 mg L−1) were investigated for 14 days at 4 °C and 23 °C. Therefore, each standard was divided into six separate vials. Three aliquots were stored at 4 °C and three at 23 °C. All standards were measured once a day.
2.2.5. LOD and LOQ
A calibration model ranging between 0.25 and 1.50 mg L
−1 was established by diluting the standard stock solution (500 mg L
−1) with 20 vol.% MeOH in water. Three identical dilution series were prepared, and each standard concentration was measured three times. The LOD and LOQ were calculated according to DIN 32645:2008-11 [
49].
2.3. Synthesis of 3,3′-(Hexane-1,6-diyl)bis(1-vinylimidazolium) Bromide
The dicationic crosslinker, 3,3′-(hexane-1,6-diyl)bis(1-vinylimidazolium) bromide [C
6-bis-VIM] [Br], was synthesized according to Wang et al. [
40]. For the implementation, 1-vinylimidazol (4.2 mL, 47 mmol) and 1,6-dibromohexane (3.2 mL, 21.2 mmol) were dissolved in 10 mL of MeOH. The mixture was stirred at 60 °C for 24 h. Subsequently, 100 mL EtOAc was added to precipitate the product. After cooling the mixture to 4 °C, the solid was filtered off, washed with 20 mL EtOAc three times, and dried under high vacuum to a constant weight. Yield: 7.9 g (86%) white, crystalline powder—
1H NMR (300 MHz, DMSO): δ 9.73 (s, 2H), 8.26 (s, 2H), 8.01 (s, 2H), 7.34 (dd,
J = 15.7, 8.8 Hz, 2H), 6.01 (d,
J = 15.6 Hz, 2H), 5.42 (d,
J = 8.7 Hz, 2H), 4.23 (t,
J = 7.2 Hz, 4H), 1.85 (s, 4H), 1.31 (s, 4H).
2.4. Synthesis of Crosslinked Vinylimidazole Polymers
The synthesis of vinylimidazole-based co-polymers was achieved according to a published article from the literature [
42]. For the implementation of the free-radical polymerization procedure, certain quantities of VIM (0.77 mL, 8.50 mmol), 1-propanol (2.24 mL, 37.26 mmol), 1-decanol (0.48 mL, 3.05 mmol), water (1.20 mL, 66.72 mmol) and the crosslinker (1.85 mmol) were mixed in a sealable amber glass vial. Subsequently, the initiator AAPH (0.121 g, 0.45 mmol) was added and the mixture ultrasonified. After flushing with nitrogen for 10 min, the vial was placed into an oil bath at 70 °C for 24 h. In the next step, the obtained monolith was grinded using a mortar and pestle and washed with water, MeOH and Et
2O. Subsequently the white powder was dried in a vacuum drying oven at 30 °C (150 mbar) until a constant weight.
Three differently crosslinked polymers were synthesized with the crosslinkers [C6-bis-VIM] [Br] (0.800 g, 1.85 mmol), EGDMA (0.35 mL, 1.85 mmol) and DVB (0.26 mL, 1.85 mmol) according to the previously described procedure, keeping the same monomer to crosslinker molar ratio of 4.59 to 1.
Furthermore, several poly(n-VIM/C
6-bis-VIM) polymers were prepared by changing the ratio between the monomer and crosslinker, by using the described polymerization procedure above. The molar sum of the monomer and crosslinker (10.35 mmol) was kept constant, whereas their molar ratio was varied between 1.15 to 1 and 13.80 to 1. All synthesized poly(n-VIM/C
6-bis-VIM) polymers are summarized in
Table S1. At the monomer to crosslinker molar ratios below 2.30 to 1, 100 µL ACN was added to the mixture before sonification to avoid phase separation.
