Simultaneous Determination of Methyl Nicotinate and Three Salicylic Acid Derivatives in Pain Relief Spray Using HPLC–DAD
Abstract
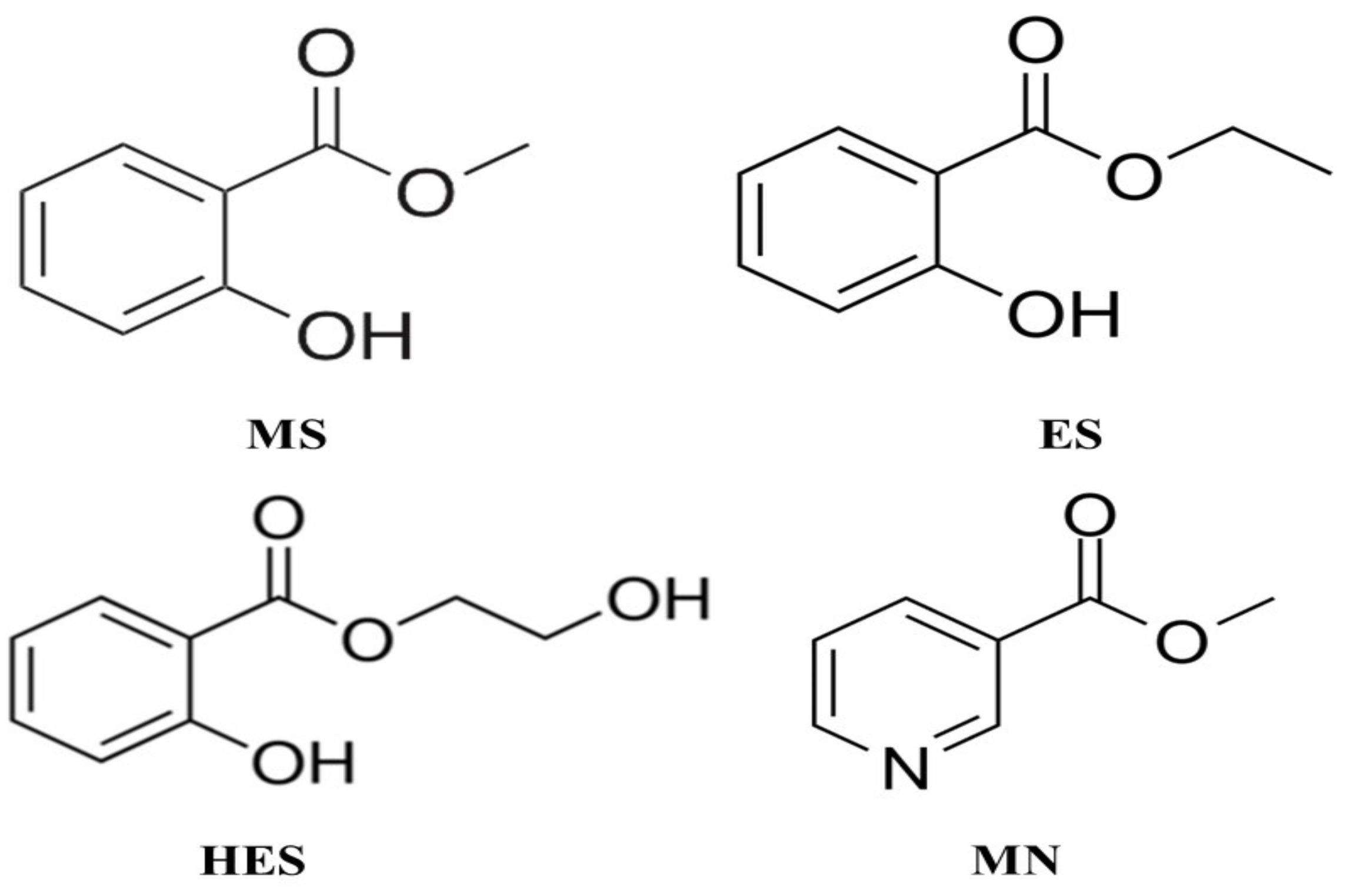
:1. Introduction
2. Materials and Methods
2.1. Instrument
2.2. Chemicals and Materials
2.3. Preparation of Stock Solutions
2.4. Preparation of Spray Solution
2.5. Chromatographic Conditions
2.6. Validation of Assay Approach
3. Result
3.1. Development and Optimization Processes
3.2. Method Validation
3.2.1. Linearity and Calibration Curve
3.2.2. LOD and LOQ Assessment
3.2.3. Accuracy and Precision
3.2.4. System Suitability Testing (SST)
3.3. Application of the Method
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mason, L.; Moore, R.A.; Derry, S.; Edwards, J.E.; McQuay, H.J. Systematic Review of Topical Capsaicin for the Treatment of Chronic Pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauwels, J.; D’Autry, W.; Van den Bossche, L.; Dewever, C.; Forier, M.; Vandenwaeyenberg, S.; Wolfs, K.; Hoogmartens, J.; Van Schepdael, A.; Adams, E. Optimization and Validation of Liquid Chromatography and Headspace-Gas Chromatography Based Methods for the Quantitative Determination of Capsaicinoids, Salicylic Acid, Glycol Monosalicylate, Methyl Salicylate, Ethyl Salicylate, Camphor and l-Menthol in A. J. Pharm. Biomed. Anal. 2012, 60, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D.; Whitehouse, M.W. Anti-Inflammatory/Anti-Pyretic Salicylic Acid Esters with Low Gastric Ulcerogenic Activity. Agents Actions 1980, 10, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Miles, S. Methyl Salicylate. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–6. ISBN 978-0-08-055232-3. [Google Scholar]
- Michel, P.; Granica, S.; Magiera, A.; Rosińska, K.; Jurek, M.; Poraj, Ł.; Olszewska, M.A. Salicylate and Procyanidin-Rich Stem Extracts of Gaultheria Procumbens L. Inhibit Pro-Inflammatory Enzymes and Suppress Pro-Inflammatory and Pro-Oxidant Functions of Human Neutrophils Ex Vivo. Int. J. Mol. Sci. 2019, 20, 1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horak, J.; Hemmer, W.; Focke, M.; Götz, M.; Jarisch, R. Contact Dermatitis from Anti-Inflammatory Gel Containing Hydroxyethyl Salicylate. Contact Dermat. 2002, 47, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Kučera, M.; Kolar, P.; Barna, M.; Kučera, A.; Hladiková, M. Arnica/Hydroxyethyl Salicylate Combination Spray for Ankle Distortion: A Four-Arm Randomised Double-Blind Study. Pain Res. Treat. 2011, 2011, 365625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Spiegeleer, B.; Van den Bossche, W.; De Moerloose, P.; Stevens, H. Determination of Methyl Nicotinate in Pharmaceutical Creams by High-Performance Thin-Layer Chromatography. Chromatographia 1985, 20, 249–252. [Google Scholar] [CrossRef]