2.5. DSPE Protocol for Phenolic Acids
For the extraction of phenolic acids from aqueous samples, a four-step DSPE protocol was established including sorbent conditioning, sample loading, washing and analyte elution. In the first step, 10.0 ± 0.2 mg of solid sorbent was weighed into a 2 mL centrifuge tube. Subsequently, 0.5 mL of conditioning solution was added, and the mixture shaken on a thermomixer (5 min, 1200 rpm, 25 °C). After centrifugation (5 min, 14,000 rpm, 23 °C) the conditioning solution was discarded, and the polymer washed with 1 mL of 5 vol.% MeOH in a water solution by applying the same shaking and centrifugation settings as before. An amount of 1 mL of the sample was added, and the mixture shaken (15 min, 1200 rpm, 25 °C) and centrifuged (5 min, 14,000 rpm, 23 °C) with the supernatant analyzed by HPLC-DAD. The residual sorbent was washed with 1 mL of 5 vol.% MeOH in a water solution. The analyte elution was obtained by adding 1 mL of eluting solution and shaking (15 min, 1200 rpm, 25 °C). After centrifugation (5 min, 14,000 rpm, 23 °C) the supernatant was analyzed.
To maximize the phenolic acid recoveries, the conditioning and eluting solution were optimized. Furthermore, the effect of the extraction time on the adsorption equilibrium was investigated to minimize the duration of the extraction process. These DSPE experiments were performed with the poly(n-VIM/C6-bis-VIM) sorbent with a monomer to crosslinker ratio of 11.50 to 1.
2.5.1. Optimization of the Conditioning Solution
Five different conditioning solutions were applied including a solution of 5 vol.% MeOH in water containing 0.5% (
w/
w) LiCl, three phosphate buffers with different pH values (6.6, 7.0 and 7.7) and a 5 vol.% MeOH in water solution. All applied conditioning solutions are summarized in
Table S2. The experiments were performed according to the four-step DSPE protocol in
Section 2.5. The loading was performed with 1 mL of 500 mg L
−1 phenolic acid standard mix. After shaking and centrifugation the supernatant was analyzed.
2.5.2. Optimization of the Eluting Solution
The elution was optimized by testing various eluting solutions containing different quantities of TFA, LiCl, ACN and water. All applied eluting solutions are provided in
Table S3. DSPE experiments were carried out according to
Section 2.5. The sorbent was conditioned with a 0.5 mL phosphate buffer (pH 7.0) and the loading was performed with 1 mL of 500 mg L
−1 phenolic acid standard mix. After washing, 1 mL of the eluting solution was added, the mixture shaken, centrifuged, and subsequently analyzed.
2.5.3. Effect of the Extraction Time
To investigate the effect of the extraction time on the adsorption equilibrium, analyte extraction was performed for different time periods (1.0, 2.5, 5.0, 10.0 and 20.0 min). DSPE experiments were carried out according to
Section 2.5. The conditioning was performed with a 0.5 mL phosphate buffer pH 7.0 and the sample consisted of 1 mL of 500 mg L
−1 phenolic acid standard mix. The obtained supernatant after loading was analyzed.
2.6. Effect of the Crosslinker on DSPE Efficiency
To compare the differently crosslinked polymers with each other, the extraction experiments were performed according to
Section 2.5. As a solid sorbent, polymers with a monomer to crosslinker ratio of 4.59 to 1 were used. The conditioning was carried out with a 0.5 mL phosphate buffer pH 7.0 and loading with 1 mL of 500 mg L
−1 phenolic acid standard mix. The elution was performed with 1 mL 2 vol.% TFA, 1.5% (
w/
w) LiCl and 50 vol.% ACN in water. The supernatant after loading and the eluting solution were both analyzed.
2.7. Effect of the Monomer to Crosslinker Ratio on DSPE Efficiency
The effect of polymer composition on the extraction efficiency was investigated by performing DSPE experiments with poly(n-VIM/C
6-bis-VIM) polymers with different monomer to crosslinker ratios. DSPE experiments were carried out according to
Section 2.5. The conditioning was performed with a 0.5 mL phosphate buffer pH 7.0 and loading with 1 mL of 500 mg L
−1 phenolic acid standard mix. The elution was carried out with 1 mL 2 vol.% TFA, 1.5% (
w/
w) LiCl and 50 vol.% ACN. The supernatant after loading and the eluting solution were analyzed.
2.8. Sorption Capacity
Different standard concentrations (10, 20, 50, 100, 250 and 500 mg L
−1) were applied during the sample loading to investigate the effect of the concentration on the extraction recovery. The DSPE experiments were performed according to
Section 2.5 with the poly(n-VIM/C
6-bis-VIM) sorbent with a monomer to crosslinker ratio of 11.50 to 1. The elution was performed with 1 mL 2 vol.% TFA, 1.5% (
w/
w) LiCl and 50 vol.% ACN in water. The supernatant after loading and the eluting solution were subsequently analyzed.
2.9. Polymer Reusability
The reusability of the poly(n-VIM/C
6-bis-VIM) polymer with a monomer to crosslinker ratio of 11.50 to 1, was investigated by performing several DSPE cycles in succession. Firstly, 10.0 ± 0.2 mg of solid sorbent was weighed into a 2 mL centrifuge tube. Sorbent conditioning and sample loading were performed according to
Section 2.5. with a 0.5 mL phosphate buffer pH 7.0 and 1 mL of 100 mg L
−1 phenolic acid standard mix. The sorbent was subsequently washed four times with 1 mL of 2 vol.% TFA, 1.5% (
w/
w) LiCl and 50 vol.% ACN in water and once with 1 mL of pure MeOH, with shaking (5 min, 1200 rpm, 25 °C) and centrifuging (5 min, 14,000 rpm, 23 °C) after every step. Finally, the polymer was dried at 60 °C and reused for the next DSPE cycle. The described procedure was carried out for a total of five times.
2.10. Sorbent Comparison Study
The poly(n-VIM/C6-bis-VIM) polymers with a monomer to crosslinker ratio of 11.50 to 1 was compared with the commercially available anion exchange sorbent Oasis® MAX from WatersTM. Therefore, the SPE cartridges were equipped with a polyethylene frit (20 µm) and 30.0 ± 0.2 mg of poly(n-VIM/C6-bis-VIM) was added. The Oasis® MAX was purchased as pre-packed cartridges containing 30.0 mg of solid sorbent.
Experiments were conducted with an adjustable air pressure machine using a SPE procedure similar to the DSPE procedure described in
Section 2.5. Firstly, the sorbent was conditioned with 0.5 mL of phosphate buffer (pH 7.0) and 1 mL 5 vol.% MeOH in water. Subsequently, 1 mL of phenolic acid standard mix (100 mg L
−1) was added. Then the sorbent was washed once with 1 mL 5 vol.% MeOH in water. Elution was accomplished by applying 1 mL of 1.5% (
w/
w) LiCl and 50 vol.% ACN in water.
In addition, the recommended SPE standard protocol for Oasis
® MAX from Waters
TM was also tested [
50]. In this case, the conditioning was performed with 1 mL MeOH and 1 mL water, loading with 1 mL of phenolic acid standard mix (100 mg L
−1) and washing with 1 mL 5 vol.% NH
3 in water and 1 mL MeOH. The elution of analytes was performed with 2 vol.% FA in MeOH.
The solutions obtained after loading and elution were centrifuged (5 min, 14,000 rpm, 23 °C) and an aliquot of the supernatants was subsequently analyzed.
3. Results and Discussion
A validated HPLC-DAD method was established for the quantification of different phenolic acids from the aqueous samples. In order to assess the accuracy, reliability and repeatability of the obtained data, SPE experiments and HPLC-DAD measurements were all carried out at least in triplicate.
3.1. Method Validation
The HPLC-DAD method was successfully validated and included linearity of calibration, repeatability, processed sample stability, long-term stability as well as the calculation of LOD and LOQ.