- Jumbelic, L.C.; Liebel, F.T.; Southall, M.D. Establishing a Minimal Erythema Concentration of Methyl Nicotinate for Optimum Evaluation of Anti-Inflammatories. Skin Pharmacol. Physiol. 2006, 19, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Abounassif, M.A.; Abdel-Moety, E.M.; Gad-Kariem, R.A. HPLC-Quantification of Diethylamine Salicylate and Methyl Nicotinate in Ointments. J. Liq. Chromatogr. Relat. Technol. 1992, 15, 625–636. [Google Scholar] [CrossRef]
- Parker, D.; Martinez, C.; Stanley, C.; Simmons, J.; McIntyre, I.M. The Analysis of Methyl Salicylate and Salicylic Acid from Chinese Herbal Medicine Ingestion. J. Anal. Toxicol. 2004, 28, 214–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, T.; Mayersohn, M. Simultaneous Quantitative Analysis of Methyl Salicylate, Ethyl Salicylate and Salicylic Acid from Biological Fluids Using Gas Chromatography–Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 1998, 718, 69–75. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research, U.S. Food and Drug Administration, FDA. Reviewer Guidance, Validation of Chromatographic Methods; FDA: Silver Spring, MD, USA, 1994. [Google Scholar]

| Analyte | Regression Equation | R | Linear Range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| MN | Y = 0.968 x − 0.713 | 0.999 | 0.03–100 | 0.0144 | 0.0478 |
| HES | Y = 2.485 x + 0.315 | 0.996 | 0.05–50 | 0.0455 | 0.1516 |
| MS | Y = 3.151 x + 0.083 | 0.998 | 0.03–50 | 0.0087 | 0.0289 |
| ES | Y = 2.926 x + 0.579 | 0.998 | 0.03–50 | 0.0061 | 0.0204 |
| Analytes | Conc. Added (µg/mL) | Intra-Day | Inter-Day | ||||
|---|---|---|---|---|---|---|---|
| Conc. Found (µg/mL) ± SD | Recovery (%) | RSD (%) | Conc. Found (µg/mL) ± SD | Recovery (%) | RSD (%) | ||
| MN | 0.07 | 0.068 ± 0.002 | 97.25 | 2.672 | 0.067 ± 0.003 | 95.38 | 3.979 |
| 0.5 | 0.510 ± 0.008 | 101.91 | 1.653 | 0.496 ± 0.011 | 99.22 | 2.115 | |
| 1 | 1.007 ± 0.059 | 100.68 | 5.886 | 1.006 ± 0.035 | 100.62 | 3.519 | |
| 5 | 5.099 ± 0.194 | 101.97 | 3.810 | 5.018 ± 0.205 | 100.36 | 4.082 | |
| 20 | 19.853 ± 0.073 | 99.263 | 0.369 | 19.85 ± 0.061 | 99.23 | 0.301 | |
| HES | 0.07 | 0.068 ± 0.001 | 97.29 | 1.380 | 0.069 ± 0.004 | 97.95 | 6.341 |
| 0.5 | 0.508 ± 0.013 | 101.68 | 2.532 | 0.503 ± 0.023 | 100.59 | 4.571 | |
| 1 | 0.982 ± 0.049 | 98.180 | 5.028 | 1.012 ± 0.033 | 101.21 | 3.218 | |