Both calibration ranges (25–250 mg L
−1 and 250–500 mg L
−1) showed a linearity in the examined concentration range with coefficient of determinations ranging from 0.9995–0.9999 and 0.9959–0.9985, respectively. For the calibration model 25–250 mg L
−1, the maximum relative standard deviation (RSD) value was 2.2%, the minimum bias −5.4 and the maximum bias 6.1%. The RSD as well as minimum and maximum bias for the calibration model 250–500 mg L
−1, were 0.9, −3.3 and 2.0%, respectively. All results are summarized in
Tables S4 and S5.
Repeatability of the applied HPLC-DAD method was investigated by calculating the interday and intraday RSD values over a period of eight days. All RSD values were far below 15%, demonstrating repeatability of the applied HPLC-DAD method. All results are given in
Tables S6 and S7.
Processed sample stability results showed that the concentration did not significantly change during a measurement period of 19 h at 10 °C in the autosampler. The maximum deviations from the initial value were ranging between 1.1 and 2.4%, respectively.
The long-term stability was investigated and showed that all phenolic acids at a concentration of 50 and 500 mg L−1 were stable for at least 14 days at 4 °C as well as 23 °C. The maximum deviations from the initial value at 4 °C were ranging from 0.9–1.8% and at 23 °C from 1.2–5.2%.
The LOD and LOQ were calculated according to DIN 32645:2008-11 and gave values ranging from 0.02–0.04 mg L
−1 and 0.08–0.12 mg L
−1, respectively. All results are given in
Table S8.
3.2. DSPE Method Optimization
An efficient DSPE protocol for the extraction of phenolic acids was established by optimizing the extraction time, the conditioning and eluting solution. Subsequently, the EGDMA, DVB and [C6-bis-VIM] [Br] crosslinked vinylimidazole polymers were compared regarding their phenolic acid extraction efficiency. It was observed that the type of crosslinker had a significant influence on the extraction recoveries. Furthermore, the influence of the monomer to crosslinker ratio of the poly(n-VIM/C6-bis-VIM) polymer on the extraction efficiency was investigated.
It was shown that by using a phosphate buffer for the conditioning, a greater percentage of analytes could be bound to the polymer than by using an aqueous solution containing 0.5% LiCl (
w/
w) and 5 vol.% MeOH or a solution of 5 vol.% MeOH in water. All results are summarized in
Table S2. In particular, the CaffA, FerA and CinA were strongly affected by the type of conditioning solution. This can probably be explained by their similar pKa values, which are significantly higher compared with ChlorA and DHB.
The amount of bound CaffA, FerA and CinA could be increased by 3.2, 6.2 and 7.7%, respectively, when using a phosphate puffer (pH 7.0) instead of 5 vol.% MeOH in water. The effect of conditioning pH on the bonding of phenolic acids was tested between 6.0 to 7.7. Higher pH values were avoided due to the instability of phenolic acids [
51].
With increasing pH value of the phosphate buffer, the adsorption of CaffA, FerA and CinA slightly increased, whereby this trend was more pronounced for FerA and CinA than for CaffA; however, the difference between pH 7.0 and 7.7 was not very pronounced. Based on the knowledge that phenolic acids can degrade at basic pH, a phosphate buffer with pH 7.0 was selected as the conditioning solution for further DSPE experiments.
The adsorption equilibrium was investigated to minimize the duration of the extraction process during the DSPE experiments. All results are given in
Table S9. As can be shown in
Figure 1 the quantity of bound analytes did not significantly change after 10 min. We decided to use an extraction time of 15 min for following the DSPE experiments to be certain that a complete adsorption equilibrium was established.
In order to optimize the elution of analytes from the solid sorbent, different eluting conditions were applied. In the preliminary experiments, MeOH and ACN with and without different concentrations of TFA were tested. Furthermore, the addition of NaCl and LiCl was investigated. It was found that higher recoveries could be achieved with ACN compared to MeOH and LiCl compared to NaCl. By adding water to the organic solvent, the solubility of the LiCl was improved and the analyte recoveries could further be increased. Based on these results, six different eluting solutions containing various quantities of TFA, LiCl, ACN and water were tested. The highest recoveries for all analytes (except DHB) could be obtained with 2 vol.% TFA, 1.5% (
w/
w) LiCl and 50 vol.% ACN in water (
Figure 2). All results are provided in
Table S3.
3.3. Effect of the Crosslinker on DSPE Efficiency
The effect of differently crosslinked polymers on the extraction efficiency towards phenolic acids is displayed in
Figure 3 and the results are summarized in
Table S10. All solid sorbents exhibited different percentage recoveries indicating the significant influence of the crosslinker. All sorbents showed the same systematic increase in recoveries following the order: CinA ˂ FerA ˂ CaffA ˂ ChlorA ˂ DHB. This circumstance could be due to the common basic structure of all three polymers, which is due to the use of the same monomer VIM. The reason why the extraction recoveries followed this particular order can probably be explained by the different pKa values. The lower the pKa, the stronger the acid, which means that the substance dissociates more strongly in water. Therefore, DHB and ChlorA with the lowest pKa values should also exhibit the highest proportion of dissociated molecules in anionic form. Since the sorbent used is an anion exchanger, more analytes could be bound to the sorbent via ionic interactions compared to the other phenolic acids. This consequently also led to higher extraction recoveries. CaffA, FerA and CinA have similar pKa values, however they exhibited different extraction recoveries. A reason could be the different number of hydroxyl groups in their chemical structures. Occurring hydrogen bonds may increase the adsorption force between the phenolic acids and sorbent, which could explain the previously stated order of recoveries.
The EGDMA crosslinked polymer exhibited the lowest extraction recoveries (52.4–85.3%) for all phenolic acids except the CinA. This can probably be explained by the absence of an aromatic moiety. Significantly better recoveries (71.9–95.1%) were obtained with the DVB crosslinked polymer. It was assumed that the EGDMA crosslinked polymer would be better suited for the extraction of phenolic acids due to its more polar structure and the possibility of forming hydrogen bonds. On the contrary, DVB has an aromatic ring structure, which enables the formation of π-π interactions with phenolic acids. This was probably the main reason for the higher recoveries with the DVB crosslinked polymer.
The polymer synthesized with [C6-bis-VIM] [Br] showed promising recovery results especially for DHB, ChlorA and CaffA, ranging between 91.1 and 99.3%. In contrast, the recoveries for FerA (71.4%) and CinA (50.8%) were significantly lower. In comparison to the EGDMA crosslinked polymer, much higher recoveries were obtained for all analytes except the CinA. In detail, 10.2% higher recoveries were obtained for FerA, 13.8% for ChlorA, 14.0% for DHB and 17.9% for CaffA. Only the recovery for CinA was 1.7% lower. Compared to the sorbent synthesized with DVB, the crosslinked [C6-bis-VIM] [Br] polymer showed significantly higher recoveries for ChlorA, CaffA and DHB. Slightly lower recoveries were found for FerA, and much lower recoveries were obtained for CinA. It is very likely that FerA and CinA, due to their higher pKa values, were mainly present as uncharged species and therefore were more affected by the non-polar interactions of the DVB. In summary, the recovery with poly(n-VIM/C6-bis-VIM) in comparison to the DVB crosslinked polymer for ChlorA was higher by 3.4%, for DHB by 4.2% and for CaffA by 5.6%. In contrast, the recoveries were 3.4% smaller for FerA and 21.1% for CinA.
Phenolic acid loading capacities (q) of the three differently crosslinked vinylimidazole polymers were calculated according to the following equation:
where c
i is the initial loading concentration, c
eq the concentration at equilibrium, V the sample volume and m the dry mass of the applied polymer. From the summarized results in
Table 1, it is evident that the type of crosslinker had a significant influence on the loading capacities of the different polymers. The EGDMA crosslinked sorbent exhibited the lowest capacities for all analytes except CinA. Polymers with the crosslinker DVB showed very high loading capacities for all phenolic acids. High capacities for ChlorA, CaffA and DHB were also obtained with the [C
6-bis-VIM] [Br] crosslinked polymer.