| 5 | 4.80 ± 0.091 | 96.08 | 1.890 | 4.719 ± 0.211 | 94.38 | 4.467 | |
| 20 | 20.06 ± 0.180 | 100.28 | 0.897 | 19.614 ± 0.709 | 98.07 | 3.616 | |
| MS | 0.07 | 0.070 ± 0.001 | 100.50 | 1.421 | 0.069 ± 0.002 | 98.73 | 2.794 |
| 0.5 | 0.503 ± 0.007 | 100.61 | 1.445 | 0.473 ± 0.008 | 94.58 | 1.782 | |
| 1 | 1.021 ± 0.011 | 102.12 | 1.121 | 0.943 ± 0.011 | 94.27 | 1.129 | |
| 5 | 4.915 ± 0.034 | 98.29 | 0.683 | 4.674 ± 0.041 | 93.48 | 0.861 | |
| 20 | 19.982 ± 0.086 | 99.91 | 0.429 | 20.082 ± 0.092 | 100.41 | 0.461 | |
| ES | 0.07 | 0.068 ± 0.002 | 97.02 | 2.949 | 0.067 ± 0.002 | 96.23 | 2.786 |
| 0.5 | 0.510 ± 0.008 | 102.05 | 1.637 | 0.492 ± 0.005 | 98.47 | 0.971 | |
| 1 | 0.993 ± 0.018 | 99.33 | 1.838 | 1.008 ± 0.012 | 100.76 | 1.191 | |
| 5 | 7.828 ± 0.076 | 96.56 | 1.580 | 4.871 ± 0.046 | 97.39 | 0.951 | |
| 20 | 19.786 ± 0.154 | 98.93 | 0.776 | 19.871 ± 0.348 | 99.36 | 1.749 | |
| Parameters | Obtained Value | Reference Value [13] | |||
|---|---|---|---|---|---|
| MN | HES | MS | ES | ||
| Resolution (Rs) | 3.195 | 15.96 | 12.125 | 9.60 | <1.5 |
| Selectivity factor (α) | 1.16 | 1.17 | 1.41 | 1.37 | < 1 |
| Tailing factor(T) | 0.941 | 0.947 | 1.00 | 1.03 | >1.5–2 or >2 |
| Capacity factor(K′) | 1.05 | 1.30 | 1.82 | 2.50 | 1–10 acceptable |
| Column efficiency (n) | 8543 | 13877 | 17918 | 17479 | Increase with efficiency of the separation |
| HETP b | 0.002 | 0.001 | 0.0008 | 0.00008 | The smaller the value the higher the column efficiency |
| Component | Taken (µg/mL) | Recovery % |
|---|---|---|
| MN | 14.57 | 97.88 ± 0.01 |
| HES | 45.55 | 92.04 ± 0.56 |
| MS | 9.11 | 101.14 ± 0.13 |
| ES | 45.55 | 94.39 ± 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, H.M. Simultaneous Determination of Methyl Nicotinate and Three Salicylic Acid Derivatives in Pain Relief Spray Using HPLC–DAD. Separations 2022, 9, 93. https://doi.org/10.3390/separations9040093
Ali HM. Simultaneous Determination of Methyl Nicotinate and Three Salicylic Acid Derivatives in Pain Relief Spray Using HPLC–DAD. Separations. 2022; 9(4):93. https://doi.org/10.3390/separations9040093
Chicago/Turabian StyleAli, Hazim M. 2022. "Simultaneous Determination of Methyl Nicotinate and Three Salicylic Acid Derivatives in Pain Relief Spray Using HPLC–DAD" Separations 9, no. 4: 93. https://doi.org/10.3390/separations9040093
APA StyleAli, H. M. (2022). Simultaneous Determination of Methyl Nicotinate and Three Salicylic Acid Derivatives in Pain Relief Spray Using HPLC–DAD. Separations, 9(4), 93. https://doi.org/10.3390/separations9040093