It is noteworthy that despite the lower loading capacities of poly(n-VIM/C6-bis-VIM) compared to the DVB crosslinked polymer, higher recoveries could be achieved for DHB, ChlorA and CaffA. This circumstance can possibly be explained by the more complete elution when using the [C6-bis-VIM] [Br] crosslinked polymer.
Furthermore, we investigated the effect of the molar ratio between VIM and [C
6-bis-VIM] [Br] on the extraction efficiency of phenolic acids. The monomer to crosslinker molar ratio was varied between 1.15 to 1.00 and 13.80 to 1.00. All applied molar ratios and results are summarized in
Table S1. It was apparent that analyte recoveries were dependent on the monomer to crosslinker ratios (
Figure 4), especially the recoveries of FerA and CinA that strongly increased with increasing monomer content, whereby a maximum was reached at a monomer to crosslinker ratio of 11.50 to 1. ChlorA and CaffA showed a similar behavior; however, the increase in recovery was not as pronounced. The DHB showed a different recovery pattern. With an increasing monomer content, the recoveries steadily decreased. In conclusion, the highest average recovery for the phenolic acids was obtained at a monomer to crosslinker ratio of 11.50 to 1 with the analyte recoveries ranging between 74.5 and 92.7%. Therefore, subsequent DSPE experiments were solely performed with this specific polymer.
By optimizing the VIM to crosslinker ratio, all loading capacities could consequently be significantly increased. The optimized [C6-bis-VIM] [Br] crosslinked polymer with a molar ratio of 11.50 to 1 exhibited the highest average loading capacity for phenolic acids. Compared to the EGDMA and DVB crosslinked polymers, higher capacities for all analytes except the CinA were achieved with this specific composition.
3.4. Sorption Capacity
DSPE experiments were performed with different loading concentrations (10–500 mg L
1) to investigate the influence of the standard concentration on the extraction efficiency. Results with the poly(n-VIM/C
6-bis-VIM) polymer with a monomer to crosslinker ratio of 11.50 to 1 are displayed in
Figure 5 and given in
Table S11.
It was observed that the concentration of phenolic acids in the sample had a direct influence on extraction recoveries. At a concentration of 10 mg L
−1 no phenolic acids were detected in the supernatant, indicating a quantitative binding; however, the highest recoveries could not be obtained in this particular case. It is possible that there existed a limited number of binding sites on the polymer that have very strong adsorption properties towards phenolic acids. Hence, a small proportion of analytes was strongly bound and was not eluted under the applied conditions. As a result, they remained attached to the polymer and significantly reduced the extraction recoveries. At analyte concentrations of 20, 50 and 100 mg L
−1, only CinA was detected in the supernatant and at 250 mg L
−1 all analytes were detected except for ChlorA. In contrast, at 500 mg L
−1 all analytes were visible in the chromatogram. The extraction recoveries obtained from the DSPE experiments with the highest sample concentration (500 mg L
−1) were limited by the loading capacity of the sorbent. Depending on the type of phenolic acid, 1.4 to 19.0% of the loaded concentration remained dissolved in the supernatant. These findings are in accordance with the results from
Section 3.3, as the binding capacities were less than 50 mg g
−1 polymer, which indicated an overloading of the sorbent. Even though a high proportion of the bound analytes could be eluted from the polymer, the recoveries were still lower than at other concentration levels. The optimal concentration range for the extraction of phenolic acids with 10.0 ± 0.2 mg of sorbent was between 20 and 250 mg L
−1, with the highest average recovery observed at 100 mg L
−1.
3.5. Polymer Reusability
The reusability of the poly(n-VIM/C
6-bis-VIM) polymer with a monomer to crosslinker ratio of 11.50 to 1 was investigated by performing multiple DSPE cycles. Therefore, after each loading step the sorbent was regenerated, dried, and reused. In
Figure 6, the quantity of bound phenolic acids after one to five DSPE cycles is displayed. Further data is given in
Table S12. The applied polymer showed excellent reusability results for DHB with a maximum adsorption decrease of 0.6%. Good reusability results were also obtained for the ChlorA and CaffA with a maximum decrease in adsorption of 5.3 and 13.4%, respectively. A considerable decrease in the adsorption performance was observed for FerA and CinA with a maximum decrease of 30.7 and 50.3%, respectively. It is noteworthy that the quantity of bound ChlorA, CaffA, FerA and CinA mainly decreased after the first DSPE cycle and remained constant after the subsequent cycles. It is possible that the washing solution containing LiCl salt was responsible for this trend by influencing the binding properties of the sorbent. Since the FerA and CinA generally exhibited the weakest binding strength, they were probably the most affected by this effect. By optimizing the washing solution, it is likely to also result in a better reusability for FerA and CinA.
In summary, it was shown that the [C6-bis-VIM] [Br] crosslinked polymer could be regenerated and reused for the majority of analytes making it a good candidate regarding sustainable analytical sample preparation.
3.6. Sorbent Comparison Study
The extraction efficiency of the novel poly(n-VIM/C
6-bis-VIM) polymer with a monomer to crosslinker ratio of 11.50 to 1 was evaluated by comparing it with Oasis
® MAX from Waters
TM. Oasis
® MAX is a strong mixed-mode anion exchange and reversed-phase polymeric sorbent with a quaternary amine as the anion exchange function. Waters
TM recommends it for the separation of acidic compounds [
50]. Therefore, it was selected as a comparative polymer in this study.
Using the developed SPE protocol, extraction recoveries above 90% could be obtained with both sorbents. In addition, no phenolic acid residues were detected in the supernatants. Significantly higher extraction recoveries for all analytes were found with the synthesized sorbent in comparison with the Oasis
® MAX. The poly(n-VIM/C
6-bis-VIM) polymer gave recovery results ranging between 97.2 and 98.5%, which were on average 5% higher than with the commercial solid sorbent. All results are given in
Table 2.
Since quantitative adsorption was achieved for both polymers, the differences in analyte recovery can only be due to differences in elution efficiency. Either the binding of phenolic acids with Oasis® MAX was too strong or the eluting conditions too weak to obtain higher recovery results.
Results with the recommended SPE standard protocol from WatersTM showed lower extraction recoveries for all phenolic acids compared to the developed protocol in this study. Furthermore, two new signals appeared in the chromatogram and the background noise increased considerably. It is very likely that the phenolic acids degraded during the washing step, due to the use of an ammonia solution and the instability of phenolic acids under alkaline conditions.
It has to be mentioned that the backpressure during the SPE experiments with the poly(n-VIM/C6-bis-VIM) polymer was very high probably due to swelling effects. Therefore, the use of DSPE was clearly at an advantage in comparison to conventional SPE.
The developed DSPE and adapted SPE procedure with the poly(n-VIM/C
6-bis-VIM) polymer were compared with different solid-phase extraction methods and sorbents described in the literature (
Table 3). It could be shown that the novel resin can compete with commercial sorbents as well as other synthesized SPE materials.
3.7. Polymer Characterization
The poly(n-VIM/C
6-bis-VIM) polymer with a monomer to crosslinker ratio of 11.50 to 1 was investigated regarding its specific surface area and surface structure. The BET measurement of the sample gave a specific surface area of 49.3 m² g
−1. The adsorption and desorption isotherm are provided in
Figure S1. Additionally, the pore size distribution calculated from the desorption branch (20 points from 0.95 to 0.3 p/p
0) of the isotherm is given in
Figure S2. The results showed that the pore size distribution ranges between 25 and 50 Å with a maximum at 35 Å. The SEM measurements provided an image of the surface structure of the co-polymer, which is given in
Figure S3. The surface is composed of many small, round, spherical components that were all about the same size. No macroscopic structural elements were observed. The recorded ATR-FTIR spectra (
Figure S4) confirmed the presence of the functional groups incorporated by the monomer and the crosslinker. The following signals in the spectra of poly(n-VIM/C
6-bis-VIM) were observed and assigned: 3083 cm
−1 N-H stretching; 2932 cm
−1 C-H stretching (aromatic); 2859 cm
−1 C-H stretching (aliphatic); 1553 cm
−1 C-N stretching; 1414 cm
−1 C-H stretching (ring); 1284 cm
−1 C-H in plane bending; 1227 cm
−1 C-H in plane bending and C-N stretching; 1156 cm
−1 C-N (aliphatic); 1083 cm
−1 C-H in plane bending (ring) and C-C stretching; 908 cm
−1 C=C bending and stretching; 818 cm
−1 C-H bending (ring); 740 cm
−1 C-H bending (ring); and 662 cm
−1 N-C stretching (ring). Especially the signal at 3083 cm
−1 is of importance as it is the characteristic frequency of imidazolium salts. Furthermore, a signal at 1176 cm
−1 was observed in the spectra of the crosslinker, which was also found in the spectra of the polymer (1156 cm
−1) and not in the spectra of the VIM. The obtained results are consistent with the structural analysis of Lippert et al. and the IR spectrum table from Merck [
52,
53].
4. Conclusions
The synthesis and optimization of new polymers is an important branch of research that has been intensively pursued for years. This has allowed SPE to be continuously improved and advanced, making it by far the most important method in analytical sample preparation. Nowadays, there is a wide range of different polymers available and every year new, even more efficient sorbents are developed and manufactured.
The preparation of novel SPE materials is feasible in particular due to the huge variety of existing monomers and crosslinkers. In this study we focused on the effect of the type of crosslinker and the ratio between the monomer and crosslinker on the extraction efficiency toward phenolic acids. We synthesized and used [C6-bis-VIM] [Br] as a crosslinker for the preparation of porous polymers. Subsequently, sorbents with the commonly used crosslinkers EGDMA and DVB were prepared and compared with the poly(n-VIM/C6-bis-VIM) polymer. It was found that the type of crosslinker had a significant influence on the adsorption properties of the polymer. This finding strongly emphasizes the importance of selecting suitable crosslinkers for the preparation of efficient SPE materials. It was observed that polymers prepared with the commonly used crosslinkers EGDMA, or DVB exhibited lower extraction recoveries for the majority of phenolic acids.
The developed DSPE procedure for the extraction of phenolic acids represents an efficient analytical sample preparation method. It is simple and fast with maximum recoveries ranging between 84.1 and 92.5%. It was also shown that the DSPE procedure gave reproducible results, as the RSD values were very low. In addition, the developed extraction procedure was adapted to cartridges. No residual phenolic acids could be found in the supernatant and the extraction recoveries were ranging between 97.2 and 98.5%. It was demonstrated that the poly(n-VIM/C6-bis-VIM) polymer could also compete with the commercially available sorbent Oasis® MAX from WatersTM. Significantly, higher extraction recoveries for all analyzed phenolic acids were obtained, which were on average 5% higher than with Oasis® MAX.
Furthermore, it was demonstrated that the poly(n-VIM/C6-bis-VIM) polymer could be regenerated and reused for the majority of analytes. Therefore, the novel sorbent is a good contender for its use in sustainable analytical sample preparation. This aspect is of great significance, as the minimization of resources is becoming more and more important in the development of new sample preparation methods.
Due to the ionic properties of [C6-bis-VIM] [Br], it is well suited for the preparation of anion exchange sorbents. Due to its physicochemical properties, [C6-bis-VIM] [Br] is not only an excellent constituent for the preparation of polymers but also because its synthesis is facile and inexpensive with high yields. Therefore, [C6-bis-VIM] [Br] could present a good alternative to conventional crosslinkers such as EGDMA and DVB, which exhibited different properties when incorporated into vinylimidazole polymers. In conclusion, this study clearly highlights the yet untapped potential of the crosslinker [C6-bis-VIM] [Br] with respect to the synthesis of new anion exchange polymers for their application in the field of SPE.